Survival of Listeria monocytogenes in Light and Full-Fat, Modified Atmosphere-Packaged, Sliced Greek Cheese over Shelf Life: Implications for Ready-to-Eat Food Safety
Abstract
:1. Introduction
2. Materials and Methods
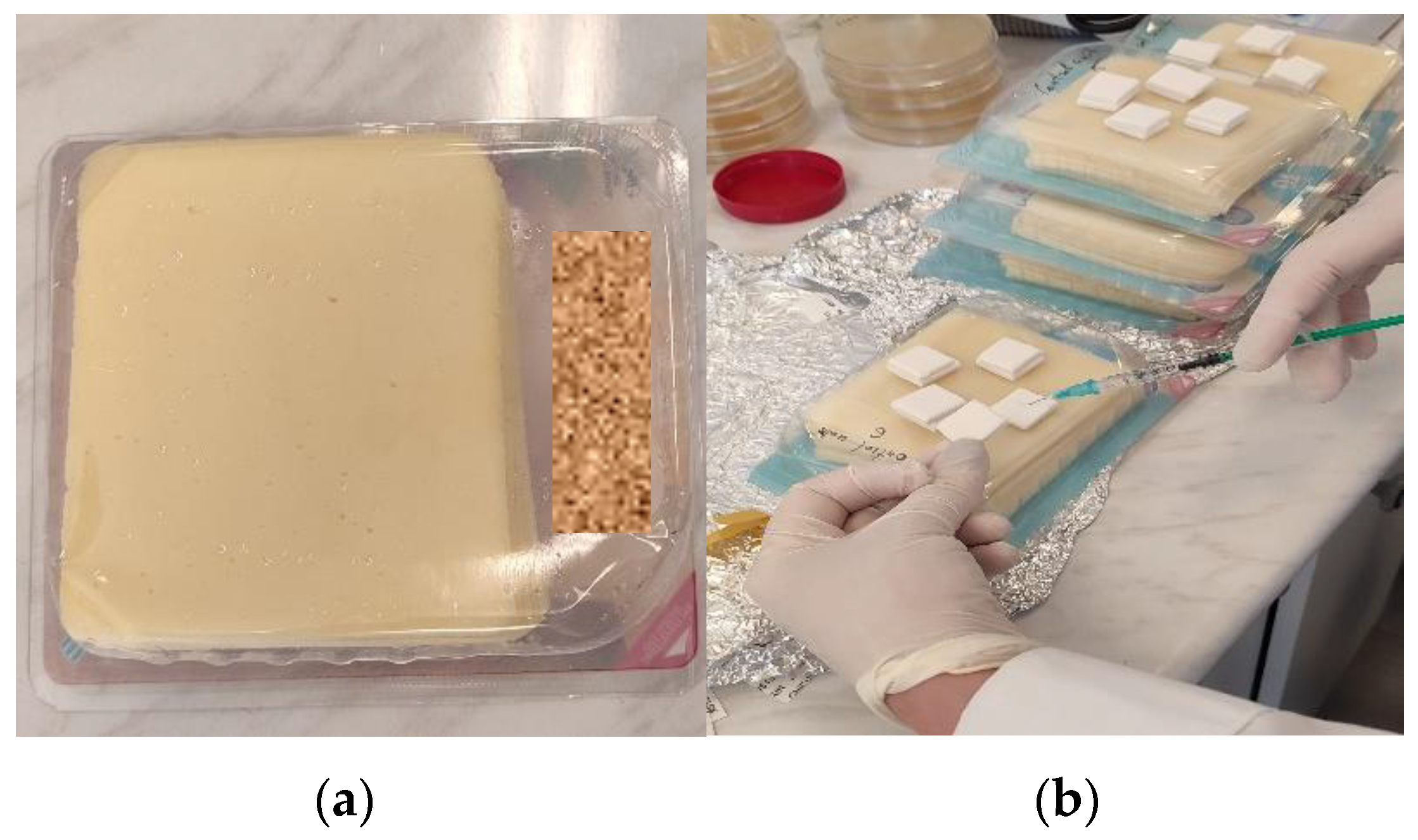
2.1. Characteristics and Storage Conditions of Cheese Food Matrix
2.2. Listeria monocytogenes Inoculum Preparation
- Strain isolated from a related environment (milk production filter): 17SEL22LM, molecular serotype IIa, clonal complex 14, sequence type 91;
- Strain isolated from raw milk cheese: 17SEL82LM, molecular serotype IVb, clonal complex 6, sequence type 6;
- Strain isolated from cheese: 09CEB411LM, molecular serotype IIa, clonal complex 26, sequence type 26.
