A Systematic Review of the Toxicokinetics of Micro- and Nanoplastics in Mammals Following Digestive Exposure
Abstract
1. Introduction
1.1. Sources of Microplastic (MP) and Nanoplastic (NP) Pollution
1.2. Plastic Types, Sizes, and Additives
1.3. Presence and Degradation of Plastics in the Environment
1.4. Identification and Toxicological Risk of Plastics
2. Microplastic and Nanoplastic Uptake
2.1. MP and NP Ingestion
2.2. Intestinal Absorbtion
2.3. MP and NP Internalization at the Molecular Level
3. Methodology
3.1. Search Strategy
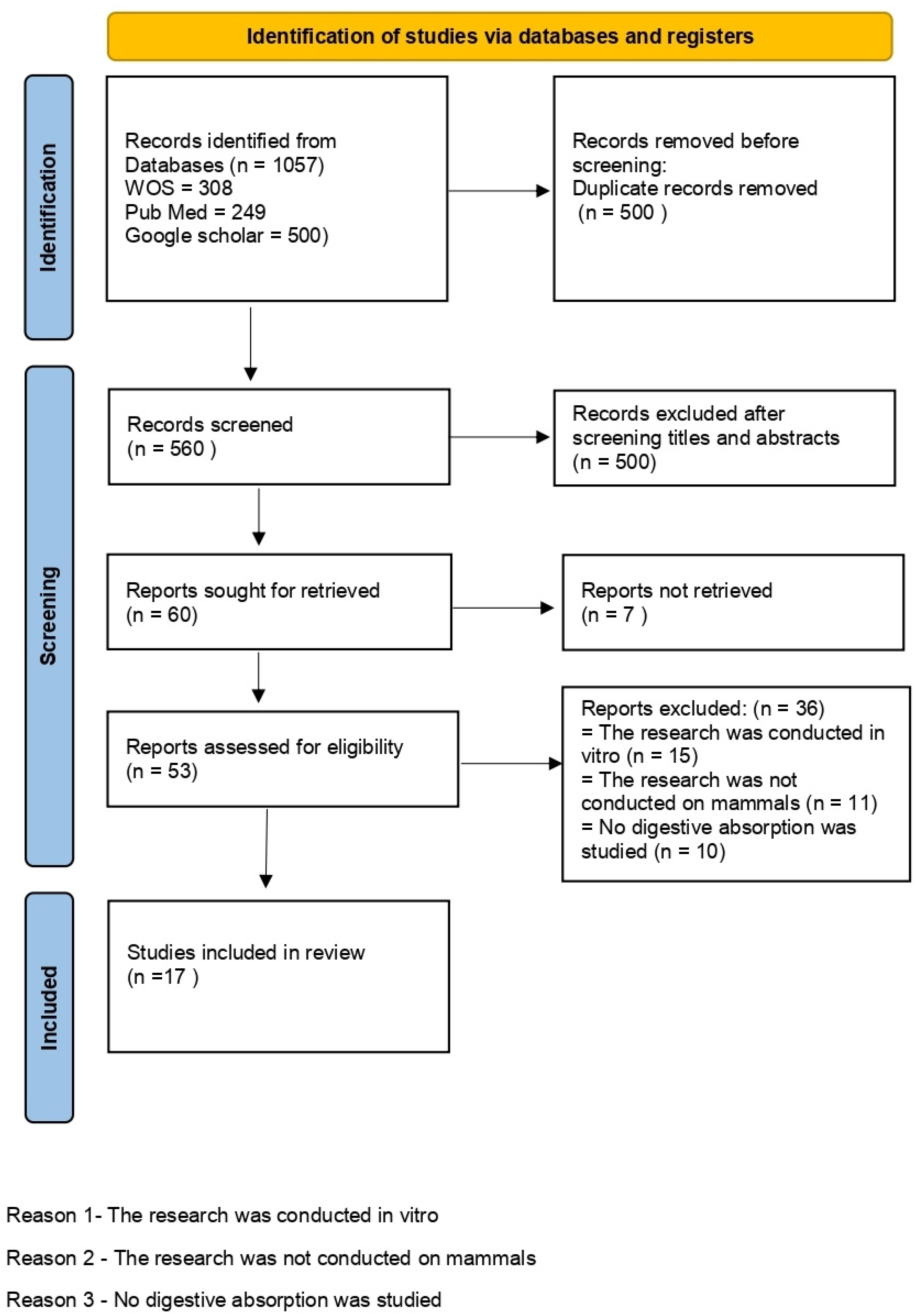
3.2. Study Selection
- -
- a total of 500 duplicate articles were removed (557 remained);
- -
- after reading the titles and abstracts, 500 were eliminated (60 articles were evaluated in full-text form);
- -
- seven articles were deleted due to a lack of irrelevant information and data (53);
- -
- reports were excluded if the research was performed in vitro (15) or not on mammals (11);
- -
- finally, 17 articles were included in the review.
