Exercise Suppresses Appetite in Obesity: A Biochemical, Metabolic, and Molecular Approach
Abstract
Featured Application
Abstract
1. Introduction
2. Exercise and Appetite-Regulatory Hormones
3. Exercise and Appetite-Reducing Metabolites, Myokines, and Hepatokines
- Increases in systemic IL-6 levels promote the production and secretion of GLP-1 and PYY from intestinal L-cells and pancreatic α-cells [139,181,237], which has a negative correlation with appetite and energy intake [139,181,238] (Figure 3). Increased GLP-1 levels elevate insulin secretion and improve glucose tolerance while also having anti-obese effects by acting on the hypothalamus [139,140,239,240]. Peripherally produced GLP-1 crosses the BBB to bind to its receptors on the hypothalamus to increase the expression and release of POMC neuropeptide, followed by diminished energy intake and body weight [140,241,242]. Additionally, central GLP-1 stimulates the expression of hypothalamic IL-6 and IL-6 receptor α (IL-6Rα) mRNA by neurons and glial cells to increase and decrease, respectively, the expression of POMC and NPY/AgRP neuropeptides, which in turn cause hypophagic effects and appetite suppression [140,243,244,245,246] (Figure 3).
- IL-6 slows gastric emptying to reduce postprandial glycaemia to negatively impact appetite and energy intake [250] (Figure 3). Generally speaking, the effects of signals of energy balance, such as leptin, GLP-1, and amylin, are modulated by the effects of IL-6 on hampering food intake and body weight [140,205,230,247] (Figure 3).
4. Exercise, BDNF and Appetite
5. Exercise and Eating Behaviors
6. Exercise and Gastric Motility and Emptying
7. Exercise, Mental Stress, and Appetite
8. Exercise, Body Temperature, and Appetite
9. Conclusions
10. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α2δ-1 | alpha2/delta 1 |
| α-MSH | alpha-melanocyte stimulating hormone |
| AgRP | agouti-related protein |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARC | arcuate nucleus |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BHB | β-hydroxybutyrate |
| CaRF | Ca2+-responsive transcription factor |
| CAT-B | cathepsin-B |
| CCK | cholecystokinin |
| CNDP2 | carnosine dipeptidase II |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| CRH | corticotropin-releasing hormone |
| DMV | dorsal motor nucleus |
| DNMT | DNA methylation |
| ERK | extracellular signal-regulated kinase |
| ERS | endoplasmic reticulum stress |
| GDF15 | growth differentiating factor 15 |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide 1 |
| GOAT | ghrelin-O-acyltransferase; |
| GPCR | G-protein-coupled receptor |
| H3K4me3 | trimethylated histone H3 at lysine 4 |
| HAT | histone acetylation |
| HDAC | histone deacetylase |
| HPA | hypothalamic–pituitary–adrenal axis |
| IGF-1 | insulin-like growth factor 1 |
| KAT | kynurenine aminotransferase |
| Lac-Phe | N-lactoyl-phenylalanine |
| LEPRs | leptin receptors |
| LRP | lipoprotein receptor-related protein |
| MAPK | mitogen-activated protein kinase |
| MC4R | melanocortin 4 receptor |
| MCTs | monocarboxylate transporters |
| MDP | mesolimbic dopamine reward pathway |
| NF-κB | nuclear factor-kappa B |
| NPY | neuropeptide Y |
| NTS | nucleus tractus solitarius |
| PI3K | phosphoinositide 3-kinases |
| PLCγ | phospholipase C-gamma |
| POMC | pro-opiomelanocortin |
| PVH | paraventricular hypothalamic nucleus |
| PYY | peptide YY |
| SIRT1 | sirtuin 1 |
| SNS | sympathetic nervous system |
| SOCS | suppressor of cytokine signaling |
| tPA | tissue plasminogen activator |
| TRH | thyrotropin-releasing hormone |
| TrkB | tyrosine kinase B receptor |
| TRPV1 | transient receptor potential vanilloid 1 |
| TSH | thyroid-stimulating hormone |
| UCN | urocortin |
| UCP-1 | uncoupling protein 1 |
| UPR | unfolded protein response |
| VMH | ventromedial nucleus of the hypothalamus |
| VTA | ventral tegmental area |
References
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. In Obesity and Obstetrics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Jéquier, E. Pathways to obesity. Int. J. Obes. 2002, 26, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Niswender, K.D. Neuroendocrine regulation of food intake. Pediatr. Blood Cancer 2012, 58, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Gibbons, C.; Caudwell, P.; Hopkins, M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol. Behav. 2015, 152, 473–478. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The physiological control of eating: Signals, neurons, and networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef]
- Martins, C.; Morgan, L.; Truby, H. A review of the effects of exercise on appetite regulation: An obesity perspective. Int. J. Obes. 2008, 32, 1337–1347. [Google Scholar] [CrossRef]
- Campbell, J.N.; Macosko, E.Z.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.; Resch, J.M.; McCarroll, S.A. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef]
- Peruzzo, B.; Pastor, F.E.; Blázquez, J.L.; Schöbitz, K.; Peláez, B.; Amat, P.; Rodríguez, E.M. A second look at the barriers of the medial basal hypothalamus. Exp. Brain Res. 2000, 132, 10–26. [Google Scholar] [CrossRef]
- Cone, R.; Cowley, M.A.; Butler, A.; Fan, W.; Marks, D.; Low, M. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. 2001, 25, S63–S67. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, G.; De Boer, P.; Luiten, P.; Van Willigen, J. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 1989, 31, 785–797. [Google Scholar] [CrossRef] [PubMed]
- van der Kooy, D.; Koda, L.Y.; McGinty, J.F.; Gerfen, C.R.; Bloom, F.E. The organization of projections from the cortes, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J. Comp. Neurol. 1984, 224, 1–24. [Google Scholar] [CrossRef]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1187–1209. [Google Scholar] [CrossRef]
- Blundell, J.E. Perspective on the central control of appetite. Obesity 2006, 14, 160S. [Google Scholar] [CrossRef]
- Hellström, P.M.; Geliebter, A.; Näslund, E.; Schmidt, P.T.; Yahav, E.K.; Hashim, S.A.; Yeomans, M.R. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br. J. Nutr. 2004, 92, S47–S57. [Google Scholar] [CrossRef]
- Bryant, E.J.; King, N.; Blundell, J.E. Disinhibition: Its effects on appetite and weight regulation. Obes. Rev. 2008, 9, 409–419. [Google Scholar] [CrossRef]
- Martins, C.; Robertson, M.D.; Morgan, L.M. Effects of exercise and restrained eating behaviour on appetite control. Proc. Nutr. Soc. 2008, 67, 28–41. [Google Scholar] [CrossRef]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Caudwell, P.; Hopkins, M.; Byrne, N.M.; Colley, R.; Hills, A.P.; Stubbs, J.R.; Blundell, J.E. Metabolic and behavioral compensatory responses to exercise interventions: Barriers to weight loss. Obesity 2007, 15, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Lluch, A.; Stubbs, R.; Blundell, J. High dose exercise does not increase hunger or energy intake in free living males. Eur. J. Clin. Nutr. 1997, 51, 478–483. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hill, J.O.; Jacobsen, D.J.; Potteiger, J.; Sullivan, D.K.; Johnson, S.L.; Heelan, K.; Hise, M.; Fennessey, P.V.; Sonko, B. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The Midwest Exercise Trial. Arch. Intern. Med. 2003, 163, 1343–1350. [Google Scholar] [CrossRef]
- Coyle, E.F. Physical activity as a metabolic stressor. Am. J. Clin. Nutr. 2000, 72, 512S–520S. [Google Scholar] [CrossRef]
- Kenneth, M.; Fadia, H. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J. Appl. Physiol. 2001, 90, 345–357. [Google Scholar]
- Prior, B.M.; Yang, H.; Terjung, R.L. What makes vessels grow with exercise training? J. Appl. Physiol. 2004, 97, 1119–1128. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Klein, S. Physiological factors that regulate the use of endogenous fat and carbohydrate fuels during endurance exercise. Nutr. Res. Rev. 2003, 16, 97–108. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Donges, C.E.; Duffield, R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism 2013, 62, 235–243. [Google Scholar] [CrossRef]
- Balaguera-Cortes, L.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 958–966. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: The modulating effect of adiposity, sex, and habitual physical activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Deighton, K.; Broom, D.R.; Wasse, L.K.; Douglas, J.A.; Burns, S.F.; Cordery, P.A.; Petherick, E.S.; Batterham, R.L.; Goltz, F.R. Individual variation in hunger, energy intake and ghrelin responses to acute exercise. Med. Sci. Sports Exerc. 2017, 49, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.-H.; Lyu, X.; Zushin, P.-J.H. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A.; King, J.; Clayton, D.; Jackson, A.; Sargeant, J.; Thackray, A.; Davies, M.J.; Stensel, D. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int. J. Obes. 2017, 41, 1737–1744. [Google Scholar] [CrossRef]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef]
- Caruso, L.; Zauli, E.; Vaccarezza, M. Physical Exercise and Appetite Regulation: New Insights. Biomolecules 2023, 13, 1170. [Google Scholar] [CrossRef]
- Blundell, J.E.; Beaulieu, K. The complex pattern of the effects of prolonged frequent exercise on appetite control, and implications for obesity. Appetite 2023, 183, 106482. [Google Scholar] [CrossRef]
- Martins, C.; Kulseng, B.; King, N.; Holst, J.; Blundell, J. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metab. 2010, 95, 1609–1616. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Gutin, B.; Ramsey, L.; Barbeau, P.; Cannady, W.; Ferguson, M.; Litaker, M.; Owens, S. Plasma leptin concentrations in obese children: Changes during 4-mo periods with and without physical training. Am. J. Clin. Nutr. 1999, 69, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Council on Sports Medicine and Fitness; Council on School Health. Active healthy living: Prevention of childhood obesity through increased physical activity. Pediatrics 2006, 117, 1834–1842. [Google Scholar]
- Zouhal, H.; Saeidi, A.; Kolahdouzi, S.; Ahmadizad, S.; Hackney, A.C.; Abderrahmane, A.B. Exercise and training effects on appetite-regulating hormones in individuals with obesity. In Endocrinology of Physical Activity and Sport; Springer: Berlin/Heidelberg, Germany, 2020; pp. 535–562. [Google Scholar]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Zouhal, H.; Sellami, M.; Saeidi, A.; Slimani, M.; Abbassi-Daloii, A.; Khodamoradi, A.; El Hage, R.; Hackney, A.C.; Ben Abderrahman, A. Effect of physical exercise and training on gastrointestinal hormones in populations with different weight statuses. Nutr. Rev. 2019, 77, 455–477. [Google Scholar] [CrossRef]
- Hopkins, M.; Blundell, J.E. Energy balance, body composition, sedentariness and appetite regulation: Pathways to obesity. Clin. Sci. 2016, 130, 1615–1628. [Google Scholar] [CrossRef]
- Rocha, J.; Paxman, J.; Dalton, C.; Winter, E.; Broom, D.R. Effects of a 12-week aerobic exercise intervention on eating behaviour, food cravings, and 7-day energy intake and energy expenditure in inactive men. Appl. Physiol. Nutr. Metab. 2016, 41, 1129–1136. [Google Scholar] [CrossRef]
- Ataeinosrat, A.; Haghighi, M.M.; Abednatanzi, H.; Soltani, M.; Ghanbari-Niaki, A.; Nouri-Habashi, A.; Amani-Shalamzari, S.; Mossayebi, A.; Khademosharie, M.; Johnson, K.E. Effects of three different modes of resistance training on appetite hormones in males with obesity. Front. Physiol. 2022, 13, 827335. [Google Scholar] [CrossRef]
- Morash, M.G.; Gagnon, J.; Nelson, S.; Anini, Y. Tissue distribution and effects of fasting and obesity on the ghrelin axis in mice. Regul. Pept. 2010, 163, 62–73. [Google Scholar] [CrossRef]
- Abdemur, A.; Slone, J.; Berho, M.; Gianos, M.; Szomstein, S.; Rosenthal, R.J. Morphology, localization, and patterns of ghrelin-producing cells in stomachs of a morbidly obese population. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 122–126. [Google Scholar] [CrossRef]
- Zouhal, H.; Lemoine-Morel, S.; Mathieu, M.-E.; Casazza, G.A.; Jabbour, G. Catecholamines and obesity: Effects of exercise and training. Sports Med. 2013, 43, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Dougherty, K.A.; Frye, B.R.; Duke, K.M.; Williams, N.I. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity 2007, 15, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R29–R35. [Google Scholar] [CrossRef]
- Broom, D.R.; Miyashita, M.; Wasse, L.K.; Pulsford, R.; King, J.A.; Thackray, A.E.; Stensel, D.J. Acute effect of exercise intensity and duration on acylated ghrelin and hunger in men. J. Endocrinol. 2017, 232, 411–422. [Google Scholar] [CrossRef]
- Kawano, H.; Mineta, M.; Asaka, M.; Miyashita, M.; Numao, S.; Gando, Y.; Ando, T.; Sakamoto, S.; Higuchi, M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite 2013, 66, 26–33. [Google Scholar] [CrossRef]
- De Vriese, C.; Gregoire, F.; Lema-Kisoka, R.; Waelbroeck, M.; Robberecht, P.; Delporte, C. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 2004, 145, 4997–5005. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef]