2.3. Inoculation of Cheese Food Matrix and Homogenization Procedure
2.4. Experimental Design
2.5. Data Analysis
3. Results
3.1. “Food Control Samples”
3.2. “Control Units”
3.2.1. Physicochemical Analysis of “Control Units” in Three Batches of Greek Light Semi-Hard Sliced Cheese, Packed Under Modified Atmosphere (MAP)
3.2.2. Physicochemical Analysis of “Control Units” in Three Batches of Greek Full-Fat Semi-Hard Sliced Cheese, Packed Under Modified Atmosphere (MAP)
3.3. Evaluation of Lm Growth in Light Semi-Hard Sliced Cheeses, Packed Under Modified Atmosphere (MAP)
3.4. Evaluation of Lm Growth in Full-Fat Semi-Hard Sliced Cheeses, Packed Under Modified Atmosphere (MAP)
3.5. Microbiological Analysis
3.5.1. Microbiological Analysis of Background Microbial Flora of Light Semi-Hard Sliced Cheeses, Packed Under Modified Atmosphere (MAP)
3.5.2. Microbiological Analysis of Background Microbial Flora of Full-Fat Semi-Hard Sliced Cheeses, Packed Under Modified Atmosphere (MAP)
4. Discussion
4.1. pH and Water Activity (Aw)
4.2. Modified Atmosphere Package
4.3. Mesophilic Aerobic Count and Lactic Acid Bacteria
4.4. Listeria monocytogenes Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/ (accessed on 5 April 2025).
- Food Standards Agency; Pearson, K.; Chobanova, S.; Kintz, E.; Food Standards Scotland. The Risk to Vulnerable Consumers from Listeria monocytogenes in Ready to Eat Smoked Fish; Food Standards Scotland: Aberdeen, UK, 2023.
- Wadhwa Desai, R.; Smith, M.A. Pregnancy-Related Listeriosis. Birth Defects Res. 2017, 109, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M. Listeriosis: Clinical Presentation. FEMS Immunol. Med. Microbiol. 2003, 35, 173–175. [Google Scholar] [CrossRef]
- Koopmans, M.M.; Brouwer, M.C.; Vázquez-Boland, J.A.; van de Beek, D. Human Listeriosis. Clin. Microbiol. Rev. 2022, 36, e00060-19. [Google Scholar] [CrossRef]
- Shahamat, M.; Seaman, A.; Woodbine, M. Survival of Listeria monocytogenes in High Salt Concentrations. Zentralblatt Für Bakteriol. 1 Abt Orig. Med. Mikrobiol. Infekt. Parasitol. 1980, 246, 506–511. [Google Scholar] [CrossRef]
- Li, X.; Hospital, X.F.; Hierro, E.; Fernández, M.; Sheng, L.; Wang, L. Formation of Listeria monocytogenes Persister Cells in the Produce-Processing Environment. Int. J. Food Microbiol. 2023, 390, 110106. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Deng, L.; Feng, F.; Wang, L.; Zhou, Q.; Luo, Q. SigB Plays a Major Role in Listeria monocytogenes Tolerance to Bile Stress. Int. J. Food Microbiol. 2011, 145, 238–243. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm Formation and Persistence in Food-Processing Environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Ramires, T.; Kleinubing, N.R.; Iglesias, M.A.; Vitola, H.R.S.; Núncio, A.S.P.; Kroning, I.S.; Moreira, G.M.S.G.; Fiorentini, Â.M.; da Silva, W.P. Genetic Diversity, Biofilm and Virulence Characteristics of Listeria monocytogenes in Salmon Sushi. Food Res. Int. 2021, 140, 109871. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Lado, B.H.; Yousef, A.E. Characteristics of Listeria monocytogenes Important to Food Processors. In Listeria, Listeriosis, and Food Safety; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-11537-0. [Google Scholar]
- Schjørring, S.; Gillesberg Lassen, S.; Jensen, T.; Moura, A.; Kjeldgaard, J.S.; Müller, L.; Thielke, S.; Leclercq, A.; Maury, M.M.; Tourdjman, M.; et al. Cross-Border Outbreak of Listeriosis Caused by Cold-Smoked Salmon, Revealed by Integrated Surveillance and Whole Genome Sequencing (WGS), Denmark and France, 2015 to 2017. Eurosurveillance 2017, 22, 17-00762. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef]
- Magalhaes, R.; Almeida, G.; Ferreira, V.; Santos, I.; Silva, J.; Mendes, M.M.; Pita, J.; Mariano, G.; Mancio, I.; Sousa, M.M.; et al. Cheese-Related Listeriosis Outbreak, Portugal, March 2009 to February 2012. Eurosurveillance 2015, 20, 21104. [Google Scholar] [CrossRef]
- Amato, E.; Filipello, V.; Gori, M.; Lomonaco, S.; Losio, M.N.; Parisi, A.; Huedo, P.; Knabel, S.J.; Pontello, M. Identification of a Major Listeria monocytogenes Outbreak Clone Linked to Soft Cheese in Northern Italy—2009–2011. BMC Infect. Dis. 2017, 17, 342. [Google Scholar] [CrossRef]