4. Results
Inclusion and Exclusion Criteria
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | microplastic |
| NP | nanoplastic |
| PS | polystyrene |
| PS MP | polystyrene microplastic |
| PE | polyethylene |
| OPERs | organophosphorus flame retardants |
| wk | week |
| PAEs | phthalate esters |
| PS-COOH | carboxylated polystyrene |
| PS-NH2 | charged aminated polystyrene |
| ALP | alkaline phosphatase |
| AST | aspartate transaminase |
| T-Bil | total bilirubin |
| CK | creatine kinase |
| r-GT | r-glutamine transferase |
| SCr | serum creatinine |
| TNF-α | tumor necrosis factor alpha |
| IL-1 β | interleukin-β |
| IFN-γ | interferon gamma |
| PP | polypropylene |
| TLR4 | toll-like receptor 4 |
| NF-kB | nuclear factor kappa light chain enhancer of activation B |
| BUN | blood urea nitrogen |
| CRE | creatinine |
| IL-6 | interleukin-6 |
| PET | polyethylene terephthalate |
| PEHD | polyethylene high-density |
| PVC | polyvinyl chloride |
| PU | polyurethane |
| PP | polypropylene |
| EPS | expandable polystyrene |
| PCBs | polychlorinated biphenyls |
| PAHs | polycyclic aromatic hydrocarbons |
| UV | ultraviolet |
| μ-FTIR | micro-Fourier transform infrared spectroscopy |
| EFSA | European Food Safety Authority |
| DNA | deoxyribonucleic acid |
| M | microfold cells |
| GALT | intestinal epithelial cells in gut-associated lymphoid |
| GI | gastrointestinal |
| BMDM | bone marrow-derived macrophages |
| 293T | renal epithelial cells |
| L929 | fibroblasts |
References
- Ritchie, H.; Roser, M. Plastic Pollution. Our World in Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 10 September 2023).
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Sharma, S.; Kundu, A.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Sustainable environmental management and related biofuel technologies. J. Environ. Manag. 2020, 273, 111096. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wick, P.; Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 2021, 16, 491–500. [Google Scholar] [CrossRef]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.A. Nanoplastics in the aquatic environment. Microplastic Contam. Aquatic Environ. 2018, 13, 379–399. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and water pollution and human health: What should cardiologists worry about? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics contamination of groundwater: Current evidence and future perspectives. A review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Rane, N.R.; Bankole, P.O.; Krishnaiah, P.; Ahn, Y.; Park, Y.K.; Yadav, K.K.; Amin, M.A.; Jeon, B.H. An assessment of micro-and nanoplastics in the biosphere: A review of detection, monitoring, and remediation technology. Chem. Eng. J. 2022, 430, 132913. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Li, H.X.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro-and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef] [PubMed]
- Lenaker, P.L.; Baldwin, A.K.; Corsi, S.R.; Mason, S.A.; Reneau, P.C.; Scott, J.W. Vertical distribution of microplastics in the water column and surficial sediment from the Milwaukee River Basin to Lake Michigan. Environ. Sci. Technol. 2019, 53, 12227–12237. [Google Scholar] [CrossRef]
- Lithner, D.; Nordensvan, I.; Dave, G. Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile–butadiene–styrene, and epoxy to Daphnia magna. Environ. Sci. Pollut. Res. 2012, 19, 1763–1772. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Galgani, F.; Fleet, D.; Van Franeker, J.; Katsanevakis, S.; Maes, T.; Mouat, J.; Oosterbaan, L.; Poitou, I.; Hanke, G.; Thompson, R.; et al. Marine Strategy Framework Directive-Task Group 10 Report Marine Litter; Office for Official Publications of the European Communities: Luxembourg, 2010. [Google Scholar] [CrossRef]
- Fuhr, L.; Schächtele, K.; Matthew, F. The Plastic Atlas 2019; Heinrich Boll Foundation: Berlin, Germany, 2019; Available online: https://za.boell.org/en/2019/11/06/plastic-atlas-facts-and-figures-about-world-synthetic-polymers (accessed on 16 January 2024).
