Abstract
Background: This retrospective clinical cohort study aimed to evaluate, over an 18-month follow-up period, implant survival rates, marginal bone loss, peri-implant parameters, and surgical and prosthetic complications in immediately loaded post-extraction implants used for single or partial implant-prosthetic rehabilitations. Methods: Ninety-nine met the inclusion criteria and received a total of 147 implants. Follow-up assessments were conducted at one week and at three, six, and twelve months after prosthesis delivery. Clinical and radiographic parameters were evaluated by three independent practitioners. The variables considered included smoking, systemic conditions, implant site, and prosthetic type. Results: The implant survival rate was 95.92% at 18 months. Failures were more frequent in smokers, patients with systemic diseases, and in the posterior maxilla. Marginal bone loss increased over time, with higher values in posterior regions and in patients with systemic conditions or smoking habits. Peri-implant clinical parameters values negatively increased in smokers. Surgical complications occurred only in smokers and patients with systemic conditions, more frequently in fixed bridge rehabilitations. Prosthetic complications were limited, more common in posterior regions and multi-unit restorations. Conclusions: Immediately loaded post-extraction implants demonstrated high reliability. Careful patient selection and structured follow-up are essential to reduce complications and ensure long-term success.
1. Introduction
Implant-prosthetic rehabilitation with immediately loaded post-extraction implants aims to reduce the overall treatment time, preserve both hard and soft tissues, and limit the extent of post-extraction bone remodeling [1]. Alveolar bone loss following tooth extraction remains a significant clinical concern, as it can negatively impact implant integration and compromise long-term prosthetic outcomes [2]. Immediate implant placement within the extraction socket helps to counteract these effects by minimizing bone resorption and supporting tissue stability, especially in the anterior maxilla where aesthetics are critical [3].
However, the clinical success of immediate post-extraction implant placement depends on several factors, including the quality of the residual bone, the ability to achieve primary stability, and the appropriate biomechanical management of immediate loading [4]. In particular, success is closely linked to the stabilization of the implant within the alveolar socket and the immediate placement of a prosthesis with a controlled and well-distributed occlusal load [5]. Primary stability is considered a critical condition for successful osseointegration, as it reduces micromovements that may interfere with the healing process [6].
Numerous clinical investigations have demonstrated that the measurement of the implant stability quotient (ISQ) represents a reliable parameter for predicting implant prognosis in immediate loading protocols [7]. Furthermore, comparative studies between immediately loaded and delayed implants have suggested that, when performed under optimal conditions, the long-term survival rates of both approaches are comparable [8]. This supports the predictability and safety of immediate protocols when case selection and surgical execution are carefully managed.
Patient selection remains one of the most crucial elements in the predictability of this technique. As reported by Tettamanti et al., several risk factors for implant failure must be considered, including the quantity and quality of the residual bone, the presence of preexisting infections, and the proper management of the gap between the implant surface and the alveolar walls [9]. The use of biomaterials in these contexts has been shown to enhance bone regeneration and to limit post-extraction ridge resorption [10].
Additionally, Testori et al., in their retrospective clinical study, reported no statistically significant differences in long-term survival between immediate and delayed implants when standardized surgical and prosthetic protocols were followed [11]. Immediate placement is often preferred in the aesthetic zone, where the preservation of soft tissue contours and bone volume is essential. Conversely, in cases of significant bone dehiscence or active infection, a delayed approach may be more appropriate [12,13].
Recent evidence by Yu et al. has demonstrated that alveolar ridge preservation techniques using bone grafts or synthetic substitutes can improve both aesthetic and functional outcomes in immediate post-extraction implants [14]. Furthermore, long-term clinical studies have reported success rates exceeding 95% for immediately loaded implants over follow-up periods longer than five years, supporting the reliability of this approach [15,16]. Nonetheless, potential complications such as marginal bone resorption and peri-implant soft tissue inflammation may compromise the overall success of the treatment [17,18].
Current analyses of soft tissue behavior indicate that immediate loading does not negatively affect the biological stability of the peri-implant environment [19].
This retrospective clinical cohort study aimed to evaluate, over an 18-month follow-up period, implant survival rates, marginal bone loss, peri-implant parameters, and surgical and prosthetic complications in immediately loaded post-extraction implants used for single or partial implant-prosthetic rehabilitations.
2. Materials and Methods
2.1. Study Design
The current retrospective clinical cohort study was conducted at the Department of Dentistry and Dental Prosthetics, IRCCS Ospedale San Raffaele, Milan, Italy. The study adhered to the ethical principles outlined in the Declaration of Helsinki and received ethics committee approval (approval number: 190/INT/2021). In addition, the research was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, available at the following link: http://www.strobe-statement.org/ (accessed on 4 January 2024).
2.2. Setting
Patient recruitment and data collection were conducted between January and December 2024. During the initial recruitment phase, 113 patients undergoing implant-prosthetic rehabilitation with immediately loaded post-extraction implants were evaluated.
Follow-up assessments were performed at one week for suture removal; at three and six months following provisional prosthetic loading; and at three, six, and twelve months after final prosthesis placement.
The minimum follow-up period was 18 months; therefore, only patients treated from January 2021 onward were included in the study.
2.3. Partecipants
The patients considered for the study were selected according to the following inclusion and exclusion criteria.
2.3.1. Inclusion Criteria
- Age over 18 years;
- Single or partial implant-prosthetic rehabilitation with post-extraction implants subjected only to immediate loading;
- Screw-retained prostheses;
- Surgical procedure performed by an operator with over 20 years of experience in implantology;
- Prosthetic rehabilitation performed by an operator with over 20 years of experience in prosthodontics;
- Minimum follow-up of 18 months;
- Absence of systemic diseases or presence of compensated systemic diseases;
- Smoking patients;
- Patients rehabilitated with implants featuring an internal hexagonal connection from the same manufacturer;
- Placement of implants in basal bone without the use of biomaterials for bone regeneration.
2.3.2. Exclusion Criteria
- Failure to adhere to recall and hygiene maintenance protocols during the follow-up period;
- Incomplete clinical and radiographic documentation;
- Patients with uncompensated systemic diseases developed during the follow-up period;
- Patients enrolled in concomitant research protocols.