- Palacios, A.; Otto, M.; Flaherty, E.; Boyle, M.M.; Malec, L.; Holloman, K.; Low, M.; Wellman, A.; Newhart, C.; Gollarza, L.; et al. Multistate Outbreak of Listeria monocytogenes Infections Linked to Fresh, Soft Hispanic-Style Cheese—United States, 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 709–712. [Google Scholar] [CrossRef]
- Heiman, K.E.; Garalde, V.B.; Gronostaj, M.; Jackson, K.A.; Beam, S.; Joseph, L.; Saupe, A.; Ricotta, E.; Waechter, H.; Wellman, A. Multistate Outbreak of Listeriosis Caused by Imported Cheese and Evidence of Cross-Contamination of Other Cheeses, USA, 2012. Epidemiol. Infect. 2016, 144, 2698–2708. [Google Scholar] [CrossRef]
- Lachmann, R.; Halbedel, S.; Lüth, S.; Holzer, A.; Adler, M.; Pietzka, A.; Al Dahouk, S.; Stark, K.; Flieger, A.; Kleta, S.; et al. Invasive Listeriosis Outbreaks and Salmon Products: A Genomic, Epidemiological Study. Emerg. Microbes Infect. 2022, 11, 1308–1315. [Google Scholar] [CrossRef]
- Halbedel, S.; Sperle, I.; Lachmann, R.; Kleta, S.; Fischer, M.A.; Wamp, S.; Holzer, A.; Lüth, S.; Murr, L.; Freitag, C.; et al. Large Multicountry Outbreak of Invasive Listeriosis by a Listeria monocytogenes ST394 Clone Linked to Smoked Rainbow Trout, 2020 to 2021. Microbiol. Spectr. 2023, 11, e0352022. [Google Scholar] [CrossRef]
- European Food Safety Authority. Prolonged Multi-Country Outbreak of Listeria monocytogenes ST173 Linked to Consumption of Fish Products. EFSA Support. Publ. 2024, 21, 8885E. [Google Scholar] [CrossRef]
- Chen, Y.I.; Burall, L.S.; Macarisin, D.; Pouillot, R.; Strain, E.; DE Jesus, A.J.; Laasri, A.; Wang, H.; Ali, L.; Tatavarthy, A.; et al. Prevalence and Level of Listeria monocytogenes in Ice Cream Linked to a Listeriosis Outbreak in the United States. J. Food Prot. 2016, 79, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate Outbreak of Listeriosis Associated with Cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef]
- McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Lai, S.; Painset, A.; Willis, C. An Outbreak of Human Listeriosis Associated with Frozen Sweet Corn Consumption: Investigations in the UK. Int. J. Food Microbiol. 2021, 338, 108994. [Google Scholar] [CrossRef]
- Ward, S.; Bedale, W.; Glass, K.A. Listeria monocytogenes Outbreaks Related to Commercially Produced Caramel Apples: Developments in Sanitation, Product Formulation, and Packaging: A Review. J. Food Prot. 2022, 85, 1287–1299. [Google Scholar] [CrossRef]
- Self, J.L.; Conrad, A.; Stroika, S.; Jackson, A.; Whitlock, L.; Jackson, K.A.; Beal, J.; Wellman, A.; Fatica, M.K.; Bidol, S.; et al. Multistate Outbreak of Listeriosis Associated with Packaged Leafy Green Salads, United States and Canada, 2015–2016. Emerg. Infect. Dis. 2019, 25, 1461–1468. [Google Scholar] [CrossRef]
- Cabal, A.; Allerberger, F.; Huhulescu, S.; Kornschober, C.; Springer, B.; Schlagenhaufen, C.; Wassermann-Neuhold, M.; Fötschl, H.; Pless, P.; Krause, R.; et al. Listeriosis Outbreak Likely Due to Contaminated Liver Pâté Consumed in a Tavern, Austria, December 2018. Eurosurveillance 2019, 24, 1900274. [Google Scholar] [CrossRef]
- Kvistholm Jensen, A.; Nielsen, E.M.; Björkman, J.T.; Jensen, T.; Müller, L.; Persson, S.; Bjerager, G.; Perge, A.; Krause, T.G.; Kiil, K.; et al. Whole-Genome Sequencing Used to Investigate a Nationwide Outbreak of Listeriosis Caused by Ready-to-Eat Delicatessen Meat, Denmark, 2014. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 64–70. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of Listeriosis—Northeastern United States, 2002. Morb. Mortal. Wkly. Rep. 2002, 51, 950–951. [Google Scholar]
- Halbedel, S.; Wilking, H.; Holzer, A.; Kleta, S.; Fischer, M.A.; Lüth, S.; Pietzka, A.; Huhulescu, S.; Lachmann, R.; Krings, A.; et al. Large Nationwide Outbreak of Invasive Listeriosis Associated with Blood Sausage, Germany, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1456–1464. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G.; et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Belias, A.; Sullivan, G.; Wiedmann, M.; Ivanek, R. Factors That Contribute to Persistent Listeria in Food Processing Facilities and Relevant Interventions: A Rapid Review. Food Control 2022, 133, 108579. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Cadavez, V.; Guillier, L.; Sanaa, M. A Critical Review of Risk Assessment Models for Listeria monocytogenes in Dairy Products. Foods 2023, 12, 4436. [Google Scholar] [CrossRef]