- Gholipour, M.; Kordi, M.R.; Taghikhani, M.; Ravasi, A.A.; Gaeini, A.A.; Tabrizi, A. Possible role for growth hormone in suppressing acylated ghrelin and hunger ratings during and after intermittent exercise of different intensities in obese individuals. Acta Medica Iran. 2014, 52, 29–37. [Google Scholar]
- Vanderheyden, L.W.; McKie, G.L.; Howe, G.J.; Hazell, T.J. Greater lactate accumulation following an acute bout of high-intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J. Appl. Physiol. 2020, 128, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Hashemipour, M.; Mohammadifard, N.; Alikhassy, H.; Adeli, K. Short-and long-term relationships of serum ghrelin with changes in body composition and the metabolic syndrome in prepubescent obese children following two different weight loss programmes. Clin. Endocrinol. 2008, 69, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Gueugnon, C.; Mougin, F.; Nguyen, N.U.; Bouhaddi, M.; Nicolet-Guénat, M.; Dumoulin, G. Ghrelin and PYY levels in adolescents with severe obesity: Effects of weight loss induced by long-term exercise training and modified food habits. Eur. J. Appl. Physiol. 2012, 112, 1797–1805. [Google Scholar] [CrossRef]
- Leidy, H.; Gardner, J.; Frye, B.; Snook, M.; Schuchert, M.; Richard, E.; Williams, N. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J. Clin. Endocrinol. Metab. 2004, 89, 2659–2664. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; McTiernan, A.; Frayo, R.S.; Schwartz, R.S.; Rajan, K.B.; Yasui, Y.; Tworoger, S.S.; Cummings, D.E. Human plasma ghrelin levels increase during a one-year exercise program. J. Clin. Endocrinol. Metab. 2005, 90, 820–825. [Google Scholar] [CrossRef]
- Morishima, T.; Kurihara, T.; Hamaoka, T.; Goto, K. Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin. Physiol. Funct. Imaging 2014, 34, 90–97. [Google Scholar] [CrossRef]
- Bowyer, K.P.; Carson, J.A.; Davis, J.M.; Wang, X. The influence of exercise training dose on fasting acylated ghrelin concentration in older women. J. Behav. Med. 2019, 42, 567–572. [Google Scholar] [CrossRef]
- Fatouros, I.; Tournis, S.; Leontsini, D.; Jamurtas, A.; Sxina, M.; Thomakos, P.; Manousaki, M.; Douroudos, I.; Taxildaris, K.; Mitrakou, A. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J. Clin. Endocrinol. Metab. 2005, 90, 5970–5977. [Google Scholar] [CrossRef]
- Fazelifar, S.; Ebrahim, K.; Sarkisian, V. Effect of exercise training and detraining on serum leptin levels in obese young boys. Med. Sport 2013, 66, 325–337. [Google Scholar]
- Hopkins, M.; Gibbons, C.; Caudwell, P.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Blundell, J.E.; Finlayson, G. Fasting leptin is a metabolic determinant of food reward in overweight and obese individuals during chronic aerobic exercise training. Int. J. Endocrinol. 2014, 2014, 323728. [Google Scholar] [CrossRef]
- Kissileff, H.R.; Thornton, J.C.; Torres, M.I.; Pavlovich, K.; Mayer, L.S.; Kalari, V.; Leibel, R.L.; Rosenbaum, M. Leptin reverses declines in satiation in weight-reduced obese humans. Am. J. Clin. Nutr. 2012, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ara, I.; Perez-Gomez, J.; Vicente-Rodríguez, G.; Chavarren, J.; Dorado, C.; Calbet, J. Serum free testosterone, leptin and soluble leptin receptor changes in a 6-week strength-training programme. Br. J. Nutr. 2006, 96, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Hildebrandt, T.; Schlögl, H.; Seitz, J.; Denecke, S.; Vieira, D.; Gradl-Dietsch, G.; Peters, T.; Antel, J.; Lau, D. The role of hypoleptinemia in the psychological and behavioral adaptation to starvation: Implications for anorexia nervosa. Neurosci. Biobehav. Rev. 2022, 141, 104807. [Google Scholar] [CrossRef]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef]
- Bouret, S.; Levin, B.E.; Ozanne, S.E. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol. Rev. 2015, 95, 47–82. [Google Scholar] [CrossRef]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef]
- Oswal, A.; Yeo, G. Leptin and the control of body weight: A review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity 2010, 18, 221. [Google Scholar] [CrossRef]
- Liu, J.; Lai, F.; Hou, Y.; Zheng, R. Leptin signaling and leptin resistance. Med. Rev. 2022, 2, 363–384. [Google Scholar] [CrossRef]
- Hegyi, K.; Fülöp, K.; Kovács, K.; Tóth, S.; Falus, A. Leptin-induced signal transduction pathways. Cell Biol. Int. 2004, 28, 159–169. [Google Scholar] [CrossRef]
- Engin, A. Diet-induced obesity and the mechanism of leptin resistance. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 381–397. [Google Scholar]
- Shimizu, H.; Oh, S.; Okada, S.; Mori, M. Leptin resistance and obesity. Endocr. J. 2007, 54, 17–26. [Google Scholar] [CrossRef]
- Bjørbæk, C.; Elmquist, J.K.; Frantz, J.D.; Shoelson, S.E.; Flier, J.S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1998, 1, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, K.W.; Kim, M.-S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Howard, S.; LoGrasso, P.V. Pharmacological inhibition of c-Jun N-terminal kinase reduces food intake and sensitizes leptin’s anorectic signaling actions. Sci. Rep. 2017, 7, 41795. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Sabio, G. Brain JNK and metabolic disease. Diabetologia 2021, 64, 265–274. [Google Scholar] [CrossRef]
- Reseland, J.E.; Anderssen, S.A.; Solvoll, K.; Hjermann, I.; Urdal, P.; Holme, I.; Drevon, C.A. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am. J. Clin. Nutr. 2001, 73, 240–245. [Google Scholar] [CrossRef]
- Abbenhardt, C.; McTiernan, A.; Alfano, C.M.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; Toriola, A.T.; Potter, J.D. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med. 2013, 274, 163–175. [Google Scholar] [CrossRef]
- Pérusse, L.; Collier, G.; Gagnon, J.; Leon, A.S.; Rao, D.; Skinner, J.S.; Wilmore, J.H.; Nadeau, A.; Zimmet, P.Z.; Bouchard, C. Acute and chronic effects of exercise on leptin levels in humans. J. Appl. Physiol. 1997, 83, 5–10. [Google Scholar] [CrossRef]
- Kraemer, R.; Johnson, L.; Haltom, R.; Kraemer, G.; Hebert, E.; Gimpel, T.; Castracane, V. Serum leptin concentrations in response to acute exercise in postmenopausal women with and without hormone replacement therapy. Proc. Soc. Exp. Biol. Med. 1999, 221, 171–177. [Google Scholar]
- Paolisso, G.; Rizzo, M.R.; Mone, C.M.; Tagliamonte, M.R.; Gambardella, A.; Riondino, M.; Carella, C.; Varricchio, M.; D’Onofrio, F. Plasma sex hormones are significantly associated with plasma leptin concentration in healthy subjects. Clin. Endocrinol. 1998, 48, 291–297. [Google Scholar] [CrossRef]
- Dagogo-Jack, S.; Tykodi, G.; Umamaheswaran, I. Inhibition of cortisol biosynthesis decreases circulating leptin levels in obese humans. J. Clin. Endocrinol. Metab. 2005, 90, 5333–5335. [Google Scholar] [CrossRef]
- Wabitsch, M.; Bo Jensen, P.; Blum, W.F.; Christoffersen, C.T.; Englaro, P.; Heinze, E.; Rascher, W.; Teller, W.; Tornqvist, H.; Hauner, H. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes 1996, 45, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cerro, A.; Garcia-Luna, P.P.; Astorga, R.; Parejo, J.; Peino, R.; Dieguez, C.; Casanueva, F.F. Serum leptin levels in male marathon athletes before and after the marathon run. J. Clin. Endocrinol. Metab. 1998, 83, 2376–2379. [Google Scholar] [CrossRef] [PubMed]
- Karamouzis, I.; Karamouzis, M.; Vrabas, I.S.; Christoulas, K.; Kyriazis, N.; Giannoulis, E.; Mandroukas, K. The Effects of Marathon Swimming on Serum Leptin and Plasma Neuropeptide Y Levels. 2002. Available online: https://pubmed.ncbi.nlm.nih.gov/11939485/ (accessed on 26 May 2025).
- de Assis, G.G.; Murawska-Ciałowicz, E. Exercise and weight management: The role of leptin—A systematic review and update of clinical data from 2000–2022. J. Clin. Med. 2023, 12, 4490. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Williams, T.D.; Dobbs, W.C. The effect of chronic exercise training on leptin: A systematic review and meta-analysis of randomized controlled trials. Sports Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.S.; Jun, D.W.; Hong, S.M. Exercise enhances insulin and leptin signaling in the cerebral cortex and hypothalamus during dexamethasone-induced stress in diabetic rats. Neuroendocrinology 2005, 82, 282–293. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Lopes-Araújo, A.; Royes, L.F.F.; Crespo-Lopez, M.E. Exercise reshapes the brain: Molecular, cellular, and structural changes associated with cognitive improvements. Mol. Neurobiol. 2023, 60, 6950–6974. [Google Scholar] [CrossRef]
- Barrios-Correa, A.A.; Estrada, J.A.; Contreras, I. Leptin signaling in the control of metabolism and appetite: Lessons from animal models. J. Mol. Neurosci. 2018, 66, 390–402. [Google Scholar] [CrossRef]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef]
- Khanh, D.V.; Choi, Y.-H.; Moh, S.H.; Kinyua, A.W.; Kim, K.W. Leptin and insulin signaling in dopaminergic neurons: Relationship between energy balance and reward system. Front. Psychol. 2014, 5, 846. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Lee, W.-J.; Funahashi, T.; Tanaka, S.; Matsuzawa, Y.; Chao, C.-L.; Chen, C.-L.; Tai, T.-Y.; Chuang, L.-M. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J. Clin. Endocrinol. Metab. 2001, 86, 3815–3819. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.P.; Dengel, D.; Leon, A.; Schmitz, K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int. J. Obes. 2007, 31, 996–1003. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, K.S.; Yau, M.-h.; Xu, A. Post-translational modifications of adiponectin: Mechanisms and functional implications. Biochem. J. 2008, 409, 623–633. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef]
- Guillod-Maximin, E.; Roy, A.-F.; Vacher, C.-M.; Aubourg, A.; Bailleux, V.; Lorsignol, A.; Pénicaud, L.; Parquet, M.; Taouis, M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J. Endocrinol. 2009, 200, 93. [Google Scholar] [CrossRef]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.-B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Polito, R.; Nigro, E.; Messina, A.; Monaco, M.L.; Monda, V.; Scudiero, O.; Cibelli, G.; Valenzano, A.; Picciocchi, E.; Zammit, C. Adiponectin and orexin-A as a potential immunity link between Adipose tissue and central nervous system. Front. Physiol. 2018, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-K.; Song, S.-E.; Oh, J.U.; Hwang, M.; Cho, H.-W.; Bae, J.-H.; Im, S.-S.; Kim, J.-I.; Song, D.-K. Orexin A-induced inhibition of leptin expression and secretion in adipocytes reducing plasma leptin levels and hypothalamic leptin resistance. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 1407–1418. [Google Scholar] [CrossRef]
- Inutsuka, A.; Yamanaka, A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Smart, D.; Jerman, J.; Brough, S.; Rushton, S.; Murdock, P.; Jewitt, F.; Elshourbagy, N.; Ellis, C.; Middlemiss, D.; Brown, F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999, 128, 1–3. [Google Scholar] [CrossRef]
- Ha, J.; Kwak, S.; Kim, K.Y.; Kim, H.; Cho, S.Y.; Kim, M.; Lee, J.-Y.; Kim, E. Relationship between adipokines, cognition, and brain structures in old age depending on obesity. J. Gerontol. Ser. A 2023, 78, 120–128. [Google Scholar] [CrossRef]
- Matafome, P.; Eickhoff, H.; Letra, L.; Seiça, R. Neuroendocrinology of adipose tissue and gut–brain axis. Obes. Brain Funct. 2017, 19, 49–70. [Google Scholar]
- Ueda, S.-y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357. [Google Scholar] [CrossRef]
- Ueda, S.-y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Nakao, H.; Fujimoto, S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 2009, 201, 151. [Google Scholar] [CrossRef]
- Holliday, A.; Blannin, A. Appetite, food intake and gut hormone responses to intense aerobic exercise of different duration. J. Endocrinol. 2017, 235, 193–205. [Google Scholar] [CrossRef]
- Martins, C.; Stensvold, D.; Finlayson, G.; Holst, J.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, N. Effect of moderate-and high-intensity acute exercise on appetite in obese individuals. Med. Sci. Sports Exerc. 2015, 47, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Glucagon-like peptide-1 and the islet β-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology 2003, 144, 5145–5148. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of glucose homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. J. Diabetes Res. 2011, 2011, 279530. [Google Scholar] [CrossRef]
- Shah, M.; Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 2014, 15, 181–187. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Roth, C.L. Fasting glucagon-like peptide-1 and its relation to insulin in obese children before and after weight loss. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 608–612. [Google Scholar] [CrossRef]
