Abstract
This research aims to explore the interdisciplinary connection between the field of neurology and artificial intelligence (AI) through machine learning (ML) algorithms. The central objective is to evaluate the current state of research in the Neuro-ML field and identify gaps in the literature that require additional approaches. To achieve this objective, 10 analyses were introduced that analyze the distribution of articles based on keywords, countries, years, publishers, and ML algorithms used in the context of neurological diseases. Surveys were also conducted to identify the diseases most frequently studied through ML algorithms. Thus, it was found that Alzheimer’s disease (37 articles for Support Vector Regression—SVR; 31 for Random Forest—RF), Parkinson’s disease (46 articles for SVM and 48 for RF), and multiple sclerosis (9 articles for SVM) are the most studied diseases in the field of Neuro-ML. The study analyzes Alzheimer’s, Parkinson’s, and multiple sclerosis in detail by focusing on diagnosis. The overall results highlight an increase in researchers’ interest in applying ML in neurology, with models such as SVM (597 articles), Artificial Neural Network (525 articles), and RF (457 articles) being the most used. The results highlighted three major gaps: the underrepresentation of rare diseases, the lack of standardization in evaluating the performance of ML models, and the lack of exploration of algorithms with greater implementation difficulty, such as Extreme Gradient Boosting and Multilayer Perceptron. The value analysis of the performance metrics of ML models demonstrates the ability to correctly classify neuro-degenerative diseases, with high accuracy in some cases (for example, 97.46% accuracy in Alzheimer’s diagnosis), but there may still be improvements. Future directions include exploring rare diseases, investigating underutilized algorithms, and developing standardized protocols for evaluating the performance of ML models, which will facilitate the comparison of results across different studies.
1. Introduction
This article explores the implications of artificial intelligence (AI) in diagnosing neurological disorders. This topic adopts an interdisciplinary approach, aiming to assess the extent to which specialized literature documents the application of AI algorithms in the field of neurology. The topic is important due to the current challenges in diagnosis. For example, nonspecific symptoms or difficulties in early identification are areas of interest in the context of AI applied to the neurological field. Thus, works from the specialized literature that address neurological diseases related to AI tools will be identified. The tools in this field can accelerate the diagnostic process, directly impacting the patient.
Nowadays, AI tools are used in numerous domains [1,2,3,4] including medicine to help reduce human errors and personalize treatments. Other benefits introduced by AI tools include the increased volume of medical data requiring advanced computational solutions specific to imaging, genomics, and the clinical sector [5,6,7,8].
This paper reviews machine learning (ML) applications in neurological diseases, especially neurodegenerative diseases. This study addresses the following research questions (RQ):
- RQ1: What ML methods are used to diagnose neurodegenerative diseases?
- RQ2: What are the performances achieved by using ML methods compared to traditional ones in diagnosing neurodegenerative diseases?
- RQ3: What are the main challenges of ML algorithms used in the field of neurological diseases (Neuro-ML)?
- RQ4: What are the main limitations of Neuro-ML?
The Web of Science (WOS) database was used to identify the research stage in the field. The research encompasses both general searches and targeted searches for specific diseases. The selection criteria for the articles include studies published between 1 January 2020 and 27 March 2025 that employ ML for diagnosis, prediction, classification, and prognosis, corresponding to the 5 years analyzed.
The research includes 10 analyses to evaluate the applicability of Neuro-ML. These cover aspects such as keyword distribution, which highlights research priorities, and country analysis, which underscores global leaders in Neuro-ML. Year and editor analyses reveal the level of interest and the role of platforms in disseminating results. Comparing ML algorithms using WOS and a customized method proposed by the authors offers complementary perspectives on the popularity of ML models. Identifying underrepresented ML algorithms highlights gaps in the literature concerning Neuro-ML. The analysis of disease distribution outlines the concentration of research on certain conditions. Additionally, the research also includes peer-reviewed articles from previous studies. These analyses identify gaps in the literature and guide future research directions.
The methodology used represents a bibliometric and meta-analytic analysis of the existing literature. The authors defined four research questions and conducted 10 analyses, including keyword analysis, analysis of publications’ geographical and temporal distribution, identification of ML algorithms used in neuroscience, and analysis of research gaps:
- For RQ1, the authors extensively analyzed the specialized literature using WOS. The search results focused on the degree of use of ML algorithms in diagnosing neurological diseases. Thus, the ML models were classified based on their frequency of use and the performance reported in various studies. Subsequently, the distributions of these algorithms were compared based on customized procedures and data provided by WOS.
- For RQ2, the methodology aimed at evaluating the performance of the ML algorithms. Therefore, the authors analyzed accuracy, sensitivity, F1-Score, and area under the curve (AUC). These metrics were compared with the results reported in traditional studies. The analysis was conducted based on Alzheimer’s, Parkinson’s, and MS diseases, emphasizing the ability of ML models to reduce diagnostic errors and accelerate the diagnostic process.
- For RQ3, the challenges identified in the critical literature analysis were highlighted by the low standardization level in evaluating ML models’ performance. Unbalanced or limited datasets and the difficulty of implementing complex algorithms have led to the under-analysis of rare diseases and the exploration of underutilized algorithms in the literature.
- For RQ4, the limitations highlighted that the comparative analyses between the customized methods and the results provided by WOS are similar. Thus, it was found that some articles include vaguely irrelevant terms. This aspect can lead to incorrect interpretations. Additionally, this research identifies gaps in the literature regarding rare diseases and the lack of standardized protocols for validating ML models.
The paper is structured into six sections. Section 2 presents a critical study of the specialized literature, which includes the classification of neurological diseases, the classification of ML algorithms, the identification of ML models in the identification of neurological diseases, and the accuracy of existing models in the literature. Section 3 describes the methodology used to identify gaps in the literature. The results of the 10 analyses conducted are presented in Section 4. Section 5 focuses on distributing articles based on ML algorithms in neurological research, identifying research gaps, and future work. Conclusions are outlined in Section 5.
2. Literature Review
Neurological diseases affect the brain, spinal cord, and peripheral nerves. This disorder generates signs and symptoms that influence cognition, behavior, movement, and overall functioning. The most common category of neurological disease corresponds to neurodegenerative diseases. In this category, Parkinson’s disease (PD) and Alzheimer’s disease (AD) are included. They are characterized by the progressive loss of neurons and the associated functional decline [9,10]. These conditions manifest through cognitive, motor, and even behavioral symptoms [11]. The symptoms significantly mark the lives of the affected individuals. AD, for example, is a condition where cognitive decline occurs long before neurological symptoms become evident [12].
2.1. Neurological Disease Classification
Neurological diseases encompass a wide range of disorders that affect the nervous system. These diseases are classified into neurodegenerative, inflammatory, and infectious diseases. Neurodegenerative diseases are characterized by the progressive degeneration of the nervous system’s structure and function. The most common neurodegenerative diseases are [13,14]:
- AD is a form of dementia through which the subject loses their memory, exhibits cognitive decline, and experiences behavioral changes. AD is marked by progressive cognitive decline generated by the accumulation of beta-amyloid and tau tangles in the brain. Thus, memory deterioration occurs gradually, and behavioral changes often accompany these changes in language and daily functions. In ref. [15], it is mentioned that genetic clinical examinations identify specific loci associated with the rapid progression of this disease.
- PD is a disorder that predominantly affects motor function. This function is lost due to the dopaminergic neurons in the substantia nigra. In this class, there are forms with early-onset and late-onset symptoms. The pathological features include Lewy bodies, which consist of aggregated alpha-synuclein [16]. PD is classified based on its clinical characteristics, including tremor, rigidity, and bradykinesia [17].
- Amyotrophic lateral sclerosis (ALS) is a disease that leads to the degeneration of mo-tor neurons. The result of this disease is paralysis [18]. The degeneration of upper and lower motor neurons leads to muscle atrophy and, ultimately, respiratory failure. This condition is linked to genetic components represented by mutations in the SOD1 gene [19].
- Frontotemporal dementia (FTD) is a progressive dementia that degenerates the frontal and temporal lobes of the brain. FTD encompasses several forms, including behavioral variant FTD and semantic variant primary progressive aphasia. These are differentiated by clinical presentation and underlying pathology. Genetic mutations, particularly in the MAPT and GRN genes, further classify the familial forms of FTD [20].
- Huntington’s disease (HD) is a hereditary condition characterized by motor dysfunction and cognitive decline. This disease is an autosomal dominant neurodegenerative disorder. It is primarily associated with the expansion of CAG repeats in the HTT gene. This leads to progressive motor dysfunction, cognitive decline, and psychiatric symptoms [21]. To prevent this disease, genetic testing is necessary for early diagnosis [22].
- Lewy body dementia (LBD) is a form of dementia that involves the accumulation of Lewy bodies in the brain. It is classified as a synucleinopathy, primarily characterized by the presence of alpha-synuclein aggregates [23]. It has symptoms similar to Alzheimer’s and PDs.
- Prion diseases are a unique category of transmissible neurodegenerative disorders. It includes conditions such as Creutzfeldt–Jakob disease (CJD). CJD genetic variants involve specific prion protein (PRNP) gene mutations and are associated with distinct phenotypic subtypes [24]. The disease is characterized by spongiform changes in brain tissue and an abnormal prion protein [25].
