Emotional Dysregulation and Cognitive Disengagement Syndrome: Exploring Their Relationship Through the Lens of Twin Studies
Abstract
1. Introduction
2. Behavioral Genetics
2.1. Quantitative Genetics
2.1.1. Twin Studies
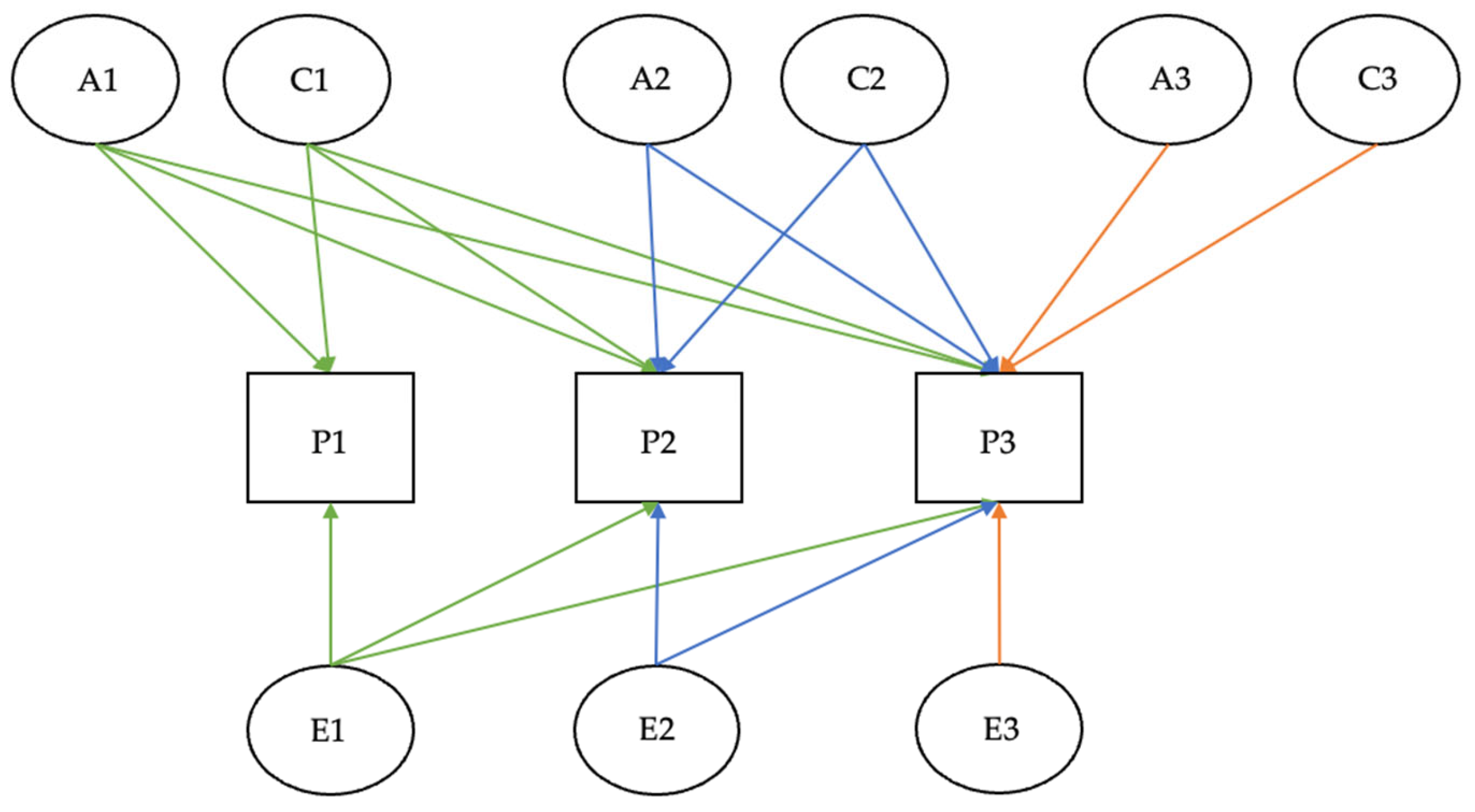
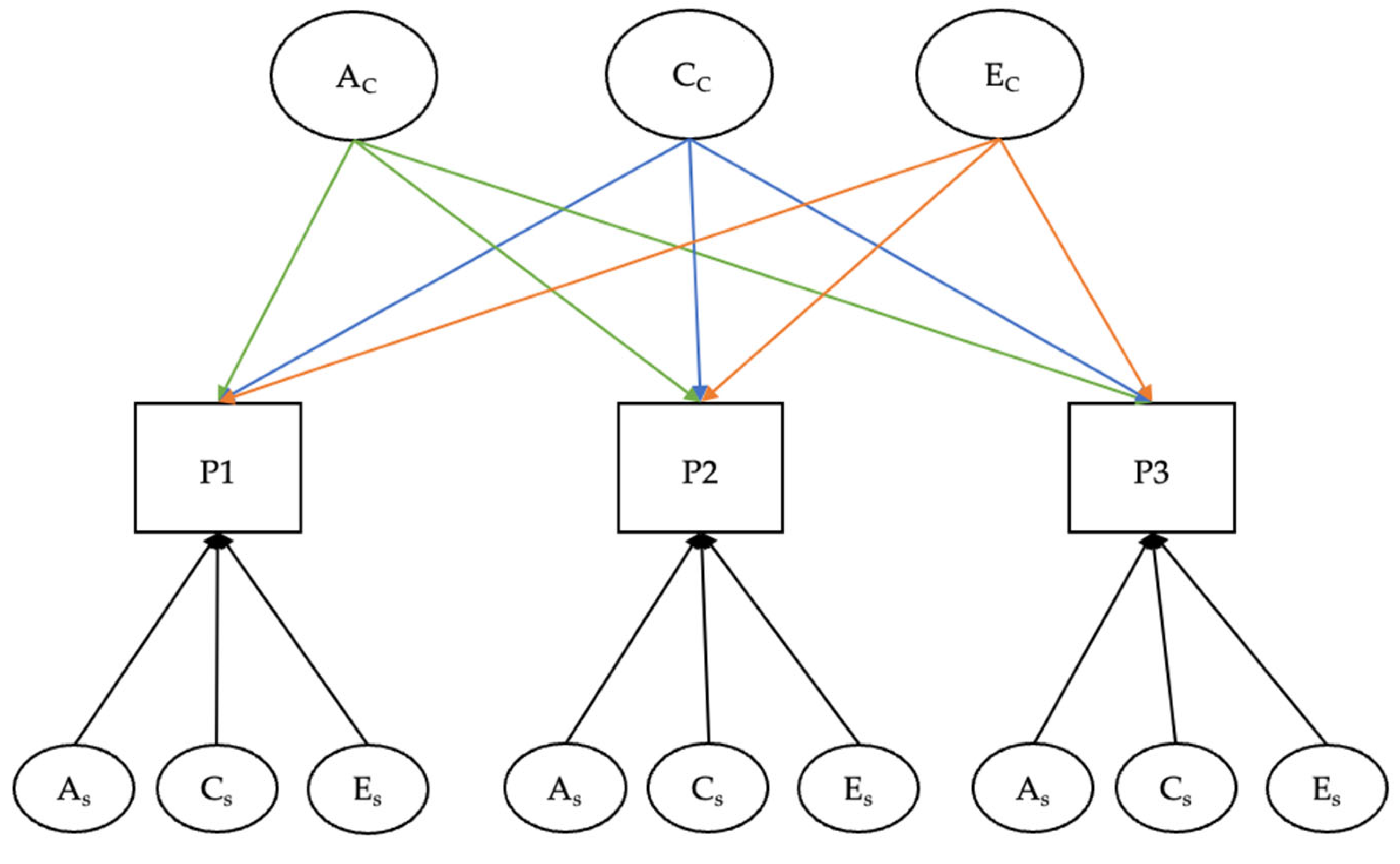
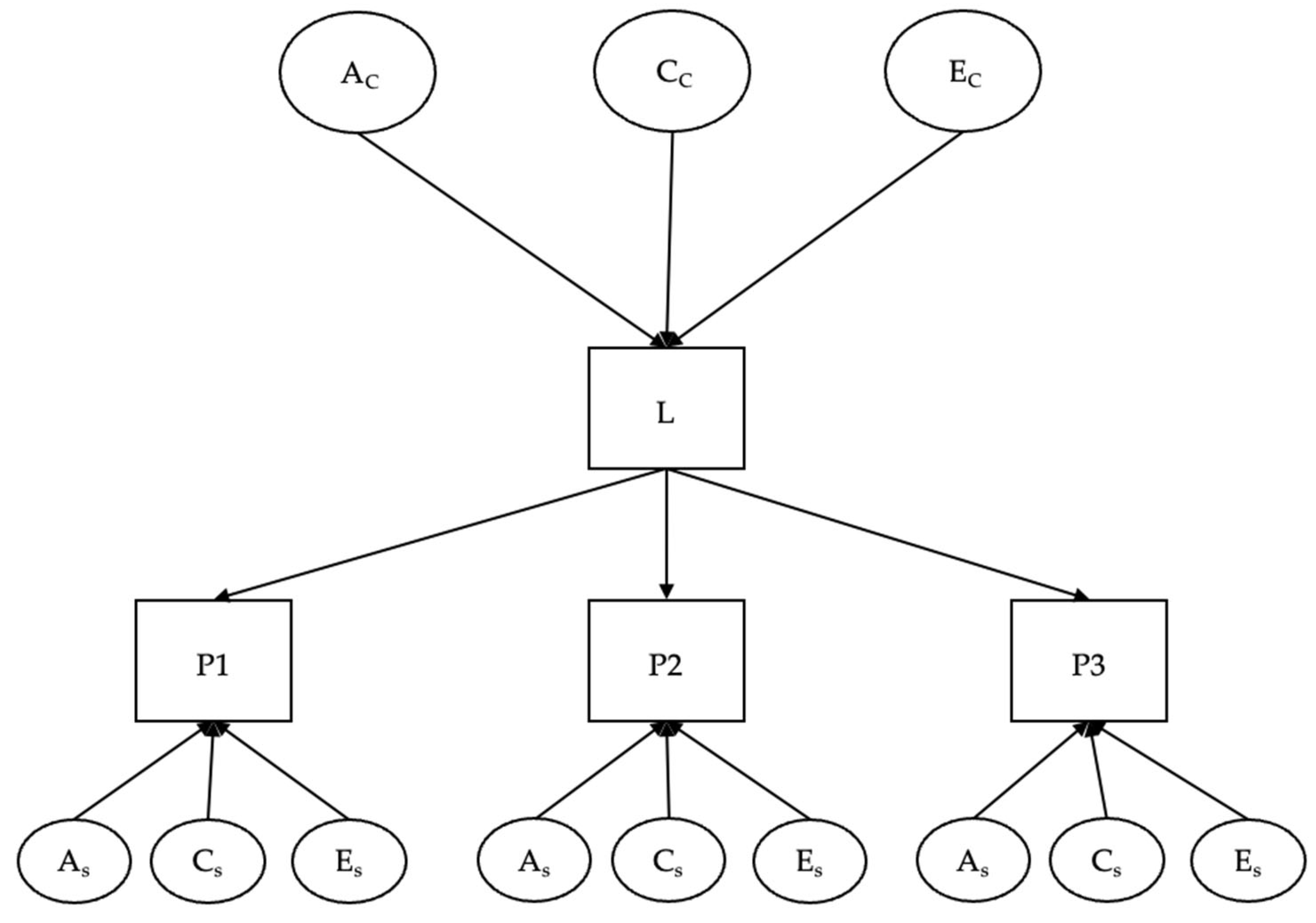
2.1.2. Twin Studies: Statistical Methods and Models
3. Emotional Regulation, Dysregulation, and Twin Studies
3.1. Emotional Regulation and Emotional Dysregulation: Definitions and Comorbidities
3.2. Twin Studies on Emotional Dysregulation
4. Cognitive Disengagement Syndrome (CDS) and Twin Studies
4.1. CDS: Symptoms, Impairments, and Differentiation with ADHD
4.2. CDS: Comorbidities
4.3. Twin Studies on CDS
5. The Association Between CDS and ED
6. Discussion: The Critical Role of Twin Studies in Examining the Association Between CDS and ED
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| CDS | Cognitive Disengagement Syndrome |
| ED | Emotional Dysregulation |
| rGE | Genotype-Environment correlation |
| A | Additive genetic effects |
| C | Shared environmental effects |
| E | Unique environmental effects |
| MZ | Monozygotic |
| DZ | Dizygotic |
| EEA | Equal Environment Assumption |
| SEM | Structural Equation Models |
| ER | Emotion Regulation |
| EF | Executive Functions |
| RDoC | Research Domain Criteria |
| ALS | Affective Lability Scale |
| AIM | Affect Intensity Measure |
| BRISC | Brief Risk-Resilience Index for Screening |
| SCT | Sluggish Cognitive Tempo |
| INP | Inattentive Problems |
| HIP | Hyperactivity-Impulsivity Problems |
| IN | Inattention |
| HI | Hyperactivity |
| DBT | Dialectical Behavior Therapy |
Appendix A
References
- Becker, S.P.; Garner, A.A.; Byars, K.C. Sluggish Cognitive Tempo in Children Referred to a Pediatric Sleep Disorders Center: Examining Possible Overlap with Sleep Problems and Associations with Impairment. J. Psychiatr. Res. 2016, 77, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Distinguishing Sluggish Cognitive Tempo from ADHD in Children and Adolescents: Executive Functioning, Impairment, and Comorbidity. J. Clin. Child Adolesc. Psychol. 2013, 42, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Calhoun, S.L.; Kallus, R.; Baweja, R.; Waschbusch, D.A. Cognitive Disengagement Syndrome (Formerly Sluggish Cognitive Tempo) and Comorbid Symptoms in Child Autism, ADHD, and Elementary School Samples. J. Psychopathol. Behav. Assess. 2024, 46, 857–865. [Google Scholar] [CrossRef]
