Abstract
Currently, there is a growing demand for ready-to-eat, prepared, high-quality, preservative-free products. However, the shelf-life of these products is often so short that a relatively high percentage of these products cannot be sold and end up as food waste. In this study, pork loin with different marinades (paprika and herbs) was treated with different high hydrostatic pressures (0 MPa, 300 MPa, 450 MPa and 600 MPa) and a bioactive component (piperine) and then the quality of the meat was examined after 0, 4, 8, 12 and 14 days of cold storage. Changes were monitored using color, pH, texture and microbiological analyses. Both pressure, piperine enrichment, storage time and the interactions of different factors had a significant effect on the quality of the loin samples with different marinades. Due to the denaturation of myoglobin, meat slices were less red and lighter after HHP treatment. The addition of piperine reduced this lightness. The pH increased with increasing pressure and decreased with storage time. HHP treatment significantly increased meat hardness, with samples treated at 600 MPa being 19% harder than those treated at 450 MPa. Microbiological results indicated that HHP at 450 MPa and 600 MPa effectively reduced anaerobic total live plate counts, ensuring satisfactory sensory and microbiological quality throughout storage. Piperine fortification also resulted in a more favorable microbiological status during storage without any perceptible change in quality properties during storage. These findings underscore the effectiveness of HHP and piperine enrichment in enhancing the safety and quality of marinated meats.
1. Introduction
One-third of food made for human consumption is lost or wasted worldwide according to the Food and Agricultural Organization (FAO) of the United Nations (UN) [1]. This food waste from households can be decreased by extending the shelf-life even by only a couple of days [2,3,4,5,6]. Efforts to increase shelf-life to reduce food wastage have already appeared in other studies [7,8,9]. So, for the purpose of sustainability, it is necessary to research and develop preservation technologies that meet modern consumer needs. Such preservation technologies include high-hydrostatic-pressure treatment and the addition of bioactive components instead of high-temperature heat treatment and the addition of artificial preservatives. This is particularly important for perishable foods such as pork.
Due to the nature of meat—high water and protein content—it is a highly perishable raw material [10]. Partly to increase shelf-life and comply with consumer expectations, meat preparations are made from fresh meat. “Meat preparations” means fresh meat, including meat that has been reduced to fragments, which has had foodstuffs, seasonings or additives added to it or which has undergone processes insufficient to modify the internal muscle fiber structure of the meat and thus to eliminate the characteristics of fresh meat according to Regulation 853/2004/EC and Regulation 854/2004/EC [11]. In addition to microbiological deterioration, physical deterioration has to be considered. Physical deterioration refers to those types of deterioration which are associated with a specific change in the meat composition (e.g., color deterioration). Long-term storage leads to a loss of muscle reductive capacity, resulting in a brownish metmyoglobin which increasingly determines the color of the meat [12]. The distance between the filaments that form myofibrils can be reduced by low pH and high temperature, which causes denaturation of myosin. These changes can lead to lightening of the meat by reducing the solubility of proteins. This is not beneficial because consumers choose food based on its nutritional and organoleptic qualities [13]. Consumers are increasingly aware and believe that food contributes directly to their health [14]. This explains the significant increase in demand for minimally processed foods [15]. In minimal processes, several areas have to be investigated, including not only food safety but also spoilage processes, physico-chemical changes and nutritional properties, where improvements are challenging [16]. High-hydrostatic-pressure (HHP) treatment is a nonthermal process that can inactivate and eliminate pathogenic and food spoilage microorganisms, thus improving food safety and extending product shelf-life while retaining the characteristics of fresh, preservative-free, minimally processed foods [17,18].
The traditional methods used to preserve meat include the following: chilling/refrigeration, freezing, drying, curing, smoking and thermal processing (cooking, baking or canning). These result in processed meat products. However, raw meat preservation techniques have long been used, such as the addition of essential oils or the addition of antioxidants (for instance, flavoring agents) [19]. The latter two are practically the equivalent of a marinade. In contrast to classical methods, there are minimal processing methods, which include the following: (1) thermal methods, such as sous vide processing, infrared heating, microwave heating and ohmic heating; (2) nonthermal methods, such as high-pressure processing, ionizing radiation, pulsed electric field, ultrasound and packaging; (3) natural antimicrobials, such as enzymes, lactoferrin, oleoresins, essential oils, herbal extracts, chitosan, etc.; and (4) the hurdle concept [20]. It can be seen that there is an overlap between classical preservation methods and minimal processing. However, minimal processing can only be defined as a method that preserves the quality of the raw material as much as possible with the least possible loss of nutrients [20].