2.4. Implant-Prosthetic Rehabilitation
Patients underwent surgery only after adequate oral hygiene control, i.e., after obtaining a full-mouth plaque score of 20% or less.
The pre-operative case planning included Level I and II radiographic investigations (orthopantomography, apical sectoral intraoral X-rays, and sectoral Cone Beam CT) to assess the length and diameter of the implant/implant fixture(s). All patients underwent digital impressions with the Medit i700 intraoral scanner (Medit Corp., Seoul, Republic of Korea) for the fabrication of the provisional prosthetic restoration.
Starting the day before surgery, all patients had taken antibiotic therapy (1 g of amoxicillin/clarithromycin in allergic patients every 12 h for a total of six days).
All extractions and implant placement procedures had been performed under loco-regional anesthesia with Articaine 1:100,000 (Ubistesin™, 3M ESPE, St. Paul, MN, USA). The flap was performed with a crestal incision that ensured the presence of at least one millimeter of keratinized mucosa per side and two release incisions that allowed a full view of the operative field and total exposure of the buccal cortical so as to intercept any bony dehiscences and/or wall fractures.
Only TTi-type implants from the TT line (Winsix, Biosafin, Cefla, Imola, Italy) featuring an internal hexagonal connection and a cylindrical body with a conical apex were inserted.
To ensure primary stability, each fixture was engaged 3–4 mm apical to the alveolus with an insertion torque of 25 Ncm or more. Each implant was placed one millimeter apical to the crestal level to compensate for the physiological process of post-extraction bone remodeling.
Any gaps between the implant and vestibular wall were filled using collagen hemostatic sponges (Spongostan, Ethicon, Somerville, NJ, USA).
Flap adaptation and suturing were performed using 3-0 non-absorbable sutures (Vicryl™, Ethicon, Johnson & Johnson, New Brunswick, NJ, USA).
Post-operative therapy included, in addition to the prescription of antibiotics, analgesics as needed (Ibuprofen 600 mg, Brufen—Mylan S.p.A., Milan, Italy) and rinses with chlorhexidine 0.20% (Curasept—Curasept S.p.A., Saronno, Italy) to be administered three times a day for ten days after surgery.
Titanium abutments (Winsix, Biosafin, Cefla, Imola, Italy) were screwed to each implant, which allowed the poly-methyl-methacrylate (PMMA) temporary obtained from the previously taken digital impression to be relined. Relining was performed using cold acrylic resin (Unifast Trad™, GC Dental, Aichi, Japan).
After finishing, the temporary was screwed in and the occlusal contacts adjusted with articulating paper (Bausch Articulating Paper, Nashua, NH, USA), so as to remove any pre-contacts in occlusion and laterality.
Six months later, using scan bodies (Winsix, Biosafin, Cefla, Imola, Italy), a definitive digital impression was taken with a Medit i700 intraoral scanner (Medit Corp., Seoul, Republic of Korea) for the fabrication of the definitive prosthetic restoration in layered ceramic or monolithic zirconia, tightened with a manual ratchet at 20 N.
The holes corresponding to the implant fixtures were sealed by the application of polytetrafluoroethylene (PTFE) (Teflon™, DuPont de Nemours, Wilmington, DE, USA) and dimethacrylate-based composite resin, such as bisphenol A-glycidyl methacrylate (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA) (Ceram.x® SphereTEC™ One, Dentsply Sirona, Charlotte, NC, USA).
2.5. Variables
The patients underwent professional oral hygiene sessions every six months after provisional prosthetic loading. The follow-up protocol included clinical and radiographic checks (intraoral X-rays) at one week after surgery for suture removal; at three and six months after provisional prosthetic loading; and then at three, six and twelve months after placement of the definitive prosthesis.
The clinical outcomes analyzed included implant survival, marginal bone loss, peri-implant clinical parameters, and the incidence of any surgical and prosthetic complications. Data were collected between January 2024 and December 2024 by three independent practitioners (dentists).
The following variables were considered: presence or absence of systemic disease; smoking; implant site (maxillary upper anterior, maxillary upper posterior, mandibular anterior, and mandibular posterior); and type of prosthetic rehabilitation (single crown or bridge).
2.5.1. Implant Survival Rate
The implant survival rate was measured according to the number of implants lost from the time of provisional prosthetic loading until twelve months after placement of the definitive prosthesis, for a total observation period of eighteen months.
2.5.2. Peri-Implant Marginal Bone Loss
Peri-implant marginal bone loss (MBL) was assessed by analyzing intraoral radiographs acquired three, six, and twelve months after definitive prosthetic loading. The images were obtained using the long cone technique associated with a centering system (XCP, Dentsply International, RINN), ensuring the perpendicularity of the radiographic beam to the long axis of the implant and minimizing geometric distortions and angular variations.
Image processing was performed using Digora Optime software (Soredex, Tuusula, Finland), using the dedicated measurement tool. To ensure an accurate assessment of bone loss, an image calibration was first performed, using the known implant diameter as the reference unit (pixels/mm).
Measurements were made by comparing the changes in marginal bone height over time. The bone level was determined by taking the most coronal portion of the implant fixture and the contact point between the implant and the marginal bone ridge as reference points. To standardize the analysis, a virtual reference line tangent to the shoulder of the implant was considered, from which a line parallel to the long axis of the fixture was drawn to the most coronal point where the bone came into contact with the implant, both mesially and distally (Figure 1).
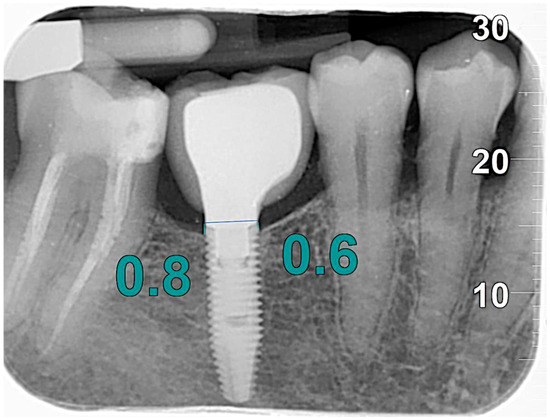
Figure 1.
MBL measurements.