- Spinocerebellar ataxia (SCA) is a reference disease in this class of neurodegenerative diseases. This represents a group of genetic disorders characterized by the degeneration of the part of the brain responsible for coordinating movements [26,27]. SCA represents a diverse group of inherited neurodegenerative disorders. It is characterized by progressive ataxia caused by the degeneration of the cerebellum and its connections. These diseases are classified into hereditary ataxias associated with specific genetic mutations or sporadic forms without a clear genetic basis [28].
- Multiple sclerosis (MS) is not a degenerative disease. It is an inflammatory disease that affects the central nervous system. It is characterized by the presence of lesions and inflammation, leading to chronic neurodegeneration. MS is stratified into clinical subcategories, including relapsing–remitting and progressive forms, reflecting the spectrum of disease behavior [29,30].
As a result, neurological diseases are classified into neurodegenerative diseases (including AD, ALS, FTD, and HD), movement disorders (such as PD and MS), prion diseases (such as CJD), and ataxias (including SCA). Each classification enables targeted treatment approaches.
2.2. Machine Learning Classification
ML is a component of AI responsible for developing algorithms that learn from data. These algorithms improve performance in certain tasks requiring prior experience by establishing input–output correlations or identifying patterns [31,32]. ML algorithms are classified into supervised, unsupervised, and hybrid learning algorithms. Supervised learning algorithms are further subclassified into classification and regression algorithms, as can be seen in Figure 1. Unsupervised learning is classified into clustering, dimensional reduction, and other techniques, while generative models and autoencoders are classified as hybrid. In supervised learning, models are trained on labeled datasets. These models learn from previous experiences and make predictions using previously unseen data [33]. This category of learning includes a multitude of algorithms, among which are those presented in Figure 1.

Figure 1.
Classification of ML algorithms [34,35].
Clustering and dimensionality-reduction methods allow algorithms to identify patterns in the provided data, even if they are unlabeled. This type of problem is compatible with situations where the datasets do not have labels [36,37]. The classification of ML also includes multi-labeling when instances belong to multiple classes. Consequently, ML includes a multitude of algorithms that are chosen based on the type of problem being modeled. Numerous ML algorithms have been studied and integrated into the medical field for diagnostics [38,39]. They are preferred in assisting diagnosis due to their ability to interpret interdependencies of a non-linear nature [40] in both numerical form and in images [41] or audio [42].
2.3. Machine Learning Models Related to Neurological Diseases
ML algorithms are intensively studied for the classification of neurodegenerative diseases. Ref. [43] employs an algorithm that combines Random Forest (RF), Logistic Regression (LR), and Gaussian Naive Bayes to enhance diagnostic performance on imbalanced datasets. The paper optimizes classifying neurodegenerative conditions by integrating Canny segmentation for brain scan analysis. Ref. [32] demonstrates the successful application of gait analysis using the Naive Bayes (NB) classifier for diagnosing PD. In this case, an accuracy of 96.3% was achieved. Ref. [44] uses Support Vector Machine (SVM) algorithms to distinguish PD from hereditary ataxias.
Classification strategies associated with neurodegenerative conditions are adapted to specific biomarkers and imaging techniques. Ref. [45] analyzes the retinal vasculature for the classification of AD. It observes that vascular changes are correlated with disease progression. Neurodegenerative diseases exhibit various pathologies that can be classified based on unique protein aggregation profiles. In this way, specific biomarkers are recognized for high-accuracy classification [46]. Ref. [47] highlights how the spatial distribution of neuronal degeneration distinguishes between different neurodegenerative diseases. The experiments were conducted to integrate spatial data with Magnetic Resonance Imaging (MRI) and improve diagnostic capabilities. Besides value correlations, there are also approaches in ML that deal with imaging technologies. Ref. [48] examines applying deep learning (DL) methods to retinal images. This research aims to detect AD using retinal colorimetry. The results of this research are supported by ref. [49], which demonstrated that changes in retinal structures correlate with neurodegenerative conditions. These findings confirm the use of ML techniques in establishing clinical diagnoses [50].
AD requires the evaluation of cognitive impairment through various biomarkers. The accuracy of diagnosing AD has been improved by integrating neuroimaging data with clinical evaluations into ML algorithms [51]. These algorithms can identify patterns associated with neuronal degeneration that are difficult to recognize through standard clinical examinations [52]. Ref. [53] uses RF and NB to analyze clinical and cognitive data to diagnose AD with the highest possible accuracy. Similarly, ref. [54] integrates MRI biomarkers in diagnosing AD. Also addressing the issue of neuroimaging signatures for AD, ref. [55] analyzes the Extreme Gradient Boosting (XGBoost) model. The Convolutional Neural Network (CNN) model detects subtle brain structural changes related to AD [56]. There is also a multimodal approach where MRI, PET, and DTI are combined in the classification issue [57]. The analysis of cellular-level changes in AD pathology is studied in [58] through ML models.
ALS and FTD affect movement, cognition, and behavior. ML applications in these disorders relate to the analysis of genetic data and phenotypic variations to classify ALS subtypes or to differentiate FTD from other types of dementia. Ref. [51] mentions that the methodologies applied for Alzheimer’s and Parkinson’s can also be adapted for this disease. HD is combined with ML techniques in assessing genetic predisposition and tracking disease progression. And in this case, the same models can be used as in the case of AD and PD.
ALS manifests through muscle weakness, atrophy, and eventually respiratory failure [59,60]. Refs. [61,62] confirms the scientific advancements in the study of specific clinical manifestations of ALS. This is due to the ability to process complex datasets such as genetic, proteomic, and clinical data. For example, refs. [63,64] have highlighted that identifying ALS-associated biomarkers facilitates patient risk stratification. The generation of biomarkers through ML approximates omics data, such as proteomics and metabolomics. Through these techniques, the accuracy of diagnosis is improved [63,65]. Predictive ML models identify major variations in biomarkers throughout the course of the disease. This ability leads to faster interventions [63,66]. The application of ML in ALS integrates multiple clinical parameters for staging disease progression. For example, models have been proposed to evaluate the effects of neurofilament levels and metabolic biomarkers on disease trajectories [66,67]. This approach is the path to personalized treatments that correlate the individual needs of the patient with the stage of the disease and the biomarker profile [67,68,69].
Prion diseases, associated with neurodegeneration and infectivity, use ML applications for diagnosis. Ref. [70] used CNN for the histopathological classification of these diseases, while ref. [71] integrated diffusion MRI (dMRI) with ML also for diagnosis. Ref. [72] has shown the importance of microglia in modulating progression through the analysis of cellular interactions with ML models.
FTD is characterized by behavioral and linguistic alterations in individuals under 65 years. Diagnosing FTD is difficult due to clinical overlaps with other types of dementia or psychiatric disorders. This aspect leads to diagnostic errors [73,74,75]. For this reason, integrating ML techniques represents an alternative to reducing errors. They use MRI datasets, cognitive assessments, and electroencephalograms (EEGs) [76,77,78,79]. For example, MRI-based models analyze brain regions affected by atrophy. The objective of ML models is to classify the type of disease based on neurodegenerative profiles [78,80,81]. Refs. [82,83] propose EEG as a real-time tool for obtaining anomalies in brain activities characteristic of FTD.
MS is also studied through ML. The models analyze MRI data and patient-reported symptoms to classify the severity of the disease [50]. ML models are also suitable for predicting potential relapses [53]. MS is a disease that manifests unpredictably, which complicates traditional diagnostic approaches. ML models can solve this problem by leveraging complex datasets that include neurological imaging and biomarker analysis [84,85]. MRI analysis is a time-consuming process prone to human errors [86]. Refs. [87,88] have demonstrated that CNNs and RF can automate the detection of MS-associated biomarkers from neurological imaging and blood samples. For example, ref. [89] demonstrated the ability of CNNs to differentiate MS from other neurological conditions through advanced quantitative MRI analysis. The data used by the models correspond to cerebrospinal fluid (CSF) and serum biomarkers [85,87]. The identification of certain cytokine patterns associated with MS was facilitated by ML techniques [90,91].
PD is researched in ref. [17] to analyze vocal signals through ML models. Similarly, ref. [92] analyzes body movement, while ref. [93] examines multimodal stages to predict their progression. Refs. [94,95] report improvements in prediction through SVM models and ensemble learning. Refs. [96,97] mention that the integration of wearable devices improves prediction by generating data collected from the wearer. The research results presented in ref. [98] managed to predict both the prevalent and incident forms of PD.
The predictive accuracy in images was 71%. This finding is also supported by ref. [17], through PD prediction in retinal imaging and enhanced vocal signals. SVM and RF algorithms are used to classify PD through dysphonic features [98]. Besides imaging techniques, voice signal analysis represents another method for early detection of PD. Advances in this field have led to a classification accuracy of over 90% in spectrogram analysis [99]. Ref. [100] reports that ML algorithms surpass human performance in diagnosing PD. This is possible by analyzing MRI characteristics that indicate neurodegenerative changes associated with the disease. Metabolic imaging uses ML to differentiate Parkinsonian disorders based on metabolic indices derived from imaging [101]. The ability of ML models to extract information from previously underutilized imaging data allows clinicians to monitor disease progression [102]. Based on fundus photographs, these algorithms are also used to identify neurological dysfunctions in PD patients. The models contribute to developing new associations that guide clinical evaluations [103,104,105].