- Farber, J.M.; Daley, E. Fate of Listeria monocytogenes on Modified-Atmosphere Packaged Turkey Roll Slices. J. Food Prot. 1994, 57, 1098–1100. [Google Scholar] [CrossRef]
- Culliney, P.; Schmalenberger, A. Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging. Foods 2020, 9, 1211. [Google Scholar] [CrossRef]
- Whitley, E.; Muir, D.; Waites, W.M. The Growth of Listeria monocytogenes in Cheese Packed under a Modified Atmosphere. J. Appl. Microbiol. 2001, 88, 52–57. [Google Scholar] [CrossRef]
- Parry, R.T. Principles and Applications of Modified Atmosphere Packaging of Foods; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-1-4615-2137-2. [Google Scholar]
- Brown, S.R.; Forauer, E.C.; D’Amico, D.J. Effect of Modified Atmosphere Packaging on the Growth of Spoilage Microorganisms and Listeria monocytogenes on Fresh Cheese. J. Dairy Sci. 2018, 101, 7768–7779. [Google Scholar] [CrossRef]
- Barmpalia-Davis, I.M.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Effect of Fat Content on Survival of Listeria monocytogenes during Simulated Digestion of Inoculated Beef Frankfurters Stored at 7 °C. Food Microbiol. 2009, 26, 483–490. [Google Scholar] [CrossRef]
- Roig-Sagués, A.X.; Velázquez, R.M.; Montealegre-Agramont, P.; López-Pedemonte, T.J.; Briñez-Zambrano, W.J.; Guamis-López, B.; Hernandez-Herrero, M.M. Fat Content Increases the Lethality of Ultra-High-Pressure Homogenization on Listeria monocytogenes in Milk. J. Dairy Sci. 2009, 92, 5396–5402. [Google Scholar] [CrossRef]
- Wang, L.L.; Johnson, E.A. Inhibition of Listeria monocytogenes by Fatty Acids and Monoglycerides. Appl. Environ. Microbiol. 1992, 58, 624–629. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Belletti, N.; Aymerich, T.; Garriga, M. Modeling the Protective Effect of Aw and Fat Content on the High Pressure Resistance of Listeria monocytogenes in Dry-Cured Ham. Food Res. Int. 2015, 75, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Epidemiological Data for Listeriosis in Greece 2004–2023. Available online: https://eody.gov.gr/wp-content/uploads/2024/03/epidemiological-data-for-listeriosis-greece-2004-2023.pdf (accessed on 17 March 2025).
- Available online: https://eody.gov.gr/enimerotiko-deltio-eody-ioynios-2024/ (accessed on 1 July 2024).
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj/eng (accessed on 17 March 2025).
- Álvarez-Ordóñez, A.; Leong, D.; Hickey, B.; Beaufort, A.; Jordan, K. The Challenge of Challenge Testing to Monitor Listeria monocytogenes Growth on Ready-to-Eat Foods in Europe by Following the European Commission (2014) Technical Guidance Document. Food Res. Int. 2015, 75, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Lanni, L.; Morena, V.; Scattareggia Marchese, A.; Destro, G.; Ferioli, M.; Catellani, P.; Giaccone, V. Challenge Test as Special Tool to Estimate the Dynamic of Listeria monocytogenes and Other Foodborne Pathogens. Foods 2021, 11, 32. [Google Scholar] [CrossRef]
- Gérard, A.; El-Hajjaji, S.; Van Coillie, E.; Bentaïb, A.; Daube, G.; Sindic, M. Determination of the Growth Potential of Listeria monocytogenes in Various Types of Belgian Artisanal Cheeses by Challenge Tests. Food Microbiol. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Collu, D.; Marras, L.; Sanna, A.; Carrucciu, G.; Pinna, A.; Carraro, V.; Sanna, G.; Coroneo, V. Evaluation of Growth Potential and Growth Dynamics of Listeria monocytogenes on Ready-to-Eat Fresh Fruit. Ital. J. Food Saf. 2021, 10, 9337. [Google Scholar] [CrossRef]
- Vasileiadi, N.; Lappa, A.; Koukouvinos, C.; Tsironi, T.; Mandilara, G. Challenge Test for Assessing the Growth Potential of Listeria monocytogenes in Greek Soft Cheese (Anthotyros). Appl. Sci. 2022, 12, 12349. [Google Scholar] [CrossRef]
- Vasileiadi, N.; Tsironi, T.; Mandilara, G.D. Assessing Listeria monocytogenes Growth in Artificially Inoculated Sea-Farmed Product—Raw Sea Bass (Dicentrarchus labrax) Fillet, Produced in Greece. Microorganisms 2024, 12, 1970. [Google Scholar] [CrossRef]
- EURL Lm TECHNICAL GUIDANCE DOCUMENT on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes; Version 4. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 1 July 2021).