2.5.3. Peri-Implant Parameters
Peri-implant clinical parameters were monitored at three, six, and twelve months after definitive prosthetic loading and included peri-implant probing depth (PPD), plaque index (PI), bleeding on probing (BoP), and presence of suppuration. Probing depth was measured using a millimeter-calibrated periodontal probe (UNC-15, Hu-Friedy, Chicago, IL, USA), with measurements taken at six sites for each implant: mesial, central, and distal on both the buccal and lingual/palatal sides. The plaque index was determined by applying a plaque detector (Plaque Test™, Ivoclar Vivadent, Schaan, Liechtenstein), expressing the value as a percentage based on the proportion of implant surfaces covered by visible bacterial biofilm. Bleeding on probing was recorded ten seconds after probe removal and expressed in percentage values in relation to the six measurement sites for each implant, representing an indicator of peri-implant tissue inflammatory response. Suppuration was assessed by probing the peri-implant sulcus and direct observation of any purulent exudate discharge and recorded as a dichotomous variable (present/absent).
2.5.4. Surgical Complications
Surgical complications were retrospectively analyzed from the patients’ clinical records, including post-operative pain, oedema, post-operative bleeding, and paresthesia. Data were extracted from the clinical records and operative reports, with no possibility of prospective integration, limiting the evaluation to the recording of events reported by clinicians in the period between provisional prosthetic loading and 12 months after insertion of the definitive prosthesis.
2.5.5. Prosthetic Complications
The analysis of prosthetic complications included unscrewing of the provisional prosthetic screw, fracture of the provisional prosthetic framework, unscrewing of the definitive prosthetic screw, and partial chipping of the ceramic of the definitive prosthetic crown. These complications were analyzed from the insertion of the provisional until 12 months after the definitive prosthesis.
2.6. Bias
The present retrospective observational study has potential methodological limitations inherent to the study design, which must be considered when interpreting the results.
A potential selection bias stems from the application of inclusion criteria that limited the study population to patients with a minimum follow-up of 18 months and those who strictly adhered to recall and hygiene maintenance protocols. Although this selection ensures the integrity and quality of the data collected, it may limit the generalizability of the results to patients with less compliance with follow-up visits or less adequate levels of oral hygiene.
Detection bias was controlled by assigning the clinical and radiographic evaluations to three independent examiners, who were not involved in the clinical management of the patients. Radiographic image acquisition was standardized, with calibration to known reference units, and peri-implant parameter measurements were performed with certified instruments and predefined protocols. However, the two-dimensional imaging methodology used has inherent limitations, including distortion phenomena and angular variability, which may affect the accuracy of marginal bone loss assessment. The possibility of intra- and inter-image discrepancies in measurements represents a further element of potential variability in the results.
Operator bias is a further limitation of the study. The surgical and prosthetic procedures were performed exclusively by clinicians with more than 20 years of experience in implantology and prosthetics, ensuring a high level of standardization and predictability of the clinical outcome. However, this condition could affect the applicability of the results to clinical settings where the level of experience of the operators is lower or variable, leading to potential differences in implant success rates and prosthetic complications.
Lastly, a selection bias related to the systemic conditions of the patients must be considered. The sample analyzed included smoking patients and subjects with compensated systemic diseases but excluded individuals with uncompensated systemic diseases potentially affecting osseointegration and the peri-implant response. This selection may have reduced the biological variability of the cohort, limiting the translatability of the results to populations with more complex systemic conditions.
2.7. Study Size
The sample size for this study was determined through a priori power analysis to ensure an optimal balance between Type I (α = 0.05) and Type II (β) error rates. The calculation was based on an estimated effect size of 0.5 and was performed using the following formula:
where:
n = 2 × (Zα/2 + Zβ)2 × σ2IES2n = IES22 × (Zα/2 + Zβ)2 × σ2
- n = required sample size per group;
- Zα/2 = Z-score corresponding to the desired Type I error rate (α = 0.05, thus Zα/2 ≈ 1.96);
- Zβ = Z-score corresponding to the desired power (1 - β; for 80% power, Zβ ≈ 0.84);
- σ2 = variance of the outcome variable;
- IES = minimum expected intervention effect size (difference between group means expressed in units of standard deviation).
Following this analysis, a total of 113 participants were included, yielding a statistical power of 0.92. This level of power is considered highly acceptable in clinical research, ensuring a sufficient probability of detecting meaningful differences while maintaining robust inferential validity. The final sample size was deemed appropriate to support the study’s objectives while further minimizing the risk of Type II errors.
2.8. Statistical Methods
All statistical tests were conducted at a significance level of p = 0.05.
Statistical analysis of the collected data was conducted using the latest version of IBM SPSS Statistics 29.0 software (IBM Corp., Armonk, NY, USA). All variables were subjected to a descriptive analysis, with calculation of the mean and standard deviation for continuous variables and incidence for categorical variables.
The continuous variables analyzed are the MBL, measured in millimeters, the PPD, measured in millimeters, and the PI and the BoP, both expressed as percentages. Categorical variables included the presence of suppuration; surgical complications (post-operative pain, oedema, bleeding, and paresthesia); prosthetic complications (unscrewing of the prosthetic screw, fracture of the provisional restoration, unscrewing of the final screw, and chipping of the ceramic); type of prosthetic rehabilitation (single crown or bridge); implant site (anterior maxillary, posterior maxillary, anterior mandible, and posterior mandible); presence of systemic pathologies; and smoking.
The independent variables considered for the analysis of each parameter were implant site, type of prosthetic rehabilitation, presence of systemic disease, and smoking. These variables were treated as categorical factors, because they divided the subjects into discrete categories, allowing comparisons between different groups of patients. The implant site was classified into four categories (anterior maxillary, posterior maxillary, anterior mandible, and posterior mandible), while the type of prosthetic rehabilitation was considered as a dichotomous variable (single crown or bridge). Similarly, the presence of systemic pathologies and smoking was analyzed as binary variables (Yes/No).