The use of bioactive compounds is gaining increasing attention in scientific communities and among food manufacturers as well. The volatile terpenes, terpenoids and phenylpropanoids have been widely shown to have preservative effects: CARV, 1,8-cineole, eugenol, carvone, p-cymene, γ-terpinene, thymol, cinnamaldehyde, geraniol, menthol, chavicol, citral, estragole, terpineol, eugenyl acetate, geranyl acetate, citronellol and vanillin can be used effectively in a wide range of foods [21,22,23,24,25]. Their effects on increasing shelf-life, inhibiting lipid and protein oxidation and discoloration and inhibiting microbial growth in fresh meat, minced meat and fish have been observed. These are on the Council of Europe’s positive list, classified as Generally Recognized as Safe (GRAS) by the US Food and Drug Administration (FDA) and registered as a flavoring and food additive by the European Commission [26,27]. Some bioactive compounds may react with meat constituents (thiols, sulfhydryl groups or proteins), or the fat content of the meat may form a protective layer around the contaminant microorganism or bind the lipophilic part of the bioactive compound [28,29]. As with essential oils, bioactive compounds can be used alone or in combination with other bioactive compounds and/or a preservation method in meat, although it is important to carefully select the right type of bioactive compound and its concentration [30]. There is 2–7.4% of piperine in black pepper, which is one of the most widely used spices all over the world. Piperine has numerous pharmacological effects; it has been found to have antiproliferative, antitumor, antiangiogenesis, antioxidant, antidiabetic, antiobesity, cardioprotective, antimicrobial, antiaging and immunomodulatory effects in various in vitro and in vivo experimental trials [31]. Piperine has a proven antioxidant and antimicrobial effect [32]. A summary is given in Table 1 about the effects of investigated treatments.

Table 1.
Summary diagram about the important effects of HHP treatment and piperine addition.
HHP treatment and enrichment with bioactive components are innovative, minimal processing preservation methods that have not yet been investigated in combination with storage experiments on marinated meat. As meat preservation is especially important and meat is considered to be a relatively valuable raw material, it was appropriate to investigate the effect of HHP treatment and bioactive component addition on the properties of this material during storing. Thus, the aim of the research was to investigate the combination of a natural bioactive compound and different levels of HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) on pork meat with different marinades (paprika or herbs) to increase shelf-life. Different marinades have been studied because the changes to the raw meat are masked by the marinades, so more beneficial properties can be achieved besides those caused by the treatments. To examine texture, color, pH and microbiological characteristics, measurements of the meats were carried out during storage on days 0, 4, 8, 12 and 14.
2. Materials and Methods
2.1. Materials
Commercially available pork meat parts (SPAR Magyarország Kft., Bicske, Hungary) were used in this research. The country of birth, fattening and slaughtering of pigs (Sus scrofa domestica) was Hungary, a member of the European Union. The slaughtering of livestock and the deboning, cutting and vacuum-packaging of meat was carried out in an industrial factory. The samples came from different pigs. All samples were taken from the short loin of the pig. Due to its relatively homogeneous muscle structure and intersection, the short loin is one of the most favorable meat cuts for parallel measurements with variation of several parameters and components. It was essential for the measurements that the geometric dimensions of the samples were identical. To achieve this, the meat was cut into equal slices of 2 cm thickness. It was important that the samples were fresh meat from slaughter animals to determine the starting microbiology and pH (pH 5.56 ± 0.02) values. The loin chops were marinated in two different marinades to flavor them and increase their shelf-life. Marinades were commercially available fine spice oils from Almi (Almi, Oftering, Austria), which contain spices, salt and oil. Half of the samples were marinated with herbs and the other half with paprika. The ratio of the marinades was 10 g (100 g)−1 of the meat weight. To half of the samples, piperine (94-62-2) (Merck, Darmstadt, Germany), the main active ingredient of pepper, was added as a bioactive component in addition to the marinade in case of both flavorings. Samples prepared without the bioactive component and those enriched with the bioactive component were marked in the rest of the paper as follows: BA− and BA+. In the BA+ sample groups, the concentration of piperine was 5 × 10−4 g (100 g)−1. The piperine was mixed into the oil marinade.
2.2. High-Hydrostatic-Pressure Processing
Meat samples were treated at 300 MPa, 450 MPa and 600 MPa by high-hydrostatic-pressure (HHP) processing for 5 min at room temperature using RESATO FPU-100-2000 equipment with a vessel volume of 2 L (Resato International B.V., Assen, The Netherlands). The pressure-transmitting fluid was supplied by the manufacturer (Resato PG fluid, Assen, The Netherlands). The initial temperature of the pressure-transmitting fluid was 21 ± 0.5 °C. The pressure build-up rate was 100 MPa/min. The treatment time did not include the build-up and decompression times. The hydrostatic pressure level of 600 MPa is a generally applied pressure value in the industry and has a strong effect on both meat structure and microbiological parameters. The lower 300 MPa pressure value is considered a mild pressure treatment, minimally affecting the textural attributes and the protein structure of meat [33]. Control samples were not treated with HHP. They were later marked with a treatment value of 0 MPa. The sample groups and pressure treatments are shown in Table 2.

Table 2.
Summary diagram of sample groups which were stored for 0, 4, 8, 12 and 14 days.