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2021, 407, 124399. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Banerjee, A.; Shelver, W.L. Micro-and nanoplastic-mediated pathophysiological changes in rodents, rabbits, and chickens: A review. J. Food Prot. 2021, 84, 1480–1495. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; International Maritime Organisation: London, UK, 2015; 96p. [Google Scholar]
- Ribeiro, F.; O’Brien, J.W.; Galloway, T.; Thomas, K.V. Accumulation and fate of nano-and micro-plastics and associated contaminants in organisms. TrAC Trends Anal. Chem. 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Schröter, L.; Ventura, N. Nanoplastic toxicity: Insights and challenges from experimental model systems. Small 2022, 18, 2201680. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Presence of microplastics and nanoplastics in food, with particulari focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar]
- Jahnke, A.; Arp, H.P.H.; Escher, B.I.; Gewert, B.; Gorokhova, E.; Kühnel, D.; Ogonowski, M.; Potthoff, A.; Rummel, C.; Schmitt-Jansen, M.; et al. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 2017, 4, 85–90. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano-and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 325–340. [Google Scholar]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef]
- Ameen, A.; Stevenson, M.; Blaschke, A.P. Microplastics: Are they really a threat to groundwater systems? In Proceedings of the EGU General Assembly 2020, Online, 4–8 May 2020; p. 9069. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- van Emmerik, T.; Schwarz, A. Plastic debris in rivers. Wiley Interdiscip. Rev. Water 2020, 7, e1398. [Google Scholar] [CrossRef]
- Roman, L.; Lowenstine, L.; Parsley, L.M.; Wilcox, C.; Hardesty, B.D.; Gilardi, K.; Hindell, M. Is plastic ingestion in birds as toxic as we think? Insights from a plastic feeding experiment. Sci. Total Environ. 2019, 665, 660–667. [Google Scholar] [CrossRef]
- Kühn, S.; van Franeker, J.A.; O’Donoghue, A.M.; Swiers, A.; Starkenburg, M.; van Werven, B.; Foekema, E.; Hermsen, E.; Egelkraut-Holtus, M.; Lindeboom, H. Details of plastic ingestion and fibre contamination in North Sea fishes. Environ. Pollut. 2020, 257, 113569. [Google Scholar] [CrossRef] [PubMed]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S. Can biodegradable plastics solve plastic solid waste accumulation? In Plastics to Energy; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 403–423. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Handbook of Polymers; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene nanoparticles perturb lipid membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. CB 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Yoo, J.W.; Doshi, N.; Mitragotri, S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, M.; Białowąs, M.; Stankevičiūtė, M.; Chomiczewska, A.; Pažusienė, J.; Jonko-Sobuś, K.; Hallmann, A.; Urban-Malinga, B. Effects of chronic exposure to microplastics of different polymer types on early life stages of sea trout Salmo trutta. Sci. Total Environ. 2020, 740, 139922. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.S.M.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- McAloose, D.; Newton, A.L. Wildlife cancer: A conservation perspective. Nat. Rev. Cancer 2009, 9, 517–526. [Google Scholar] [CrossRef]