- Stinson, S.E.; Jonsson, A.E.; Lund, M.A.; Frithioff-Bøjsøe, C.; Aas Holm, L.; Pedersen, O.; Ängquist, L.; Sørensen, T.I.; Holst, J.J.; Christiansen, M. Fasting plasma GLP-1 is associated with overweight/obesity and cardiometabolic risk factors in children and adolescents. J. Clin. Endocrinol. Metab. 2021, 106, 1718–1727. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Parkinson, J.R.; Chaudhri, O.B.; Kuo, Y.-T.; Field, B.C.; Herlihy, A.H.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R.; Bell, J.D. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1, oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging (MEMRI). Neuroimage 2009, 44, 1022–1031. [Google Scholar] [CrossRef]
- Knauf, C.; Cani, P.D.; Perrin, C.; Iglesias, M.A.; Maury, J.F.; Bernard, E.; Benhamed, F.; Grémeaux, T.; Drucker, D.J.; Kahn, C.R. Brain glucagon-like peptide–1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J. Clin. Investig. 2005, 115, 3554–3563. [Google Scholar] [CrossRef] [PubMed]
- Rüttimann, E.B.; Arnold, M.; Hillebrand, J.J.; Geary, N.; Langhans, W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 2009, 150, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Kanoski, S.E.; De Jonghe, B.C.; Leichner, T.M.; Alhadeff, A.L.; Fortin, S.M.; Arnold, M.; Langhans, W.; Grill, H.J. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like peptide-1 and the central/peripheral nervous system: Crosstalk in diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Shirazi, R.; Palsdottir, V.; Collander, J.; Anesten, F.; Vogel, H.; Langlet, F.; Jaschke, A.; Schürmann, A.; Prévot, V.; Shao, R. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc. Natl. Acad. Sci. USA 2013, 110, 16199–16204. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin and the hormone concept. Endocr. Connect. 2021, 10, R139–R150. [Google Scholar] [CrossRef]
- Desai, A.; Dong, M.; Harikumar, K.; Miller, L. Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor. Int. J. Obes. Suppl. 2016, 6, S22–S27. [Google Scholar] [CrossRef]
- Skibicka, K.P.; Dickson, S.L. Enteroendocrine hormones—Central effects on behavior. Curr. Opin. Pharmacol. 2013, 13, 977–982. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Otsuki, M. Interaction among fat, lipase, CCK, and gastric emptying. J. Gastroenterol. 1999, 34, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Giardino, W.J. Neural circuit mechanisms of the cholecystokinin (CCK) neuropeptide system in addiction. Addict. Neurosci. 2022, 3, 100024. [Google Scholar] [CrossRef]
- Raiteri, M.; Paudice, P.; Vallebuona, F. Release of cholecystokinin in the central nervous system. Neurochem. Int. 1993, 22, 519–527. [Google Scholar] [CrossRef]
- Noble, F.; Wank, S.A.; Crawley, J.N.; Bradwejn, J.; Seroogy, K.B.; Hamon, M.; Roques, B.P. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol. Rev. 1999, 51, 745–781. [Google Scholar] [CrossRef]
- Dufresne, M.; Seva, C.; Fourmy, D. Cholecystokinin and gastrin receptors. Physiol. Rev. 2006, 86, 805–847. [Google Scholar] [CrossRef]
- Shillabeer, G.; Davison, J. Proglumide, a cholecystokinin antagonist, increases gastric emptying in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1987, 252, R353–R360. [Google Scholar] [CrossRef]
- Moran, T.H.; McHugh, P.R. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1982, 242, R491–R497. [Google Scholar] [CrossRef]
- Scarpignato, C.; Varga, G.; Corradi, C. Effect of CCK and its antagonists on gastric emptying. J. Physiol.-Paris 1993, 87, 291–300. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Cao, S.; Liu, B.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. The mechanism of the gut-brain axis in regulating food intake. Nutrients 2023, 15, 3728. [Google Scholar] [CrossRef]
- Raybould, H.E.; Tache, Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 1988, 255, G242–G246. [Google Scholar] [CrossRef]
- Kobelt, P.; Tebbe, J.J.; Tjandra, I.; Stengel, A.; Bae, H.-G.; Andresen, V.; van der Voort, I.R.; Veh, R.d.W.; Werner, C.R.; Klapp, B.F. CCK inhibits the orexigenic effect of peripheral ghrelin. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R751–R758. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Sliwowski, Z.; Lorens, K.; Konturek, S.; Bielanski, W.; Zoladz, J. Leptin, gastrointestinal and stress hormones in response to exercise in fasted or fed subjects and before or after blood donation. J. Physiol. Pharmacol. 2001, 52, 53–70. [Google Scholar] [PubMed]
- Bailey, D.M.; Davies, B.; Castell, L.M.; Newsholme, E.A.; Calam, J. Physical exercise and normobaric hypoxia: Independent modulators of peripheral cholecystokinin metabolism in man. J. Appl. Physiol. 2001, 90, 105–113. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Milledge, J.S.; Richards, M.; Williams, S.; Jordinson, M.; Calam, J. Elevated plasma cholecystokinin at high altitude: Metabolic implications for the anorexia of acute mountain sickness. High Alt. Med. Biol. 2000, 1, 9–23. [Google Scholar] [CrossRef]
- Hirschberg, A.L.; Lindholm, C.; Carlström, K.; Von Schoultz, B. Reduced serum cholecystokinin response to food intake in female athletes. Metabolism 1994, 43, 217–222. [Google Scholar] [CrossRef]
- Martins, C.; Kulseng, B.; Rehfeld, J.; King, N.; Blundell, J. Effect of chronic exercise on appetite control in overweight and obese individuals. Med. Sci. Sports Exerc. 2013, 45, 805–812. [Google Scholar] [CrossRef]
- Woods, S.C.; D’Alessio, D.A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008, 93, s37–s50. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Glavas, M.M.; Grayson, B.E.; Allen, S.E.; Copp, D.R.; Smith, M.S.; Cowley, M.A.; Grove, K.L. Characterization of brainstem peptide YY (PYY) neurons. J. Comp. Neurol. 2008, 506, 194–210. [Google Scholar] [CrossRef]
- Michel, M.C.; Beck-Sickinger, A.; Cox, H.; Doods, H.N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.; Westfall, T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Larhammar, D.; Salaneck, E. Molecular evolution of NPY receptor subtypes. Neuropeptides 2004, 38, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Pedragosa-Badia, X.; Stichel, J.; Beck-Sickinger, A.G. Neuropeptide Y receptors: How to get subtype selectivity. Front. Endocrinol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3–36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Brownley, K.A.; Heymen, S.; Hinderliter, A.L.; MacIntosh, B. Effect of glycemic load on peptide-YY levels in a biracial sample of obese and normal weight women. Obesity 2010, 18, 1297–1303. [Google Scholar] [CrossRef]
- Zwirska-Korczala, K.; Konturek, S.; Sodowski, M.; Wylezol, M.; Kuka, D.; Sowa, P.; Adamczyk-Sowa, M.; Kukla, M.; Berdowska, A.; Rehfeld, J. Basal and postprandial plasma levels of Pyy, Ghrelin. J. Physiol. Pharmacol. 2007, 58, 13–35. [Google Scholar]
- Gibbons, C.; Blundell, J.E.; Caudwell, P.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Finlayson, G. The role of episodic postprandial peptides in exercise-induced compensatory eating. J. Clin. Endocrinol. Metab. 2017, 102, 4051–4059. [Google Scholar] [CrossRef]
- Jones, T.E.; Basilio, J.; Brophy, P.; McCammon, M.; Hickner, R. Long-term exercise training in overweight adolescents improves plasma peptide YY and resistin. Obesity 2009, 17, 1189–1195. [Google Scholar] [CrossRef]
- Roth, C.L.; Enriori, P.J.; Harz, K.; Woelfle, J.; Cowley, M.A.; Reinehr, T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J. Clin. Endocrinol. Metab. 2005, 90, 6386–6391. [Google Scholar] [CrossRef]
- Ueda, S.-y.; Miyamoto, T.; Nakahara, H.; Shishido, T.; Usui, T.; Katsura, Y.; Yoshikawa, T.; Fujimoto, S. Effects of exercise training on gut hormone levels after a single bout of exercise in middle-aged Japanese women. Springerplus 2013, 2, 83. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Palm, S.; Bansal, A.; Austin, K.J.; Hart, A.M.; Alexander, B.M. Influence of running and walking on hormonal regulators of appetite in women. J. Obes. 2012, 2012, 730409. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.L.; Stensel, D.J. Differential acylated ghrelin, peptide YY3–36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. Metab. 2011, 96, 1114–1121. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- de Freitas, M.C.; Ricci-Vitor, A.L.; De Oliveira, J.V.N.; Quizzini, G.H.; Vanderlei, L.C.; Silva, B.S.; Zanchi, N.E.; Cholewa, J.M.; Lira, F.S.; Rossi, F.E. Appetite is suppressed after full-body resistance exercise compared with split-body resistance exercise: The potential influence of lactate and autonomic modulation. J. Strength Cond. Res. 2021, 35, 2532–2540. [Google Scholar] [CrossRef]
- Islam, H.; Townsend, L.K.; McKie, G.L.; Medeiros, P.J.; Gurd, B.J.; Hazell, T.J. Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J. Appl. Physiol. 2017, 123, 614–623. [Google Scholar] [CrossRef]
- McCarthy, S.F.; Bornath, D.P.; Jarosz, C.; Tucker, J.A.; Medeiros, P.J.; Kenno, K.A.; Hazell, T.J. Intense interval exercise induces lactate accumulation and a greater suppression of acylated ghrelin compared with submaximal exercise in middle-aged adults. J. Appl. Physiol. 2023, 134, 1177–1187. [Google Scholar] [CrossRef]
- Engelstoft, M.; Schwartz, T. Opposite regulation of ghrelin and glucagon-like peptide-1 by metabolite G-protein-coupled receptors. Trends Endocrinol. Metab. 2016, 27, 665–675. [Google Scholar] [CrossRef]
- Engelstoft, M.S.; Park, W.-m.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef]
- Buettner, C.; Pocai, A.; Muse, E.D.; Etgen, A.M.; Myers, M.G.; Rossetti, L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Ma, Y.; Sun, Y.; Zheng, G.; Wang, S.; Xing, R.; Chen, X.; Han, Y.; Wang, J.; Lu, Q.R. A GPR17-cAMP-lactate signaling axis in oligodendrocytes regulates whole-body metabolism. Cell Rep. 2019, 26, 2984–2997.e4. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Choi, P.P.; Smith, W.W.; Xu, W.; Ma, D.; Cordner, Z.A.; Liang, N.-C.; Moran, T.H. Exendin-4 reduces food intake via the PI3K/AKT signaling pathway in the hypothalamus. Sci. Rep. 2017, 7, 6936. [Google Scholar] [CrossRef]
- Cha, S.H.; Lane, M.D. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl-CoA signaling pathway. Biochem. Biophys. Res. Commun. 2009, 386, 212–216. [Google Scholar] [CrossRef]
- McCracken, M.; Ainsworth, B.; Hackney, A. Effects of the menstrual cycle phase on the blood lactate responses to exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 174–175. [Google Scholar] [CrossRef]
- McCarthy, S.F.; Islam, H.; Hazell, T.J. The emerging role of lactate as a mediator of exercise-induced appetite suppression. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E814–E819. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, H.; Zhou, J.J.; Pradhan, G.; Sun, Y.; Pan, H.L.; Li, D.P. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J. Neurochem. 2017, 142, 512–520. [Google Scholar] [CrossRef]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef]
- Jansen, R.S.; Addie, R.; Merkx, R.; Fish, A.; Mahakena, S.; Bleijerveld, O.B.; Altelaar, M.; IJlst, L.; Wanders, R.J.; Borst, P. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl. Acad. Sci. USA 2015, 112, 6601–6606. [Google Scholar] [CrossRef]
- Goltz, F.; Thackray, A.; King, J.; Dorling, J.; Atkinson, G.; Stensel, D.J. Interindividual responses of appetite to acute exercise: A replicated crossover study. Med. Sci. Sports Exerc. 2018, 50, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Grannell, A.; Kokkinos, A.; le Roux, C.W. Myokines in appetite control and energy balance. Muscles 2022, 1, 26–47. [Google Scholar] [CrossRef]
- Legård, G.E.; Pedersen, B.K. Muscle as an endocrine organ. In Muscle and Exercise Physiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–307. [Google Scholar]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Himmerich, H. Effects of IL-6 signaling pathway inhibition on weight and BMI: A systematic review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 6290. [Google Scholar] [CrossRef]
- Hidalgo, J.; Florit, S.; Giralt, M.; Ferrer, B.; Keller, C.; Pilegaard, H. Transgenic mice with astrocyte-targeted production of interleukin-6 are resistant to high-fat diet-induced increases in body weight and body fat. Brain Behav. Immun. 2010, 24, 119–126. [Google Scholar] [CrossRef]
- Bobbo, V.C.; Engel, D.F.; Jara, C.P.; Mendes, N.F.; Haddad-Tovolli, R.; Prado, T.P.; Sidarta-Oliveira, D.; Morari, J.; Velloso, L.A.; Araujo, E.P. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J. Neuroinflamm. 2021, 18, 192. [Google Scholar] [CrossRef]
- Mishra, D.; Richard, J.E.; Maric, I.; Porteiro, B.; Häring, M.; Kooijman, S.; Musovic, S.; Eerola, K.; Lopez-Ferreras, L.; Peris, E. Parabrachial interleukin-6 reduces body weight and food intake and increases thermogenesis to regulate energy metabolism. Cell Rep. 2019, 26, 3011–3026.e5. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Anorexia induced by activators of the signal transducer gp 130. Neuroreport 1996, 7, 841. [Google Scholar] [CrossRef] [PubMed]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006, 74, 443–477. [Google Scholar]