SCA is studied using ML to classify its different variants. Refs. [106,107] analyze genomic data in the diagnostic process to identify rare subtypes of SCA. Ref. [108] proposes a mathematical model for SCA37 that uses genetic variables related to repeat expansions. This paper demonstrates that ML algorithms are compatible with the issue of predicting the progression of neurodegenerative diseases. For SCA3, explainable artificial intelligence (XAI) was used to optimize the features and predict the onset of SCA [109]. In ref. [106], an unsupervised ML pipeline was applied to investigate variants of unknown significance in SCA. The result of the research highlighted the potential of ML in the clinical diagnosis of rare forms. Using MRI images, federated learning methods achieved an error rate of 13.75% in differentiating SCA2 and SCA6 genotypes [110]. Additionally, ML models are applied for the quantitative assessment of gait parameters in MS. This method is also adaptable for SCA. Ref. [111] suggests that the analysis of spatiotemporal parameters classifies the pathological forms of ataxia.
HD applications are modeled through CNNs and other DL architectures that analyze medical images, such as MRI [112,113]. These models identify distinctive patterns in neuroimaging data. The typology of ML applications models the differential diagnosis between HD and other neurodegenerative diseases, such as Alzheimer’s. ML is also studied to evaluate disease progression through imaging biomarkers and structural brain changes. Besides imaging, ML applications have also been studied for voice analysis. These analyses evaluate HD symptoms through algorithms that quantify vocal markers associated with the severity of the disease [114]. Genetic and molecular biomarkers are identified through algorithms that analyze genomic datasets [115]. The metrics used to evaluate the performance of ML models concerning HD are diagnostic accuracy, symptom monitoring, and biomarker identification [116,117].
The ML models used in LBD diagnosis include NLP techniques and algorithms that analyze cognitive, demographic, and imaging data. Horigome et al. developed an NLP model with an AUC of 0.93 and an accuracy of 90% using conversational data, while Alkenani et al. reported an accuracy of 95% and an AUC of 0.98 on the DementiaBank dataset [118]. Xu et al. used questionnaire data to predict dementia in diverse populations, achieving AUC values of 68.6% for RF, 67.7% for LR, and 66.4% for NN [119]. The datasets include DementiaBank, UK Biobank, and data collected through online tracking tools [120,121]. The standard metrics for evaluating ML models are AUC, accuracy, sensitivity, and specificity [118,122]. Integrating cerebrovascular factors, genetic markers (apolipoprotein E—APOE), and imaging data differentiates LBD from Alzheimer’s [123,124].
Table 1 presents a summary of the diseases with the dataset and the objective of the ML model. Table 1 is created based on the information extracted from the previously presented literature and highlights the importance of the dataset that must correspond to the typology of the problem to be solved.

Table 1.
Literature review summary for datasets.
The type of dataset is always correlated with its volume. The quality of the dataset directly influences the performance of the ML model. A model’s performance is evaluated using specific ML metrics. Although accuracy is one of the most commonly used metrics in the specialized literature, it is not always the most relevant—especially in the case of imbalanced datasets, where metrics such as AUC, F1-Score, sensitivity, or specificity provide a more realistic picture of performance. The correct choice of metric is essential to avoid misinterpretations and to ensure the clinical validity of the results.
There are studies in which the model’s performance is compared from the perspective of the F1-Score indicator, such as the paper case [125]. It reports the performance of AD models through the F1-Score indicator. In the opinion of the authors of this review, a pertinent evaluation should include multiple indicators, such as accuracy, F1-Score, recall, precision, etc.
Table 2 summarizes the research presented in this section. It presents the ML algorithm used, the column that mentions strengths with a value between 1 and 5, and the column weaknesses in numerical form. Value 1 represents significant strengths, while value 5 represents exceptional strengths. The third column, corresponding to weakness, evaluates the study’s limitations. Value 1 means a minor restriction, while value 5 indicates a significant issue.

Table 2.
Review studies in the Neuro-ML research area.
The taxonomy of ML algorithms presented in Figure 2 includes the main applications represented by diagnosis, prediction, treatment, and medical recommendations. The taxonomy’s structure is tree-like, with four distinct levels, each corresponding to one of the mentioned applications.
Ref. [130] discusses Intellectual and Developmental Disabilities (IDDs), including Down syndrome and autism, which involve intellectual and adaptive impairments influenced by genetic and environmental factors. Advances in sequencing, imaging, and behavioral data analysis have deepened the understanding of IDDs. ML transforms IDD research by enhancing diagnosis, identifying biomarkers, and advancing personalized treatment. ML applications improve screening, early detection, and knowledge of comorbidity. Challenges remain in implementing reliable ML-based tools in clinics, requiring further innovation to bridge gaps in IDD research and care. The rising prevalence of neurodevelopmental disorders (NDDs) in children impacts health, social life, and economies [131]. Early diagnosis remains challenging, delaying interventions. ML offers promising early detection and prediction tools, integrating AI with medicine. Ref. [131] reviews ML’s role in diagnosing and treating NDDs using supervised and unsupervised learning, providing new perspectives for improving early diagnosis and intervention strategies.
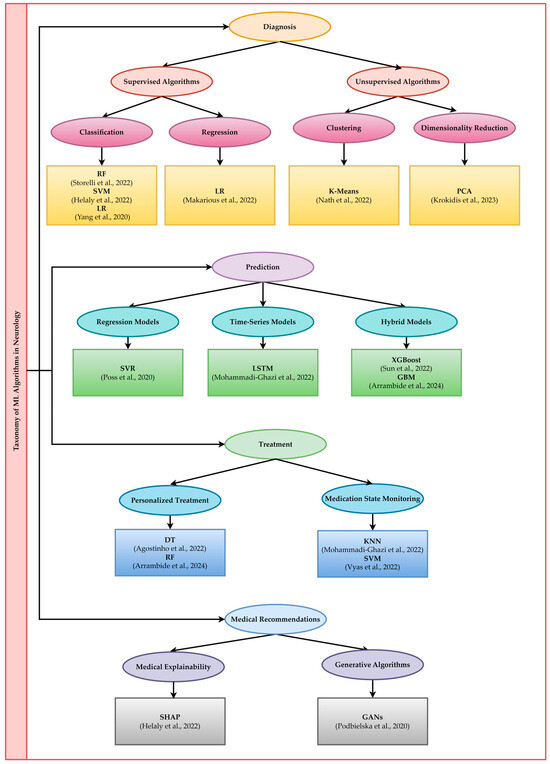
Figure 2.
Taxonomy of ML algorithms in neurology [29,51,56,57,58,65,84,85,93,100,106,132,133].
2.4. ML Models Accuracy
Accuracy is an indicator that evaluates the performance of ML models. Performance is associated with the ability of ML models to classify positive and negative cases correctly. For example, suppose the model’s objective is to identify the presence of a disease. In that case, patients with the disease are the positive cases, and those without the disease are the negative cases. The higher the accuracy, the more useful the model is in clinical practice. The models must meet a series of standards to be used in practice:
- Capacity for generalization based on a large volume of data;
- Reducing diagnostic errors;
- The ability to make medical decisions based on past experiences;
- Preventing overtraining.
All these standards are evaluated through the model’s accuracy. According to the literature, ML models have accuracy rates of over 90% in diagnosing AD using techniques such as natural language processing (NLP) and acoustic analysis. CNN and SVM have high performance in classifying HD, ALS, and PD. Table 3 presents a synthesis of results obtained in scientific papers from the specialized literature to obtain an overview of the accuracy levels for different diseases.

Table 3.
Accuracy according to different diseases.
Table 3 shows that some models have good results, but there is room for improvement. Moreover, some diseases have not been studied sufficiently from the perspective of ML. For these reasons, numerous challenges and gaps in the literature highlight the need for further research to address these diseases.
Considering the necessity of continuing research to address diseases that are not intensively explored, the authors analyze and highlight the ML algorithms that are not sufficiently explored. In addition, the authors also present the gaps in the literature concerning the current state of research concerning the most studied diseases (AD and PD).
3. Methodology
Ref. [145] specifies the prevalence of AD, PD, and MS in 1990 and 2019, according to Figure 3. AD’s prevalence increased by approximately 161% in 2019 compared to 1990. For PD, the prevalence increased by approximately 155%, and for MS by approximately 72%. The increase in prevalence indicates the need for modern tools equipped with AI to assist medical staff in diagnosis, prediction, treatment, and recommendations.

Figure 3.
Prevalence of neurological disorders in 1990 and 2019.
The criteria for classifying gaps in the specialized literature have been established based on three dimensions extracted from the analysis of the results:
- The representativeness of neurological diseases is determined by the number of articles identified through WOS queries or customized keyword analysis. If the result returned by WOS was extremely low or nonexistent, it was considered an “underexplored disease”. The quantitative criterion was based on a limit of 5 articles published in the period 2020–2025.