- Becker, S.P.; Willcutt, E.G.; Leopold, D.R.; Fredrick, J.W.; Smith, Z.R.; Jacobson, L.A.; Burns, G.L.; Mayes, S.D.; Waschbusch, D.A.; Froehlich, T.E.; et al. Report of a Work Group on Sluggish Cognitive Tempo: Key Research Directions and a Consensus Change in Terminology to Cognitive Disengagement Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 2023, 62, 629–645. [Google Scholar] [CrossRef]
- Kofler, M.J.; Irwin, L.N.; Sarver, D.E.; Fosco, W.D.; Miller, C.E.; Spiegel, J.A.; Becker, S.P. What Cognitive Processes Are “Sluggish” in Sluggish Cognitive Tempo? J. Consult. Clin. Psychol. 2019, 87, 1030–1042. [Google Scholar] [CrossRef]
- Hartman, C.A.; Willcutt, E.G.; Rhee, S.H.; Pennington, B.F. The Relation Between Sluggish Cognitive Tempo and DSM-IV ADHD. J. Abnorm. Child Psychol. 2004, 32, 491–503. [Google Scholar] [CrossRef]
- Sesso, G.; Milone, A.; Drago, F.; Viglione, V.; Berloffa, S.; Boldrini, S.; Loriaux, N.; Valente, E.; Molesti, A.; Placini, F.; et al. A Novel Multidimensional Questionnaire for the Assessment of Emotional Dysregulation in Adolescents: Reactivity, Intensity, Polarity and Stability Questionnaire–Youth Version (RIPoSt–Y). J. Affect. Disord. 2021, 291, 359–367. [Google Scholar] [CrossRef]
- Cai, R.Y.; Hardan, A.Y.; Phillips, J.M.; Frazier, T.W.; Uljarević, M. Brief Report: Emotion Regulation Influences on Internalizing and Externalizing Symptoms Across the Normative-Clinical Continuum. Front. Psychiatry 2021, 12, 693570. [Google Scholar] [CrossRef]
- Plomin, R.; DeFries, J.C.; Knopik, V.S.; Neiderhiser, J.M. Genetica del Comportamento, 6th ed.; Raffaello Cortina Editore: Milano, Italy, 2018; ISBN 978-88-6030-685-2. [Google Scholar]
- Fuller, J.L.; Thompson, W.R. Behavior Genetics, 1st ed.; John Wiley & Sons: New York, NY, USA, 1960. [Google Scholar]
- Plomin, R. Behavioral Genetics, 5th ed.; Worth Publishers: New York, NY, USA, 2008; ISBN 978-1-4292-0577-1. [Google Scholar]
- Scaini, S.; Medda, E.; Battaglia, M.; De Giuli, G.; Stazi, M.A.; D’Ippolito, C.; Fagnani, C. A Twin Study of the Relationships between Cognitive Disengagement Syndrome and Anxiety Phenotypes in Childhood and Adolescence. Res. Child Adolesc. Psychopathol. 2023, 51, 949–960. [Google Scholar] [CrossRef]
- Plomin, R. L’impronta Genetica: Come il DNA ci Rende Quelli che Siamo; Cortina: Milano, Italy, 2019; ISBN 978-88-328-5103-8. [Google Scholar]
- Plomin, R.; Bergeman, C.S. The Nature of Nurture: Genetic Influence on “Environmental” Measures. Behav. Brain Sci. 1991, 14, 373–386. [Google Scholar] [CrossRef]
- Kendler, K.S. Genetic Epidemiology in Psychiatry: Taking Both Genes and Environment Seriously. Arch. Gen. Psychiatry 1995, 52, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S. Twin Studies of Psychiatric Illness: Current Status and Future Directions. Arch. Gen. Psychiatry 1993, 50, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; Fulker, D.W.; Corley, R.; DeFries, J.C. Nature, Nurture, and Cognitive Development from 1 to 16 Years: A Parent-Offspring Adoption Study. Psychol. Sci. 1997, 8, 442–447. [Google Scholar] [CrossRef]