- Stenlöf, K.; Wernstedt, I.; Fjällman, T.; Wallenius, V.; Wallenius, K.; Jansson, J.-O. Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J. Clin. Endocrinol. Metab. 2003, 88, 4379–4383. [Google Scholar] [CrossRef]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.; Velloso, L.c.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Hunschede, S.; Kubant, R.; Akilen, R.; Thomas, S.; Anderson, G.H. Decreased appetite after high-intensity exercise correlates with increased plasma interleukin-6 in normal-weight and overweight/obese boys. Curr. Dev. Nutr. 2017, 1, e000398. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Fernández-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca2+ signals and an IL-6 autocrine loop. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; Van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef]
- Chan, M.S.; Carey, A.L.; Watt, M.J.; Febbraio, M.A. Cytokine gene expression in human skeletal muscle during concentric contraction: Evidence that IL-8, like IL-6, is influenced by glycogen availability. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R322–R327. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Keller, C.; Steensberg, A.; Pilegaard, H.; Osada, T.; Saltin, B.; Pedersen, B.K.; Neufer, P.D. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: Influence of muscle glycogen content. FASEB J. 2001, 15, 2748–2750. [Google Scholar] [CrossRef]
- Fischer, C.P.; Plomgaard, P.; Hansen, A.K.; Pilegaard, H.; Saltin, B.; Pedersen, B.K. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E1189–E1194. [Google Scholar] [CrossRef]
- Keller, C.; Steensberg, A.; Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Pedersen, B.K. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J. Appl. Physiol. 2005, 99, 2075–2079. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Steensberg, A.; Keller, C.; Starkie, R.L.; Nielsen, H.B.; Krustrup, P.; Ott, P.; Secher, N.H.; Pedersen, B.K. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. 2003, 549, 607–612. [Google Scholar] [CrossRef]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000, 529 Pt 1, 237–242. [Google Scholar] [CrossRef]
- Starkie, R.; Arkinstall, M.; Koukoulas, I.; Hawley, J.; Febbraio, M. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Päth, G.n.; Bornstein, S.R.; Gurniak, M.; Chrousos, G.P.; Scherbaum, W.A.; Hauner, H. Human breast adipocytes express interleukin-6 (IL-6) and its receptor system: Increased IL-6 production by β-adrenergic activation and effects of IL-6 on adipocyte function. J. Clin. Endocrinol. Metab. 2001, 86, 2281–2288. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Keller, C.; Richard, A.-M.; Saha, A.K.; Luo, Z.; Xiang, X.; Giralt, M.; Ritov, V.B.; Menshikova, E.V.; Kelley, D.E. Interleukin-6 regulation of AMP-activated protein kinase: Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 2006, 55, S48–S54. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engström-Ruud, L.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Brüning, J.C. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef]
- Wallenius, K.; Wallenius, V.; Sunter, D.; Dickson, S.L.; Jansson, J.-O. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem. Biophys. Res. Commun. 2002, 293, 560–565. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Razi, O.; Parnow, A.; Rashidi, I.; Pakravan, N.; Nedaei, S.E.; Motl, R.W. Aerobic training improves blood-brain barrier and neuronal apoptosis in experimental autoimmune encephalomyelitis. Iran. J. Basic Med. Sci. 2022, 25, 245. [Google Scholar]
- Jüttler, E.; Tarabin, V.; Schwaninger, M. Interleukin-6 (IL-6): A possible neuromodulator induced by neuronal activity. Neurosci. 2002, 8, 268–275. [Google Scholar]
- Vallières, L.; Rivest, S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1β. J. Neurochem. 1997, 69, 1668–1683. [Google Scholar] [CrossRef]
- Schöbitz, B.; de Kloet, E.R.; Sutanto, W.; Holsboer, F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur. J. Neurosci. 1993, 5, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Seelig, E.; Timper, K.; Coslovsky, M.; Soederlund, L.; Lyngbaek, M.P.; Wewer Albrechtsen, N.J.; Schmidt-Trucksäss, A.; Hanssen, H.; Frey, W.O. GLP-1 secretion is regulated by IL-6 signalling: A randomised, placebo-controlled study. Diabetologia 2020, 63, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Almada, C.; Cataldo, L.; Smalley, S.; Diaz, E.; Serrano, A.; Hodgson, M.; Santos, J. Plasma levels of interleukin-6 and interleukin-18 after an acute physical exercise: Relation with post-exercise energy intake in twins. J. Physiol. Biochem. 2013, 69, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Marliss, E.B.; Vranic, M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: Implications for diabetes. Diabetes 2002, 51, S271–S283. [Google Scholar] [CrossRef]
- Kastin, A.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. 2003, 27, 313–318. [Google Scholar] [CrossRef]
- Hunter, K.; Hölscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Quintana, A.; Erta, M.; Ferrer, B.; Comes, G.; Giralt, M.; Hidalgo, J. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun. 2013, 27, 162–173. [Google Scholar] [CrossRef]
- Senaris, R.; Trujillo, M.; Navia, B.; Comes, G.; Ferrer, B.; Giralt, M.; Hidalgo, J. Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way. J. Neuroendocrinol. 2011, 23, 675–686. [Google Scholar] [CrossRef]
- Schéle, E.; Benrick, A.; Grahnemo, L.; Egecioglu, E.; Anesten, F.; Pálsdóttir, V.; Jansson, J.O. Inter-relation between interleukin (IL)-1, IL-6 and body fat regulating circuits of the hypothalamic arcuate nucleus. J. Neuroendocrinol. 2013, 25, 580–589. [Google Scholar] [CrossRef]
- Vallières, L.; Rivest, S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology 1999, 140, 3890–3903. [Google Scholar] [CrossRef]
- Le Foll, C.; Johnson, M.D.; Dunn-Meynell, A.A.; Boyle, C.N.; Lutz, T.A.; Levin, B.E. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 2015, 64, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Le Foll, C.; Dunn-Meynell, A.A.; Levin, B.E. IL-6 ameliorates defective leptin sensitivity in DIO ventromedial hypothalamic nucleus neurons. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R764–R770. [Google Scholar] [CrossRef] [PubMed]
- Sadagurski, M.; Norquay, L.; Farhang, J.; D’Aquino, K.; Copps, K.; White, M. Human IL6 enhances leptin action in mice. Diabetologia 2010, 53, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Lehrskov, L.L.; Lyngbaek, M.P.; Soederlund, L.; Legaard, G.E.; Ehses, J.A.; Heywood, S.E.; Albrechtsen, N.J.W.; Holst, J.J.; Karstoft, K.; Pedersen, B.K. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018, 27, 1201–1211.e3. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W.-W. The GDF15-GFRAL pathway in health and metabolic disease: Friend or foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Tsai, V.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: Implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Brown, D.A.; Hance, K.W.; Rogers, C.J.; Sansbury, L.B.; Albert, P.S.; Murphy, G.; Laiyemo, A.O.; Wang, Z.; Cross, A.J.; Schatzkin, A. Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): A potential screening tool for the prevention of colon cancer? Cancer Epidemiol. Biomark. Prev. 2012, 21, 337–346. [Google Scholar] [CrossRef]
- Hsiao, E.C.; Koniaris, L.G.; Zimmers-Koniaris, T.; Sebald, S.M.; Huynh, T.V.; Lee, S.-J. Characterization of growth-differentiation factor 15, a transforming growth factor β superfamily member induced following liver injury. Mol. Cell. Biol. 2000, 20, 3742–3751. [Google Scholar] [CrossRef]
- Corre, J.; Hébraud, B.; Bourin, P. Concise review: Growth differentiation factor 15 in pathology: A clinical role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Campderrós, L.; Moure, R.; Cairó, M.; Gavaldà-Navarro, A.; Quesada-López, T.; Cereijo, R.; Giralt, M.; Villarroya, J.; Villarroya, F. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity 2019, 27, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.-C.; Roberts, G.P.; Meek, C.L. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019, 29, 707–718.e708. [Google Scholar] [CrossRef]
- Dostálová, I.; Roubíček, T.; Bártlová, M.; Mráz, M.; Lacinová, Z.; Haluzíková, D.; Kaválková, P.; Matoulek, M.; Kasalický, M.; Haluzík, M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: The influence of very low calorie diet. Eur. J. Endocrinol. 2009, 161, 397–404. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Kowalska, I.; Nikolajuk, A.; Adamska, A.; Otziomek, E.; Gorska, M.; Straczkowski, M. Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity. Clin. Endocrinol. 2012, 76, 46–50. [Google Scholar] [CrossRef]
- Sarkar, S.; Legere, S.; Haidl, I.; Marshall, J.; MacLeod, J.B.; Aguiar, C.; Lutchmedial, S.; Hassan, A.; Brunt, K.R.; Kienesberger, P. Serum GDF15, a promising biomarker in obese patients undergoing heart surgery. Front. Cardiovasc. Med. 2020, 7, 103. [Google Scholar] [CrossRef]
- Böttner, M.; Suter-Crazzolara, C.; Schober, A.; Unsicker, K. Expression of a novel member of the TGF-β superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999, 297, 103–110. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Zhong, Y. Hepatic GDF15 is regulated by CHOP of the unfolded protein response and alleviates NAFLD progression in obese mice. Biochem. Biophys. Res. Commun. 2018, 498, 388–394. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Smith, B.K.; Mohammadi-Shemirani, P.; Morrow, M.R.; Gutgesell, R.M.; Lu, R.; Raphenya, A.R.; Kabiri, M.; McArthur, A.G. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 2019, 1, 1202–1208. [Google Scholar] [CrossRef]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Ost, M. Stress-induced FGF21 and GDF15 in obesity and obesity resistance. Trends Endocrinol. Metab. 2021, 32, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci. Transl. Med. 2017, 9, eaan8732. [Google Scholar] [CrossRef]
- L’homme, L.; Sermikli, B.P.; Staels, B.; Piette, J.; Legrand-Poels, S.; Dombrowicz, D. Saturated fatty acids promote GDF15 expression in human macrophages through the PERK/eIF2/CHOP signaling pathway. Nutrients 2020, 12, 3771. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Tchou, I.; Margeli, A.; Tsironi, M.; Skenderi, K.; Barnet, M.; Kanaka-Gantenbein, C.; Papassotiriou, I.; Beris, P. Growth-differentiation factor-15, endoglin and N-terminal pro-brain natriuretic peptide induction in athletes participating in an ultramarathon foot race. Biomarkers 2009, 14, 418–422. [Google Scholar] [CrossRef]
- Galliera, E.; Lombardi, G.; Marazzi, M.G.; Grasso, D.; Vianello, E.; Pozzoni, R.; Banfi, G.; Corsi Romanelli, M.M. Acute exercise in elite rugby players increases the circulating level of the cardiovascular biomarker GDF-15. Scand. J. Clin. Lab. Investig. 2014, 74, 492–499. [Google Scholar] [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 2021, 12, 1041. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Mosconi, G.; Chiariello, A.; Cappuccilli, M.; Totti, V.; Santoro, A.; Franceschi, C.; Salvioli, S. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front. Immunol. 2020, 11, 915. [Google Scholar] [CrossRef]
- Zhang, H.; Fealy, C.E.; Kirwan, J.P. Exercise training promotes a GDF15-associated reduction in fat mass in older adults with obesity. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E829–E836. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.S.; Klein, A.B.; Færch, K.; Beaulieu, K.; Rosenkilde, M.; Gram, A.S.; Sjödin, A.; Torekov, S.; Stallknecht, B.; Clemmensen, C. Effects of acute exercise and exercise training on plasma GDF15 concentrations and associations with appetite and cardiometabolic health in individuals with overweight or obesity–A secondary analysis of a randomized controlled trial. Appetite 2023, 182, 106423. [Google Scholar] [CrossRef] [PubMed]
- Sabaratnam, R.; Kristensen, J.M.; Pedersen, A.J.; Kruse, R.; Handberg, A.; Wojtaszewski, J.F.; Højlund, K. Acute Exercise Increases GDF15 and Unfolded Protein Response/Integrated Stress Response in Muscle in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 1754–1764. [Google Scholar] [CrossRef]
- Kleinert, M.; Bojsen-Møller, K.N.; Jørgensen, N.B.; Svane, M.S.; Martinussen, C.; Kiens, B.; Wojtaszewski, J.F.; Madsbad, S.; Richter, E.A.; Clemmensen, C. Effect of bariatric surgery on plasma GDF15 in humans. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E615–E621. [Google Scholar] [CrossRef]
- Enarsson, M.; Feldreich, T.; Byberg, L.; Nowak, C.; Lind, L.; Ärnlöv, J. Association between cardiorespiratory fitness and circulating proteins in 50-year-old Swedish men and women: A cross-sectional study. Sports Med.-Open 2021, 7, 52. [Google Scholar] [CrossRef]
- Gil, C.I.; Ost, M.; Kasch, J.; Schumann, S.; Heider, S.; Klaus, S. Role of GDF15 in active lifestyle induced metabolic adaptations and acute exercise response in mice. Sci. Rep. 2019, 9, 20120. [Google Scholar] [CrossRef]