2.3. Storage Experiment
The meat was tested on five measurement days (day 0, day 4, day 8, day 12 and day 14) following the pressure treatments, where day 0 is the day of pressure treatment. The samples were stored in vacuum packaging at 3 ± 1 °C until the day of the measurement, which was 4, 8, 12 or 14 days later. At least three parallel samples from each sample group were measured on each measurement day. The measuring points were chosen based on the observation that the shelf-life of fresh meat is usually four days, the shelf-life of vacuum-packed meat is usually seven days and the shelf-life of prepared vacuum-packed meat is usually 12 days in Central Europe, so it was relevant to measure any changes from these points. Two days were added to this to measure any changes beyond the average shelf-life.
2.4. Texture Measurement
The texture of the samples was examined by a Stable Micro System (SMS) TA.XT Plus texture analyzer (Stable Micro System, Godalming, Surrey, UK) equipped with a 50 kg force load cell. A 2 mm diameter needle probe (P/2N) was used for the puncture test. The direction of penetration was parallel to the fibers in the middle of the loin slices. The distance the probe moved in the sample was 10 mm, half the thickness of the sample. The test speed was 10 mm s−1. Both the pre-test and test speeds were 2 mm/s. The force was recorded as a function of distance. Two texture parameters were examined. (1) The hardness [N] was the maximum force measured during penetration [33]. (2) The penetration work [W] was the work performed up to the maximum distance of penetration. The work was examined because the maximum force exerted by the probe head may not be evaluated at maximum penetration due to the inhomogeneity of the biological samples, and therefore a better picture of the sample texture may be obtained by examining the work performed during full penetration. Six parallel samples were measured from all samples groups.
2.5. Color Measurement
A Minolta CR-400 (Konica Minolta, INC., Osaka, Japan) chroma meter was used for the reflection color measurement. The instrument measures the CIE Lab color characteristics. The ratio of three different wavelength lights was plotted in a coordinate system called CIE Lab color space. The color results were quantified by the device. The instrument was calibrated with a standard white etalon. Each sample (N = 6) was measured three times on a white plate with the same illumination. The measured attributes were as follows: redness/greenness (a*), yellowness/blueness (b*) and lightness (L*).
Hue angle (hab) was also an important color attribute, which was calculated according to Equation (1) [34]:
2.6. pH Measurement
When testing prepared meats and meat products during the storage experiment, pH is an important property, as it can indicate the growth of acid-forming microorganisms and the decomposition of meat components. An HI9810362 HALO 2 pH tester (HANNA Instruments, Woonsocket, RI, USA) was used for pH measurement. The device was calibrated before each measurement series. To measure pH, the meat was tempered to room temperature. Each parallel sample (N = 6) was measured three times and their average was used for the evaluation.
2.7. Microbiological Analysis
There is a widely used standard method for the detection of Clostridium perfringens, which is a sulfite-reducing anaerobic bacteria. Clostridium perfringens is a foodborne pathogen that induces food poisoning and enteric infections in humans and animals. It can be detected in raw and processed meat and spices [35]. The anaerobic total live plate count of meat samples was measured according to a modified EN ISO 7937-2005, which originally was fit for Clostridium perfringens, in an accredited laboratory. A representative sample was taken from which a homogenized sample was measured.
2.8. Experimental Design and Statistical Analysis
There were four factors in the experimental design: (1) marinade flavoring, (2) piperine enrichment, (3) HHP treatment and (4) storage time. The types of marinade flavoring were the (1) herb and (2) paprika flavorings. The levels of piperine enrichment were (1) BA− and (2) BA+. The levels of HHP treatment were (1) 0 MPa, (2) 300 MPa, (3) 450 MPa and (4) 600 MPa. The levels of storage time were (1) 0 day, (2) 4 days, (3) 8 days, (4) 12 days and (5) 14 days. Nearly a full factorial experimental design was used. However, only BA− samples were measured at 0 MPa HHP treatment from samples with both marinade flavorings.
Measurement results were evaluated by IBM SPSS v27 (IBM, Armonk, New York, NY, USA). Microsoft Excel 365 version 2010 (build: 13328.20356) was used for representation. To detect the effect of these four factors on texture (hardness and penetration work), color (L*, a*, b* and hab) and pH, multivariate analysis of variance (MANOVA) was carried out. The value of the unexplained variance rate (Wilks’s lambda) was evaluated. The homogenous groups were separated by Tukey’s HSD post hoc test for the evaluation (α = 0.05). The homogeneity of variances was checked by Levene’s test (p < 0.01 in case of each dependent variant). The normality of residuals was checked by d’Agostino’s test.
3. Results and Discussion
3.1. Texture
According to Tukey’s post hoc test, the meat prepared with different marinades differed significantly (p = 0.05) in terms of hardness: the samples marinated with paprika were on average 15% harder, but the samples enriched with bioactive components did not differ significantly (p = 0.05) from the nonenriched samples. The four groups of samples from the control and three different HHP treatments differed significantly (p = 0.05) from each other: the marinated but non-HHP-treated sample groups were on average 24% harder than the raw sample, the sample groups treated at 300 MPa were on average 22% harder than the non-HHP-treated sample groups and the sample groups treated at 450 MPa were on average 25% harder than the sample groups treated at 300 MPa, while the sample groups treated at 600 MPa were on average 19% harder than the sample groups treated at 450 MPa. This can be explained by coagulation of the meat proteins. The hardness of the raw samples differed significantly (p = 0.05) from the other sample groups based on all four factors. The effect of storage time was also observed in the samples, clearly showing the softening, texture-tenderizing effect of the marinades. The 12- and 14-day sample groups were significantly (p = 0.05) softer than the other sample groups, but not significantly different from each other (p = 0.05). The 7-day sample groups were on average 19% harder than the other sample groups, and the 0-day and 4-day sample groups were significantly (p = 0.05), on average, 13% harder than the 7-day sample groups. The sample groups for the first two measurement days did not differ significantly (p = 0.05) from each other. The hardness results for the different sample groups are illustrated in Table 3.