- The degree of utilization of ML algorithms classifies advanced algorithms as “underexplored” if they appear in fewer than 20 relevant studies or are not frequently used with the analyzed diseases. This quantitative criterion is based on the thresholds set by the WOS platform and customized analysis.
- Standardization of evaluation metrics is necessary due to the lack of direct comparability between studies, which was identified when the analyzed articles did not report the same indicators (precision, AUC, sensitivity, specificity). This aspect complicates the cross-sectional evaluation of model performance.
The methodology for assessing researchers’ interest in Neuro-AI using ML is evaluated through an initial search on the WOS platform. This search included the keyword “machine learning” or “ML” abbreviation, as depicted in Figure 4. The results were exported for this search, and a map was created based on bibliographic data using the authors’ keywords. The minimum number of occurrences for each keyword was set to 20. For each keyword, the co-occurrence and the number of selected keywords were calculated. The identified clusters enabled a comprehensive evaluation of the key concepts relevant to this type of analysis. Keeping the same settings, a map was also generated based on bibliographic data relating to the authors’ countries of origin for co-authorship. This analysis aims to identify the countries with the highest level of interest in neurodegenerative diseases. Also, in this category of statistics, the distribution of research by year and the distribution of researchers by publications were identified. The two statistics highlight the interest in Neuro-ML and the publications encouraging such research.
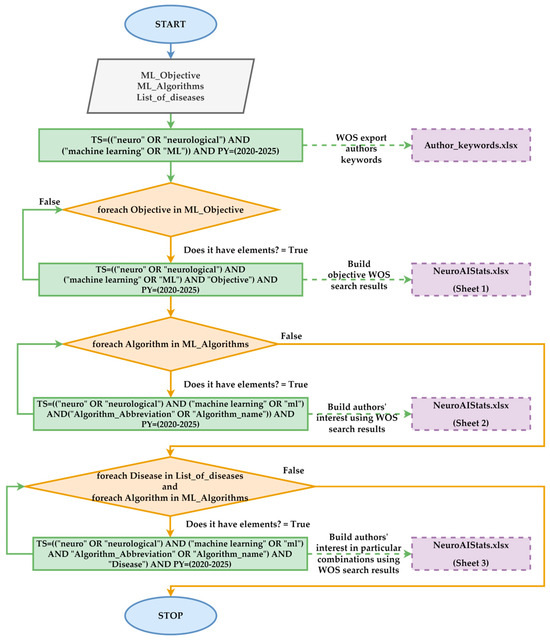
Figure 4.
Flow procedure employed in evaluating the number of articles in different scenarios.
Statistics were generated based on the NeuroAIStats.xlsx file (available in the Supplementary Materials section) regarding the most used ML algorithms in the neural domain for the four objectives specific to this AI component. Subsequently, statistics were generated for each ML algorithm from a total of 46 identified. The algorithms that provided over 100 results in WOS were extracted. In the end, statistics were generated for the neural diseases presented in the literature review section in relation to each previously selected ML algorithm.
Subsequently, a filtered search was conducted by including the terms “diagnosis”, “prediction”, “classification”, and “prognosis”. These words are associated with ML algorithms’ objectives in Neuro-AI, which involve pattern recognition. Next, a list of 46 algorithms specific to ML models was created. Then, for each algorithm, a new set of searches was conducted that included the name of the ML algorithm, the name of the algorithm itself, and “neuro AI”, as shown in Figure 4. The search aims to identify the field’s most extensively studied ML algorithms. The previous WOS search results identified the algorithms that yielded over 100 articles in the Neuro-AI field. All identified algorithms were combined to construct WOS queries, with each neurodegenerative disease identified in the literature aiming to identify research gaps related to specific algorithms. Figure 4 presents the logical diagram used in this category, which focuses on the statistical evaluation of Neuro-AI research related to ML algorithms in WOS.
The procedure presented in Figure 4 generates two files, based on which detailed analyses will be conducted. The Author_keywords.xlsx file was generated from the WOS platform via export, and the NeuroAIStats.xlsx file was built using the execution of WOS queries. Both files, along with the article, are publicly available for analysis.
The second type of analysis included extracting all the results generated by the initial search in WOS from the Author_keywords.xlsx file. For all the results, the author’s keywords were extracted. Subsequently, a program was implemented in C#, version 7.3 that searched all previously evaluated ML algorithms individually, using the authors’ keywords. In this way, the frequency of occurrence of each algorithm was constructed within the general search that included the term “ML.”
Figure 5 illustrates the workflow used to compute the authors’ keyword frequency. The results are saved in Sheet 4 of the NeuroAIStats.xlsx file. In this approach, the algorithms effectively addressed in the specialized paper were identified compared to the results provided by the WOS-specific search.

Figure 5.
Flow procedure to evaluate the appearance frequency of each algorithm in the research papers (2020–2025).
Summarizing the presented methodology, 10 analyses are identified and explored in detail:
- Analysis 1: The distribution of papers based on the authors’ keywords in Neuro-ML aims to identify the main themes based on research clusters in the field of Neuro-ML.
- Analysis 2: The distribution of papers by country in the Neuro-ML field evaluates the level of interest grouped by country for research in the field of neurological diseases.
- Analysis 3: The distribution of papers by year in the Neuro-ML field (2020–2024) analyzes the temporal trends of researchers’ interest in applying ML in neurology.
- Analysis 4: The distribution of works by publishers in the Neuro-ML field aims to identify the publishers that facilitate disseminating research results in the Neuro-ML field.
- Analysis 5: The Neuro-ML Distribution by Research Objectives aims to evaluate researchers’ priorities based on specific objectives, such as prediction, diagnosis, and classification.
- Analysis 6: Comparing the number of articles for ML algorithms applied in neurological research (WOS) aims to establish the most used ML algorithms in neurological research.
- Analysis 7: Comparative metrics between ML models applied in different diseases research.
- Analysis 8: Comparing the number of articles on ML algorithms applied in neurological research (custom procedure) obtains a complementary WOS perspective on using ML algorithms through customized methods.
- Analysis 9: The comparative analysis of the results between the customized method and WOS aims to compare the results obtained through two different approaches to identify the differences.
- Analysis 10: Identifying gaps and opportunities in the specialized literature aims to highlight future research directions, focusing on the underrepresentation of rare diseases and the exploration of underutilized algorithms.
The results obtained for the 10 proposed analyses are presented in the following section. Additionally, a detailed examination of the literature regarding the performance of ML algorithms will also be conducted.
4. Results
The results disseminated in this section pertain from 1 January 2020 to 27 March 2025. These results provide an overview of the current state of research in the field of Neuro-ML and help researchers clarify future research directions based on the shortcomings identified in the following analyses.
4.1. Distribution of Research Papers Across Authors’ Keywords in Neuro-ML
The network map, generated using VOSviewer 1.6.20 based on WOS data, reflects the thematic structure of research published between 1 January 2020 and 27 March 2025 on the application of ML in Neuro-AI. The visual analysis of the network is presented in Figure 6, where five distinct clusters are observable.

Figure 6.
Distribution of research papers in Neuro-ML by authors’ keywords.
These were generated by setting the minimum number of occurrences of a keyword to 50, allowing the results to provide interpretable data. This setting generated the identification of 37 keywords grouped into four clusters. Each cluster represents a research direction that can be interpreted as follows:
- The first cluster (colored in red in Figure 6) comprises 16 terms, with neurological diseases (such as cerebrospinal diseases, inflammations, MS, Alzheimer’s, etc.) as central elements. This cluster highlights the significance of ML technologies in Neuro-AI diagnostics.
- The second cluster (colored in green), comprising 11 terms, is associated with ML techniques such as Artificial Neural Networks (ANNs), RF, and SVM. This cluster suggests that researchers are investigating various methods for analyzing neurological data.
- The third cluster (colored in blue) contains seven terms. This cluster features DL as its central element, highlighting the importance of feature selection and feature extraction techniques in electroencephalograms and epilepsy, particularly when using classification methods.
- The last cluster (colored in yellow) contains three terms correlating the MRI algorithm with neuroimaging and PD.
This network illustrates the connection between elements from ML and neurology.
4.2. Distribution of Research Papers Across Countries in Neuro-ML
The results from the map analysis, based on bibliographic data for co-authorship and the authors’ countries of origin, are presented in Figure 7. The results show a large number of research studies in the field of Neuro-ML for China, the USA, and India. In Europe, England, Germany, and Italy stand out. Australia, Canada, Japan, South Korea, and Saudi Arabia report an average number of research studies in the field.
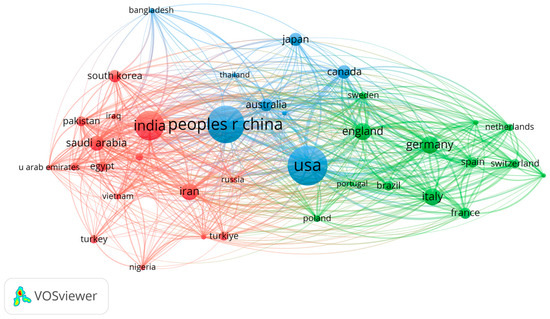
Figure 7.