- Liu, J.; Kumar, S.; Heinzel, A.; Gao, M.; Guo, J.; Alvarado, G.F.; Reindl-Schwaighofer, R.; Krautzberger, A.M.; Cippà, P.E.; McMahon, J. Renoprotective and immunomodulatory effects of GDF15 following AKI invoked by ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2020, 31, 701–715. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Liu, W.; Huang, X.; Xu, J.; Zhou, Y.; Jiang, L.; Yang, J. Elevated circulating growth differentiation factor 15 is related to decreased heart rate variability in chronic kidney disease patients. Ren. Fail. 2021, 43, 340–346. [Google Scholar] [CrossRef]
- Connelly, P.W.; Yan, A.T.; Nash, M.M.; Lok, C.E.; Gunaratnam, L.; Prasad, G.R. Growth differentiation factor 15 is decreased by kidney transplantation. Clin. Biochem. 2019, 73, 57–61. [Google Scholar] [CrossRef]
- Fairlie, W.; Moore, A.; Bauskin, A.; Russell, P.; Zhang, H.P.; Breit, S. MIC-1 is a novel TGF-β superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 1999, 65, 2–5. [Google Scholar] [CrossRef]
- Townsend, L.K.; Weber, A.J.; Day, E.A.; Shamshoum, H.; Shaw, S.J.; Perry, C.G.; Kemp, B.E.; Steinberg, G.R.; Wright, D.C. AMPK mediates energetic stress-induced liver GDF15. FASEB J. 2021, 35, e21218. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-H.; Koch, P.D.; Luan, H.H.; Tu, H.-C.; Shimada, K.; Ngan, I.; Ventura, R.; Jiang, R.; Mitchison, T.J. Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat. Metab. 2021, 3, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.M.; Dethlefsen, M.M.; Tøndering, A.S.; Lassen, S.B.; Meldgaard, J.N.; Ringholm, S.; Pilegaard, H. PGC-1α in hepatic UPR during high-fat high-fructose diet and exercise training in mice. Physiol. Rep. 2018, 6, e13819. [Google Scholar] [CrossRef]
- Kristensen, C.M.; Jessen, H.; Ringholm, S.; Pilegaard, H. Muscle PGC-1α in exercise and fasting-induced regulation of hepatic UPR in mice. Acta Physiol. 2018, 224, e13158. [Google Scholar] [CrossRef]
- Plomgaard, P.; Hansen, J.S.; Townsend, L.K.; Gudiksen, A.; Secher, N.H.; Clemmesen, J.O.; Støving, R.K.; Goetze, J.P.; Wright, D.C.; Pilegaard, H. GDF15 is an exercise-induced hepatokine regulated by glucagon and insulin in humans. Front. Endocrinol. 2022, 13, 1037948. [Google Scholar] [CrossRef]
- Hughey, C.C.; James, F.D.; Bracy, D.P.; Donahue, E.P.; Young, J.D.; Viollet, B.; Foretz, M.; Wasserman, D.H. Loss of hepatic AMP-activated protein kinase impedes the rate of glycogenolysis but not gluconeogenic fluxes in exercising mice. J. Biol. Chem. 2017, 292, 20125–20140. [Google Scholar] [CrossRef]
- Takekoshi, K.; Fukuhara, M.; Quin, Z.; Nissato, S.; Isobe, K.; Kawakami, Y.; Ohmori, H. Long-term exercise stimulates adenosine monophosphate–activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver. Metabolism 2006, 55, 1122–1128. [Google Scholar] [CrossRef]
- Camacho, R.C.; Donahue, E.P.; James, F.D.; Berglund, E.D.; Wasserman, D.H. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E405–E408. [Google Scholar] [CrossRef]
- Hoene, M.; Lehmann, R.; Hennige, A.M.; Pohl, A.K.; Häring, H.U.; Schleicher, E.D.; Weigert, C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J. Physiol. 2009, 587, 241–252. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Yan, Z. AMPK and the adaptation to exercise. Annu. Rev. Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.-W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Yoo, J.H.; Kang, S.; Woo, J.H.; Shin, K.O.; Kim, K.B.; Cho, S.Y.; Roh, H.T.; Kim, Y.I. The effects of 12 weeks regular aerobic exercise on brain-derived neurotrophic factor and inflammatory factors in juvenile obesity and type 2 diabetes mellitus. J. Phys. Ther. Sci. 2014, 26, 1199–1204. [Google Scholar] [CrossRef]
- Tsai, V.W.-W.; Manandhar, R.; Jørgensen, S.B.; Lee-Ng, K.K.M.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Husaini, Y.; Lin, S.; Sainsbury, A. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS ONE 2014, 9, e100370. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Sabatini, P.V.; Frikke-Schmidt, H.; Arthurs, J.; Gordian, D.; Patel, A.; Rupp, A.C.; Adams, J.M.; Wang, J.; Beck Jørgensen, S.; Olson, D.P. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl. Acad. Sci. USA 2021, 118, e2021357118. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; Hultman, K.; Galaske, J.W.; Jørgensen, S.B.; Myers, M.G., Jr.; Seeley, R.J. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol. Metab. 2019, 21, 13–21. [Google Scholar] [CrossRef]
- Borner, T.; Wald, H.S.; Ghidewon, M.Y.; Zhang, B.; Wu, Z.; De Jonghe, B.C.; Breen, D.; Grill, H.J. GDF15 induces an aversive visceral malaise state that drives anorexia and weight loss. Cell Rep. 2020, 31, 107543. [Google Scholar] [CrossRef]
- Herrstedt, J. The latest consensus on antiemetics. Curr. Opin. Oncol. 2018, 30, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul. Pept. 2009, 156, 24–27. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Banno, R.; Arima, H.; Hayashi, M.; Goto, M.; Watanabe, M.; Sato, I.; Ozaki, N.; Nagasaki, H.; Ozaki, N.; Oiso, Y. Central administration of melanocortin agonist increased insulin sensitivity in diet-induced obese rats. FEBS Lett. 2007, 581, 1131–1136. [Google Scholar] [CrossRef]
- Zarjevski, N.; Cusin, I.; Vettor, R.; Rohner-Jeanrenaud, F.; Jeanrenaud, B. Intracerebroventricular administration of neuropeptide Y to normal rats has divergent effects on glucose utilization by adipose tissue and skeletal muscle. Diabetes 1994, 43, 764–769. [Google Scholar] [CrossRef]
- Wang, D.; Townsend, L.K.; DesOrmeaux, G.J.; Frangos, S.M.; Batchuluun, B.; Dumont, L.; Kuhre, R.E.; Ahmadi, E.; Hu, S.; Rebalka, I.A. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023, 619, 143–150. [Google Scholar] [CrossRef]
- Macia, L.; Tsai, V.W.-W.; Nguyen, A.D.; Johnen, H.; Kuffner, T.; Shi, Y.-C.; Lin, S.; Herzog, H.; Brown, D.A.; Breit, S.N. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS ONE 2012, 7, e34868. [Google Scholar]
- Tsai, V.; Zhang, H.; Manandhar, R.; Lee-Ng, K.; Lebhar, H.; Marquis, C.; Husaini, Y.; Sainsbury, A.; Brown, D.; Breit, S. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int. J. Obes. 2018, 42, 561–571. [Google Scholar] [CrossRef]
- Chrysovergis, K.; Wang, X.; Kosak, J.; Lee, S.-H.; Kim, J.S.; Foley, J.F.; Travlos, G.; Singh, S.; Baek, S.J.; Eling, T.E. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int. J. Obes. 2014, 38, 1555–1564. [Google Scholar] [CrossRef]
- Chung, H.K.; Ryu, D.; Kim, K.S.; Chang, J.Y.; Kim, Y.K.; Yi, H.-S.; Kang, S.G.; Choi, M.J.; Lee, S.E.; Jung, S.-B. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017, 216, 149–165. [Google Scholar] [CrossRef]
- Choi, M.J.; Jung, S.-B.; Lee, S.E.; Kang, S.G.; Lee, J.H.; Ryu, M.J.; Chung, H.K.; Chang, J.Y.; Kim, Y.K.; Hong, H.J. An adipocyte-specific defect in oxidative phosphorylation increases systemic energy expenditure and protects against diet-induced obesity in mouse models. Diabetologia 2020, 63, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Parmar, A.; Murphy, E.; Carper, D.; Lair, B.; Maes, P.; Vion, J.; Boulet, N.; Fontaine, C.; Marquès, M. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 2020, 5, e131870. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Zhu, M.Q.; Xie, B.C.; Shi, X.C.; Liu, H.; Zhang, R.X.; Xia, B.; Wu, J.W. Camptothecin effectively treats obesity in mice through GDF15 induction. PLoS Biol. 2022, 20, e3001517. [Google Scholar] [CrossRef]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P. Antibody-mediated inhibition of GDF15–GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef]
- Crovesy, L.; Rosado, E.L. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition 2019, 67, 110547. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Esvald, E.-E.; Tuvikene, J.; Kiir, C.S.; Avarlaid, A.; Tamberg, L.; Sirp, A.; Shubina, A.; Cabrera-Cabrera, F.; Pihlak, A.; Koppel, I. Revisiting the expression of BDNF and its receptors in mammalian development. Front. Mol. Neurosci. 2023, 16, 1182499. [Google Scholar] [CrossRef]
- Bartkowska, K.; Turlejski, K.; Djavadian, R. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467. [Google Scholar] [CrossRef]
- Schmidt-Kastner, R.; Wetmore, C.; Olson, L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience 1996, 74, 161–183. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.; Bruce, C.R.; Krabbe, K.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Kemi, C.; Grunewald, J.; Eklund, A.; Olgart Höglund, C. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir. Res. 2006, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Mulders, S.M.; Gelpke, M.D.S.; Hsueh, A.J. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc. Natl. Acad. Sci. USA 2005, 102, 9206–9211. [Google Scholar] [CrossRef]
- Jamali, A.; Shahrbanian, S.; Morteza Tayebi, S. The effects of exercise training on the brain-derived neurotrophic factor (BDNF) in the patients with type 2 diabetes: A systematic review of the randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 633–643. [Google Scholar] [CrossRef]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef]
- Tran, P.V.; Akana, S.F.; Malkovska, I.; Dallman, M.F.; Parada, L.F.; Ingraham, H.A. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J. Comp. Neurol. 2006, 498, 637–648. [Google Scholar] [CrossRef]
- Unger, T.J.; Calderon, G.A.; Bradley, L.C.; Sena-Esteves, M.; Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007, 27, 14265–14274. [Google Scholar] [CrossRef]
- Bariohay, B.; Lebrun, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology 2005, 146, 5612–5620. [Google Scholar] [CrossRef]
- Gray, J.; Yeo, G.S.; Cox, J.J.; Morton, J.; Adlam, A.-L.R.; Keogh, J.M.; Yanovski, J.A.; El Gharbawy, A.; Han, J.C.; Tung, Y.L. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 2006, 55, 3366–3371. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; Sommer, W.; Metsis, M.; Strömberg, I.; Agnati, L.F.; Fuxe, K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J. Neuroendocrinol. 2006, 18, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.; de Jong, J.; de Kloet, E.R.; Vreugdenhil, E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998, 813, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Rage, F.; Silhol, M.; Tapia-Arancibia, L. IL-1β regulation of BDNF expression in rat cultured hypothalamic neurons depends on the presence of glial cells. Neurochem. Int. 2006, 49, 433–441. [Google Scholar] [CrossRef]
- Schnydrig, S.; Korner, L.; Landweer, S.; Ernst, B.; Walker, G.; Otten, U.; Kunz, D. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci. Lett. 2007, 429, 69–73. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef]
- de Lima, N.S.; De Sousa, R.A.L.; Amorim, F.T.; Gripp, F.; Diniz e Magalhaes, C.O.; Henrique Pinto, S.; Peixoto, M.F.D.; Monteiro-Junior, R.S.; Bourbeau, K.; Cassilhas, R.C. Moderate-intensity continuous training and high-intensity interval training improve cognition, and BDNF levels of middle-aged overweight men. Metab. Brain Dis. 2022, 37, 463–471. [Google Scholar] [CrossRef]
- Inoue, D.S.; Monteiro, P.A.; Gerosa-Neto, J.; Santana, P.R.; Peres, F.P.; Edwards, K.M.; Lira, F.S. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020, 10, 13493. [Google Scholar] [CrossRef]
- Rodriguez, A.L.; Whitehurst, M.; Fico, B.G.; Dodge, K.M.; Ferrandi, P.J.; Pena, G.; Adelman, A.; Huang, C.-J. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. 2018, 243, 1153–1160. [Google Scholar] [CrossRef]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Health Sci. 2017, 6, 225–230. [Google Scholar] [CrossRef]
- Kim, B.; Kang, S. Regular leisure-time physical activity is effective in boosting neurotrophic factors and alleviating menopause symptoms. Int. J. Environ. Res. Public Health 2020, 17, 8624. [Google Scholar] [CrossRef] [PubMed]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996, 726, 49–56. [Google Scholar] [CrossRef]
- Kim, K.; Sung, Y.-H.; Seo, J.-H.; Lee, S.-W.; Lim, B.-V.; Lee, C.-Y.; Chung, Y.-R. Effects of treadmill exercise-intensity on short-term memory in the rats born of the lipopolysaccharide-exposed maternal rats. J. Exerc. Rehabil. 2015, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.; Millam, J.; Delany, M.; Fry, D. Regulation of brain-derived neurotrophic factor messenger RNA levels in avian hypothalamic slice cultures. Neuroscience 2000, 99, 373–380. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Lin, E.-J.D.; Wang, C.; Choi, E.Y.; Riban, V.; Lin, B.; During, M.J. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 2010, 142, 52–64. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Recruiting adaptive cellular stress responses for successful brain ageing. Nat. Rev. Neurosci. 2012, 13, 209–216. [Google Scholar] [CrossRef]
- Koppel, I.; Aid-Pavlidis, T.; Jaanson, K.; Sepp, M.; Pruunsild, P.; Palm, K.; Timmusk, T. Tissue-specific and neural activity-regulated expression of human BDNF gene in BAC transgenic mice. BMC Neurosci. 2009, 10, 68. [Google Scholar] [CrossRef]
- Marini, A.; Jiang, X.; Wu, X.; Pan, H.; Guo, Z.; Mattson, M.; Blondeau, N.; Novelli, A.; Lipsky, R. Preconditioning and neurotrophins: A model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids 2007, 32, 299–304. [Google Scholar] [CrossRef]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef]