- ISO 20976-1:2019; Microbiology of the Food Chain—Requirements and Guidelines for Conducting Challenge Tests of Food and Feed Products—Part 1: Challenge Tests to Study Growth Potential, Lag Time and Maximum Growth Rate. International Organization for Standardization: Geneva, Switzerland, 2019.
- Bonanno, L.; Bergis, H.; Bonanno, L.; Bergis, H. Temperatures of the Food Cold Chain at Consumer Level in Europe; Version 2; Unit Salmonella & Listeria, Anses, Laboratory for Food Safety, EURL Lm: Maisons-Alfort, France, 2021. [Google Scholar]
- Bonanno, L.; Bergis, H.; Gnanou-Besse, N.; Asséré, A.; Danan, C. Which Domestic Refrigerator Temperatures in Europe—Focus on Shelf-Life Studies Regarding Listeria monocytogenes (Lm) in Ready-to-Eat (RTE) Foods. Food Microbiol. 2024, 123, 104595. [Google Scholar] [CrossRef]
- ISO 11290-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/60314.html (accessed on 17 March 2025).
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/60313.html (accessed on 17 March 2025).
- ISO 4833-1:2013; Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 17 March 2025).
- ISO 15214; Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony Count Technique at 30 °C. ISO: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/26853.html (accessed on 6 April 2025).
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Pouillot, R.; Klontz, K.C.; Chen, Y.; Burall, L.S.; Macarisin, D.; Doyle, M.; Bally, K.M.; Strain, E.; Datta, A.R.; Hammack, T.S.; et al. Infectious Dose of Listeria monocytogenes in Outbreak Linked to Ice Cream, United States, 2015. Emerg. Infect. Dis. 2016, 22, 2113–2119. [Google Scholar] [CrossRef]
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Hartnett, E.; Chapman, B.; Donnelly, C.W.; Goodburn, K.E.; et al. Alternative Approaches to the Risk Management of Listeria monocytogenes in Low Risk Foods. Food Control 2021, 123, 107601. [Google Scholar] [CrossRef]
| Type of Units | Type of Analysis | Number of Units and Day of Analysis per Batch * | |
|---|---|---|---|
| Test Units | Enumeration of Lm | 8 | 3 Test Units on “Day 0”, 1 Test Unit on each of 4 intermediate dates, and 1 Test Unit on “Day End” |
| Food Control Samples | Detection of Lm | 5 | 5 on “Day 0” |
| Measurement of physicochemical characteristics | |||
| Enumeration of the associated microflora | |||
| MAP measurement | |||
| MAP measurement | 2 | 2 on “Day End” | |
| Control Units | Measurement of physicochemical characteristics | 4 | 2 on “Day 0” and 2 on “Day End” |
| Enumeration of the associated microflora | |||
| MAP measurement | |||
| Temperature control | 1 | Throughout the test | |
| Total number of units per Batch | 20 | ||
| Total number of all examined samples of the 3 Batches of sliced light cheese and the 3 Batches of sliced full-fat cheese | 60 + 60 = 120 | ||
| Batch 1 Food Control Samples | Batch 2 Food Control Samples | Batch 3 Food Control Samples | ||||||||||||||||