Table 3.
Hardness [N] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).
The penetration work value of raw meat samples was also significantly different (p = 0.05) from the penetration work value of marinated samples. However, the penetration work values of marinated samples with different flavorings and of samples enriched and not enriched with bioactive components were not significantly different (p = 0.05). By analyzing the penetration work of the samples treated at different pressures, results similar to the hardness analysis were obtained. However, in this case, the raw samples were not significantly different (p = 0.05) from the marinated but not HHP-treated samples. This was probably because the marinade induced exudate formation and a harder crust was formed on the surface of the meat, which increased the maximum force, namely the hardness, but the work performed during the total penetration was not significantly different. Sample groups treated with HHP at different pressures were significantly (p = 0.05) different from each other based on penetration work. In ascending order, there was an average difference of 37%, 24% and 17% in penetration work between the sample groups treated at 0 MPa, 300 MPa, 450 MPa and 600 MPa. An interesting observation was that the effect of storage time was not clear, partly due to the large standard deviation of the biological samples, but the trend was similar to the differences observed for hardness. The penetration work results for the different sample groups are shown in Table 4.

Table 4.
Penetration work [W] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).
Previous studies tested HHP-treated post-rigor meat in the range of 130–520 MPa, 100–600 MPa and 200–800 MPa. A disintegration of the myofibrils was observed as a result of the pressure, but the meat did not become softer or more tender. Overall, the pressure-treated meats were found to be firmer than the untreated meats, and in addition, the firmer the meat, the higher the pressure [36,37,38,39]. These results are similar to the results of this study. The hardening of meat following pressure treatment at room temperature or lower temperatures can be explained by irreversible structural changes in the myofibrils [40]. The change is related to the applied pressure and the pressure at which the structural change starts depends on the animal species [41].
3.2. Color
According to the results of Tukey’s post hoc test, the marinated sample groups with different flavorings were significantly different from each other and from the raw samples (p = 0.05) in terms of a*, b* and hab. In terms of L* value, only the herb-marinated samples differed significantly (p = 0.05) by on average 13% from the raw and paprika-marinated samples. The differences were 64–96% for a* and b*, which is a color difference clearly visible to the naked eye. It is interesting to note that in the case of the green herb marinade, a value close to 0 for a* was measured, which means that the sample is neither green nor red. This was probably due to the fact that the measured spots had approximately the same proportion of green herb pieces and red meat surfaces. All color measurement results are well explained by the coloring effect of the natural coloring substances in the marinades (herbs or paprika). In the case of hab, the adequacy of the color measurement can be clearly validated, as the raw samples showed the pink color typical of raw meat, the paprika samples showed orange and the herb samples showed yellowish-green hues. There was no significant difference between the color factors of the BA+ and BA− samples (p = 0.05), except for the factor a*, where the BA+ samples were on average 13% redder than the BA− samples. This is basically due to the orange-red color of piperine. Although this difference is not visible to the naked eye, it is very detectable by instrumental measurement. Storage time also had an effect on the color of the samples. The 0-day samples were significantly different (p = 0.05) from the samples from other measurement days, which were not significantly different (p = 0.05) from each other. The relative difference between the two homogenous groups was on average 4–11% depending on the color factor, which is nominally small. The color factors of the HHP-treated sample groups were not significantly different (p = 0.05) from each other, but were significantly different (p = 0.05) from the non-HHP-treated (0 MPa) sample groups. This difference was mostly observed for two color factors: an average relative difference of 10% for L* and a relative difference of almost 40% for b*. Both can be explained by the coagulation of meat proteins and coloring carotenoids darkening the meat during treatment. The HHP treatment and the piperine addition by itself had a strong and significant effect on the colors. However, the interaction effect of these two factors, although significant, with a Wilks’s lambda value of 0.836, was the least impactful of the dependent variables. This can be explained by the fact that, in the case of color, the two effects did not strengthen or weaken each other, as they mainly affected other color factors. In addition, piperine did not degrade or change color more strongly in reaction to HHP treatment. The results of the color factors are presented in Table 5, Table 6, Table 7 and Table 8.

Table 5.
Lightness (L*) [-] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).

Table 6.
Redness/greenness (a*) [-] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).

Table 7.
Yellowness/blueness (b*) [-] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).

Table 8.
Hue angle (hab) [-] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).