- Leger, C.; Quirié, A.; Méloux, A.; Fontanier, E.; Chaney, R.; Basset, C.; Lemaire, S.; Garnier, P.; Prigent-Tessier, A. Impact of Exercise Intensity on Cerebral BDNF Levels: Role of FNDC5/Irisin. Int. J. Mol. Sci. 2024, 25, 1213. [Google Scholar] [CrossRef] [PubMed]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Ivy, A.S.; Russo-Neustadt, A.A. Nitric oxide synthesis is required for exercise-induced increases in hippocampal BDNF and phosphatidylinositol 3′ kinase expression. Brain Res. Bull. 2006, 68, 257–268. [Google Scholar] [CrossRef]
- Nishijima, T.; Soya, H. Evidence of functional hyperemia in the rat hippocampus during mild treadmill running. Neurosci. Res. 2006, 54, 186–191. [Google Scholar] [CrossRef]
- Cefis, M.; Quirié, A.; Pernet, N.; Marie, C.; Garnier, P.; Prigent-Tessier, A. Brain-derived neurotrophic factor is a full endothelium-derived factor in rats. Vasc. Pharmacol. 2020, 128, 106674. [Google Scholar] [CrossRef]
- Bayas, A.; Hummel, V.; Kallmann, B.; Karch, C.; Toyka, K.; Rieckmann, P. Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine 2002, 19, 55–58. [Google Scholar] [CrossRef]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Li, J.; Kambayashi, J.-i.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef]
- Benchenane, K.; Berezowski, V.; Ali, C.; Fernández-Monreal, M.; López-Atalaya, J.P.; Brillault, J.; Chuquet, J.; Nouvelot, A.; MacKenzie, E.T.; Bu, G. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor–related protein-mediated transcytosis. Circulation 2005, 111, 2241–2249. [Google Scholar] [CrossRef]
- Nicole, O.; Docagne, F.; Ali, C.; Margaill, I.; Carmeliet, P.; MacKenzie, E.T.; Vivien, D.; Buisson, A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001, 7, 59–64. [Google Scholar] [CrossRef]
- Anfray, A.; Drieu, A.; Hingot, V.; Hommet, Y.; Yetim, M.; Rubio, M.; Deffieux, T.; Tanter, M.; Orset, C.; Vivien, D. Circulating tPA contributes to neurovascular coupling by a mechanism involving the endothelial NMDA receptors. J. Cereb. Blood Flow Metab. 2020, 40, 2038–2054. [Google Scholar] [CrossRef]
- Ding, Q.; Ying, Z.; Gómez-Pinilla, F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience 2011, 192, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Burdette, J.H.; Laurienti, P.J.; Espeland, M.A.; Morgan, A.R.; Telesford, Q.; Vechlekar, C.D.; Hayaska, S.; Jennings, J.J.; Katula, J.A.; Kraft, R.A. Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2010, 2, 1695. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef]
- Wareski, P.; Vaarmann, A.; Choubey, V.; Safiulina, D.; Liiv, J.; Kuum, M.; Kaasik, A. PGC-1α and PGC-1β regulate mitochondrial density in neurons. J. Biol. Chem. 2009, 284, 21379–21385. [Google Scholar] [CrossRef]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef]
- Dadkhah, M.; Saadat, M.; Ghorbanpour, A.M.; Moradikor, N. Experimental and clinical evidence of physical exercise on BDNF and cognitive function: A comprehensive review from molecular basis to therapy. Brain Behav. Immun. Integr. 2023, 3, 100017. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.-Y.; Mao, Y.-W.; Gräff, J.; Guan, J.-S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.-H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Ghaedi, K.; Megraw, T.L.; Curtiss, J.; Shirani Faradonbeh, M.; Vaziri, P.; Nasr-Esfahani, M.H. Does PGC1α/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? Neuromol. Med. 2016, 18, 1–15. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Goncalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.; Wales, T.E.; Zhou, H.; Draga-Coletă, S.-V.; Gorgulla, C.; Blackmore, K.A.; Mittenbühler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q. Irisin acts through its integrin receptor in a two-step process involving extracellular Hsp90α. Mol. Cell 2023, 83, 1903–1920.e1912. [Google Scholar]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.-c. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629. [Google Scholar] [CrossRef]
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef]
- Marquez, C.M.S.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Brüning, J. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef]
- Mikoshiba, K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J. Neurochem. 2007, 102, 1426–1446. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Nakashima, T.; Takemoto-Kimura, S.; Aoki, K.; Morishita, Y.; Asahara, H.; Ohya, K.; Yamaguchi, A.; Takai, T. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat. Med. 2006, 12, 1410–1416. [Google Scholar] [CrossRef]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef]
- Kang, K.; Xu, P.; Wang, M.; Chunyu, J.; Sun, X.; Ren, G.; Xiao, W.; Li, D. FGF21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress. Biomed. Pharmacother. 2020, 129, 110439. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-i.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Masaki, T.; Chiba, S.; Ando, H.; Fujiwara, K.; Shimasaki, T.; Mitsutomi, K.; Katsuragi, I.; Kakuma, T.; Sakata, T. Hypothalamic brain-derived neurotrophic factor regulates glucagon secretion mediated by pancreatic efferent nerves. J. Neuroendocrinol. 2013, 25, 302–311. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Zhuang, Y.; Feng, J.; Ying, Z.; Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011, 33, 383–390. [Google Scholar] [CrossRef]
- Avila, A.M.; Burnett, B.G.; Taye, A.A.; Gabanella, F.; Knight, M.A.; Hartenstein, P.; Cizman, Z.; Di Prospero, N.A.; Pellizzoni, L.; Fischbeck, K.H. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Investig. 2007, 117, 659–671. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 2004, 53, 1208–1214. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Butler, C.W.; Berchtold, N.C.; Matheos, D.P.; Wood, M.A.; Cotman, C.W. Exercise reduces H3K9me3 and regulates brain derived neurotrophic factor and GABRA2 in an age dependent manner. Front. Aging Neurosci. 2021, 13, 798297. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ogawa, Y.; Ebihara, K.; Yamanaka, M.; Tsuchida, A.; Taiji, M.; Noguchi, H.; Nakao, K. Antiobesity and antidiabetic effects of brain-derived neurotrophic factor in rodent models of leptin resistance. Int. J. Obes. 2003, 27, 557–565. [Google Scholar] [CrossRef]
- Krabbe, K.; Nielsen, A.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.; Lindegaard, B.; Petersen, A.; Taudorf, S. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Garcia, C.; Chen, M.; Garza, A.; Cotman, C.; Russo-Neustadt, A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience 2003, 119, 721–732. [Google Scholar] [CrossRef]
- Ivy, A.; Rodriguez, F.; Garcia, C.; Chen, M.; Russo-Neustadt, A. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol. Biochem. Behav. 2003, 75, 81–88. [Google Scholar] [CrossRef]
- Sutoo, D.e.; Akiyama, K. Regulation of brain function by exercise. Neurobiol. Dis. 2003, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Russo-Neustadt, A.A.; Alejandre, H.; Garcia, C.; Ivy, A.S.; Chen, M.J. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology 2004, 29, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Okada, K.; Sato, A.; Uchida, S. Extracellular release of acetylcholine, noradrenaline and serotonin increases in the cerebral cortex during walking in conscious rats. Neurosci. Lett. 1993, 161, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.N.; Undieh, A.S. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport 2009, 20, 606–610. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef]
- de Queiroz, K.B.; Guimaraes, J.B.; Coimbra, C.C.; Rodovalho, G.V.; Carneiro, C.M.; Evangelista, E.A.; Guerra-Sá, R. Endurance training increases leptin expression in the retroperitoneal adipose tissue of rats fed with a high-sugar diet. Lipids 2014, 49, 85–96. [Google Scholar] [CrossRef]
- Halaas, J.L.; Boozer, C.; Blair-West, J.; Fidahusein, N.; Denton, D.A.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8878–8883. [Google Scholar] [CrossRef]
- Komori, T.; Morikawa, Y.; Nanjo, K.; Senba, E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 2006, 139, 1107–1115. [Google Scholar] [CrossRef]
- Wisse, B.E.; Schwartz, M.W. The skinny on neurotrophins. Nat. Neurosci. 2003, 6, 655–656. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R992–R1002. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.M.; Kanoski, S.E.; Hayes, M.R.; Grill, H.J. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E1252–E1260. [Google Scholar] [CrossRef] [PubMed]
- Kernie, S.G.; Liebl, D.J.; Parada, L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef]
- Yan, Q.; Radeke, M.J.; Matheson, C.R.; Talvenheimo, J.; Welcher, A.A.; Felnstein, S.C. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 1997, 378, 135–157. [Google Scholar] [CrossRef]
- Marmigère, F.; Rage, F.; Tapia-Arancibia, L. GABA–glutamate interaction in the control of BDNF expression in hypothalamic neurons. Neurochem. Int. 2003, 42, 353–358. [Google Scholar] [CrossRef]
- Nawa, H.; Carnahan, J.; Gall, C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: Partial disagreement with mRNA levels. Eur. J. Neurosci. 1995, 7, 1527–1535. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R1003–R1012. [Google Scholar] [CrossRef]
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Hefti, F. BDNF and NGF treatment in lesioned rats: Effects on cholinergic function and weight gain. Neuroreport 1992, 3, 405–408. [Google Scholar] [CrossRef]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.A.; Byerly, M.S. A mechanism underlying mature-onset obesity: Evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 286, R994–R1004. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Han, J.C.; Liu, Q.-R.; Jones, M.; Levinn, R.L.; Menzie, C.M.; Jefferson-George, K.S.; Adler-Wailes, D.C.; Sanford, E.L.; Lacbawan, F.L.; Uhl, G.R. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008, 359, 918–927. [Google Scholar] [CrossRef]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef]
- Pandit, M.; Behl, T.; Sachdeva, M.; Arora, S. Role of brain derived neurotropic factor in obesity. Obes. Med. 2020, 17, 100189. [Google Scholar] [CrossRef]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Mattson, M.P. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 2003, 144, 2446–2453. [Google Scholar] [CrossRef]
- Toriya, M.; Maekawa, F.; Maejima, Y.; Onaka, T.; Fujiwara, K.; Nakagawa, T.; Nakata, M.; Yada, T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010, 22, 987–995. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Wellman, C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995, 131, 229–238. [Google Scholar] [CrossRef]
- Nonomura, T.; Tsuchida, A.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchia, H. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. J. Diabetes Res. 2001, 2, 201–209. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tsuchida, A.; Itakura, Y.; Nonomura, T.; Ono, M.; Hirota, F.; Inoue, T.; Nakayama, C.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000, 49, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Boyuk, B.; Degirmencioglu, S.; Atalay, H.; Guzel, S.; Acar, A.; Celebi, A.; Ekizoglu, I.; Simsek, C. Relationship between levels of brain-derived neurotrophic factor and metabolic parameters in patients with type 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 978143. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Itakura, Y.; Inoue, T.; Tsuchida, A.; Nakagawa, T.; Noguchi, H.; Taiji, M. Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism 2006, 55, 1286–1292. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ono-Kishino, M.; Sugaru, E.; Yamanaka, M.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes/Metab. Res. Rev. 2002, 18, 185–191. [Google Scholar] [CrossRef]
- Givalois, L.; Naert, G.; Rage, F.; Ixart, G.; Arancibia, S.; Tapia-Arancibia, L. A single brain-derived neurotrophic factor injection modifies hypothalamo–pituitary–adrenocortical axis activity in adult male rats. Mol. Cell. Neurosci. 2004, 27, 280–295. [Google Scholar] [CrossRef]
- Guerra-Crespo, M.; Ubieta, R.; Joseph-Bravo, P.; Charli, J.L.; Pérez-Martínez, L. BDNF increases the early expression of TRH mRNA in fetal TrkB+ hypothalamic neurons in primary culture. Eur. J. Neurosci. 2001, 14, 483–494. [Google Scholar] [CrossRef]
- Cordeira, J.W.; Felsted, J.A.; Teillon, S.; Daftary, S.; Panessiti, M.; Wirth, J.; Sena-Esteves, M.; Rios, M. Hypothalamic dysfunction of the thrombospondin receptor α2δ-1 underlies the overeating and obesity triggered by brain-derived neurotrophic factor deficiency. J. Neurosci. 2014, 34, 554–565. [Google Scholar] [CrossRef]
- Fargali, S.; Sadahiro, M.; Jiang, C.; Frick, A.L.; Indall, T.; Cogliani, V.; Welagen, J.; Lin, W.-J.; Salton, S.R. Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis. J. Mol. Neurosci. 2012, 48, 654–659. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-m. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Mainardi, M.; Scabia, G.; Vottari, T.; Santini, F.; Pinchera, A.; Maffei, L.; Pizzorusso, T.; Maffei, M. A sensitive period for environmental regulation of eating behavior and leptin sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 16673–16678. [Google Scholar] [CrossRef]