2.8.1. Multivariate Logistic Regression
To identify predictors of marginal bone loss, peri-implant parameters, implant survival rate, and surgical and prosthetic complications, a multivariate logistic regression was performed. The independent variables included in the model were implant site, type of prosthetic rehabilitation, presence of systemic disease, and smoking. The results were expressed as odds ratios (ORs) with 95% confidence intervals. To assess the influence of systemic and clinical variables on the main implant outcomes, a series of null hypotheses were formulated predicting the absence of statistically significant associations between the covariables analyzed and the clinical outcomes considered. Specifically, it was hypothesized that the presence of systemic pathology, smoking habit, implant site (anterior or posterior maxilla and anterior or posterior mandible), and type of prosthetic rehabilitation (single crown or bridge) did not significantly influence any of the following dependent variables: implant survival, marginal bone loss, peri-implant clinical parameters (such as probing depth and bleeding on probing), and incidence of surgical or prosthetic complications. More specifically, a specific null hypothesis was tested for each combination of independent variable and clinical outcome, assuming that any differences observed in the data were attributable to chance. The level of statistical significance (α) was set at 0.05; therefore, a p-value below this threshold was considered indicative of the rejection of the null hypothesis and the existence of a statistically significant association between the independent variable and the outcome under investigation.
2.8.2. Implant Survival Rate
The implant survival rate was calculated by Kaplan–Meier analysis, considering implant loss as a censored event. The comparison was performed using the log-rank test, including the defined independent variables. The null hypothesis was that the implant survival rate was not influenced by the independent variables (p > 0.05).
2.8.3. Peri-Implant Marginal Bone Loss
MBL was assessed by repeated measures analysis of variance (ANOVA for repeated measures) to compare the values recorded at 3, 6, and 12 months after definitive prosthetic loading. In case of statistical significance, post hoc comparisons were performed with Bonferroni correction. The null hypothesis was that marginal bone loss did not vary significantly over time or in relation to the independent variables (p > 0.05).
2.8.4. Peri-Implant Clinical Parameters
PPD, PI, BoP, and suppuration were compared at different follow-ups by the Friedman’s test for non-parametric data, followed by Wilcoxon’s test for pairwise comparisons with correction for multiple errors. Defined independent variables were considered. The null hypothesis was that the peri-implant clinical parameters did not vary significantly over time or in relation to the independent variables (p > 0.05).
2.8.5. Surgical Complications
Surgical complications (post-operative pain, edema, bleeding, and paresthesia) were analyzed as dichotomous variables (present/absent). Their association with the independent variables was assessed by means of Pearson’s chi-square test or Fisher’s exact test. The null hypothesis was that there was no significant association between surgical complications and the independent variables (p > 0.05).
2.8.6. Prosthetic Complications
The prosthetic complications (unscrewing of the prosthetic screw, fracture of the temporary restoration, unscrewing of the definitive screw, and chipping of the ceramic) were analyzed as dichotomous variables (present/absent). Their association with the independent variables was assessed by means of Pearson’s chi-square test or Fisher’s exact test. The null hypothesis was that there was no significant association between prosthetic complications and the independent variables (p > 0.05).
3. Results
Of the 113 patients recruited, 11 were excluded due to non-adherence to follow-up sessions as they were residents in a different city from the reference site (IRCCS Ospedale San Raffaele, Milan, Italy) and 3 due to systemic pathologies not compensated for during the follow-up period.
The study included a total of 99 patients, of whom 54 were male and 45 female. The mean age of the participants was 57 years, ranging from 45 to 70 years.
Of the 99 patients, 51 underwent single implant-prosthetic rehabilitation, while 48 were rehabilitated with bridges supported by two distal implants. A total of 147 TTi line implants (Winsix, Biosafin, Cefla, Imola, Italy) were placed.
There were 6 smokers with systemic diseases, 13 smokers with systemic diseases, and 16 non-smokers with systemic diseases.
3.1. Implant Survival Rate
Six implants were lost during the follow-up period, resulting in an implant survival rate of 95.92%. Details of the patients in whom implant failure had occurred were reported as follows (Table 1).

Table 1.
Implant failures.
During follow-up, six implants were lost, resulting in an implant survival rate of 95.92%. The Kaplan–Meier analysis showed a statistically significant correlation between implant failure, smoking (p = 0.04), and systemic pathology (p = 0.04), as well as a higher incidence of implant loss in the posterior sectors, particularly in the posterior maxilla (p = 0.03). The type of rehabilitation did not significantly influence implant failure (p = 0.09).
3.2. Peri-Implant Marginal Bone Loss
Marginal bone loss showed a statistically significant progression over time (p < 0.05).
Single implants had a mean MBL of 0.30 ± 0.11 mm at 3 months, 0.50 ± 0.13 mm at 6 months, and 0.75 ± 0.15 mm at 12 months. Bridge-supported implants showed lower values: 0.27 ± 0.10 mm at 3 months, 0.45 ± 0.12 mm at 6 months, and 0.70 ± 0.14 mm at 12 months. The posterior maxilla showed the greatest bone loss (0.34 ± 0.12 mm at 3 months, 0.54 ± 0.14 mm at 6 months, and 0.80 ± 0.16 mm at 12 months), while the anterior mandible showed the least bone loss (0.22 ± 0.09 mm at 3 months, 0.38 ± 0.10 mm at 6 months, and 0.58 ± 0.12 mm at 12 months). Logistic regression showed a significant association between MBL and the implant site (p = 0.03), smoking (p = 0.04), and the presence of systemic pathology (p = 0.03), while no significant correlation was found with type of rehabilitation (p = 0.07).
3.3. Peri-Implant Clinical Parameters
The peri-implant probing depth increased significantly over time (p < 0.05). In single implants, the mean values increased from 2.80 ± 0.16 mm at 3 months to 3.10 ± 0.18 mm at 6 months and to 3.35 ± 0.20 mm at 12 months. In bridge-supported implants, the values were slightly lower (2.65 ± 0.14 mm at 3 months, 2.90 ± 0.16 mm at 6 months, and 3.15 ± 0.18 mm at 12 months). The PPD was significantly higher in the posterior maxilla (p = 0.02) and significantly influenced by smoking (p = 0.04), while no significant associations were observed with the type of rehabilitation (p = 0.09) and the presence of systemic pathology (p = 0.06).
Plaque index (PI) and bleeding on probing (BoP) showed a statistically significant increase over time (p < 0.0001). The highest values were found in the posterior maxilla and posterior mandible (p = 0.03). Smoking showed a significant correlation with both parameters (p = 0.03). No significant correlation was found with the type of rehabilitation (p = 0.08) and the presence of systemic diseases (p = 0.07).