The increase in the lightness factor (L*) is one of the most common color changes reported in the previous study results and is typically observed in treatments above 200 MPa in case of pork and other mammal meat [41,42,43]. Increasing the lightness of meat is associated with protein coagulation, which results in the loss of solubility of sarcoplasmic and myofibrillar proteins [44], furthermore with denaturation of myoglobin where the heme group in myoglobin is displaced or completely released from muscle cells [45].
3.3. pH
All samples had a slightly alkaline pH. Tukey’s post hoc test found significant (p = 0.05) differences between sample groups. The nature of the marinades significantly (p = 0.05) influenced the pH of the meat. Meat marinated with paprika was on average 4% less alkaline than raw meat, and herb-marinated samples were on average 0.6% less alkaline than paprika-marinated samples. It is very interesting that the very weak alkaline effect of piperine was also significantly (p = 0.05) shown: the BA+ sample groups were on average 0.6% more alkaline than the BA− sample groups. By coagulating proteins with alkaline pH, the pressure treatment shifted the pH of the samples towards neutral. All groups of samples treated with HHP at different pressures were significantly different (p = 0.05). Although this difference was statistically significant, there was on average a relative difference of 0.5–0.8% between the pH of the different pressure-treated sample groups. During storage, the pH also increased, which can be explained by the decomposition of alkaline substances and the effect of acids produced by growing microorganisms. Tukey’s post hoc test significantly (p = 0.05) separated four homogeneous groups, which were the following in ascending order of pH: (1) 14-day and 12-day sample groups; (2) 12-day and 7-day sample groups; (3) 7-day and 4-day sample groups; and (4) 4-day and 0-day sample groups. The average difference between the expected values of the homogeneous groups was 0.15–0.2%, which is not a big change. The pH results are shown in Table 9.

Table 9.
pH [-] results of meat sample groups made with different marinade flavoring (herbs or paprika), without or with piperine enrichment (BA− or BA+), with different HHP treatment (0 MPa, 300 MPa, 450 MPa or 600 MPa) and with different storage time (0 day, 4 days, 7 days, 12 days or 14 days).
Other authors have obtained similar results for pH when post-mortem beef was treated at 200–300 MPa at room temperature, resulting in a slight increase of 0.2 units in pH [46,47]. The change in pH was explained by a change in protein conformation and the release of the imidazolium group of histidine under pressure.
3.4. Overall Results of MANOVA
As a result of MANOVA, an unexplained variance rate was concluded (Table 10). The lower the Wilks’s lambda value, the greater the proportion of the dependent variables (marinade flavoring, piperine enrichment, HHP treatment and storage time) that can be explained by the setting of the independent variables (texture attributes, color factors and pH). Although the effect of each factor and the interaction of each factor with all other factors was highly significant, only Wilks’s lambda values below 0.5 are considered to be relevant. The effects of marinade flavoring and HHP treatment were very strong with Wilks’s lambda values below 0.1, but the effects of piperine enrichment and storage time were slightly strong. This means that marinade flavoring and HHP treatment had the greatest effect on the measured techno-functional properties. These were the main determinants of the properties measured.

Table 10.
Wilks’s lambda (unexplained variance rate) results of independent variables from MANOVA.
3.5. Microbiology
Currently, the main objective of pressure treatment was to increase the microbiological safety of food by inactivating microorganisms (for instance, Clostridium perfringens and Listeria monocytogenes) without altering the organoleptic properties. The change in pH values is indirectly related to microbiological deterioration and lactic acid production, which is characteristic of anaerobic storage conditions. The paprika-flavored pork short loin samples without the bioactive component showed an increase in anaerobic total live plate count with increasing storage time, but it is worth noting that with increasing pressure, the samples showed lower lg (CFU g−1) values (Figure 1). At the end of the storage period, adequate sensory and microbiological properties were observed at pressures of 450 and 600 MPa.
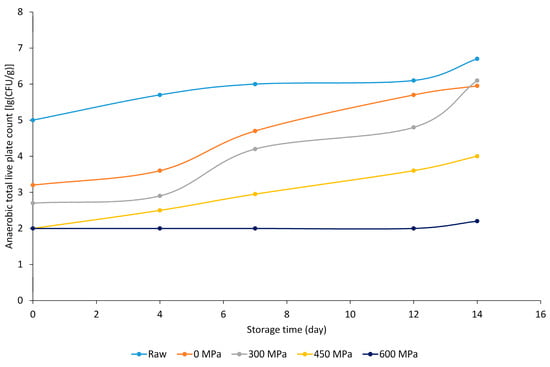
Figure 1.
Anaerobic total live plate count [lg (CFU g−1)] results of paprika-marinated BA− meat sample groups.
The paprika-flavored marinade contained 0.39 ppm methyl eugenol, as described in its specification, which, in addition to the presence of piperine, enhanced the antimicrobial effect, as clearly seen in the lg (CFU g−1) values (Figure 2). The values decreased with increasing pressure. As storage time progressed, samples treated at 300–450–600 MPa also showed positive organoleptic and microbiological properties.