Distribution of research papers in Neuro-ML by countries.
4.3. Distribution of Research Papers Across Years in Neuro-ML (2020–2024)
The results presented in Figure 8 correspond to the distribution of research conducted each year within the 2020–2024 interval. The year 2025 was excluded from the analysis because it corresponds to the current year. In Figure 8, an upward trend in the number of articles is observed, confirming the growing interest of researchers in Neuro-ML.
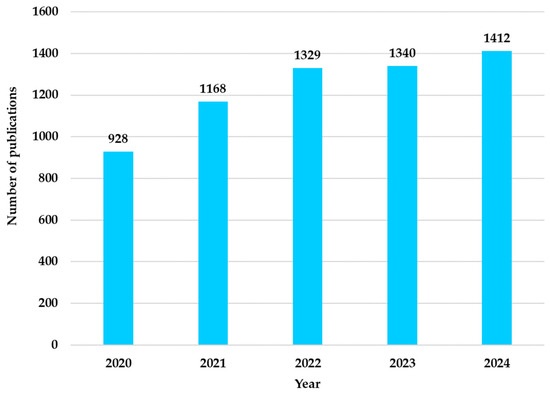
Figure 8.
Distribution of research papers in Neuro-ML by year (2020–2024).
4.4. Distribution of Research Papers Across Publishers in the Neuro-ML Domain
Figure 9 presents the distribution of the number of articles by publisher in which the intensity of the blue color directly correlates with the number of published articles. For the construction of these results, the Author_keywords.xlsx file was used. The obtained statistics are presented in Sheet 5 of the NeuroAIStats.xlsx file. It lists each publisher and the number of articles out of a total of 6542. The results show that MDPI is indisputably the leader in publications in the Neuro-ML field (647 articles), followed by Elsevier (543), Springer (378), Frontiers Media SA (373), and Wiley (323).

Figure 9.
Distribution of research papers in Neuro-ML by publisher (2020–2025).
4.5. Distribution of Neuro-ML Across Objective Research
These results confirm that MDPI ranks among the top publications that encourage research in the field of Neuro-ML and support the concise dissemination of results, thereby preventing the obsolescence of research findings. The encouraging attitude of this publisher leads many researchers to use the platform for documentation and disseminating results. For these reasons, the paper will include a section on research results disseminated in MDPI.
The results for executing WOS queries in the Neuro-ML field are presented in Figure 10. Thus, the increased interest in this field is evident, with most articles focusing on predictions. The 1595 articles aim to anticipate the progression of diseases or the outcomes of treatments. Regarding diagnosis and classification, they account for almost equal results. This suggests that these objectives identify and classify neurological conditions. Regarding the prognosis, 359 results were provided. Due to the complexity of the field, the number of results about this objective is relatively low. These numerical values underscore the significance of modern neurological research in the field of ML.
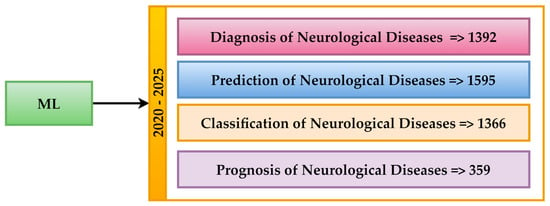
Figure 10.
Distribution of Neuro-ML across objective research (2020–2025).
4.6. Comparison of Article Counts for ML Algorithms Applied in Neurological Research Based on WOS
In Figure 11, the results obtained from running the WOS queries for the combinations of the 46 ML algorithms are presented. The results presented in Figure 11 indicate an uneven distribution of articles utilizing ML models. In neurological research, most articles address the SVM model. It provided 597 articles. The reason for the intensive study of this model comes from its ability to classify and predict membership in a specific category through the processing of complex data.
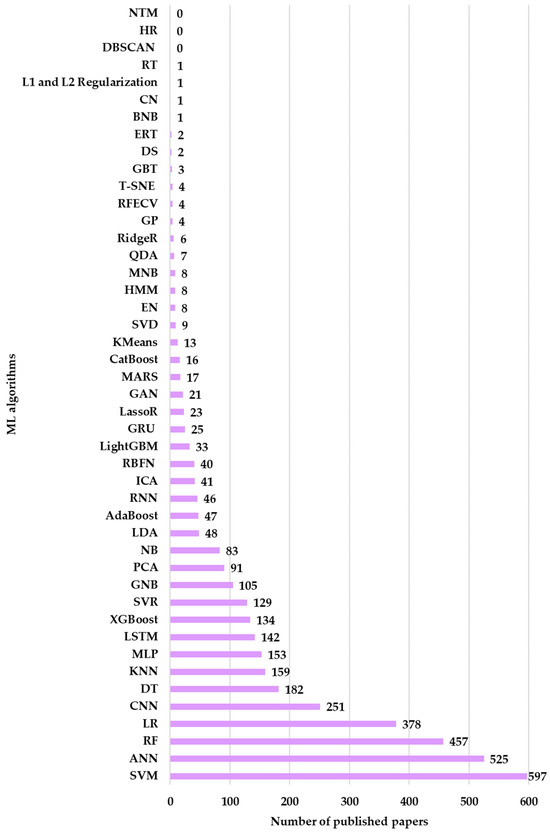
Figure 11.
Distribution of research papers in Neuro-ML by the ML algorithm employed (2020–2025).
Additionally, ANN has generated 525 articles, which are being studied due to its ability to identify patterns that allow it to associate them with specific classes. This advantage makes it suitable for non-linear approaches specific to neurological conditions. The third algorithm intensively studied in the neurological field is RF. It generated 457 articles, while LR generated 378 articles. The two algorithms are suitable for neurological research due to their ability to explore heterogeneous data and their training settings that prevent overfitting. The CNN (251) and Long Short-Term Memory (LSTM) (142) models are less studied. This is due to the need for complex calculations, which require increased computational resources. Moreover, these algorithms require large trained datasets to generalize. Models such as Decision Tree (DT) (182), 159, and Multilayer Perceptron (MLP) (153) have moderate usage. Models such as XGBoost (134), SVR (129), and Gaussian (105) are among the least studied in the category of results, which have generated over 100 articles in a neurological context.
4.7. Distribution of ML Algorithms Across Specific Neurological Diseases Relevant in Research
This distribution will be further used to identify researchers’ interest in studying these algorithms in certain neurological diseases. After all the WOS queries were run and the results were centralized in Sheet 3 of the NeuroAIStats.xlsx file, the results that provided values between 1 and 5 were excluded, as they were considered irrelevant. The remaining results, after filtering, showed exceptionally valuable information. Figure 12 shows that the most studied diseases in the context of ML are Alzheimer’s, Parkinson’s, and MS.
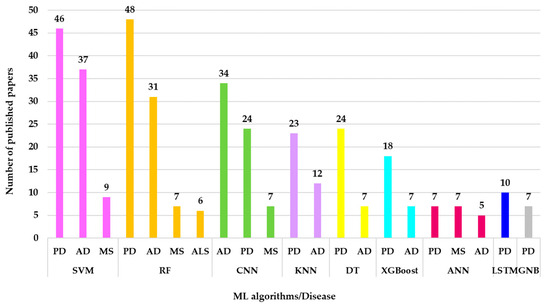
Figure 12.
Distribution of ML algorithms across specific neurological diseases relevant in research (2020–2025).
The results in Figure 12 show that SVM (pink color) is the most used model, with 46 articles for PD, 37 for AD, and 9 for MS. RF (orange color) follows with 48 articles for PD, 31 for AD, 7 for MS, and 6 for ALS. CNN (light green color) reports 34 articles in the context of AD, 42 for PD, and 7 for MS, with a preference for medical image analysis. KNN (lavender color) stands out with 23 articles for PD and 12 for AD, suitable for classification problems. DT (yellow color) has 24 articles for PD and 7 for AD. Other algorithms, such as XGBoost (turquoise color), ANN (burgundy color), LSTM (dark blue color), MLP (gray color), etc., have rarely been studied and have few or no results. For example, MLP appears with only 1 article for MS and LBD. Rare diseases, such as ALS and prion diseases, show low or zero values for most models. This aspect highlights a research gap. GNB, although rarely used, appears in 7 articles about PD.
The value of these results indicates the following gaps in the literature:
- The reduced volume of datasets required for the other ML algorithms generated a low number of results;
- The algorithms are not suitable for the issues of neural diseases;
- The lack of popularity of these algorithms causes researchers’ interest in them to be low.
Figure 13 shows that AD, PD, and MS have generated a consistent number of articles in the specialized literature, even though their number is still relatively small compared to the large number of cases existing globally. The results presented in Figure 13 are obtained through WOS queries, including the search terms “ML” or “Machine Learning”, the disease name, and the year constraint.