- Cordeira, J.; Rios, M. Weighing in the role of BDNF in the central control of eating behavior. Mol. Neurobiol. 2011, 44, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Vanevski, F.; Xu, B. Molecular and neural bases underlying roles of BDNF in the control of body weight. Front. Neurosci. 2013, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, L.A.; Blue, M.E.; Siuciak, J.A.; Altar, C.A. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J. Neurosci. 1995, 15, 7929–7939. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Clark, M.S.; Rind, H.B.; Whittemore, S.R.; Russo, A.F. BDNF induction of tryptophan hydroxylase mRNA levels in the rat brain. J. Neurosci. Res. 1998, 52, 149–158. [Google Scholar] [CrossRef]
- Feijó, F.d.M.; Bertoluci, M.C.; Reis, C. Serotonin and hypothalamic control of hunger: A review. Rev. Da Assoc. Médica Brasileira 2011, 57, 74–77. [Google Scholar] [CrossRef]
- Harrold, J.A.; G Halford, J.C. The hypothalamus and obesity. Recent Pat. CNS Drug Discov. 2006, 1, 305–314. [Google Scholar] [CrossRef]
- Korner, J.; Wardlaw, S.L.; Liu, S.-M.; Conwell, I.M.; Leibel, R.L.; Chua, S.C., Jr. Effects of Leptin Receptor Mutation on Agrp Gene Expression in Fed and Fasted Lean and Obese (LA/N-fa f) Rats. Endocrinology 2000, 141, 2465–2471. [Google Scholar] [CrossRef]
- Byerly, M.S.; Simon, J.; Lebihan-Duval, E.; Duclos, M.J.; Cogburn, L.A.; Porter, T.E. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1180–R1189. [Google Scholar] [CrossRef]
- Cao, L.; Lin, E.-J.D.; Cahill, M.C.; Wang, C.; Liu, X.; During, M.J. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat. Med. 2009, 15, 447–454. [Google Scholar] [CrossRef]
- Richard, D. Involvement of corticotropin-releasing factor in the control of food intake and energy expenditure. Ann. N. Y. Acad. Sci. 1993, 697, 155–172. [Google Scholar] [CrossRef]
- Spina, M.; Merlo-Pich, E.; Chan, R.K.; Basso, A.M.; Rivier, J.; Vale, W.; Koob, G.F. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 1996, 273, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Huang, Q.; Timofeeva, E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int. J. Obes. 2000, 24, S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Zapanti, E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: The role of corticotropin releasing hormone. Nutr. Neurosci. 2004, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Risbrough, V.; Oakley, R.H.; Olivares-Reyes, J.A.; Dautzenberg, F.M. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann. N. Y. Acad. Sci. 2009, 1179, 120–143. [Google Scholar] [CrossRef]
- Milner, T.A.; Reis, D.; Pickel, V.; Aicher, S.; Giuliano, R. Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: Implications for central cardiovascular regulation. J. Comp. Neurol. 1993, 333, 151–167. [Google Scholar] [CrossRef]
- Reyes, B.A.; Valentino, R.J.; Xu, G.; Van Bockstaele, E.J. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur. J. Neurosci. 2005, 22, 93–106. [Google Scholar] [CrossRef]
- Brown, M.R.; Fisher, L.A.; Rivier, J.; Spiess, J.; Rivier, C.; Vale, W. Corticotropin-releasing factor: Effects on the sympathetic nervous system and oxygen consumption. Life Sci. 1982, 30, 207–210. [Google Scholar] [CrossRef]
- Naert, G.; Ixart, G.; Tapia-Arancibia, L.; Givalois, L. Continuous icv infusion of brain-derived neurotrophic factor modifies hypothalamic–pituitary–adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience 2006, 139, 779–789. [Google Scholar] [CrossRef]
- Clapham, J.C. Central control of thermogenesis. Neuropharmacology 2012, 63, 111–123. [Google Scholar] [CrossRef]
- Campbell, R.E.; Grove, K.L.; Smith, M.S. Distribution of corticotropin releasing hormone receptor immunoreactivity in the rat hypothalamus: Coexpression in neuropeptide Y and dopamine neurons in the arcuate nucleus. Brain Res. 2003, 973, 223–232. [Google Scholar] [CrossRef]
- Sternson, S.M.; Shepherd, G.M.; Friedman, J.M. Topographic mapping of VMH→ arcuate nucleus microcircuits and their reorganization by fasting. Nat. Neurosci. 2005, 8, 1356–1363. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Levine, A.; Billington, C.; Kotz, C.M. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R1037–R1045. [Google Scholar] [CrossRef]
- Levin, B.E. Neurotrophism and energy homeostasis: Perfect together. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R988–R991. [Google Scholar] [CrossRef]
- Lechan, R.M.; Fekete, C. The TRH neuron: A hypothalamic integrator of energy metabolism. Prog. Brain Res. 2006, 153, 209–235. [Google Scholar]
- Shintani, M.; Tamura, Y.; Monden, M.; Shiomi, H. Thyrotropin-releasing hormone induced thermogenesis in Syrian hamsters: Site of action and receptor subtype. Brain Res. 2005, 1039, 22–29. [Google Scholar] [CrossRef]
- Tsuchida, A.; Nonomura, T.; Ono-Kishino, M.; Nakagawa, T.; Taiji, M.; Noguchi, H. Acute effects of brain-derived neurotrophic factor on energy expenditure in obese diabetic mice. Int. J. Obes. 2001, 25, 1286–1293. [Google Scholar] [CrossRef]
- Zhang, Z.; Boelen, A.; Kalsbeek, A.; Fliers, E. TRH neurons and thyroid hormone coordinate the hypothalamic response to cold. Eur. Thyroid. J. 2018, 7, 279–288. [Google Scholar] [CrossRef]
- Iwen, K.A.; Oelkrug, R.; Brabant, G. Effects of thyroid hormones on thermogenesis and energy partitioning. J. Mol. Endocrinol. 2018, 60, R157–R170. [Google Scholar] [CrossRef]
- Bauer, C.S.; Tran-Van-Minh, A.; Kadurin, I.; Dolphin, A.C. A new look at calcium channel α2δ subunits. Curr. Opin. Neurobiol. 2010, 20, 563–571. [Google Scholar] [CrossRef]
- Davies, A.; Hendrich, J.; Van Minh, A.T.; Wratten, J.; Douglas, L.; Dolphin, A.C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007, 28, 220–228. [Google Scholar] [CrossRef]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Özkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999, 11, 4389–4397. [Google Scholar] [CrossRef]
- Rada, P.; Avena, N.M.; Hoebel, B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005, 134, 737–744. [Google Scholar] [CrossRef]
- Bassareo, V.; De Luca, M.A.; Di Chiara, G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J. Neurosci. 2002, 22, 4709–4719. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Cordeira, J.W.; Frank, L.; Sena-Esteves, M.; Pothos, E.N.; Rios, M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 2010, 30, 2533–2541. [Google Scholar] [CrossRef]
- Okazawa, H.; Murata, M.; Watanabe, M.; Kamei, M.; Kanazawa, I. Dopaminergic stimulation up-regulates the in vivo expression of brain-derived neurotrophic factor (BDNF) in the striatum. FEBS Lett. 1992, 313, 138–142. [Google Scholar] [CrossRef]
- Numan, S.; Seroogy, K.B. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: A double-label in situ hybridization study. J. Comp. Neurol. 1999, 403, 295–308. [Google Scholar] [CrossRef]
- Freeman, A.; Soghomonian, J.-J.; Pierce, R. Tyrosine kinase B and C receptors in the neostriatum and nucleus accumbens are co-localized in enkephalin-positive and enkephalin-negative neuronal profiles and their expression is influenced by cocaine. Neuroscience 2003, 117, 147–156. [Google Scholar] [CrossRef]
- Pu, L.; Liu, Q.-s.; Poo, M.-m. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat. Neurosci. 2006, 9, 605–607. [Google Scholar] [CrossRef]
- Tsao, D.; Thomsen, H.K.; Chou, J.; Stratton, J.; Hagen, M.; Loo, C.; Garcia, C.; Sloane, D.L.; Rosenthal, A.; Lin, J.C. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology 2008, 149, 1038–1048. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Melanson, E.L.; Salzberg, A.K.; Bechtell, J.L.; Tregellas, J.R. The effects of exercise on the neuronal response to food cues. Physiol. Behav. 2012, 105, 1028–1034. [Google Scholar] [CrossRef]
- Evero, N.; Hackett, L.C.; Clark, R.D.; Phelan, S.; Hagobian, T.A. Aerobic exercise reduces neuronal responses in food reward brain regions. J. Appl. Physiol. 2012, 112, 1612–1619. [Google Scholar] [CrossRef]
- De Castro, J.M. How can eating behavior be regulated in the complex environments of free-living humans? Neurosci. Biobehav. Rev. 1996, 20, 119–131. [Google Scholar] [CrossRef]
- Broberger, C. Brain regulation of food intake and appetite: Molecules and networks. J. Intern. Med. 2005, 258, 301–327. [Google Scholar] [CrossRef]
- Pessoa, L.; McMenamin, B. Dynamic networks in the emotional brain. Neuroscientist 2017, 23, 383–396. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tanaka, M.; Ishii, A.; Watanabe, Y. Immediate neural responses of appetitive motives and its relationship with hedonic appetite and body weight as revealed by magnetoencephalography. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2013, 19, 631. [Google Scholar]
- Yoshikawa, T.; Tanaka, M.; Ishii, A.; Watanabe, Y. Suppressive responses by visual food cues in postprandial activities of insular cortex as revealed by magnetoencephalography. Brain Res. 2014, 1568, 31–41. [Google Scholar] [CrossRef][Green Version]
- Crabtree, D.R.; Chambers, E.S.; Hardwick, R.M.; Blannin, A.K. The effects of high-intensity exercise on neural responses to images of food. Am. J. Clin. Nutr. 2014, 99, 258–267. [Google Scholar] [CrossRef]
- Killgore, W.D.; Kipman, M.; Schwab, Z.J.; Tkachenko, O.; Preer, L.; Gogel, H.; Bark, J.S.; Mundy, E.A.; Olson, E.A.; Weber, M. Physical exercise and brain responses to images of high-calorie food. Neuroreport 2013, 24, 962–967. [Google Scholar] [CrossRef]
- Geliebter, A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol. Behav. 1988, 44, 665–668. [Google Scholar] [CrossRef]
- Yau, A.M.; McLaughlin, J.; Maughan, R.J.; Gilmore, W.; Evans, G.H. The effect of short-term dietary fructose supplementation on gastric emptying rate and gastrointestinal hormone responses in healthy men. Nutrients 2017, 9, 258. [Google Scholar] [CrossRef]
- Näslund, E.; Hellström, P.M.; Kral, J.G. The gut and food intake: An update for surgeons. J. Gastrointest. Surg. 2001, 5, 556–567. [Google Scholar] [CrossRef]
- Powley, T.L.; Spaulding, R.A.; Haglof, S.A. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: Chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol. 2011, 519, 644–660. [Google Scholar] [CrossRef]
- Grundy, D. Neuroanatomy of visceral nociception: Vagal and splanchnic afferent. Gut 2002, 51, i2–i5. [Google Scholar] [CrossRef]
- Powley, T.L.; Phillips, R.J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 2004, 82, 69–74. [Google Scholar] [CrossRef]
- Jones, K.L.; Doran, S.M.; Hveem, K.; Bartholomeusz, F.; Morley, J.E.; Sun, W.M.; Chatterton, B.E.; Horowitz, M. Relation between postprandial satiation and antral area in normal subjects. Am. J. Clin. Nutr. 1997, 66, 127–132. [Google Scholar] [CrossRef]
- Santangelo, A.; Peracchi, M.; Conte, D.; Fraquelli, M.; Porrini, M. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br. J. Nutr. 1998, 80, 521–527. [Google Scholar] [CrossRef]
- Beckoff, K.; MacIntosh, C.G.; Chapman, I.M.; Wishart, J.M.; Morris, H.A.; Horowitz, M.; Jones, K.L. Effects of glucose supplementation on gastric emptying, blood glucose homeostasis, and appetite in the elderly. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R570–R576. [Google Scholar] [CrossRef]
- Halawi, H.; Camilleri, M.; Acosta, A.; Vazquez-Roque, M.; Oduyebo, I.; Burton, D.; Busciglio, I.; Zinsmeister, A.R. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G442–G447. [Google Scholar] [CrossRef]
- Meyer-Gerspach, A.C.; Wölnerhanssen, B.; Beglinger, B.; Nessenius, F.; Napitupulu, M.; Schulte, F.H.; Steinert, R.E.; Beglinger, C. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol. Behav. 2014, 129, 265–271. [Google Scholar] [CrossRef]
- Carrio, I.; Estorch, M.; Serra-Grima, R.; Ginjaume, M.; Notivol, R.; Calabuig, R.; Vilardell, F. Gastric emptying in marathon runners. Gut 1989, 30, 152–155. [Google Scholar] [CrossRef]
- Davis, J.; Camilleri, M.; Eckert, D.; Burton, D.; Joyner, M.; Acosta, A. Physical activity is associated with accelerated gastric emptying and increased ghrelin in obesity. Neurogastroenterol. Motil. 2020, 32, e13879. [Google Scholar] [CrossRef]
- Horner, K.M.; Byrne, N.M.; Cleghorn, G.J.; King, N.A. Influence of habitual physical activity on gastric emptying in healthy males and relationships with body composition and energy expenditure. Br. J. Nutr. 2015, 114, 489–496. [Google Scholar] [CrossRef]
- Horner, K.; Byrne, N.; Cleghorn, G.; Näslund, E.; King, N. The effects of weight loss strategies on gastric emptying and appetite control. Obes. Rev. 2011, 12, 935–951. [Google Scholar] [CrossRef]
- Noakes, T.D.; Rehrer, N.J.; Maughan, R.J. The importance of volume in regulating gastric emptying. Med. Sci. Sports Exerc. 1991, 23, 307–313. [Google Scholar] [CrossRef]
- Costill, D.; Saltin, B. Factors limiting gastric emptying during rest and exercise. J. Appl. Physiol. 1974, 37, 679–683. [Google Scholar] [CrossRef]