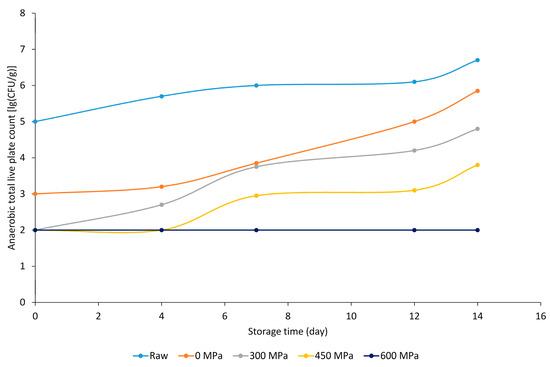
Figure 2.
Anaerobic total live plate count [lg (CFU g−1)] results of paprika-marinated BA+ meat sample groups.
In the case of the herb-flavored samples prepared without bioactive ingredients, lg (CFU g−1) values decreased significantly with increasing pressure but increased with increasing storage time (Figure 3). Samples treated at 600 MPa were also below the detection limit in this case.
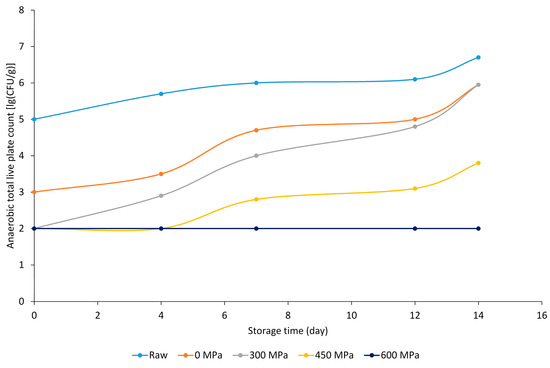
Figure 3.
Anaerobic total live plate count [lg (CFU g−1)] results of herb-marinated BA− meat sample groups.
The herb-flavored marinade contained 0.12 ppm methyl eugenol, as described in its specification, and samples prepared with the addition of the bioactive substance also showed a confirmed antimicrobial effect in this case. Anaerobic total live plate count decreased significantly with increasing pressure but increased with increasing storage time (Figure 4). Also, in this case, samples treated at 600 MPa gave the lowest values. Even samples treated at 300 MPa, 450 MPa and 600 MPa were still within the microbiological breakthrough limit at the end of the storage period.
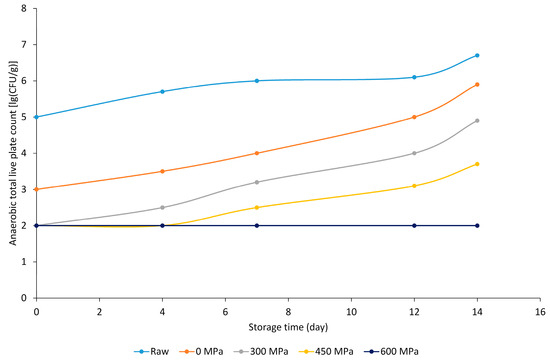
Figure 4.
Anaerobic total live plate count [lg (CFU g−1)] results of herb-marinated BA+ meat sample groups.
As the treatment pressure increased, a significant antibacterial effect was observed. However, it is important to mention that partly due to the adiabatic temperature increase caused by HHP treatment [48] and partly due to the high pressure, the structure of the myofibrils changes, which has an impact on the quality of the meat [49]. This effect was described through the texture change and color change shown above. For this reason, it is important to consider the treatment parameters that are chosen for the different types of prepared (marinated) meats. The results are in line with previous studies which also found that 400 and 600 MPa were the most effective treatment pressures. These previous studies observed 6-7 log Bacillus cereus and 5 log Clostridium botulinum proteolytic Type A and Type B inactivation by HHP treatment with 600 MPa pressure [18].
4. Conclusions
The modern consumer is interested in the origin, composition and preservation methods used by food companies. Health-conscious eating and the consumption of food made from the most natural ingredients and in the most natural way possible have been given a prominent role. For this reason, minimal processing such as HHP and natural preservatives and bioactive substances such as spicy marinades and piperine are gaining ground. The study provided comprehensive insights into the effects of various marinades, HHP treatments and storage times on the texture, color, pH and microbiological properties of marinated meat (short loin slices).
This study demonstrated the significant effect of HHP treatment and piperine enrichment on the texture, color, pH and microbiological properties of marinated meat. HHP treatment notably increased the hardness and altered the color of the samples, with higher pressures leading to more pronounced changes due to protein coagulation. Piperine enrichment slightly enhanced the redness of the meat and influenced the pH, albeit to a lesser extent than HHP. Importantly, HHP treatment significantly improved the microbiological safety of the samples, with pressures of 450 MPa and 600 MPa effectively reducing microbial counts while maintaining acceptable organoleptic properties. The presence of piperine further reduced the growth of microorganisms in the products. However, the piperine enrichment and storage time did not have strong effects on techno-functional properties, which is beneficial for consumers. Overall, the combination of HHP treatment and piperine bioactive component enrichment offers a promising approach to improving both the safety and quality of marinated meats.