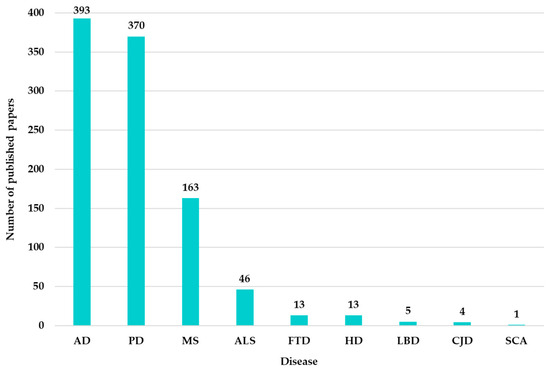
Figure 13.
Distribution of research papers in the Neuro-ML by disease (2020–2025).
As shown in Figure 13, very few articles appear for other diseases, such as ALS, FTD, and so on, compared to the other three diseases identified as most investigated.
4.8. Comparison of Article Counts for ML Algorithms Applied in Neurological Research Based on a Custom-Made Procedure
The results presented in Figure 14 reflect the frequency with which each of the 46 evaluated algorithms is mentioned in the keywords of authors in the field of neurology. This customized statistic was created for comparative analysis, and WOS returned the results based on the queries constructed for each algorithm. Figure 14 shows that ANN is the most used algorithm, with 97 occurrences. This is followed by SVM, 92 occurrences, and RF, 63 occurrences. CNN appears 57 times, followed by RF (63), LSTM (29), DT (27), XGBoost (24), SVR (22), and LR (20). Algorithms with fewer than 20 results are excluded from the analysis, as the WOS analysis was configured with the same occurrence threshold of 20.

Figure 14.
Comparison of paper counts for ML algorithms applied in neurological research based on a custom-made procedure and WOS (2020–2025).
4.9. Comparative Analysis of Custom-Made vs. WOS Results for ML Algorithms in Neurology
Figure 15 presents a comparative analysis of the results obtained using the custom-made method and those from WOS for the same algorithms.
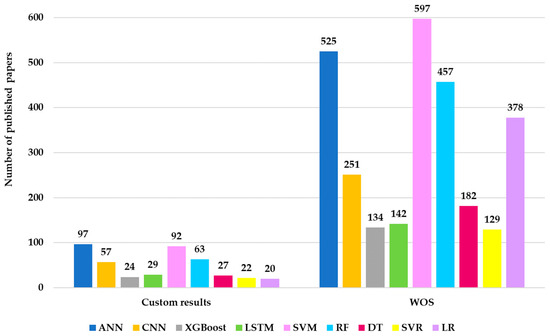
Figure 15.
Comparative analysis of custom-made versus WOS results for ML algorithms in neurology (2020–2025).
The differences between the results come from the method of searching and indexing. The WOS platform considers the context in which the terms appear, the relationships between words, and the field of research. This does not mean that the custom-made method is inferior, but it imposes strict search restrictions, resulting in a rigorous filtering of articles. For example, suppose an article mentions “ANN” in a neurological context. In that case, WOS will include the article in the results even if it is not explicitly related to the authors’ keywords. Conversely, the custom-made search will not include the article in the results. For these reasons, the custom-made method is included in this research. The results obtained from this research ensure the inclusion of the mentioned algorithm in the study.
4.10. Review of ML on AD
Ref. [126] analyzes multi-stage diagnosis of AD using two types of biomarkers: blood gene expression and clinical data, both obtained from the AD Neuroimaging Initiative (ADNI). The genomic expression dataset includes 744 samples, while the clinical dataset includes 2000 samples. The evaluated models include DL, SVM, gradient boosting (GB), and RF. The maximum receiver operating characteristic (ROC) area under the curve (AUC) metric values for clinical data are 0.989 (cognitively normal—CogN), 0.927 (mild cognitive impairment—MCI), 0.907 (dementia); and for gene expression data, 0.763 (CogN), 0.761 (MCI), 0.706 (dementia). The respective F1-Score obtained for clinical data is: 0.971 (CogN), 0.939 (MCI), 0.886 (dementia), and for gene expression: 0.71 (CogN), 0.77 (MCI), 0.53 (dementia).
Ref. [146] describes a study that explores natural compounds (NC) for the prevention of AD using a mix of bioinformatics and deep neural analysis. The evaluated model is Random Forest Regression (RFR) to predict the IC50 (pIC50) values of ligands interacting with target proteins associated with AD. The dataset was obtained from chemical databases, but the exact size is not specified. Testing metrics include excellent recall performance. Other details (such as the dataset size) were not found.
Discussions about using ML algorithms to predict brain age and assess the risk of Alzheimer’s, taking into account the apolipoprotein E4 (APOE4) genotype and gender, are presented in ref. [147]. Three ML models are evaluated, namely XGBoost, RF, and LR. The dataset consists of volumetric MRI data and medical records from 1100 individuals without cognitive impairments and 602 Alzheimer’s patients. The introduced metrics include brain age difference (BAD) and integrated difference (ID). The RF model was the most accurate, and the BAD ranged between 6.5 and 10 years.
Ref. [148] analyzes the differences in progression from MCI to Alzheimer’s in men and women using Random Survival Forests (RSF) and Shapley Additive Explanations (SHAP), evaluating the contributions of dementia biomarkers. The evaluated models are separate RSFs for men (M-RSF) and women (F-RSF). The dataset used is not specified, nor is its size. The obtained performance metrics are 0.87 for M-RSF and 0.79 for F-RSF. The results show differences in the influence of variables between sexes, with a higher risk of progression in women.
Ref. [149] discusses the application of ML techniques to improve the diagnosis and prediction of AD using MRI data. It evaluates models such as the Deep Belief Network (DBN) with the Moonflower Optimization Algorithm (MOA) and compares them with others like RF, SVM, KNN, LR, and DT. The dataset from the ADNI, which comprises 1029 individuals, is used. The DBN-MOA achieved an accuracy of 97.46%, an f-Score of 93.19%, a recall of 95.79%, and a precision of 94.62%. The paper highlights the benefit of integrating demographic and medical data with MRI features. Additional advancements could include EfficientNet models and data augmentation.
The challenges of ML with imbalanced datasets, particularly in the context of high-dimensional longitudinal medical data, are discussed in ref. [128]. An RF-based feature selection algorithm is evaluated and applied to imbalanced neuroimaging data for classifying AD, MCI, and normal individuals (NIs). The public ADNI dataset is used, but the exact size is not specified. The algorithm achieves higher accuracy and AUC (area under the ROC curve) values, with the best results in the NI-AD group data.
Ref. [150] addresses the detection of AD and its stages using multimodal techniques that combine Positron Emission Tomography (PET) and MRI imaging. Ensemble classification models, including SVM, RF, and GB, are evaluated with feature selection based on RF. The dataset includes three classes: AD, MCI, and CogN. The size of the dataset is not specified. The metric values obtained from tests include 99% accuracy for binary classifications AD vs. CogN and MCI vs. CogN, 91% for AD vs. MCI, and 96% accuracy for the multi-class classification AD vs. MCI vs. CogN. The size of the dataset is not available.
Ref. [127] introduces RF for AD staging. It achieves 99.69% sensitivity and 99.61% F1-Score, outperforming existing methods. The study highlights challenges such as limited datasets and high computational complexity, which can be mitigated through data augmentation. Another issue is related to the feature extraction. The results surpass those of state-of-the-art methods and demonstrate the potential for clinical use.
Ref. [151] evaluates the efficiency of dual-phase 18F-florbetaben (FBB) imaging (dual FBB) compared to delay-phase FBB (dFBB) in diagnosing amyloid deposition in the brain using ML. Four classification models are evaluated: SVM, NB, LR, and RF. RF achieved the best results, with an F1-Score of 78.06% and AUC of 0.8724 for composite standardized uptake value ratio (SUVR) and F1-Score of 78.54% and AUC of 0.8456 for regional SUVR.
Ref. [152] developed an ontology to diagnose AD and the evaluation of some ML models. An AD dataset from Kaggle and the Open Access Series of Imaging Studies (OASIS) is used. The evaluated models include LR, XGBoost, GB, Stochastic Gradient Descent (SGD), MLP, SVM, KNN, RF, and CNN. The best metric values are: MLP achieves an accuracy of 92.12%, while CNN achieves an accuracy of 94.61%.
Ref. [153] addresses the evaluation of predictive genes and molecular pathways for the progression of AD and PD using human RNA sequencing transcriptomic data. The evaluated models include differential gene expression and an ML algorithm based on RFs. The analyzed dataset comes from Brodmann Area 9 in the dorsolateral prefrontal cortex, whole blood, and mononuclear cells from peripheral blood. The size of the dataset is not specified. The results suggest the existence of distinct pathogenetic mechanisms for AD and PD, with no significant overlap between the two diseases. The common ML metrics are not mentioned.
Ref. [154] discusses evaluating dementia conversion in patients with MCI using non-selective metabolomic profiling. The evaluated models include an RF model. The data comes from a set of 48 patients: 19 MCI patients who progressed to AD (P-MCI) and 29 stable MCI patients (S-MCI). A panel of 20 metabolites was identified for classification, achieving an accuracy of 73.5% for classifying the three groups and 80.3% for identifying P-MCI versus S-MCI. Indole-3-propionic acid was identified as a predictor of AD progression.