| Day 0 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 |
| 6.23 | 0.74 | 1.02 | 17.40 | 1.00 | 22.30 | 6.02 | 0.73 | 1.20 | 17.30 | 0.10 | 22.90 | 5.75 | 0.72 | 0.78 | 16.70 | 0.20 | 23.60 | |
| 5.94 | 0.79 | 0.88 | 16.00 | 1.40 | 22.70 | 5.95 | 0.65 | 1.00 | 17.60 | 2.80 | 23.50 | 5.68 | 0.80 | 0.88 | 16.30 | 0.30 | 25.60 | |
| 6.04 | 0.68 | - | - | 2.30 | 21.20 | 5.86 | 0.69 | - | - | 3.50 | 19.60 | 5.92 | 0.76 | - | - | 0.10 | 19.70 | |
| 6.15 | 0.77 | - | - | 2.10 | 23.40 | 5.87 | 0.74 | - | - | 0.40 | 25.80 | 5.90 | 0.81 | - | - | 0.40 | 24.40 | |
| 6.07 | 0.81 | - | - | 2.90 | 19.40 | 5.90 | 0.66 | - | - | 3.20 | 22.40 | 6.20 | 0.76 | - | - | 0.30 | 22.50 | |
| Average | 6.09 | 0.76 | 0.95 | 16.70 | 1.94 | 21.80 | 5.92 | 0.69 | 1.10 | 17.45 | 2.00 | 22.84 | 5.89 | 0.77 | 0.83 | 16.50 | 0.26 | 23.16 |
| ±SD | 0.11 | 0.05 | 0.10 | 0.99 | 0.75 | 1.56 | 0.07 | 0.04 | 0.14 | 0.21 | 1.62 | 2.23 | 0.20 | 0.04 | 0.07 | 0.28 | 0.11 | 2.24 |
| Batch 1 Food Control Samples | Batch 2 Food Control Samples | Batch 3 Food Control Samples | ||||||||||||||||
| Day End | %MAP O2 | %MAP CO2 | %MAP O2 | %MAP CO2 | %MAP O2 | %MAP CO2 | ||||||||||||
| 0.30 | 32.40 | 0.50 | 25.90 | 0.20 | 34.10 | |||||||||||||
| 0.40 | 28.70 | 0.20 | 30.60 | 0.40 | 27.30 | |||||||||||||
| Average | 0.35 | 30.55 | 0.35 | 28.25 | 0.30 | 30.70 | ||||||||||||
| ±SD | 0.07 | 2.62 | 0.21 | 3.32 | 0.14 | 4.81 | ||||||||||||
| Batch 1 Food Control Samples | Batch 2 Food Control Samples | Batch 3 Food Control Samples | ||||||||||||||||
| Day 0 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 |
| 5.63 | 0.730 | 0.93 | 27.20 | 1.20 | 21.40 | 5.71 | 0.785 | 0.72 | 28.40 | 0.80 | 20.40 | 5.89 | 0.786 | 1.00 | 28.60 | 0.80 | 19.60 | |
| 5.84 | 0.754 | 0.84 | 31.00 | 0.80 | 19.30 | 5.79 | 0.761 | 0.82 | 29.30 | 0.70 | 19.80 | 5.96 | 0.774 | 0.86 | 29.50 | 0.60 | 18.50 | |
| 5.89 | 0.782 | - | - | 0.50 | 20.10 | 5.84 | 0.710 | - | - | 1.10 | 25.50 | 6.02 | 0.789 | - | - | 1.30 | 20.40 | |
| 6.23 | 0.748 | - | - | 1.60 | 21.50 | 5.77 | 0.743 | - | - | 0.60 | 22.80 | 5.92 | 0.793 | - | - | 0.80 | 20.00 | |
| 6.11 | 0.763 | - | - | 0.60 | 20.80 | 5.89 | 0.692 | - | - | 0.90 | 21.70 | 6.17 | 0.820 | - | - | 0.50 | 24.70 | |
| Average | 5.94 | 0.755 | 0.89 | 29.10 | 0.94 | 20.62 | 5.80 | 0.738 | 0.77 | 28.85 | 0.82 | 22.04 | 5.99 | 0.792 | 0.93 | 29.05 | 0.80 | 20.64 |
| ±SD | 0.24 | 0.019 | 0.06 | 2.69 | 0.46 | 0.93 | 0.07 | 0.038 | 0.07 | 0.64 | 0.19 | 2.26 | 0.11 | 0.017 | 0.10 | 0.64 | 0.31 | 2.38 |
| Batch 1 Food Control Samples | Batch 2 Food Control Samples | Batch 3 Food Control Samples | ||||||||||||||||
| Day End | %MAP O2 | %MAP CO2 | %MAP O2 | %MAP CO2 | %MAP O2 | %MAP CO2 | ||||||||||||
| 0.10 | 33.30 | 0.40 | 27.60 | 0.30 | 32.80 | |||||||||||||
| 0.30 | 29.10 | 0.20 | 31.50 | 0.60 | 28.40 | |||||||||||||
| Average | 0.20 | 31.20 | 0.30 | 29.55 | 0.45 | 30.60 | ||||||||||||
| ±SD | 0.14 | 2.97 | 0.14 | 2.76 | 0.21 | 3.11 | ||||||||||||