- Erren, T.; Zeuß, D.; Steffany, F.; Meyer-Rochow, B. Increase of wildlife cancer: An echo of plastic pollution? Nat. Rev. Cancer 2009, 9, 842. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Li, Y.; He, X.; Wang, R.; Xu, J.; Gao, G. The accumulation of microplastics in fish from an important fish farm and mariculture area, Haizhou Bay, China. Sci. Total Environ. 2019, 696, 133948. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, S. Contamination of table salts from Turkey with microplastics. Food Addit. Contam. Part A 2018, 35, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Laohaprapanon, S. Quality assessment for methodological aspects of microplastics analysis in bottled water–A critical review. Food Control 2021, 130, 108285. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded milks–are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Philipp, C.; Unger, B.; Fischer, E.K.; Schnitzler, J.G.; Siebert, U. Handle with care—Microplastic particles in intestine samples of seals from German waters. Sustainability 2020, 12, 10424. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Cabellos, J.; Delpivo, C.; Fernández-Rosas, E.; Vázquez-Campos, S.; Janer, G. Contribution of M-cells and other experimental variables in the translocation of TiO2 nanoparticles across in vitro intestinal models. NanoImpact 2017, 5, 51–60. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Coméra, C.; Cartier, C.; Gaultier, E.; Catrice, O.; Panouille, Q.; El Hamdi, S.; Tirez, K.; Nelissen, I.; Théodorou, V.; Houdeau, E. Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: An in vivo and ex vivo study in mice. Part. Fibre Toxicol. 2020, 17, 26. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro-and nano-plastics and human health. Mar. Anthropog. Litter 2015, 1, 343–366. [Google Scholar] [CrossRef]
- Pappo, J.; Ermak, T.H.; Steger, H.J. Monoclonal antibody-directed targeting of fluorescent polystyrene microspheres to Peyer’s patch M cells. Immunology 1991, 73, 277. [Google Scholar]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef] [PubMed]
- Brody, H. The gut microbiome. Nature 2020, 577, S5. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 723–731. [Google Scholar] [CrossRef]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. The importance of contamination control in airborne fibers and microplastic sampling: Experiences from indoor and outdoor air sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2020, 159, 111522. [Google Scholar] [CrossRef]
- Peda, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano-and microplastics for freshwater ecosystems: A critical review. TrAC Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Zhu, Q.; Zhang, Y. Tissue accumulation of microplastics and toxic effects: Widespread health risks of microplastics exposure. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; Springer: Berlin/Heidelberg, Germany, 2020; pp. 321–341. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Hodges, G.M.; Carr, E.A.; Hazzard, R.A.; Carr, K.E. Uptake and translocation of microparticles in small intestine: Morphology and quantification of particle distribution. Dig. Dis. Sci. 1995, 40, 967–975. [Google Scholar] [CrossRef]