Suppuration was documented in two patients and was significantly associated with smoking (p = 0.02) and the presence of systemic pathologies (p = 0.03), whereas no correlations were found with the implant site (p = 0.08) and type of rehabilitation (p = 0.09).
3.4. Surgical Complications
No cases of post-operative pain were reported, while there were nine cases of edema, four of which were also associated with post-operative bleeding, reported in a total of seven patients (Table 2).

Table 2.
Surgical complications.
No patients reported paresthesia. All surgical complications occurred exclusively in patients with systemic diseases (p = 0.02) and smokers (p = 0.03). Furthermore, a significant correlation was observed with the type of prosthetic rehabilitation (p = 0.04), with a higher prevalence of complications in patients rehabilitated with bridges supported by two distal implants than in those undergoing single implant-prosthetic rehabilitation. In contrast, there was no significant correlation in relation to implant site (p = 0.08).
3.5. Prosthetic Complications
Five cases of unscrewing of the provisional prosthetic screw, two fractures of the provisional restoration, and only one case of unscrewing of the definitive prosthetic screw were reported. No cases of chipping were recorded (Table 3).

Table 3.
Prosthetic complications.
The chi-square test showed a significant association between prosthetic complications and implant site (p = 0.03), with a higher incidence in the posterior region, and with the type of rehabilitation (p = 0.04), being more frequent in bridges. No statistically significant correlation was found with smoking (p = 0.07) and the presence of systemic pathologies (p = 0.08).
3.6. Multivariate Logistic Regression
Multivariate logistic regression analysis showing the association between independent variables (smoking, systemic disease, implant site, and type of rehabilitation) and main clinical outcomes (implant failure, marginal bone loss, peri-implant clinical parameters, and surgical and prosthetic complications). The odds ratios (ORs), 95% confidence intervals (CI), and p-values are reported (Table 4).

Table 4.
Multivariate logistic regression analysis according to the OR, CI, and p-values.
3.7. Null Hypothesis
3.7.1. Implant Survival Rate
Over the 18-month follow-up period, implant survival was significantly influenced by a number of systemic and clinical variables. In particular, a smoking habit showed a statistically significant association with implant failure (p = 0.04), leading to the rejection of the null hypothesis that excluded its impact. Similarly, the presence of systemic disease also proved to be a significant determinant of implant loss (p = 0.04), leading to the rejection of the null hypothesis. An equally significant role was observed for the implant site, with a higher incidence of failure in the posterior maxilla (p = 0.03), confirming the influence of local anatomy on treatment outcome. In contrast, the type of rehabilitation, i.e., the use of single crowns as opposed to bridges on distal implants, did not show a statistically significant effect on implant survival (p = 0.09), leading to the acceptance of the null hypothesis concerning this variable.
3.7.2. Marginal Bone Loss
Marginal bone loss showed a significant development over time and was influenced by several factors. Smoking was associated with increased bone loss (p = 0.04), leading to rejection of the null hypothesis. The presence of systemic disease also showed a negative influence on peri-implant bone maintenance (p = 0.03), thus rejecting the null hypothesis. Similarly, the implant site proved to be decisive, with the highest resorption values observed in the posterior maxilla (p = 0.03), confirming a significant correlation. Conversely, the type of rehabilitation did not show a statistically significant association with MBL (p = 0.07), and therefore, the null hypothesis was accepted for this variable.
3.7.3. Peri-Implant Clinical Parameters
The analysis of the clinical parameters, in particular PPD, PI, BoP, and suppuration, revealed significant associations with some of the covariables analyzed. The probing depth was significantly greater in smoking patients (p = 0.04) and in posterior areas, particularly in the maxilla (p = 0.02), leading to the rejection of the null hypothesis in both cases. On the other hand, no statistically significant association was found with the presence of systemic pathology (p = 0.06) nor with the type of rehabilitation (p = 0.09), confirming the null hypothesis for these two variables.
Concerning the plaque index and bleeding on probing, there was also a significant correlation with smoking (p = 0.03) and implant site (p = 0.03), while there were no significant associations with the presence of systemic pathology (p = 0.07) nor with the type of rehabilitation (p = 0.08). About suppuration, the data showed a significant association with both smoking (p = 0.02) and systemic pathologies (p = 0.03), while neither implant site (p = 0.08) nor type of rehabilitation (p = 0.09) showed significant effects. Overall, the null hypotheses were rejected for smoking and systemic pathologies in relation to suppuration but accepted for site and type of rehabilitation.
3.7.4. Surgical Complications
Post-operative complications, although generally moderate, were significantly more frequent in smokers (p = 0.03) and in patients with systemic diseases (p = 0.02), leading to the rejection of the corresponding null hypotheses. The type of restoration also showed a significant association with the incidence of surgical complications (p = 0.04), with a higher frequency in patients treated with bridges on two distal implants. The implant site, on the other hand, did not reach statistical significance (p = 0.08), confirming the null hypothesis in this case.
3.7.5. Prosthetic Complications
Regarding prosthetic complications, there was a significant association with the implant site (p = 0.03), particularly in the posterior region, and with the type of rehabilitation (p = 0.04), with a higher incidence in patients with bridges than in those with single crowns. In both cases, the null hypothesis was rejected. In contrast, neither smoking (p = 0.07) nor the presence of systemic diseases (p = 0.08) showed a statistically significant influence on the occurrence of these complications, leading to the acceptance of the corresponding null hypotheses.
4. Discussion
The objective of this retrospective clinical cohort study was to evaluate, at 18 months of follow-up, the implant survival rate, marginal bone loss, peri-implant parameters, surgical and prosthetic complications of immediately loaded post-extractive implants in single or partial implant-prosthetic rehabilitations.
The implant survival rate was 95.92%. Implant failure was statistically associated with smoking, systemic pathology, and site of implant placement, with the greatest loss of fixtures recorded in the posterior upper jaw.
This result is consistent with the data reported by Mura et al. in their retrospective 5-year follow-up clinical study of immediately loaded post-extractive implants. The study included 45 patients treated with rough surface implants, and the survival rate of 97.8% confirmed the long-term reliability of post-extractive implants [20].