- Koeppen-Schomerus, G.; Spinath, F.M.; Plomin, R. Twins and Non-Twin Siblings: Different Estimates of Shared Environmental Influence in Early Childhood. Twin Res. Hum. Genet. 2003, 6, 97–105. [Google Scholar] [CrossRef]
- Rende, R.; Plomin, R. Nature, nurture and the development of psychopathology. In Developmental Psychopathology. Volume 1: Theory and Method; Cicchetti, D., Cohen, D.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 291–314. ISBN 0-471-23736-1. [Google Scholar]
- Fagnani, C.; Brescianini, S.; Medda, E.; Stazi, M.A. Metodi statistici per lo studio dei gemelli. Ann. dell’Istituto Super. Sanità 2006, 42, 86–93. [Google Scholar]
- Nikstat, A.; Riemann, R. On the Etiology of Internalizing and Externalizing Problem Behavior: A Twin-Family Study. PLoS ONE 2020, 15, e0230626. [Google Scholar] [CrossRef]
- Cosgrove, V.E.; Rhee, S.H.; Gelhorn, H.L.; Boeldt, D.; Corley, R.C.; Ehringer, M.A.; Young, S.E.; Hewitt, J.K. Structure and Etiology of Co-Occurring Internalizing and Externalizing Disorders in Adolescents. J. Abnorm. Child Psychol. 2011, 39, 109–123. [Google Scholar] [CrossRef]
- Willoughby, E.A.; Polderman, T.J.C.; Boutwell, B.B. Behavioural Genetics Methods. Nat. Rev. Methods Primers 2023, 3, 10. [Google Scholar] [CrossRef]
- Rijsdijk, F. Independent Pathway Model. In Wiley StatsRef: Statistics Reference Online; Kenett, R.S., Longford, N.T., Piegorsch, W.W., Ruggeri, F., Eds.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-44511-2. [Google Scholar]
- Brockman, R.; Ciarrochi, J.; Parker, P.; Kashdan, T. Emotion Regulation Strategies in Daily Life: Mindfulness, Cognitive Reappraisal and Emotion Suppression. Cogn. Behav. Ther. 2017, 46, 91–113. [Google Scholar] [CrossRef]
- Gross, J.J. Antecedent- and Response-Focused Emotion Regulation: Divergent Consequences for Experience, Expression, and Physiology. J. Personal. Soc. Psychol. 1998, 74, 224–237. [Google Scholar] [CrossRef]
- Gross, J.J. Emotion Regulation: Current Status and Future Prospects. Psychol. Inq. 2015, 26, 1–26. [Google Scholar] [CrossRef]
- Rogier, G.; Garofalo, C.; Velotti, P. Is Emotional Suppression Always Bad? A Matter of Flexibility and Gender Differences. Curr. Psychol. 2019, 38, 411–420. [Google Scholar] [CrossRef]
- Gross, J.J.; Levenson, R.W. Emotional Suppression: Physiology, Self-Report, and Expressive Behavior. J. Personal. Soc. Psychol. 1993, 64, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Doebel, S. Rethinking Executive Function and Its Development. Perspect. Psychol. Sci. 2020, 15, 942–956. [Google Scholar] [CrossRef]