- Chang, X.; Xue, Y.; Li, J.; Zou, L.; Tang, M. Potential health impact of environmental micro-and nanoplastics pollution. J. Appl. Toxicol. 2020, 40, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.H.; Etherington, G.; Shutt, A.L.; Youngman, M.J. A study of aerosol deposition and clearance from the human nasal passage. Ann. Occup. Hyg. 2002, 46 (Suppl. S1), 309–313. [Google Scholar] [CrossRef]
- Asgharian, B.; Hofmann, W.; Miller, F.J. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J. Aerosol Sci. 2001, 32, 817–832. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Monti, D.M.; Guarnieri, D.; Napolitano, G.; Piccoli, R.; Netti, P.; Fusco, S.; Arciello, A. Biocompatibility, uptake and endocytosis pathways of polystyrene nanoparticles in primary human renal epithelial cells. J. Biotechnol. 2015, 193, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Harush-Frenkel, O.; Rozentur, E.; Benita, S.; Altschuler, Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules 2008, 9, 435–443. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailänder, V. Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- Firdessa, R.; Oelschlaeger, T.A.; Moll, H. Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: Relevance for drug delivery systems. Eur. J. Cell Biol. 2014, 93, 323–337. [Google Scholar] [CrossRef]
- Levin, R.; Grinstein, S.; Schlam, D. Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Citi, S. The molecular organization of tight junctions. J. Cell Biol. 1993, 121, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T. Clathrin. Annu. Rev. Biochem. 2000, 69, 699–727. [Google Scholar] [CrossRef]
- Damm, E.M.; Pelkmans, L.; Kartenbeck, J.; Mezzacasa, A.; Kurzchalia, T.; Helenius, A. Clathrin-and caveolin-1–independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005, 168, 477–488. [Google Scholar] [CrossRef]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef]
- Yacobi, N.R.; Malmstadt, N.; Fazlollahi, F.; DeMaio, L.; Marchelletta, R.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. Am. J. Respir. Cell Mol. Biol. 2010, 42, 604–614. [Google Scholar] [CrossRef]
- Jiang, X.; Musyanovych, A.; Röcker, C.; Landfester, K.; Mailänder, V.; Nienhaus, G.U. Specific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cells. Nanoscale 2011, 3, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dausend, J.; Hafner, M.; Musyanovych, A.; Röcker, C.; Landfester, K.; Mailänder, V.; Nienhaus, G.U. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules 2010, 11, 748–753. [Google Scholar] [CrossRef]
- Hillery, A.; Jani, P.; Florence, A. Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles. J. Drug Target. 1994, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Prüst, M.; Meijer, J.; Westerink, R.H. The plastic brain: Neurotoxicity of micro-and nanoplastics. Part. Fibre Toxicol. 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 1. [Google Scholar] [CrossRef]