In addition, Mustapha et al. performed a systematic review and meta-analysis of 292 studies that evaluated a total of 35,511 implants in smokers and 114,597 implants in non-smokers. The analysis showed that the rate of implant failure in smokers was statistically higher than in non-smokers, with reduced implant stability and increased marginal bone loss [21].
Similarly, Chrcanovic et al. conducted a review of 34 studies, showing that smoking is a primary risk factor for implant failure, regardless of implant site and type of load applied [22].
Regarding peri-implant marginal bone loss, logistic regression showed a significant association between MBL and implant site, smoking, and the presence of systemic diseases, while no significant correlation was found with the type of rehabilitation.
Cristalli et al. conducted a prospective clinical study of 45 patients undergoing single post-extraction implants with immediate loading. The study used standardized digital radiographs to monitor the MBL and showed an average marginal bone loss of 0.35 mm at one year, with a more significant impact on smokers than on non-smokers [23].
Vázquez Álvarez et al. conducted a retrospective study on a cohort of 150 patients with a 5-year follow-up, evaluating peri-implant bone loss in relation to various risk factors. The analysis revealed that smoking, uncontrolled diabetes, and posterior implant sites were significantly associated with greater marginal bone loss. Specifically, diabetic patients exhibited a mean marginal bone loss (MBL) of 1.2 mm at 5 years compared to 0.8 mm in non-diabetic patients [24].
Agrawal et al. conducted a clinical-radiographic study on 80 patients, comparing marginal bone loss between immediately loaded and delayed-loaded implants. The results indicated that immediately loaded implants exhibited a mean MBL of 0.85 mm at 12 months compared to 0.72 mm in delayed-loading implants; however, this difference was not statistically significant. Nevertheless, in patients with systemic conditions, marginal bone loss was significantly greater, suggesting, in agreement with the findings of the present study, a negative impact of systemic conditions on the long-term stability of implants [25].
Regarding peri-implant parameters, the probing pocket depth (PPD) was significantly higher in the posterior maxilla and was notably influenced by smoking, whereas no significant associations were observed with the type of rehabilitation or the presence of systemic conditions. The plaque index (PI) and bleeding on probing (BoP) showed a statistically significant increase over time, with the highest values recorded in the posterior maxilla and posterior mandible. Smoking was significantly correlated with both parameters, whereas no significant correlation was found with the type of rehabilitation or the presence of systemic conditions. Suppuration was documented in two patients and was significantly associated with smoking and systemic conditions, while no correlation emerged with the implant site or the type of rehabilitation.
Peñarrocha et al. conducted a systematic review of the literature, analyzing 30 clinical studies to assess the effectiveness of immediate post-extractive implants and the peri-implant complications associated with this approach. The results highlighted that smoking is a critical risk factor, associated with an increased incidence of peri-implant mucositis and peri-implantitis, with a significantly higher prevalence in posterior regions. Furthermore, the study reported that patients with systemic conditions, such as diabetes mellitus and osteoporosis, exhibited more pronounced peri-implant inflammation and higher rates of marginal bone loss compared to systemically healthy individuals [10].
Apatzidou et al. conducted a review including 25 clinical and retrospective studies, focusing on the role of smoking in implant treatments. Their analysis revealed that smokers have a significantly higher risk of developing peri-implant inflammation compared to non-smokers. Moreover, the review indicated that marginal bone loss in smokers is, on average, 20–30% greater than in non-smokers after a 5-year follow-up [26].
Tettamanti et al. conducted a literature review on immediate loading post-extraction implants, including studies with a minimum follow-up of 12 months. Their findings emphasized that proper oral hygiene management and regular follow-up visits, as performed in the present study, can significantly reduce the risk of peri-implant inflammatory complications [1]. Specifically, in agreement with other studies, they highlighted that, in patients with inadequate oral hygiene, the risk of bleeding on probing and peri-implant mucositis increases by up to 40% compared to those with good oral hygiene [27,28,29].
Regarding surgical complications, no cases of postoperative pain were reported, whereas nine cases of edema were recorded, four of which were also associated with postoperative bleeding, which was observed in seven patients overall. All surgical complications occurred exclusively in patients with systemic conditions and smokers. Furthermore, a significant correlation was observed with the type of prosthetic rehabilitation, with a higher prevalence of complications in patients rehabilitated with bridges supported by two distal implants compared to those undergoing single implant-prosthetic rehabilitation. In contrast, no significant correlation was found concerning implant site.
Felice et al. conducted a randomized controlled trial on 210 patients, comparing three implant loading protocols in post-extraction single implants: immediate, immediate-delayed (6 weeks), and delayed (4 months). The results indicated that patients undergoing immediate loading had a significantly higher incidence of surgical complications compared to the other groups, with a greater prevalence in smokers and individuals with systemic compromising conditions such as diabetes mellitus and osteoporosis [4].
D’Ambrosio et al. performed an umbrella review of eight systematic reviews to assess the impact of systemic conditions on implant osseointegration. The analysis included studies with follow-up periods ranging from 1 to 10 years, evaluating conditions such as diabetes, osteoporosis, cardiovascular diseases, and bone metabolism disorders. The results indicated that uncontrolled diabetes is significantly associated with an increased risk of implant failure (OR = 2.1, p < 0.01), while osteoporosis compromises the peri-implant bone density, increasing marginal bone loss during the first 12 months post-surgery. Additionally, the review highlighted that certain medications, such as proton pump inhibitors (PPIs) and selective serotonin reuptake inhibitors (SSRIs), negatively interfere with bone regeneration, delaying healing processes and increasing the risk of early implant failure (p < 0.05) [30]. These findings underscore the importance of thorough preoperative evaluation and personalized protocols for patients with systemic conditions to minimize complications and improve implant prognosis.
Regarding prosthetic complications, a significant association was observed concerning the implant site, with a higher incidence in posterior regions, and rehabilitation type, with a greater frequency of complications in bridges compared to single implants. No statistically significant correlation was found between complications and smoking or the presence of systemic conditions.
The higher rate of complications in posterior regions can be attributed to specific biomechanical factors. Previous studies have demonstrated that the greater intensity of occlusal forces in the posterior regions, combined with the generally lower bone quality compared to anterior regions, may increase the risk of ceramic fractures, prosthetic screw loosening, and wear of restorative components [31,32]. In a systematic review, Pjetursson et al. analyzed the influence of prosthetic design and material selection on complications in implant-supported bridges in posterior regions, reporting a significantly higher incidence of fractures and mechanical failures compared to single-unit fixed prostheses [33].