Author Contributions
Conceptualization, G.J. and Z.F.; methodology, G.J. and Z.F.; validation, A.V. and K.I.H., formal analysis, G.J. and Z.F.; investigation, Z.F.; resources, A.V. and G.J.; data curation, Z.F. and T.C., writing—original draft preparation, Z.F. and T.C.; writing—review and editing, G.J. and I.D.; visualization, T.C.; supervision, L.F.F.; project administration, Z.F.; funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by the Flagship Research Groups Programme of the Hungarian University of Agriculture and Life Sciences. The authors are grateful to the Department of Livestock Products and Food Preservation Technology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Global Food Losses and Food Waste-Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Zhang, B.Y.; Tong, Y.; Singh, S.; Cai, H.; Huang, J.-Y. Assessment of carbon footprint of nano-packaging considering potential food waste reduction due to shelf life extension. Resour. Conserv. Recycl. 2019, 149, 322–331. [Google Scholar] [CrossRef]
- Cooreman-Algoed, M.; Minnens, F.; Boone, L.; Botterman, K.; Taelman, S.E.; Verbeke, W.; Devleesschauwer, B.; Hung, Y.; Dewulf, J. Consumer and food product determinants of food wasting: A case study on chicken meat. Sustainability 2021, 13, 7027. [Google Scholar] [CrossRef]
- Sumrin, S.; Gupta, S.; Asaad, Y.; Wang, Y.; Bhattacharya, S.; Foroudi, P. Eco-innovation for environment and waste prevention. J. Bus. Res. 2020, 122, 627–639. [Google Scholar] [CrossRef]
- Gogliettino, M.; Balestrieri, M.; Ambrosio, R.L.; Anastasio, A.; Smaldone, G.; Proroga, Y.T.R.; Moretta, R.; Rea, I.; De Stefano, L.; Agrillo, B.; et al. Extending the shelf-life of meat and dairy products via PET-modified packaging activated with the antimicrobial peptide MTP1. Front. Microbiol. 2020, 10, 2963. [Google Scholar] [CrossRef] [PubMed]
- Amani, P.; Gadde, L.E. Shelf Life Extension And Food Waste Reduction. In Proceedings of the 2015 International European Forum (144th EAAE Seminar), Innsbruck-Igls, Austria, 9–13 February 2015. [Google Scholar]
- Kirtil, E.; Kilercioglu, M.; Oztop, M.H. Modified Atmosphere Packaging of Foods. Ref. Modul. Food Sci. 2016, 1, 1–6. [Google Scholar]
- Soro, A.B.; Noore, S.; Hannon, S.; Whyte, P.; Bolton, D.J.; O’Donnell, C.; Tiwari, B.K. Current sustainable solutions for extending the shelf life of meat and marine products in the packaging process. Food Packag. Shelf Life 2021, 29, 100722. [Google Scholar] [CrossRef]
- Casson, A.; Giovenzana, V.; Frigerio, V.; Zambelli, M.; Beghi, R.; Pampuri, A.; Tugnolo, A.; Merlini, A.; Colombo, L.; Limbo, S.; et al. Beyond the eco-design of case-ready beef packaging: The relationship between food waste and shelf-life as a key element in life cycle assessment. Food Packag. Shelf Life 2022, 34, 100943. [Google Scholar] [CrossRef]
- Bogataj, D.; Hudoklin, D.; Bogataj, M.; Dimovski, V.; Colnar, S. Risk mitigation in a meat supply chain with options of redirection. Sustainability 2020, 12, 8690. [Google Scholar] [CrossRef]
- EUR-Lex Access to European Union Law. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32004R0853 (accessed on 3 September 2024).