- Halse, M.; Steinsbekk, S.; Bjørklund, O.; Hammar, Å.; Wichstrøm, L. Emotions or Cognitions First? Longitudinal Relations between Executive Functions and Emotion Regulation in Childhood. Child Dev. 2024, 95, 1508–1521. [Google Scholar] [CrossRef]
- Feldman, R. The Development of Regulatory Functions from Birth to 5 Years: Insights from Premature Infants. Child Dev. 2009, 80, 544–561. [Google Scholar] [CrossRef]
- Faraone, S.V.; Rostain, A.L.; Blader, J.; Busch, B.; Childress, A.C.; Connor, D.F.; Newcorn, J.H. Practitioner Review: Emotional Dysregulation in Attention-deficit/Hyperactivity Disorder—Implications for Clinical Recognition and Intervention. Child Psychol. Psychiatry 2019, 60, 133–150. [Google Scholar] [CrossRef]
- Abravanel, B.T.; Sinha, R. Emotion Dysregulation Mediates the Relationship between Lifetime Cumulative Adversity and Depressive Symptomatology. J. Psychiatr. Res. 2015, 61, 89–96. [Google Scholar] [CrossRef]
- Herd, T.; Kim-Spoon, J. A Systematic Review of Associations Between Adverse Peer Experiences and Emotion Regulation in Adolescence. Clin. Child Fam. Psychol. Rev. 2021, 24, 141–163. [Google Scholar] [CrossRef]
- Moore, A.A.; Lapato, D.M.; Brotman, M.A.; Leibenluft, E.; Aggen, S.H.; Hettema, J.M.; York, T.P.; Silberg, J.L.; Roberson-Nay, R. Heritability, Stability, and Prevalence of Tonic and Phasic Irritability as Indicators of Disruptive Mood Dysregulation Disorder. J. Child Psychol. Psychiatry 2019, 60, 1032–1041. [Google Scholar] [CrossRef]
- Aldao, A.; Gee, D.G.; De Los Reyes, A.; Seager, I. Emotion Regulation as a Transdiagnostic Factor in the Development of Internalizing and Externalizing Psychopathology: Current and Future Directions. Dev. Psychopathol. 2016, 28, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Ong, A.D.; Seroczynski, A.D.; Bergeman, C.S. Affective Intensity and Lability: Heritability in Adult Male Twins. J. Affect. Disord. 2012, 136, 1011–1016. [Google Scholar] [CrossRef]
- Harvey, P.D.; Greenberg, B.R.; Serper, M.R. The Affective Lability Scales: Development, Reliability, and Validity. J. Clin. Psychol. 1989, 45, 786–793. [Google Scholar] [CrossRef]
- Larsen, R.J.; Diener, E. Affect Intensity as an Individual Difference Characteristic: A Review. J. Res. Personal. 1987, 21, 1–39. [Google Scholar] [CrossRef]
- McRae, K.; Rhee, S.H.; Gatt, J.M.; Godinez, D.; Williams, L.M.; Gross, J.J. Genetic and Environmental Influences on Emotion Regulation: A Twin Study of Cognitive Reappraisal and Expressive Suppression. Emotion 2017, 17, 772–777. [Google Scholar] [CrossRef]