Ref. [155] discusses the evaluation of RF compared to other ML models. A dataset consisting of 2250 brain MRI scans was used, including 687 normal controls, 1094 MCI, and 469 AD patients, provided by the ADNI database7. The classification was performed with three feature sets (63, 29, and 22 features), and RF, MLP, and CNN achieved accuracies of 90.2%, 89.6%, and 90.5%, respectively, with 63 features. With 22 features, RF had a smaller accuracy drop (−3.8%) than MLP and CNN. The brain areas identified as important in classification were the hippocampus, amygdala, and inferior lateral ventricle.
Table 4 provides a numerical summary of the previously discussed metrics to facilitate easier comparison.

Table 4.
Comparative performance metrics of the discussed literature related to AD.
Table 5 concisely evaluates each approach, highlighting its strengths and weaknesses while maintaining references to the original studies. Strengths (1–5) represent scores that reflect the method’s performance concerning the metrics analyzed in the paper. Weaknesses (1–5) reflect the severity of the technique’s limitations or weaknesses.

Table 5.
Strengths and weaknesses of AD investigated articles in the literature.
4.11. Review of ML on PD
Ref. [156] studies PD to the Dense Multiscale Sample Entropy (DM-SamEn) method for feature extraction from vertical ground reaction force (VGRF) signals. The evaluation involves three classifiers: KNN, RBFN-SVM, and MLP. The analyzed data are ground reaction force signals from patients with PD. The metrics obtained are PD diagnostic accuracy (95% CI: 97.82–98.5%) and severity assessment (95% CI: 96.3–97.3%). Limitations include the lack of external validation, limited interpretability, and data imbalance.
Ref. [157] is about the early detection of PD by analyzing spiral drawings. The evaluation focuses on hybrid models: KNN, CNN with SVM, and ResNet-50 with LR. The study uses a dataset of spiral drawings. KNN had an accuracy of 96.77% without augmentation but decreased with augmentation. ResNet-50 with LR achieved 93.55% accuracy with augmentation through rotation and flipping.
Ref. [158] analyzes PD detection based on voice changes, focusing on phonation, articulation, and prosody. PD diagnostic models were evaluated using speech signals and SVM and KNN methods. The data comes from voice recordings collected at the Medical University of Warsaw. The sequential floating forward (SFFS) method was used for descriptor selection. In 10-fold cross-validation, the KNN method achieved a recognition rate of 92.2%, with a sensitivity of 91.1% and a specificity of 93.3%.
The diagnosis of PD uses CNN in ref. [159]. This evaluates the CNN model in comparison to other ML techniques (NB, DF, SVM, and ANN). The dataset used is from the Parkinson’s Progression Markers Initiative (PPMI) and includes T2-weighted MRI images of patients with PD and healthy controls. The evaluation metrics include accuracy (98.7%), sensitivity (95.83%), specificity (96.87%), and the AUC of 94.5%.
Ref. [160] discusses the development of a model for the early detection of PD using human voices. A transformer-based model for dysphonia measurements is evaluated and compared to existing models, including Gradient-Boosted Decision Trees (GBDTs), MLP, SVM, and RF. The dataset uses tabular vocal measurements. The proposed model improves AUC by at least 1% compared to state-of-the-art.
Ref. [161] discusses dynamic brain activity in PD, focusing on dynamic regional homogeneity (dReHo). It utilizes a dataset comprising 57 patients with PD and 31 healthy controls (HCs), including resting-state functional Magnetic Resonance Imaging (rs-fMRI) scans and neuropsychological examinations. Compare regional homogeneity (ReHo) and dReHo between groups and analyze the associations between dReHo and clinical and neuropsychological measures. Evaluator models use SVM to differentiate between groups. The metric values include the correct identification of 98% of individuals by SVM (p < 0.001). The conclusion highlights abnormal brain activity in the precuneus of PD patients and its associations with neuropsychiatric symptoms.
A novel aspect is presented in ref. [162], which highlights feature selection techniques for improving model metrics. Thus, the performance for XGBoost and DNN increased by 10% and 38.18%, respectively, while SVM and DT increased by 0.91% and 7.27% after applying the feature selection methods.
Bayesian Optimization-SVM (BO-SVM) represents a model for classifying PD patients introduced in ref. [163]. The model optimizes hyperparameters for six ML models: SVM, RF, LR, NB, Ridge Classifier, and DT. The dataset uses 195 instances and 23 features to identify whether a person has PD or not. SVM achieved the best results with an accuracy of 92.3%, using Bayesian Optimization.
A dynamic system combined with wearable sensors and ML algorithms to detect balance anomalies in PD patients is presented in ref. [164]. The dataset uses kinematic measurements recorded by inertial sensors on 20 PD patients and 15 healthy subjects. Fifty-two classifiers from algorithm families such as DT, KNN, SVM, and ANN were evaluated. From the investigations, the best algorithm was Fine K-Nearest Neighbor (FKNN), with an accuracy of 95.6%, a recall of 99%, and a precision of 95.2%.
Ref. [165] discusses building a classifier to distinguish between Parkinson’s patients and healthy individuals, using gait data collected from subjects. The evaluated models include LR, SVM, DT, and KNN. The dataset is “Gait in Parkinson’s Disease” from PhysioNet, which contains data from 92 healthy subjects and 214 patients with idiopathic PD. The size of the dataset is 306 subjects. The metric values obtained from the test were 92.3% for accuracy and 0.929 for AUC.
A predictive model for cognitive degeneration in PD patients, using ML methods, is introduced in ref. [166]. The study evaluated cognitive classification models using SVM and PCA. The dataset contains clinical data, plasma biomarkers, and neuropsychological test results. The PCA-SVM model with 32 parameters achieved an accuracy of 92.3% and an AUC of 0.929, while with 13 selected features, the accuracy was 100%, and the AUC was 1.0.
Ref. [167] discusses the identification of PD based on voice disorders using a dataset from Kaggle. It evaluates comparative techniques and ML models, including an unsupervised autoencoder for feature selection and a 1D CNN for PD classification. Obtained metrics are CNN-1D with F1-Score at 92.7% and LR with F1-Score at 92.2%. Limitations include that only one dataset is analyzed without methods for balancing the dataset. The study suggests that CNN-1D offers superior performance compared to manual selection techniques [168].
Ref. [129] studies postural stability in patients with PD using a new methodology employing the empirical mode decomposition (EMD) method. The evaluation is carried out using ML models: KNN, DT, RF, and SVM. The dataset includes 60 subjects (28 healthy and 32 with PD). SVM performs 92%, while the Dempster–Shafer method has an accuracy of 96.51%. This methodology helps differentiate healthy individuals from those with PD, achieving correct classification rates of up to 96%.
The models evaluated in ref. [169] include classifiers such as KNN, SVM, and DT. The methods used achieved an accuracy of 95.4%, a sensitivity of 94.9%, a specificity of 93%, a precision of 95.2%, and an F1-Score of 95.5%.
The early detection of PD is evaluated in ref. [170] using KNN, MLP, and SVM. The dataset is imbalanced, with more uninfected patients than infected ones. Synthetic minority over-sampling technique (SMOTE), RFE, and PCA were used to address the imbalance. The obtained values include a maximum SVM accuracy of 98.2% and a maximum KNN specificity of 99%.
The potential of using voice for PD screening in resource-limited settings is investigated in ref. [171]. The dataset includes 11,942 voice recordings from 1078 participants with PD and 5453 control participants, from which 3000 balanced samples were selected for training and testing, using 10-fold cross-validation, and the remainder (8942 samples) for model validation. The obtained metrics are a sensitivity of 67.43% and a specificity of 67.25%.
Ref. [172] addresses the discrimination between essential tremors and Parkinsonian tremors. The dataset used includes measurements of lower arm tremors collected from patients with different types of tremors and healthy volunteers. The SVM method achieved 100% accuracy for a single measurement at the metacarpal area and 82.62% in general movement positions.
Ref. [173] studies ANN to differentiate PD from Parkinsonism caused by other disorders, using dopamine transporter single-photon emission computed tomography (DAT-SPECT) images. The dataset consists of abnormal DAT-SPECT images from subjects with PD and Parkinsonism. The obtained metric values have an accuracy of 86%, with a sensitivity of 81.8% and a specificity of 88.6%.
Automatic monitoring of “On”/“Off” medication states in PD patients using gait signals detected by a body-worn accelerometer is proposed in ref. [174]. Four models are evaluated: RF, SVM, KNN, and Naive Bayes. The dataset used includes 20 subjects with clear motor fluctuations. The best-performing model was RF, achieving an accuracy of 96.72%, a recall of 97.35%, and a precision of 96.92%. The limitations include using data only in a laboratory environment and focusing exclusively on the “On”/“Off” states.
Ref. [175] discusses the automatic assessment of postural instability in Parkinson’s patients using an optimized SVM algorithm. The dataset includes 42 PD patients and seven healthy subjects. Four hundred fourteen features were extracted and selected based on Pearson correlation. The model achieved 100% accuracy in differentiating patients with mild and severe postural instability from healthy individuals. The data was obtained using the inertial sensors of a smartphone mounted on the waist. The aim is home monitoring and prevention of PD falls.
Table 6 summarizes the results using each paper’s objective and evaluation metrics. The papers that do not explicitly mention the models’ performance metrics have been excluded from the results.