The increased incidence of complications in bridges compared to single implants has been further confirmed by Sailer et al., who, in a literature review, highlighted that multiple-unit fixed prostheses tend to be more vulnerable to complications than single implant crowns. This phenomenon is attributable to the distribution of masticatory forces across multiple units, which can lead to increased mechanical stress on the prosthetic structure and implant connections. Additionally, the complexity of implant bridge design implies a higher risk of ceramic material fractures and prosthetic screw loosening over time [34].
Göthberg et al. conducted a retrospective study on complications associated with fixed implant-supported prostheses, concluding that the primary cause of long-term failure is represented by technical complications, including ceramic fractures and wear of prosthetic components. The high frequency of these events in posterior restorations suggests that the biomechanical design of implant-supported fixed prostheses should carefully consider the type of material used, the distribution of occlusal forces, and the quality of the supporting bone [35].
Misch emphasized the importance of considering biomechanical forces in implant treatment planning. Excessive loading can induce micromovements at the implant level, contributing to the onset of prosthetic complications, particularly in posterior regions. The author suggests that proper occlusal load distribution and the use of high-strength materials can reduce the risk of implant rehabilitation failure [36].
In the current study, the placement of the implants was performed through basal bone, without biomaterials for bone regeneration. Any gaps between the implant surface and the buccal cortex were filled exclusively by collagen hemostatic sponges, according to a minimally invasive approach geared towards the preservation of hard and soft tissues. The results obtained in terms of implant survival rate contained marginal bone loss, and the incidences of surgical and prosthetic complications are equal to, if not superior to, other studies in the literature in which biomaterials were used to fill post-extraction sockets.
In a prospective study conducted by Crespi et al. using hydroxyapatite and collagen-based biomaterials in immediately loaded post-extractive implants, a survival rate of 94.4% at 12 months was reported, with an average marginal bone resorption of 0.78 mm [31]. Similarly, Barone et al. clinically compared alveolar filling with an autologous bone versus xenogeneic graft in post-extractive implants, finding similar survival rates but with more frequent peri-implant inflammation in sites treated with heterologous materials [37].
These data suggest that, in the presence of adequate residual vestibular bone thickness, an approach that avoids the use of biomaterials may guarantee comparable clinical results in terms of implant stability and the reduction of complications, provided that careful surgical and prosthetic management of the site is maintained. Furthermore, the absence of biomaterials could reduce the risk of chronic inflammatory reactions or suboptimal graft integration, as reported in some systematic review studies [38,39].
5. Conclusions
Within the limitations of the present study, the results confirm the reliability of immediately loaded post-extraction implants in single and partial rehabilitations while highlighting the necessity of detailed preoperative planning and regular clinical and radiographic monitoring to optimize the long-term prognosis. Patient selection, particularly in the presence of risk factors such as smoking and systemic conditions, plays a crucial role in reducing the incidence of surgical and prosthetic complications.
The absence of a correlation between smoking, systemic conditions, and prosthetic complications suggests that other factors, such as the biomechanical characteristics of the implant-supported arch and occlusal quality, may influence the longevity of implant-supported rehabilitations with immediately loaded post-extraction implants. Further prospective studies with extended follow-up periods and in-depth analyses of potential confounding variables are needed to strengthen these findings and refine the treatment strategies for patients undergoing immediate loading post-extraction implant rehabilitations.
Author Contributions
Conceptualization, M.N., B.D. and R.V.; methodology, B.D., F.C. and R.D.C.; software, B.D.; validation, B.D., M.N. and R.V.; formal analysis, B.D., F.C. and R.D.C.; investigation, B.D., F.C. and R.D.C.; resources, B.D., M.N. and R.V.; data curation, B.D., F.C. and R.D.C.; writing—original draft preparation, B.D. and F.C.; writing—review and editing, B.D., R.D.C., F.C. and R.V.; visualization, B.D., F.C. and R.D.C.; supervision, M.N. and R.V.; project administration, M.N., R.V. and R.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Ethics Committee of Vita-Salute San Raffaele University, no. 180/INT/2021, Dental School Department of Dentistry IRCCS San Raffaele Hospital, Milan, 20132, Italy.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MBL | Marginal Bone Loss |
| PPD | Probing Pocket Depth |
| PI | Plaque Index |
| BoP | Bleeding on Probing |
| PMMA | Poly-methyl-methacrylate |
| CBCT | Cone Beam Computed Tomography |
| ISQ | Implant Stability Quotient |
| PTFE | Polytetrafluoroethylene |
| Bis-GMA | Bisphenol A-Glycidyl Methacrylate |
| TEGDMA | Triethylene Glycol Dimethacrylate |
| SPSS | Statistical Package for the Social Sciences |
| OR | Odds Ratio |
| ANOVA | Analysis of Variance |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Tettamanti, L.; Andrisani, C.; Bassi, M.A.; Vinci, R.; Silvestre-Rangil, J.; Tagl, A. Post extractive implant: Evaluation of the critical aspects. Oral Implant. 2017, 10, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Milillo, L.; Fiandaca, C.; Giannoulis, F.; Ottria, L.; Lucchese, A.; Silvestre, F.; Petruzzi, M. Immediate vs non-immediate loading post-extractive implants: A comparative study of implant stability quotient (ISQ). Oral Implant. 2016, 9, 123–131. [Google Scholar] [CrossRef]
- Arora, H.; Ivanovski, S. Clinical and aesthetic outcomes of immediately placed single-tooth implants with immediate vs. delayed restoration in the anterior maxilla: A retrospective cohort study. Clin. Oral Implant. Res. 2018, 29, 346–352. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Barausse, C.; Trullenque-Eriksson, A.; Esposito, M. Immediate non-occlusal loading of immediate post-extractive versus delayed placement of single implants in preserved sockets of the anterior maxilla: 1-year post-loading outcome of a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 361–372. [Google Scholar]