- Williams, L.M.; Cooper, N.J.; Wisniewski, S.R.; Gatt, J.M.; Koslow, S.H.; Kulkarni, J.; DeVarney, S.; Gordon, E.; John Rush, A. Sensitivity, Specificity, and Predictive Power of the “Brief Risk-Resilience Index for SCreening,” a Brief Pan-Diagnostic Web Screen for Emotional Health. Brain Behav. 2012, 2, 576–589. [Google Scholar] [CrossRef]
- Mikhail, M.E.; Burt, S.A.; Neale, M.C.; Keel, P.K.; Katzman, D.K.; Klump, K.L. Comorbidity Between Internalizing Symptoms and Disordered Eating Is Primarily Driven by Genetic Influences on Emotion Regulation in Adult Female Twins. Clin. Psychol. Sci. 2024, 12, 1242–1261. [Google Scholar] [CrossRef]
- Astenvald, R.; Frick, M.A.; Neufeld, J.; Bölte, S.; Isaksson, J. Emotion Dysregulation in ADHD and Other Neurodevelopmental Conditions: A Co-Twin Control Study. Child Adolesc. Psychiatry Ment. Health 2022, 16, 92. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J.; Zhang, J.; Li, X.; McGue, M. Investigating Genetic and Environmental Contributions to Adolescent Externalizing Behavior in a Collectivistic Culture: A Multi-Informant Twin Study. Psychol. Med. 2015, 45, 1989–1997. [Google Scholar] [CrossRef]
- Barkley, R.A. Sluggish Cognitive Tempo (Concentration Deficit Disorder?): Current Status, Future Directions, and a Plea to Change the Name. J. Abnorm. Child Psychol. 2014, 42, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Waschbusch, D.A. Editorial: A Review of Sluggish Cognitive Tempo Measures That Will Benefit Researchers and Clinicians. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Krone, B.; Adler, L.A.; Anbarasan, D.; Leon, T.; Gallagher, R.; Patel, P.; Faraone, S.V.; Newcorn, J.H. Characteristics of Sluggish Cognitive Tempo among Adults with ADHD: Objective Neurocognitive Measures Align with Self-Report of Executive Function. Front. Child Adolesc. Psychiatry 2023, 2, 1188901. [Google Scholar] [CrossRef]
- Becker, S.P.; Burns, G.L.; Leopold, D.R.; Olson, R.K.; Willcutt, E.G. Differential Impact of Trait Sluggish Cognitive Tempo and ADHD Inattention in Early Childhood on Adolescent Functioning. Child Psychol. Psychiatry 2018, 59, 1094–1104. [Google Scholar] [CrossRef]
- Fredrick, J.W.; Becker, S.P.; Kofler, M.J.; Jarrett, M.A.; Burns, G.L.; Luebbe, A.M. Disentangling the Effects of Attentional Difficulties on Fears of Social Evaluation and Social Anxiety Symptoms: Unique Interactions with Sluggish Cognitive Tempo. J. Psychiatr. Res. 2020, 131, 39–46. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Becker, S.P.; Nick, T.G.; Brinkman, W.B.; Stein, M.A.; Peugh, J.; Epstein, J.N. Sluggish Cognitive Tempo as a Possible Predictor of Methylphenidate Response in Children with ADHD: A Randomized Controlled Trial. J. Clin. Psychiatry 2018, 79, 17m11553. [Google Scholar] [CrossRef]