- Doyle-McCullough, M.; Smyth, S.H.; Moyes, S.M.; Carr, K.E. Factors influencing intestinal microparticle uptake in vivo. Int. J. Pharm. 2007, 335, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Qiao, R.; Bonilla, M.M.; Yang, X.; Ren, H.; Lemos, B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J. Hazard. Mater. 2018, 357, 348–354. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Wang, J.; Wu, B.; Guo, X. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 2022, 833, 155198. [Google Scholar] [CrossRef]
- Lee, S.; Kang, K.K.; Sung, S.E.; Choi, J.H.; Sung, M.; Seong, K.Y.; Lee, J.; Kang, S.; Yang, S.Y.; Lee, S.; et al. In Vivo toxicity and pharmacokinetics of polytetrafluoroethylene microplastics in ICR mice. Polymers 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to polypropylene microplastics via oral ingestion induces colonic apoptosis and intestinal barrier damage through oxidative stress and inflammation in mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guan, J.; Feng, Y.; Nie, L.; Xu, Y.; Xu, H.; Fu, F. Polystyrene microplastics induced nephrotoxicity associated with oxidative stress, inflammation, and endoplasmic reticulum stress in juvenile rats. Front. Nutr. 2023, 9, 1059660. [Google Scholar] [CrossRef] [PubMed]
- Guševac Stojanović, I.; Drakulić, D.; Todorović, A.; Martinović, J.; Filipović, N.; Stojanović, Z. Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats. Toxics 2024, 12, 167. [Google Scholar] [CrossRef]
- Gałęcka, I.; Szyryńska, N.; Całka, J. Influence of polyethylene terephthalate (PET) microplastic on selected active substances in the intramural neurons of the porcine duodenum. Part. Fibre Toxicol. 2024, 21, 5. [Google Scholar] [CrossRef]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene microplastic-induced oxidative stress triggers intestinal barrier dysfunction via the NF-κB/NLRP3/IL-1β/MCLK pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef]
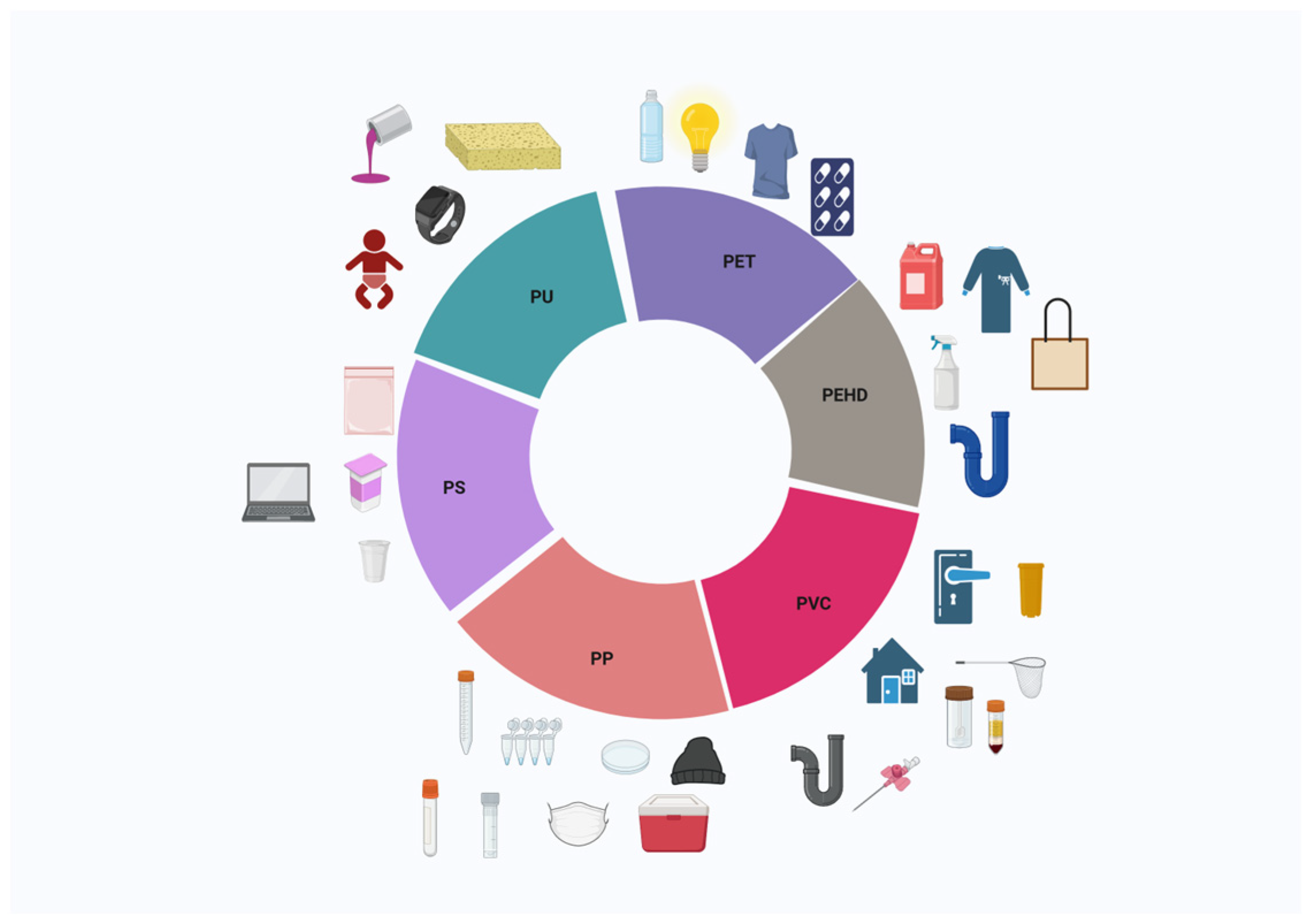
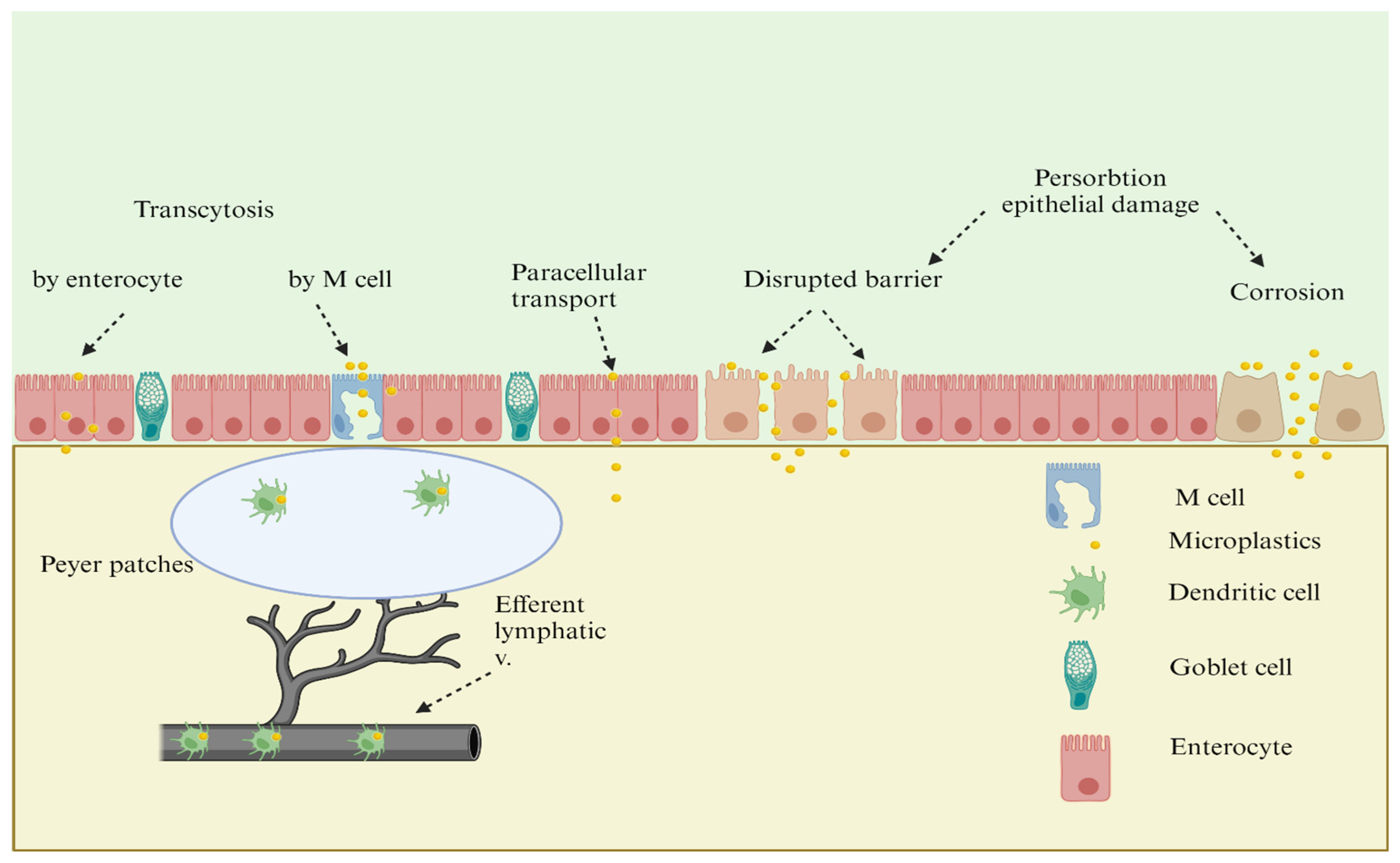
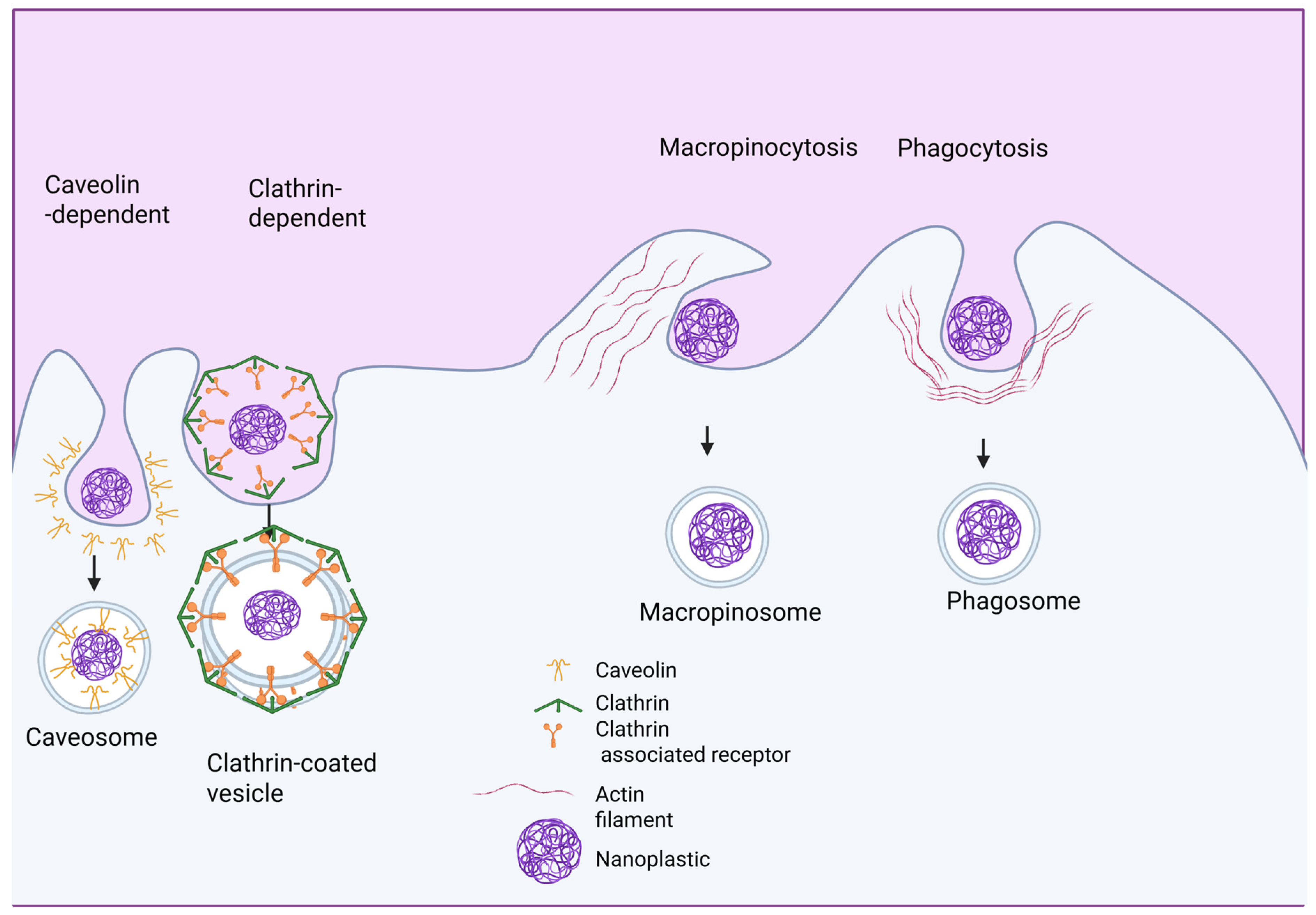
| Type of Plastic | Size | References |
|---|---|---|
| Macroplastic | ≥25 mm | [23,26,27,28] |
| Mesoplastic | 5–25 mm | [23,27,28] |
| Microplastic | 1 μm–5000 μm | [21,22,23,29,30,31,32,33,34] |
| 0.1 μm–5 mm | [19,24,32,35] | |
| 0.1 μm–5000 μm | [36] | |
| Nanoplastic | <1 μm | [21,37,38] |
| 0.001 μm–1 μm | [22,23,31] | |
| 0.001–0.1 μm | [35,36] | |
| <0.1 μm | [32,39,40,41] |
| Reference | Species | Type, Size, and Period of Exposure to Plastic | Mode of Exposure and Dose | Results |
|---|---|---|---|---|
| [120] | Rats and guinea pigs | Polystyrene latex microspheres, 2 μm | Oral gavage 1.42 to 1.95 × 109 particles in 0.25 mL dose | Particle absorption varied with age. Young adult males (7 weeks) were more affected than younger (3 weeks) and older (17 and 52 weeks) age groups. |
| Mice | Polystyrene latex microspheres, 2 μm | Oral gavage 6.84 × 108 in 0.1 mL | ||
| [121] | Mice | Polystyrene (PS) MPs of 5 μm, 20 μm; Periods studied: 1, 2, 4, 7, 14, 21, and 28 days after exposure to MPs. | Oral gavage | MPs accumulated in the liver, kidneys, and intestine, and the distribution depended on the size of the PS particles. Disruption of energy and lipid metabolism induced oxidative stress and negative neurotoxic responses. Lipid droplets and hepatic inflammation were found in mice treated with PS MPs. |