- Rehrer, N.; Beckers, E.; Brouns, F.; Ten Hoor, F.; Saris, W. Exercise and training effects on gastric emptying of carbohydrate beverages. Med. Sci. Sports Exerc. 1989, 21, 540–549. [Google Scholar] [CrossRef]
- Moore, J.; Christian, P.; Brown, J.; Brophy, C.; Datz, F.; Taylor, A.; Alazraki, N. Influence of meal weight and caloric content on gastric emptying of meals in man. Dig. Dis. Sci. 1984, 29, 513–519. [Google Scholar] [CrossRef]
- Horner, K.M.; Byrne, N.M.; King, N.A. Effect of combined interval and continuous exercise training on gastric emptying, appetite, and adaptive responses in men with overweight and obesity. Front. Nutr. 2021, 8, 654902. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Suzuki, H.; Masaoka, T.; Tanaka, K.; Mori, H.; Kanai, T. Influence of regular exercise on gastric emptying in healthy men: A pilot study. J. Clin. Biochem. Nutr. 2016, 59, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Schubert, M.M.; Desbrow, B.; Byrne, N.M.; King, N.A. Acute exercise and gastric emptying: A meta-analysis and implications for appetite control. Sports Med. 2015, 45, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. Bicarbonate buffering of lactic acid generated during exercise. J. Appl. Physiol. 1986, 60, 472–478. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Church, J.G. The effect of intravenous sodium bicarbonate on intestinal movements. Am. J. Physiol.-Leg. Content 1922, 62, 459–472. [Google Scholar] [CrossRef]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Fox, E.A.; Powley, T.L. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985, 341, 269–282. [Google Scholar] [CrossRef]
- Norgren, R.; Smith, G.P. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol. 1988, 273, 207–223. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Miselis, R.R. The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol. 1985, 238, 473–488. [Google Scholar] [CrossRef]
- Travagli, R.; Gillis, R. Hyperpolarization-activated currents, IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J. Neurophysiol. 1994, 71, 1308–1317. [Google Scholar] [CrossRef]
- Travagli, R.; Gillis, R.; Rossiter, C.; Vicini, S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol.-Gastrointest. Liver Physiol. 1991, 260, G531–G536. [Google Scholar] [CrossRef] [PubMed]
- Sivarao, D.V.; Krowicki, Z.K.; Hornby, P.J. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol. Motil. 1998, 10, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.F.; Derbenev, A.V.; Williams, K.W.; Glatzer, N.R.; Smith, B.N. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004, 1017, 208–217. [Google Scholar] [CrossRef]
- Babic, T.; Browning, K.N.; Travagli, R.A. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G21–G32. [Google Scholar] [CrossRef]
- Hautala, A.J.; Kiviniemi, A.M.; Tulppo, M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Browning, K.N.; Travagli, R.A. Central control of gastrointestinal motility. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 11–16. [Google Scholar] [CrossRef]
- Zheng, J.; Dobner, A.; Babygirija, R.; Ludwig, K.; Takahashi, T. Effects of repeated restraint stress on gastric motility in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1358–R1365. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Nakade, Y.; Mantyh, C.; Ludwig, K.; Pappas, T.N.; Takahashi, T. Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1537–R1541. [Google Scholar] [CrossRef]
- Bülbül, M.; Babygirija, R.; Ludwig, K.; Takahashi, T. Central oxytocin attenuates augmented gastric postprandial motility induced by restraint stress in rats. Neurosci. Lett. 2010, 479, 302–306. [Google Scholar] [CrossRef]
- Nakade, Y.; Tsuchida, D.; Fukuda, H.; Iwa, M.; Pappas, T.N.; Takahashi, T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R427–R432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Browning, K.N.; Toti, L.; Travagli, R.A. Vagally mediated gastric effects of brain stem α2-adrenoceptor activation in stressed rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G504–G516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Coleman, F.H.; Doheny, K.K.; Travagli, R.A. Stress adaptation upregulates oxytocin within hypothalamo-vagal neurocircuits. Neuroscience 2018, 390, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.E.; Ekkekakis, P.; Eisenmann, J.C. The physical activity, stress and metabolic syndrome triangle: A guide to unfamiliar territory for the obesity researcher. Obes. Rev. 2010, 11, 492–507. [Google Scholar] [CrossRef]
- Tsatsoulis, A.; Fountoulakis, S. The protective role of exercise on stress system dysregulation and comorbidities. Ann. N. Y. Acad. Sci. 2006, 1083, 196–213. [Google Scholar] [CrossRef]
- Schernthaner-Reiter, M.H.; Wolf, P.; Vila, G.; Luger, A. The interaction of insulin and pituitary hormone syndromes. Front. Endocrinol. 2021, 12, 626427. [Google Scholar] [CrossRef]
- Janssen, J.A. New insights into the role of insulin and hypothalamic-pituitary-adrenal (HPA) Axis in the metabolic syndrome. Int. J. Mol. Sci. 2022, 23, 8178. [Google Scholar] [CrossRef]
- Holmäng, A.; Björntorp, P. The effects of cortisol on insulin sensitivity in muscle. Acta Physiol. Scand. 1992, 144, 425–431. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.M.; Bowles, N.P.; Weil, Z.M.; Pfaff, D.W.; McEwen, B.S. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology 2010, 151, 2117–2127. [Google Scholar] [CrossRef]
- Kukucka, T.; Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Kovacova, V.; Macejova, A.; Mlyncekova, Z.; Tonhajzerova, I. Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change. Int. J. Mol. Sci. 2024, 25, 4511. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Johnson, J.D.; Solinas, G.; Jansson, P.-A. Insulin Hypersecretion as Promoter of Body Fat Gain and Hyperglycemia. Diabetes 2024, 73, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kuckuck, S.; van Der Valk, E.S.; Scheurink, A.J.; van Der Voorn, B.; Iyer, A.M.; Visser, J.A.; Delhanty, P.J.; van den Berg, S.A.; van Rossum, E.F. Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones. Obes. Rev. 2023, 24, e13539. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Foster, M.T.; Warne, J.P.; Ginsberg, A.B.; Horneman, H.F.; Pecoraro, N.C.; Akana, S.F.; Dallman, M.F. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology 2009, 150, 2325–2333. [Google Scholar] [CrossRef]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones 2005, 4, 73–89. [Google Scholar]
- Boutcher, S.H.; Nurhayati, Y.; McLaren, P.F. Cardiovascular response of trained and untrained old men to mental challenge. Med. Sci. Sports Exerc. 2001, 33, 659–664. [Google Scholar] [CrossRef]
- Brownley, K.A.; Hinderliter, A.L.; West, S.G.; Girdler, S.S.; Sherwood, A.; Light, K.C. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med. Sci. Sports Exerc. 2003, 35, 978–986. [Google Scholar] [CrossRef]
- Nabkasorn, C.; Miyai, N.; Sootmongkol, A.; Junprasert, S.; Yamamoto, H.; Arita, M.; Miyashita, K. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Public Health 2006, 16, 179–184. [Google Scholar] [CrossRef]
- Davies, C.; Few, J. Effects of exercise on adrenocortical function. J. Appl. Physiol. 1973, 35, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Chatterjee, S.; Roy, D. Impact of exercise on brain neurochemicals: A comprehensive review. Sport Sci. Health 2023, 19, 405–452. [Google Scholar] [CrossRef]
- McMurray, R.G. Exercise, Mood States, and Neuroendocrinology. In Mind-Body Maturity; Taylor & Francis: Abingdon, UK, 2019; pp. 237–254. [Google Scholar]
- Van Hook, M.J. Temperature effects on synaptic transmission and neuronal function in the visual thalamus. PLoS ONE 2020, 15, e0232451. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.; Hua, T.; Cai, J. Alpha-melanocyte-stimulating hormone-mediated appetite regulation in the central nervous system. Neuroendocrinology 2023, 113, 885–904. [Google Scholar] [CrossRef]
- Meguid, M.M.; Fetissov, S.O.; Varma, M.; Sato, T.; Zhang, L.; Laviano, A.; Rossi-Fanelli, F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 2000, 16, 843–857. [Google Scholar] [CrossRef]
- Gasmi, A.; Nasreen, A.; Menzel, A.; Gasmi Benahmed, A.; Pivina, L.; Noor, S.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 2022, 28, 210. [Google Scholar] [CrossRef]
- Chondronikola, M.; Porter, C.; Malagaris, I.; Nella, A.A.; Sidossis, L.S. Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation. Eur. J. Endocrinol. 2017, 177, 33–40. [Google Scholar] [CrossRef]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Olza, J.; Aguilera, C.M.; Gil, Á.; Ruiz, J.R. Role of exercise in the activation of brown adipose tissue. Ann. Nutr. Metab. 2015, 67, 21–32. [Google Scholar] [CrossRef]
- Dong, H.; Qin, M.; Wang, P.; Li, S.; Wang, X. Regulatory effects and mechanisms of exercise on activation of brown adipose tissue (BAT) and browning of white adipose tissue (WAT). Adipocyte 2023, 12, 2266147. [Google Scholar] [CrossRef]
- Aydin, S.; Guven, T.; Sahin, I.; Aksoy, A.; Kendir, Y.; Ilhan, M.N.; Citil, C.; Catak, Z.; Ustun, C. The effects of fever on hormone ghrelins, immunoglobulins, and heat shock protein 70 expression after swine flu vaccinations. Endocrine 2012, 42, 352–358. [Google Scholar] [CrossRef]
- Mandic, I.; Ahmed, M.; Rhind, S.; Goodman, L.; L’Abbe, M.; Jacobs, I. The effects of exercise and ambient temperature on dietary intake, appetite sensation, and appetite regulating hormone concentrations. Nutr. Metab. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Laurila, S.; Lahesmaa, M.; Rebelos, E.; Virtanen, K.A.; Schnabl, K.; Klingenspor, M.; Nummenmaa, L.; Nuutila, P. Secretin modulates appetite via brown adipose tissue-brain axis. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schnabl, K.; Gabler, S.-M.; Willershäuser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; u Din, M.; Bast-Habersbrunner, A. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell 2018, 175, 1561–1574.e1512. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.K.; Liu, S.-M.; Chua, S.C., Jr.; Schwartz, G.J.; Jo, Y.-H. Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake. PLoS Biol. 2018, 16, e2004399. [Google Scholar] [CrossRef]
- Cheng, C.Y.Y.; Chu, J.Y.S.; Chow, B.K.C. Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 2011, 36, 459–471. [Google Scholar] [CrossRef]
- Ryu, V.; Garretson, J.T.; Liu, Y.; Vaughan, C.H.; Bartness, T.J. Brown adipose tissue has sympathetic-sensory feedback circuits. J. Neurosci. 2015, 35, 2181–2190. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hirvonen, J.; Hannukainen, J.C.; Immonen, H.; Lindroos, M.M.; Salminen, P.; Nuutila, P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS ONE 2012, 7, e31089. [Google Scholar] [CrossRef]
- Laurila, S.; Sun, L.; Lahesmaa, M.; Schnabl, K.; Laitinen, K.; Klén, R.; Li, Y.; Balaz, M.; Wolfrum, C.; Steiger, K. Secretin activates brown fat and induces satiation. Nat. Metab. 2021, 3, 798–809. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Smith, G.; Gunthorpe, M.; Kelsell, R.; Hayes, P.; Reilly, P.; Facer, P.; Wright, J.; Jerman, J.; Walhin, J.-P.; Ooi, L. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, F.; Liu, S.; Colton, C.K.; Wang, C.; Cui, Y.; Cao, X.; Zhu, M.X.; Sun, C.; Wang, K. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J. Biol. Chem. 2012, 287, 7279–7288. [Google Scholar] [CrossRef]
- Fonseca, C.G.; Pires, W.; Lima, M.R.M.; Guimaraes, J.B.; Lima, N.R.V.; Wanner, S.P. Hypothalamic temperature of rats subjected to treadmill running in a cold environment. PLoS ONE 2014, 9, e111501. [Google Scholar] [CrossRef]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.-e.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 2013, 33, 3624–3632. [Google Scholar] [CrossRef]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef]
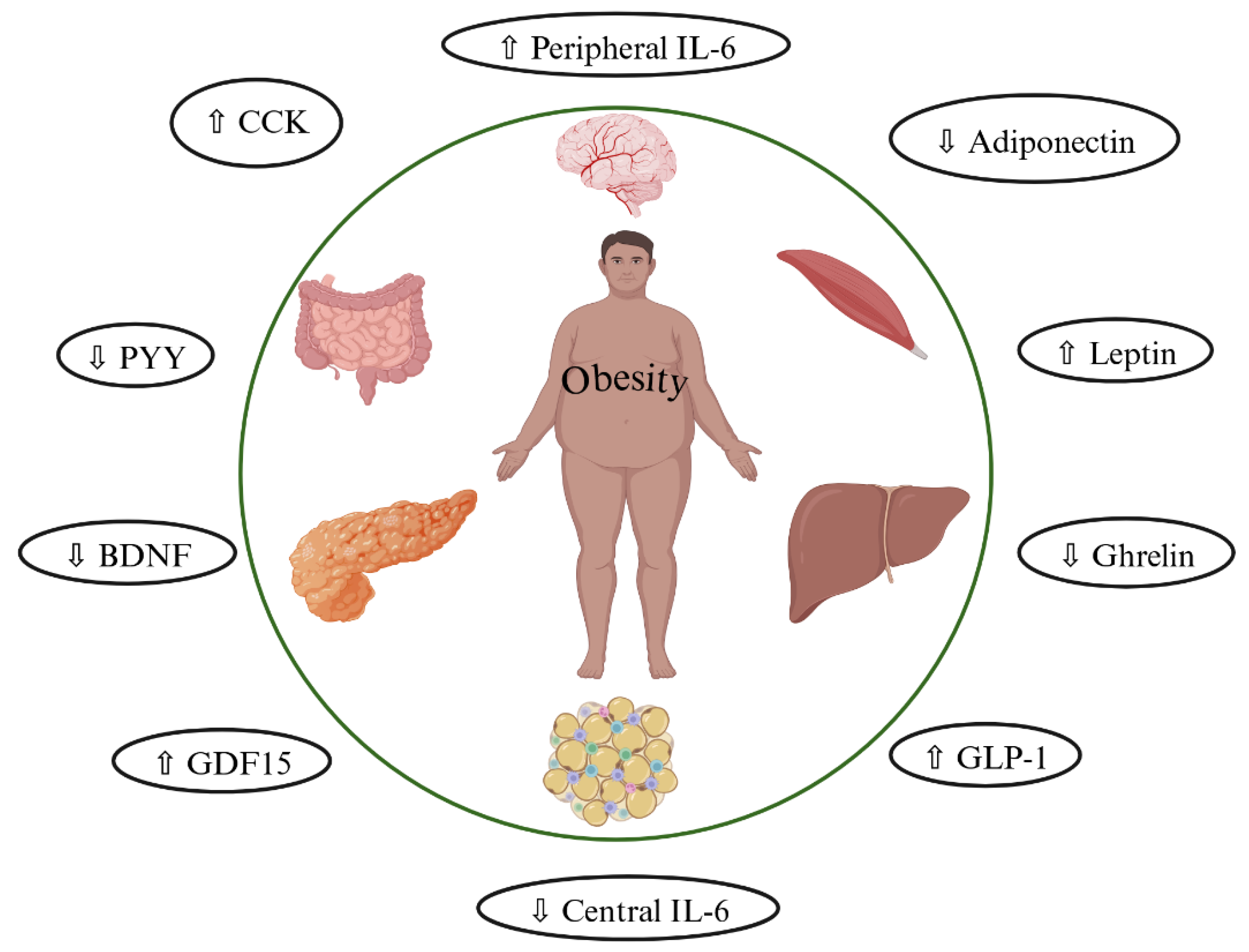
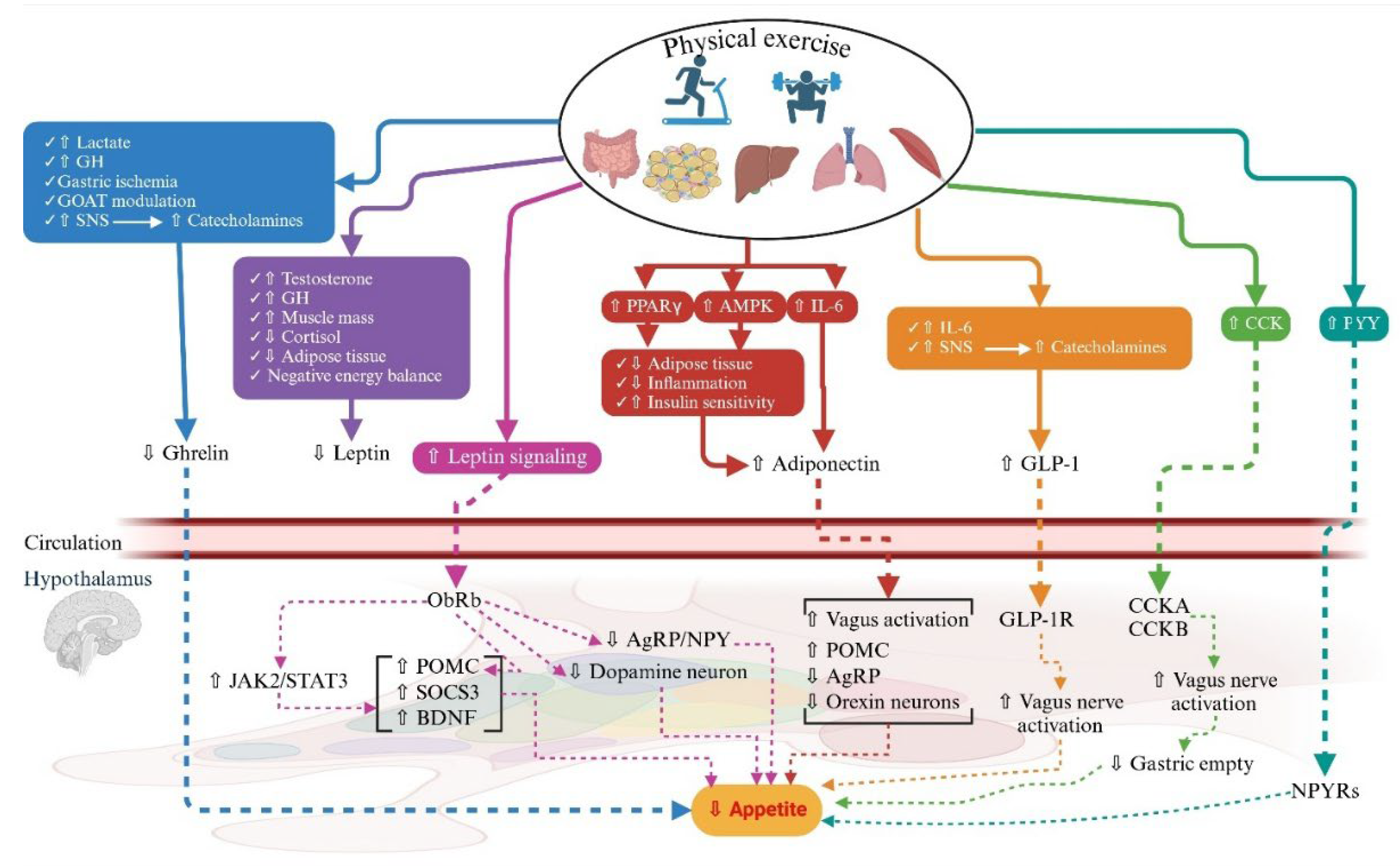
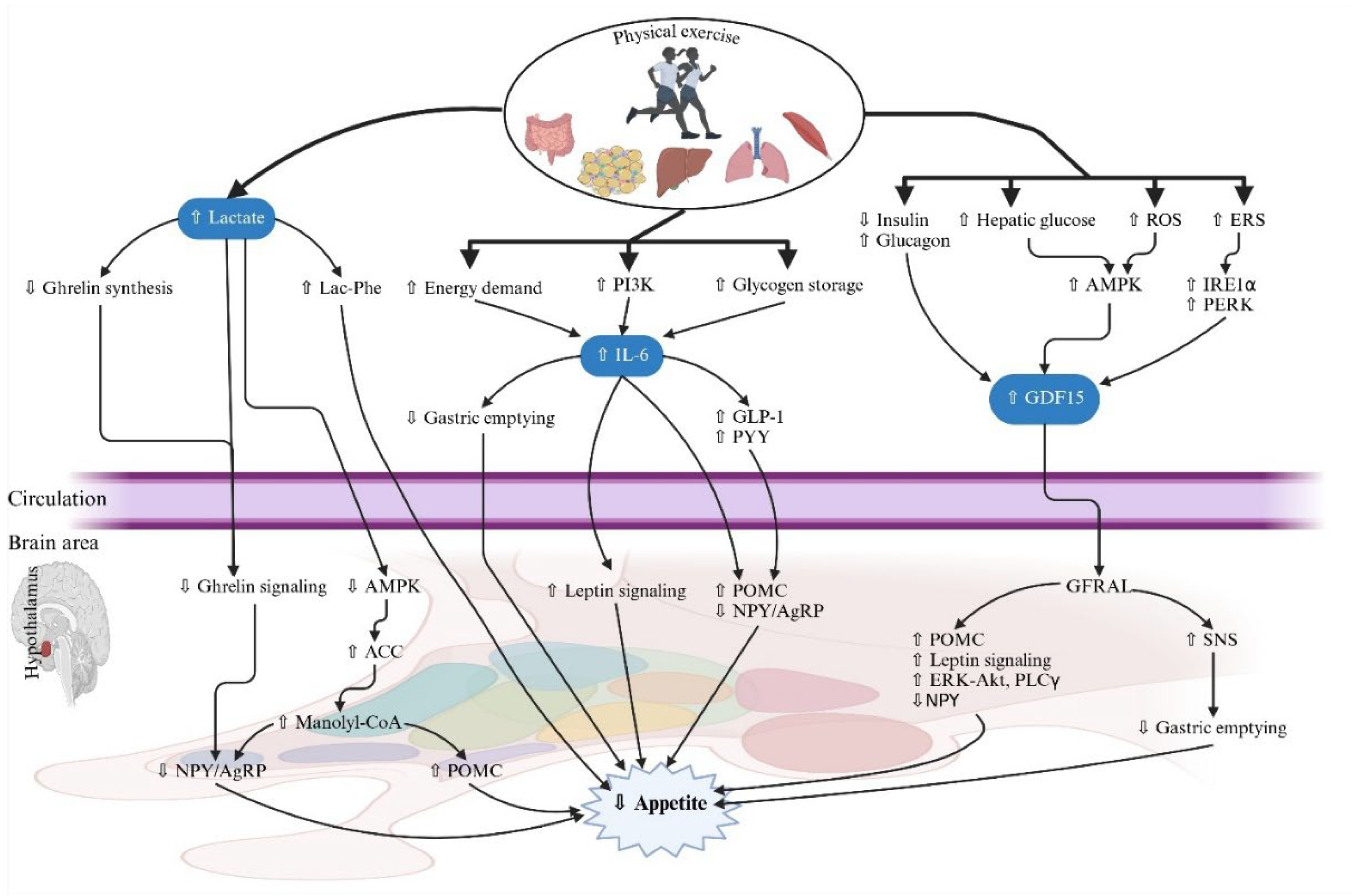

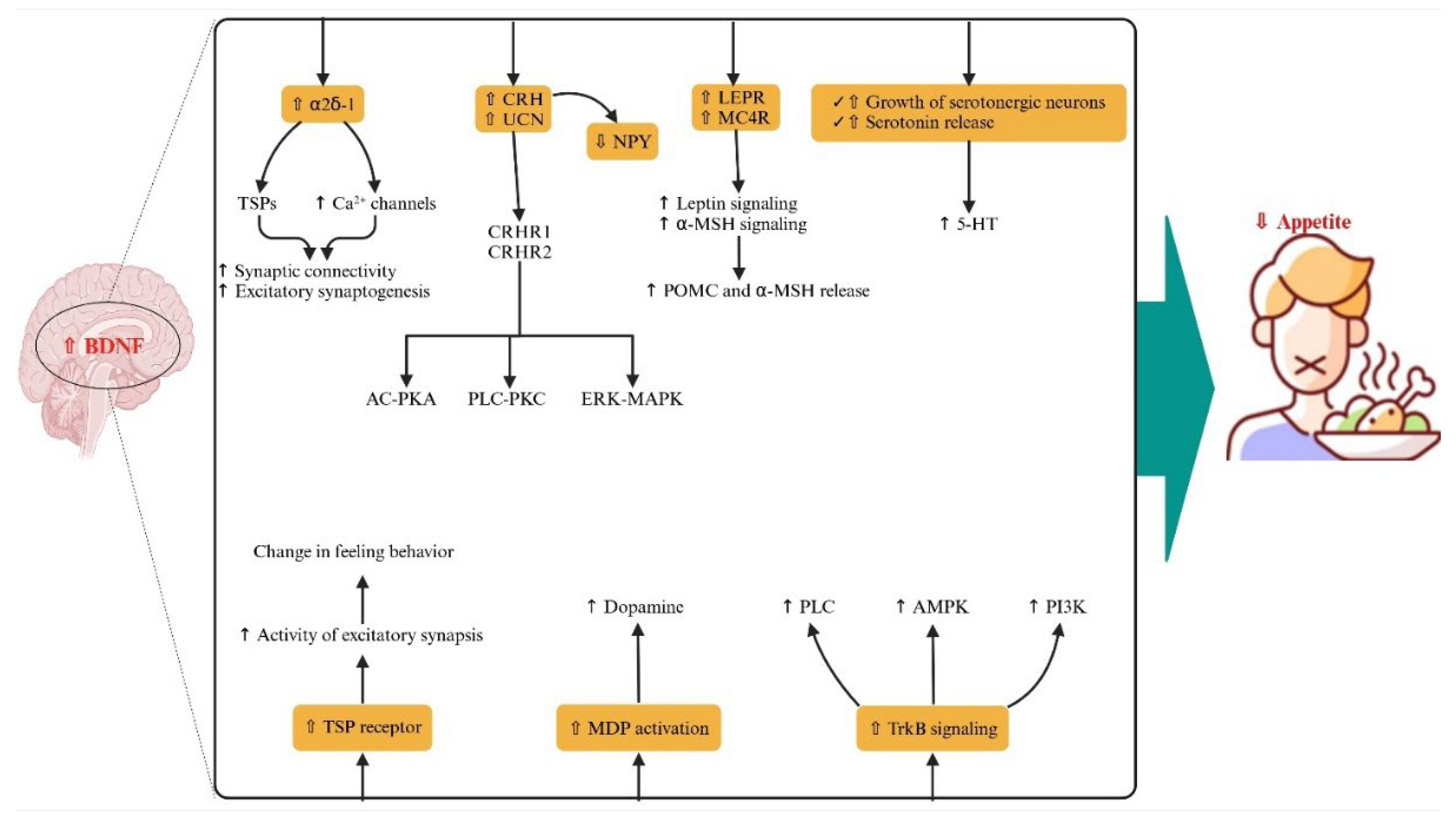
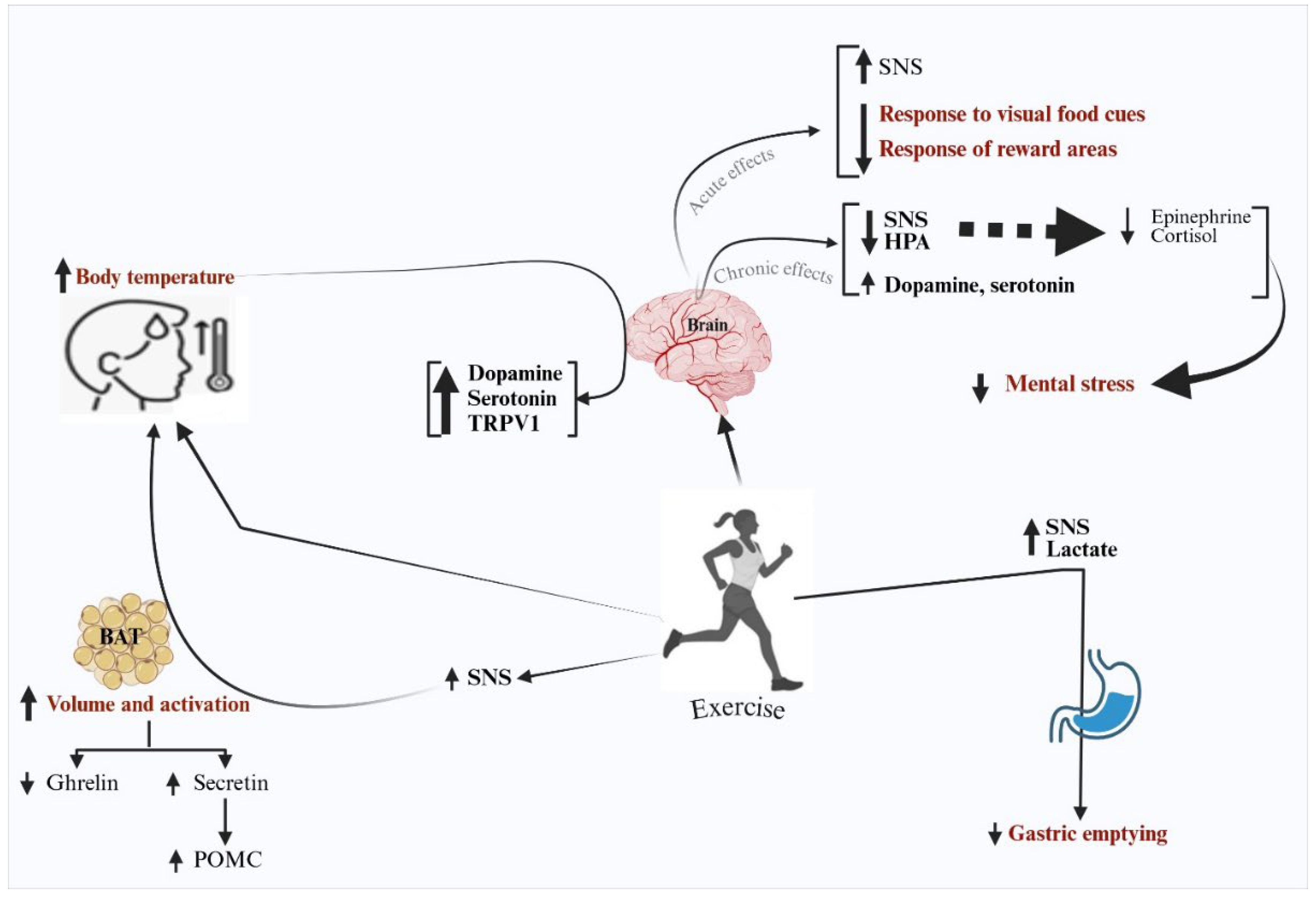
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razi, O.; Zamani, N.; Moraes, C.d.; Laher, I.; Hadjicharalambous, M. Exercise Suppresses Appetite in Obesity: A Biochemical, Metabolic, and Molecular Approach. Appl. Sci. 2025, 15, 6191. https://doi.org/10.3390/app15116191
Razi O, Zamani N, Moraes Cd, Laher I, Hadjicharalambous M. Exercise Suppresses Appetite in Obesity: A Biochemical, Metabolic, and Molecular Approach. Applied Sciences. 2025; 15(11):6191. https://doi.org/10.3390/app15116191
Chicago/Turabian StyleRazi, Omid, Nastaran Zamani, Camila de Moraes, Ismail Laher, and Marios Hadjicharalambous. 2025. "Exercise Suppresses Appetite in Obesity: A Biochemical, Metabolic, and Molecular Approach" Applied Sciences 15, no. 11: 6191. https://doi.org/10.3390/app15116191
APA StyleRazi, O., Zamani, N., Moraes, C. d., Laher, I., & Hadjicharalambous, M. (2025). Exercise Suppresses Appetite in Obesity: A Biochemical, Metabolic, and Molecular Approach. Applied Sciences, 15(11), 6191. https://doi.org/10.3390/app15116191