- Vu, A.; Thompson, L.; Willcutt, E.; Petrill, S. Sluggish Cognitive Tempo: Longitudinal Stability and Validity. ADHD Atten. Deficit Hyperact. Disord. 2019, 11, 463–471. [Google Scholar] [CrossRef]
- Servera, M.; Sáez, B.; Burns, G.L.; Becker, S.P. Clinical Differentiation of Sluggish Cognitive Tempo and Attention-Deficit/Hyperactivity Disorder in Children. J. Abnorm. Psychol. 2018, 127, 818–829. [Google Scholar] [CrossRef]
- Becker, S.P.; Burns, G.L.; Smith, Z.R.; Langberg, J.M. Sluggish Cognitive Tempo in Adolescents with and without ADHD: Differentiation from Adolescent-Reported ADHD Inattention and Unique Associations with Internalizing Domains. J. Abnorm. Child Psychol. 2020, 48, 391–406. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Chhabildas, N.; Kinnear, M.; DeFries, J.C.; Olson, R.K.; Leopold, D.R.; Keenan, J.M.; Pennington, B.F. The Internal and External Validity of Sluggish Cognitive Tempo and Its Relation with DSM–IV ADHD. J. Abnorm. Child Psychol. 2014, 42, 21–35. [Google Scholar] [CrossRef]
- Smith, Z.R.; Zald, D.H.; Lahey, B.B. Sluggish Cognitive Tempo and Depressive Symptoms in Children and Adolescents Predict Adulthood Psychopathology. J. Abnorm. Child Psychol. 2020, 48, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Seebeck, J.; Waschbusch, D.A. Relationship Between Sluggish Cognitive Tempo and Age and IQ in Preschool and School-Age Children and Adolescents with Autism and with ADHD. J. Autism Dev. Disord. 2022, 52, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Burns, G.L.; Becker, S.P. Can Sluggish Cognitive Tempo Be Distinguished from ADHD Inattention in Very Young Children? Evidence from a Sample of Korean Preschool Children. J. Atten. Disord. 2017, 21, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Callan, P.D.; Shepler, D.K.; Justice, M.D.; Oliver, C.E. Correlates of Sluggish Cognitive Tempo among Clinic-Referred Youth: Expanding Awareness of Somatic Symptoms and Stress in the Clinical Picture. J. Pediatr. Neuropsychol. 2020, 6, 143–158. [Google Scholar] [CrossRef]
- Burns, G.L.; Becker, S.P. Sluggish Cognitive Tempo and ADHD Symptoms in a Nationally Representative Sample of U.S. Children: Differentiation Using Categorical and Dimensional Approaches. J. Clin. Child Adolesc. Psychol. 2021, 50, 267–280. [Google Scholar] [CrossRef]