| [122] | Mice, male | Polyethylene (PE) and PS+ co-exposure organophosphorus flame retardant (OPERs) beads, 0.5–1.0 μm; for 90 days | Drinking water | Inflammation and lipid droplet formation in mouse liver and gut. Oxidative stress and neurotoxicity. Disruption of amino acid metabolism and energy metabolism. |
| [123] | Mice, male | Polystyrene MPs, 0.5, 50 μm, for 5 wk | Drinking water: - 1.456 × 1010 particles/L for 0.5 μm; - 1.456 × 104 particles/L for 50 μm | Reduced mucus secretion in the colon caused by intestinal microbiota dysbiosis. |
| [85] | Mice, female F0, F1 | Polystyrene pristine MPs, size 5 μm, during pregnancy and lactation (~6 weeks) | Drinking water: 100 and 1000 μg/L | Maternal metabolic disorders were linked to imbalances in gut microbiota and impaired gut barrier function. The F1 and F2 generations experienced intergenerational changes that had long-lasting metabolic effects. |
| [124] | Mice, male | PS pristine fluorescent MPs, size 5 μm, for 6 wk | Drinking water: 100 (1.456 × 106 particles/L) and 1000 μg/L (1.456 × 107 particles/L) | MP exposure caused intestinal barrier dysfunction, gut microbiota dysbiosis, and bile acid metabolism disorder. |
| [125] | Mouse | Polyethylene (PE) MPs + contaminated PAEs (phthalate esters), size 45–53 µm, for 30 days | Oral gavage 100 mg/kg/day, about 5.25 × 104 particles/day | Symptoms of inflammation and metabolic disorder in the gut including increased intestinal permeability, heightened inflammation, and altered gut microbiota. |
| [126] | Mice C57BL/6 | Polyethylene MPs, size 10–150 μm, for 5 consecutive weeks | Feed: 6, 60, and 600 μg/day | Gut bacterial overgrowth. Dysbiosis and inflammation in the small intestine. |
| [127] | Mice, C57 male, with induced acute colitis | PS MPs, size 5 µm, for 28 days | Drinking water: 500 μg/L | Mice exhibited enhanced inflammation, increased hepatic lipid peroxidation, promoted adipocyte differentiation, and hepatic metabolic disorder. |
| [128] | Mice, male, C57/BL6 | Pristine polystyrene (PS) negatively charged carboxylated (PS-COOH) and positively charged aminated polystyrene (PS-NH2), size 70 nm and 5 μm in diameter, for 28 days | Oral gavage 2 mg kg−1 0.2 mg kg−1 (for the carboxylated and aminated groups) | Marked dysbiosis of the gut microbiota. Decrease in body weight. The levels of serum ALP, AST, T-Bil, CK, r-glutamine transferase (r-GT), and creatinine (SCr) increased significantly after exposure to 2 mg kg−1 PS-NH2. Morphopathology of the stomach, duodenum, jejunum, and colon was significantly damaged after exposure to PS-NH2; crypts were damaged, intestinal villi disappeared, and the walls of the intestine and stomach became thin and showed obvious layers of inflammatory exudates after exposure to PS-NH2. Caused obvious injuries to the gut tract, leading to the decreased expression of tight junction proteins. |
| [129] | Mice, C57-BL/6, male | PS MPs, size 5 µm | Oral gavage 500 μg/L | Increased expression of inflammatory factors (TNF-α, IL-1 β, and IFN-γ) and intestinal immune imbalance. Exposure to PS MPs induced histopathological damage in colonic mucosa. |