| Batch 1 Control Units (Injected with Physiological Water) | Batch 2 Control Units (Injected with Physiological Water) | Batch 3 Control Units (Injected with Physiological Water) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 |
| 5.69 | 0.76 | 0.91 | 17.4 | 0.40 | 21.70 | 6.12 | 0.74 | 0.73 | 16.90 | 0.60 | 24.40 | 5.82 | 0.68 | 1.21 | 17.4 | 0.60 | 25.80 | |
| 5.99 | 0.80 | 0.75 | 16.2 | 2.00 | 21.70 | 6.30 | 0.70 | 0.83 | 16.30 | 0.10 | 23.60 | 5.70 | 0.72 | 1.10 | 17.0 | 0.30 | 23.70 | |
| Ave rage | 5.84 | 0.78 | 0.83 | 16.8 | 1.20 | 21.70 | 6.21 | 0.72 | 0.78 | 16.60 | 0.35 | 24.00 | 5.76 | 0.70 | 1.16 | 17.2 | 0.45 | 24.75 |
| ±SD | 0.21 | 0.03 | 0.11 | 0.85 | 1.13 | 0.00 | 0.13 | 0.03 | 0.07 | 0.42 | 0.35 | 0.57 | 0.08 | 0.03 | 0.08 | 0.28 | 0.21 | 1.48 |
| Day End | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 |
| 6.42 | 0.72 | 0.93 | NE * | 0.00 | 33.20 | 6.50 | 0.76 | 0.95 | NE * | 0.00 | 29.40 | 6.21 | 0.78 | 1.20 | NE * | 0.00 | 34.10 | |
| 6.24 | 0.65 | 0.78 | NE * | 0.00 | 32.30 | 6.40 | 0.71 | 0.91 | NE * | 0.00 | 36.70 | 6.58 | 0.72 | 0.92 | NE * | 0.80 | 29.70 | |
| Average | 6.33 | 0.69 | 0.86 | 0.00 | 32.75 | 6.45 | 0.74 | 0.93 | 0.00 | 33.05 | 6.40 | 0.75 | 1.06 | 0.40 | 31.90 | |||
| ±SD | 0.13 | 0.05 | 0.11 | 0.00 | 0.64 | 0.07 | 0.04 | 0.03 | 0.00 | 5.16 | 0.26 | 0.04 | 0.20 | 0.57 | 3.11 | |||
| Batch 1 Control Units (Injected with Physiological Water) | Batch 2 Control Units (Injected with Physiological Water) | Batch 3 Control Units (Injected with Physiological Water) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 |
| 6.14 | 0.70 | 1.20 | 28.30 | 1.50 | 20.40 | 5.78 | 0.770 | 0.87 | 29.00 | 0.70 | 19.20 | 5.92 | 0.786 | 0.95 | 27.40 | 0.30 | 18.60 | |
| 5.84 | 0.78 | 0.72 | 29.70 | 0.90 | 19.80 | 5.62 | 0.781 | 0.85 | 27.40 | 0.60 | 18.40 | 6.08 | 0.781 | 0.84 | 28.90 | 0.60 | 20.70 | |
| Average | 5.99 | 0.74 | 0.96 | 29.00 | 1.20 | 20.10 | 5.70 | 0.776 | 0.86 | 28.20 | 0.65 | 18.80 | 6.00 | 0.784 | 0.90 | 28.15 | 0.45 | 19.65 |
| ±SD | 0.21 | 0.06 | 0.34 | 0.99 | 0.42 | 0.42 | 0.11 | 0.008 | 0.01 | 1.13 | 0.07 | 0.57 | 0.11 | 0.004 | 0.08 | 1.06 | 0.21 | 1.48 |
| Day End | pH | aw | %NaCl | %Fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %fat | %MAP O2 | %MAP CO2 | pH | aw | %NaCl | %fat | %MAP O2 | %MAP CO2 |
| 6.14 | 0.75 | 0.80 | NE * | 0.70 | 25.90 | 5.92 | 0.748 | 0.90 | NE * | 0.50 | 27.40 | 6.06 | 0.810 | 0.91 | NE * | 0.10 | 31.20 | |
| 6.21 | 0.71 | 0.93 | NE * | 0.50 | 28.60 | 5.91 | 0.750 | 0.71 | NE * | 0.30 | 29.50 | 6.87 | 0.786 | 0.76 | NE * | 0.40 | 30.60 | |
| Average | 6.18 | 0.73 | 0.87 | 0.60 | 27.25 | 5.92 | 0.749 | 0.81 | 0.40 | 28.45 | 6.47 | 0.798 | 0.84 | 0.25 | 30.90 | |||
| ±SD | 0.05 | 0.03 | 0.09 | 0.14 | 1.91 | 0.01 | 0.001 | 0.13 | 0.14 | 1.48 | 0.57 | 0.017 | 0.11 | 0.21 | 0.42 | |||
| Lm (log10 CFU/g) | Lm % Change * | |||||
|---|---|---|---|---|---|---|
| Day (Storage Temperature) | Batch 1 | Batch 2 | Batch 3 | Batch 1 | Batch 2 | Batch 3 |