- Reinvall, O.; Kujala, T.; Voutilainen, A.; Moisio, A.; Lahti-Nuuttila, P.; Laasonen, M. Sluggish Cognitive Tempo in Children and Adolescents with Higher Functioning Autism Spectrum Disorders: Social Impairments and Internalizing Symptoms. Scand. J. Psychol. 2017, 58, 389–399. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Waschbusch, D.A. Relationship between Sluggish Cognitive Tempo and Sleep, Psychological, Somatic, and Cognitive Problems and Impairment in Children with Autism and Children with ADHD. Clin. Child Psychol. Psychiatry 2021, 26, 518–530. [Google Scholar] [CrossRef]
- Brewe, A.M.; Simmons, G.L.; Capriola-Hall, N.N.; White, S.W. Sluggish Cognitive Tempo: An Examination of Clinical Correlates for Adults with Autism. Autism 2020, 24, 1373–1383. [Google Scholar] [CrossRef]
- Duncan, A.; Tamm, L.; Birnschein, A.M.; Becker, S.P. Clinical Correlates of Sluggish Cognitive Tempo in Adolescents with Autism Spectrum Disorder. Autism 2019, 23, 1354–1362. [Google Scholar] [CrossRef]
- Becker, S.P.; Luebbe, A.M.; Joyce, A.M. The Child Concentration Inventory (CCI): Initial Validation of a Child Self-Report Measure of Sluggish Cognitive Tempo. Psychol. Assess. 2015, 27, 1037–1052. [Google Scholar] [CrossRef]
- Moruzzi, S.; Rijsdijk, F.; Battaglia, M. A Twin Study of the Relationships among Inattention, Hyperactivity/Impulsivity and Sluggish Cognitive Tempo Problems. J. Abnorm. Child Psychol. 2014, 42, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D.R.; Christopher, M.E.; Burns, G.L.; Becker, S.P.; Olson, R.K.; Willcutt, E.G. Attention-deficit/Hyperactivity Disorder and Sluggish Cognitive Tempo throughout Childhood: Temporal Invariance and Stability from Preschool through Ninth Grade. Child Psychol. Psychiatry 2016, 57, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.J.; Becker, S.P.; Luebbe, A.M. Does Emotion Dysregulation Mediate the Association Between Sluggish Cognitive Tempo and College Students’ Social Impairment? J. Atten. Disord. 2016, 20, 802–812. [Google Scholar] [CrossRef]
- Greene, T.; West, M.; Somer, E. Maladaptive Daydreaming and Emotional Regulation Difficulties: A Network Analysis. Psychiatry Res. 2020, 285, 112799. [Google Scholar] [CrossRef]
- Yılmaz, Y.; Bahadır, E. Cognitive Disengagement Syndrome and Attention Deficit Hyperactivity Disorder: An Examination of Relationships with Alexithymia and Emotion Regulation Difficulties. Appl. Neuropsychol. Adult 2024, 1–7. [Google Scholar] [CrossRef]
- Barkley, R.A. Distinguishing Sluggish Cognitive Tempo from Attention-Deficit/Hyperactivity Disorder in Adults. J. Abnorm. Psychol. 2012, 121, 978–990. [Google Scholar] [CrossRef]
- Araujo Jiménez, E.A.; Jané Ballabriga, M.C.; Bonillo Martin, A.; Arrufat, F.J.; Serra Giacobo, R. Executive Functioning in Children and Adolescents with Symptoms of Sluggish Cognitive Tempo and ADHD. J. Atten. Disord. 2015, 19, 507–514. [Google Scholar] [CrossRef]