| [130] | Mice, ICR | Polyethylene microplastics (PE MPs), size 10–50 μm 1. Single oral dose toxicity study, 14 days; 2. Repeated oral dose toxicity study, 28 days | Oral gavage 500, 1000, and 2000 mg/kg/day | In the toxicity experiments with a single oral dose, there were no changes. For the repeated oral dose toxicity study, the histopathological examination revealed granulomatous inflammation in the lungs and MPs in the lungs, stomach, duodenum, ileum, and serum. |
| [131] | Mice, C57BL/6 | Polypropylene (PP) MPs, size 8 and 70 m, for 28 days | Oral gavage 1, 10, and 100 mg/kg/d | Damage to tight junctions of the colon and decreased expression of ion transporters, intestinal mucus, and secretion. Induced colonic apoptosis and damage to the intestinal barrier through oxidative stress and TLR4/NF-κB inflammation. |
| [132] | Rats (juvenile) | Polystyrene MPs, size 1 μm, for 28 days | Oral gavage 2.0 mg/kg/d | Decreased rate of body weight gain and organ indices of the kidney, heart, and ovaries. Nephrotoxicity caused by disturbances of serum blood urea nitrogen (BUN), creatinine (CRE), and pro-inflammatory mediators IL-1b, IL-6, and TNF-a. |
| [133] | Rats (Wistar, male) | Polyethylene terephthalate (PET MPs), size 85 μm, for 14 days | Oral gavage 1.4 mg/kg, 35 mg/kg, and 125 mg/kg | Lack of clinical signs of toxicity. Changes in specific markers of liver, cardiac, and renal function. Increased levels of oxidative stress indicators. |
| [134] | Pigs, 8 wk old | PET (maximum size 300 μm), 28 days | Oral feed 0.1 g/day, 1 g/day, 28 days | Alterations in the enteric nervous system and the histological structure of the duodenum. Changes were more pronounced in the group of animals receiving microplastics at a dose of 1 g/day than in the group receiving 0.1 g/day. |
| [135] | Mice, C57BL/6J, male | Polystyrene MPs, size 0.2, 1, or 5 μm, for 28 days | Oral gavage Dose: 1 mg/kg body weight daily | Oxidative stress and inflammatory cell infiltration in the colons of mice and increased expression of pro-inflammatory cytokines. Increased intestinal permeability and decreased mucus secretion. At 5 μm size, PS damage was more severe than at 0.2 and 1 μm. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, R.P.; Tabaran, A.F. A Systematic Review of the Toxicokinetics of Micro- and Nanoplastics in Mammals Following Digestive Exposure. Appl. Sci. 2025, 15, 6135. https://doi.org/10.3390/app15116135
Popa RP, Tabaran AF. A Systematic Review of the Toxicokinetics of Micro- and Nanoplastics in Mammals Following Digestive Exposure. Applied Sciences. 2025; 15(11):6135. https://doi.org/10.3390/app15116135
Chicago/Turabian StylePopa, Raluca Paula, and Alexandru Flaviu Tabaran. 2025. "A Systematic Review of the Toxicokinetics of Micro- and Nanoplastics in Mammals Following Digestive Exposure" Applied Sciences 15, no. 11: 6135. https://doi.org/10.3390/app15116135
APA StylePopa, R. P., & Tabaran, A. F. (2025). A Systematic Review of the Toxicokinetics of Micro- and Nanoplastics in Mammals Following Digestive Exposure. Applied Sciences, 15(11), 6135. https://doi.org/10.3390/app15116135