- Kropf, D.H. Colour stability. Factors Affect. Color Fresh Meat. 1993, 2, 269–275. [Google Scholar]
- Norton, T.; Sun, D.W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Siddiqui, M.W.; Rahman, M.S. Minimally processed foods: Overview. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience; Springer: Cham, Switzerland, 2014; pp. 1–15. [Google Scholar]
- Dávila-Aviña, J.E.; Solís-Soto, L.Y.; Rojas-Verde, G.; Salas, N.A. Sustainability and challenges of minimally processed foods. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience; Springer: Cham, Switzerland, 2015; pp. 279–295. [Google Scholar]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing–effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hashem, M.A.; Azad, M.A.K.; Choudhury, M.S.H.; Bhuiyan, M.K.J. Techniques of meat preservation—A review. Meat Res. 2023, 3, 55. [Google Scholar] [CrossRef]
- Banerjee, R.; Verma, A.K. Minimally Processed Meat and Fish Products. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience; Springer International Publishing: Cham, Switzerland, 2014; pp. 193–250. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Amiri, S.; Moghanjougi, Z.M.; Bari, M.R.; Khaneghah, A.M. Natural protective agents and their applications as bio-preservatives in the food industry: An overview of current and future applications. Ital. J. Food Sci. 2021, 33, 55–68. [Google Scholar] [CrossRef]
- Nadarajah, D.; Han, J.H.; Holley, R.A. Inactivation of Escherichia coli O157:H7 in packaged ground beef by allyl isothiocyanate. Int. J. Food Microbiol. 2005, 99, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Chacon, P.A.; Muthukumarasamy, P.; Holley, R.A. Elimination of Escherichia coli O157:H7 from Fermented Dry Sausages at an Organoleptically Acceptable Level of Microencapsulated Allyl Isothiocyanate. Appl. Environ. Microbiol. 2006, 72, 3096–3102. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Zarai, Z.; Boujelbene, E.; Salem, N.B.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Kenesei, G.; Kiskó, G.; Dalmadi, I. Combined Sous-Vide and High Hydrostatic Pressure Treatment of Pork: Is the Order of Application Decisive When Using Minimal Processing Technologies? Appl. Sci. 2024, 14, 3583. [Google Scholar] [CrossRef]
- Csurka, T.; Varga-Tóth, A.; Kühn, D.; Hitka, G.; Badak-Kerti, K.; Alpár, B.; Surányi, J.; Friedrich, L.F.; Pásztor-Huszár, K. Comparison of techno-functional and sensory properties of sponge cakes made with egg powder and different quality of powdered blood products for substituting egg allergen and developing functional food. Front. Nutr. 2022, 9, 979594. [Google Scholar] [CrossRef]
- Issimov, A.; Baibatyrov, T.; Tayeva, A.; Kenenbay, S.; Abzhanova, S.; Shambulova, G.; Kuzembayeva, G.; Kozhakhiyeva, M.; Brel-Kisseleva, I.; Safronova, O.; et al. Prevalence of Clostridium perfringens and Detection of Its Toxins in Meat Products in Selected Areas of West Kazakhstan. Agriculture 2022, 12, 1357. [Google Scholar] [CrossRef]
- Jung, S.; de Lamballerie-Anton, M.; Ghoul, M. Modifications of ultrastructure and myofibrillar proteins of post-rigor beef treated by high pressure. LWT-Food Sci. Technol. 2000, 33, 313–319. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Changes in lysosomal enzyme activities and shear values of high pressure treated meat during ageing. Meat Sci. 2000, 56, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Ledward, D.A. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 2004, 68, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Scheibenzuber, M.; Ruß, W.; Görg, A.; Meyer-Pittroff, R. Scanning Electron Microscopic Study of High Pressure Induced Microstructural Changes of Proteins in Turkey and Pork Meat. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 19, pp. 545–550. [Google Scholar]
- Iwasaki, T.; Noshiroya, K.; Saitoh, N.; Okano, K.; Yamamoto, K. Studies of the effect of hydrostatic pressure pretreatment on thermal gelation of chicken myofibrils and pork meat patty. Food Chem. 2006, 95, 474–483. [Google Scholar] [CrossRef]
- Del Olmo, A.; Morales, P.; Ávila, M.; Calzada, J.; Nuñez, M. Effect of single-cycle and multiple-cycle high-pressure treatments on the colour and texture of chicken breast fillets. Innov. Food Sci. Emerg. Technol. 2010, 11, 441–444. [Google Scholar] [CrossRef]
- Tintchev, F.; Bindrich, U.; Toepfl, S.; Strijowski, U.; Heinz, V.; Knorr, D. High hydrostatic pressure/temperature modeling of frankfurter batters. Meat Sci. 2013, 94, 376–387. [Google Scholar] [CrossRef]
- Korzeniowski, W.; Jankowska, B.; Kwiatkowska, A. The effect of high pressure on some technological properties of pork. Electron. J. Pol. Agric. Univ. 1999, 2, 1–8. [Google Scholar]
- Goutefongea, R.; Rampon, V.; Nicolas, N.; Dumont, J. Meat colour changes under high pressure treatment. In Proceedings of the 41st Annual International Congress of Meat Science and Technology, San Antonio, TX, USA, 20–25 August 1995. [Google Scholar]
- Carlez, A.; Veciana-Nogues, T.; Cheftel, J.C. Changes in colour and myoglobin of minced beef meat due to high pressure processing. LWT-Food Sci. Technol. 1995, 28, 528–538. [Google Scholar] [CrossRef]
- Horgan, D.J.; King, N.L.; Kurth, L.B.; Kuypers, R. Collagen crosslinks and their relationship to the thermal properties of calf tendons. Arch. Biochem. Biophys. 1990, 281, 21–26. [Google Scholar] [CrossRef]
- Suzuki, A.; Watanabe, M.; Iwamura, K.; Ikeuchi, Y.; Saito, M. Effect of high pressure treatment on the ultrastructure and myofibrillar protein of beef skeletal muscle. Agric. Biol. Chem. 1990, 54, 3085–3091. [Google Scholar] [CrossRef]
- Buzrul, S.; Alpas, H.; Largeteau, A.; Bozoglu, F.; Demazeau, G. Compression heating of selected pressure transmitting fluids and liquid foods during high hydrostatic pressure treatment. J. Food Eng. 2008, 85, 466–472. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of high hydrostatic pressure on the conformational structure and gel properties of myofibrillar protein and meat quality: A review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).