- Cano-Crespo, A.; Moreno-García, I.; Servera, M.; Morales-Ortiz, M. Emotional Regulation Problems in Cognitive Disengagement Syndrome (Formerly Sluggish Cognitive Tempo), Attention Deficit and Hyperactivity Disorder, Anxiety and Depression. Span. J. Psychol. 2024, 27, e20. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S.; Becker, S.P.; Bölte, S.; Castellanos, F.X.; Franke, B.; Newcorn, J.H.; Nigg, J.T.; Rohde, L.A.; Simonoff, E. Annual Research Review: Perspectives on Progress in ADHD Science—From Characterization to Cause. Child Psychol. Psychiatry 2023, 64, 506–532. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Cicchetti, D. Emotion Dysregulation and Emerging Psychopathology: A Transdiagnostic, Transdisciplinary Perspective. Dev. Psychopathol. 2019, 31, 799–804. [Google Scholar] [CrossRef]
- Wiggs, K.K.; Froehlich, T.E.; Becker, S.P. Pharmacologic Management of Cognitive Disengagement Syndrome (CDS) and Implications for Attention-Deficit/Hyperactivity Disorder (ADHD) Treatment: Emerging Treatments and Recommendations for Future Research. CNS Drugs 2023, 37, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Linehan, M.M.; Wilks, C.R. The Course and Evolution of Dialectical Behavior Therapy. Am. J. Psychother. 2015, 69, 97–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giuli, G.; Amico, C.; De Francesco, S.; Giani, L.; Tüzün, G.; Galli, F.; Caputi, M.; Forresi, B.; Scaini, S. Emotional Dysregulation and Cognitive Disengagement Syndrome: Exploring Their Relationship Through the Lens of Twin Studies. Appl. Sci. 2025, 15, 6067. https://doi.org/10.3390/app15116067
De Giuli G, Amico C, De Francesco S, Giani L, Tüzün G, Galli F, Caputi M, Forresi B, Scaini S. Emotional Dysregulation and Cognitive Disengagement Syndrome: Exploring Their Relationship Through the Lens of Twin Studies. Applied Sciences. 2025; 15(11):6067. https://doi.org/10.3390/app15116067
Chicago/Turabian StyleDe Giuli, Gaia, Cecilia Amico, Stefano De Francesco, Ludovica Giani, Gülşah Tüzün, Federico Galli, Marcella Caputi, Barbara Forresi, and Simona Scaini. 2025. "Emotional Dysregulation and Cognitive Disengagement Syndrome: Exploring Their Relationship Through the Lens of Twin Studies" Applied Sciences 15, no. 11: 6067. https://doi.org/10.3390/app15116067
APA StyleDe Giuli, G., Amico, C., De Francesco, S., Giani, L., Tüzün, G., Galli, F., Caputi, M., Forresi, B., & Scaini, S. (2025). Emotional Dysregulation and Cognitive Disengagement Syndrome: Exploring Their Relationship Through the Lens of Twin Studies. Applied Sciences, 15(11), 6067. https://doi.org/10.3390/app15116067