| 0 | 2.08 | 2.31 | 2.23 | |||
| 24 (5 °C) | 1.93 | 1.74 | <1.60 | −7.21 | −24.68 | <−28.25 |
| 59 (7 °C) | 1.74 | 1.74 | <1.60 | −9.84 | 0.00 | 0.00 |
| 108 (7 °C) | 1.78 | 1.78 | <1.60 | 2.30 | 2.30 | 0.00 |
| 164 (7 °C) | 1.60 | <1.60 | <1.00 | −10.11 | <−10.11 | <−37.50 |
| 181 (10 °C) | <1.60 | <1.60 | <1.60 | 0.00 | 0.00 | <60 |
| Lm (log10 CFU/gr) | Lm % Change * | |||||
|---|---|---|---|---|---|---|
| Day (Storage Temperature) | Batch 1 | Batch 2 | Batch 3 | Batch 1 | Batch 2 | Batch 3 |
| 0 | 2.20 | 2.02 | 2.08 | |||
| 24 (5 °C) | 1.78 | 1.78 | 1.88 | −19.09 | −11.88 | −9.62 |
| 59 (7 °C) | 1.78 | <1.60 | 1.60 | 0.00 | <−10.11 | −14.89 |
| 108 (7 °C) | 1.85 | 1.70 | 1.85 | 3.93 | 6.25 | 15.63 |
| 164 (7 °C) | <1.00 | <1.60 | <1.60 | <−45.95 | <−5.88 | <−13.51 |
| 181 (10 °C) | <1.00 | <1.60 | <1.00 | 0.00 | 0.00 | <−37.50 |
| Day (Storage Temperature) | Mesophilic Aerobic Count in “Control Units” (log10 CFU/g) | Lactic Acid Bacteria (Lab) in “Control Units” (log10 CFU/g) | ||||
|---|---|---|---|---|---|---|
| Day 0 5 °C | 7.36 | 7.65 | 8.11 | 7.15 | 7.55 | 7.87 |
| Day End 10 °C | 7.80 | 7.77 | 7.88 | 7.02 | 7.36 | 7.66 |
| * % Change from “Day 0” to “Day End” (Day 181) | 6.05% | 1.60% | −2.85% | −1.76% | −2.56% | −2.66% |
| Day (Storage Temperature) | Mesophilic Aerobic Count in “Control Units” (log10 CFU/g) | Lactic Acid Bacteria (Lab) in “Control Units” (log10 CFU/g) | ||||
|---|---|---|---|---|---|---|
| Day 0 5 °C | 7.58 | 7.67 | 6.91 | 7.42 | 6.92 | 6.14 |
| Day End 10 °C | 6.91 | 7.60 | 7.85 | 6.07 | 6.00 | 6.04 |
| * % Change from “Day 0” to “Day End” (Day 181) | −8.82% | −0.84% | 13.65% | −18.17% | −13.27% | −1.72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadi, N.; Tsironi, T.; Mandilara, G.D. Survival of Listeria monocytogenes in Light and Full-Fat, Modified Atmosphere-Packaged, Sliced Greek Cheese over Shelf Life: Implications for Ready-to-Eat Food Safety. Appl. Sci. 2025, 15, 6109. https://doi.org/10.3390/app15116109
Vasileiadi N, Tsironi T, Mandilara GD. Survival of Listeria monocytogenes in Light and Full-Fat, Modified Atmosphere-Packaged, Sliced Greek Cheese over Shelf Life: Implications for Ready-to-Eat Food Safety. Applied Sciences. 2025; 15(11):6109. https://doi.org/10.3390/app15116109
Chicago/Turabian StyleVasileiadi, Ntina, Theofania Tsironi, and Georgia D. Mandilara. 2025. "Survival of Listeria monocytogenes in Light and Full-Fat, Modified Atmosphere-Packaged, Sliced Greek Cheese over Shelf Life: Implications for Ready-to-Eat Food Safety" Applied Sciences 15, no. 11: 6109. https://doi.org/10.3390/app15116109
APA StyleVasileiadi, N., Tsironi, T., & Mandilara, G. D. (2025). Survival of Listeria monocytogenes in Light and Full-Fat, Modified Atmosphere-Packaged, Sliced Greek Cheese over Shelf Life: Implications for Ready-to-Eat Food Safety. Applied Sciences, 15(11), 6109. https://doi.org/10.3390/app15116109