- Grandi, T.; Guazzi, P.; Samarani, R.; Grandi, G. Immediate provisionalisation of single post-extractive implants versus implants placed in healed sites in the anterior maxilla: 1-year results from a multicentre controlled cohort study. Eur. J. Oral Implantol. 2013, 6, 285–295. [Google Scholar] [PubMed]
- Esposito, M.; Zucchelli, G.; Cannizzaro, G.; Checchi, L.; Barausse, C.; Trullenque-Eriksson, A.; Felice, P. Immediate, immediate-delayed (6 weeks) and delayed (4 months) post-extractive single implants: 1-year post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 11–26. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Timing of implant placement after tooth extraction: Immediate, immediate-delayed or delayed implants? A Cochrane systematic review. Eur. J. Oral Implantol. 2010, 3, 189–205. [Google Scholar]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontol. 2000 2017, 73, 84–102. [Google Scholar] [CrossRef]
- Yu, X.; Teng, F.; Zhao, A.; Wu, Y.; Yu, D. Effects of post-extraction alveolar ridge preservation versus immediate implant placement: A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2022, 22, 101734. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Uribe, R.; Balaguer, J. Immediate implants after extraction. A review of the current situation. Med. Oral 2004, 9, 234–242. [Google Scholar]
- Testori, T.; Zuffetti, F.; Capelli, M.; Galli, F.; Weinstein, R.L.; Del Fabbro, M. Immediate versus conventional loading of post-extraction implants in the edentulous jaws. Clin. Implant Dent. Relat. Res. 2014, 16, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Oral Maxillofac. Implant. 2003, 18, 189–197. [Google Scholar]
- Roccuzzo, M.; Gaudioso, L.; Bunino, M.; Dalmasso, P. Surgical treatment of peri-implantitis in partially edentulous patients: A prospective clinical study on the effect of implant surface. Clin. Oral Implant. Res. 2010, 21, 389–395. [Google Scholar]
- Lang, N.P.; Berglundh, T.; Heitz-Mayfield, L.J.A.; Pjetursson, B.E.; Sanz, M.; Lindhe, J. Consensus statements and recommended clinical procedures regarding implant survival and complications. Clin. Oral Implant. Res. 2007, 18, 97–113. [Google Scholar]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors influencing early dental implant failures. J. Oral Rehabil. 2015, 42, 624–635. [Google Scholar] [CrossRef]
- Van Assche, N.; Quirynen, M. Tapered implants improve primary stability in bone of different density. Clin. Implant Dent. Relat. Res. 2011, 13, 175–183. [Google Scholar]
- Fugazzotto, P.A.; Beagle, J.R.; Ganeles, J.; Curtis, M.S.; Elian, N.; Eng, J.; Kan, J.; Tarnow, D.P. Success and failure rates of 1,344 6- to 9-mm-wide-diameter implants: A retrospective study. Int. J. Oral Maxillofac. Implant. 2004, 19, 775–781. [Google Scholar]
- Traini, T.; Degidi, M.; Caputi, S.; Piattelli, A.; Mangano, C. Histologic and histomorphometric evaluation of an innovative dental implant design in human-retrieved, clinically stable, successful implants. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 66–75. [Google Scholar]
- Mura, P. Immediate loading of tapered implants placed in postextraction sockets: Retrospective analysis of the 5-year clinical outcome. Clin. Implant Dent. Relat. Res. 2012, 14, 565–574. [Google Scholar] [CrossRef]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants: A systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, M.P.; Marini, R.; La Monaca, G.; Sepe, C.; Tonoli, F.; Annibali, S. Immediate loading of post-extractive single-tooth implants: A 1-year prospective study. Clin. Oral Implant. Res. 2015, 26, 1070–1079. [Google Scholar] [CrossRef]
- Vázquez Álvarez, R.; Pérez Sayáns, M.; Gayoso Diz, P.; García García, A. Factors affecting peri-implant bone loss: A post-five-year retrospective study. Clin. Oral Implant. Res. 2015, 26, 1006–1014. [Google Scholar] [CrossRef]
- Agrawal, H.; Kumar, R.; Kanteshwari, I.K.; Jaiswal, G.; Marothiya, S.; Jasuja, A.; Raje, S. Soft & Hard Tissue Assessment around Immediate & Delayed Implants: A Clinico-Radiographical Study. Mymensingh Med. J. 2020, 29, 691–700. [Google Scholar]
- Apatzidou, D.A. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol. 2000 2022, 90, 45–61. [Google Scholar] [CrossRef]
- Polizzi, E.; D’orto, B.; Tomasi, S.; Tetè, G. A micromorphological/microbiological pilot study assessing three methods for the maintenance of the implant patient. Clin. Exp. Dent. Res. 2021, 7, 156–162. [Google Scholar] [CrossRef]
- Di Petto, L.F.; Tetè, G.; Hera, M.; Polizzi, E. Aesthetic Restorations: The Role of The Dental Hygienist in Professional Maintenance: In Vitro Microbiological Study. Materials 2023, 16, 1373. [Google Scholar] [CrossRef]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S152–S157. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Dental implants placed in extraction sites grafted with different bone substitutes: Radiographic evaluation at 24 months. J. Periodontol. 2009, 80, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Sailer, I.; Merino-Higuera, E.; Spies, B.C.; Burkhardt, F.; Karasan, D. Systematic review evaluating the influence of the prosthetic material and prosthetic design on the clinical outcomes of implant-supported multi-unit fixed dental prosthesis in the posterior area. Clin. Oral Implant. Res. 2023, 34 (Suppl. S26), 86–103. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontol. 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Göthberg, C.; Bergendal, T.; Magnusson, T. Complications after treatment with implant-supported fixed prostheses: A retrospective study. Int. J. Prosthodont. 2003, 16, 201–207. [Google Scholar]
- Misch, C.E. Consideration of biomechanical stress in treatment with dental implants. Dent. Today 2006, 25, 80, 82, 84–85, quiz 85. [Google Scholar]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Covani, U. Volumetric Changes After Immediate Implant Placement With or Without Xenograft in the Esthetic Zone: A Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2013, 24, 1231–1238. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of Alveolar Ridge Preservation After Tooth Extraction: A Systematic Review and Meta-Analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Horvath, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.; Donos, N. Alveolar Ridge Preservation: A Systematic Review. Clin. Oral Investig. 2017, 21, 1433–1462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).