Table 6.
Summary of PD discussed studies.
The same comparative evaluation metrics as for AD were employed in the PD case. Strengths are rated based on performance metrics such as accuracy, sensitivity, specificity, and AUC. Higher values indicate better performance. Weaknesses are rated based on limitations like lower performance in certain metrics, lack of generalizability, or specific challenges in implementation.
4.12. Review of ML on MS
One of the main research directions in MS focuses on ML algorithms. Ref. [176] demonstrated that models using KNN-type algorithms have superior accuracy in differentiating MS lesions in MRI scans compared to traditional approaches. Refs. [85,177] predict the likelihood of disease progression and exacerbations based on neuroimaging characteristics. Ref. [178] presents a review highlighting the techniques used in identifying patterns in large datasets to assist clinicians in making informed decisions regarding managing patients’ diseases. Additionally, DL has enabled the automatic extraction of features from complex datasets, as in the case of ref. [179]. This uses CNN to learn hierarchical features that facilitate diagnosis with superior accuracy compared to traditional methods in multiple sclerosis. Ref. [180] improves the predicted values compared to conventional methods, while ref. [181] investigates integrating multimodal data such as imaging, clinical assessments, and biological markers in identifying MS.
Another direction in which studies have explored MS involves unsupervised learning to classify MS patients based on clinical and MRI data without predefined labels. This approach can lead to personalized treatment strategies. Unsupervised techniques help discover subtypes within the heterogeneous population of MS patients. The direct consequence concerns adapting the therapeutic plan for each patient. Recent models that predict future clinical outcomes are being investigated through metrics such as gait analysis and offer results that promise advancements in early disease identification.
Ref. [182] proposes an ensemble of dormant models from LSTM, DNN, and CNN optimized by the improved walrus optimization algorithm. For detecting MS lesions using MRI images, a CNN implemented with the VGG16 architecture achieved results associated with accuracy metrics of 98.44%, specificity 99.4%, and sensitivity 97.56% [183]. Transcriptomic-based approaches have shown that blood transcriptomic signatures can classify MS with an accuracy of 97% [184]. The analysis of metabolomic data using ANN achieved an accuracy of 87% and a sensitivity of 82.5%, and a specificity of 89% [185]. Regarding gait analysis, a ResNet model with regression-based normalization achieved accuracy and an F1-Score of 100% when generalized from simple walking to walking while talking [186]. For the evaluation of visual signs, the NeuroVEP system agreed with standard tests in 91% of cases [187]. Automatic detection of the central vein sign in MS lesions using a 3D convolutional neural network (CVSnet) achieved an accuracy of 91% on the test set [188]. Accelerometry-based classification achieved 87% accuracy compared to EMG [189].
ML models for biomarkers identified specific panels for the presence of oligoclonal bands (OCB) in CSF: the MS OCB+ model achieved 91% sensitivity and 94% specificity in training and 81% and 94% in validation, respectively; the MS OCB− model performed worse [190]. SVM and KNN applied to MRI differentiated MS from Neuromyelitis Optica (NMO) with precision 98%, recall 99%, F1-Score 99%, and accuracy 99% [191]. A hidden layer ANN predicted the MS diagnosis with a testing accuracy of 75% [192].
Table 7 synthesizes the MS discussed studies and outlines the strengths and weaknesses of the method’s performance employed.

Table 7.
Summary of the MS discussed studies.
The scores for strengths are assigned based on the method’s performance in terms of accuracy, sensitivity, specificity, and clinical applicability. The scores for weaknesses reflect the severity of the limitations, such as poorer performance in certain metrics, lack of generalizability, or specific implementation challenges.
4.13. Overview of Publicly Available Datasets in Neuro-ML Studies
The quality of ML models is obtained based on the quality of the dataset used for training. The quality of these data is subsequently reflected in the performance metrics used to evaluate the model’s performance. For neurological diseases, especially neurodegenerative ones, datasets are used to train algorithms, which need to be generalized in diagnosis, disease progression monitoring, and biomarker identification.
Datasets for neurological diseases are classified according to the type of data they consist of. Thus, there are four general types: imaging, vocal data, clinical data, and genetics. Using these datasets to train ML models allows for progress in Neuro-ML.
To more easily identify the diversity and source of the datasets used in this review, Table 8 summarizes the works presented in Table 4, Table 6 and Table 7. Table 8 presents the public datasets analyzed in the review. These datasets are used for training and validating ML models to diagnose and predict the three previously analyzed neurodegenerative diseases.

Table 8.
Comparative overview of datasets used in AD, PD, and MS.
Table 8 highlights that the volumes of the datasets used for medical imaging are over a thousand subjects, making them suitable for complex models associated with DL models. On the other hand, the voice and gait datasets have volumes between 200 and 300 subjects. Most MS-related datasets are small, ranging from 5 to 60 patients. There are also datasets with genomic, metabolomic, or EEG data whose size varies. The volume of the datasets is correlated with the type of data, the disease being investigated, and the kind of model analyzed.
Table 8 demonstrates the diversity of datasets used in neurodegenerative studies. They use various data types, from medical images to physiological signals and molecular data. The integration into ML models varies depending on the type of model used. The dimensions of the datasets also vary, but the performance metrics ensure a relevant evaluation of the final model obtained after training.
In the authors’ opinion, the larger the dataset, the better the performance metrics, but this general idea does not guarantee high model performance. The quality of the data in the dataset, along with the type of model, dictates the quality of the resulting model. A concrete example in this regard, from the authors’ experience, is that DL models require much larger volumes of data than traditional ML models. Therefore, the quality of the model is generally correlated with accuracy, prediction, F1-Score, and recall. Usually, performance metrics are those that are evaluated at the end of the model training and validation. For this reason, most studies analyze a wide range of models and do not specifically target a particular model. The explanation is that it cannot be known a priori which model will provide better metrics for a given dataset.
An ML model’s main objective is generalization capability in the subsequent training and validation stage. This generalization capability is technically analyzed through performance metrics. Many datasets presented in Table 8, such as ADNI or PPMI, are imbalanced regarding disease stage representation. This aspect can affect the generative capacity of the analyzed models. Voice or gait datasets, which can be downloaded from platforms like Kaggle or PhysioNet, exhibit a lack of demographic diversity. This aspect limits their applicability in real clinical scenarios. In the authors’ opinion, these aspects highlight the necessity for future studies to mention how data is collected, explicitly state the volume of existing data in the dataset, the methods applied in the data preprocessing stage, and the standardized documentation. Implementing these directives proposed by the authors will support the advancement of ML algorithms in diagnosing neurodegenerative diseases.
5. Discussion
The results presented in this paper demonstrate the interest of researchers and the trends in using ML algorithms to diagnose and classify neurological diseases. This section discusses the involvement of these algorithms in Neuro-AI research by identifying existing gaps and proposing future study directions that encourage the evolution of the Neuro-AI field.
The research included 10 types of statistical analyses in the specialized literature to evaluate the level of approach to the Neuro-ML subject rigorously.
Analysis 1: The distribution of works based on authors’ keywords in the Neuro-ML field generated five clusters, each representing a research direction. The central cluster highlighted the interest in Neuro-ML research for AD, PD, and MS. The other clusters highlighted the diversity of methodological approaches.
Analysis 2: The distribution of works by country in the field of Neuro-ML showed that China, the USA, and India are the countries most concerned with addressing these diseases through an evolutionary technological approach. At the European level, England, Germany, and Italy stand out.
Analysis 3: The distribution of works by year in Neuro-ML (2020–2024) illustrates the upward trend in the number of articles published in this field. These results confirm researchers’ growing interest in applying ML in neurology.
Analysis 4: The distribution of works by publishers in the field of Neuro-ML (2020–2025) indicates MDPI as the leader in Neuro-ML publications, with 647 articles, followed by Elsevier (543 articles), Springer (378 articles), FRONTIERS MEDIA SA (373 articles), and WILEY (323 articles).
Analysis 5: Neuro-ML distribution by research objectives highlighted that most articles focus on predictions (1595 articles), followed by diagnosis and classification, which have similar results. Prognosis is the least explored.
Analysis 6: Comparing the number of articles for ML algorithms applied in neurological research using WOS shows that SVM is the most studied model, with 597 articles, followed by ANN (525 articles), RF (457 articles), LR (378 articles), CNN (251 articles), and LSTM (142 articles). Models such as XGBoost and SVR are underrepresented.
Analysis 7: The distribution of ML algorithms on specific neurological diseases has shown that Alzheimer’s, Parkinson’s, and MS are the most studied in the context of ML. SVM dominates in all these cases, and models such as MLP appear only in a single article about MS and LBD. The underrepresentation of rare diseases, such as ALS and prion diseases, highlights a major gap in the literature.
Analysis 8: The comparison of the number of articles for ML algorithms applied in neurological research (custom procedure) showed that ANN is the most used algorithm, with 97 occurrences, followed by SVM (92), RF (63), CNN (57), and LSTM (29). Algorithms such as XGBoost and SVR appear less frequently. The customized method confirms researchers’ preference for easily implementable models. There is also a noticeable lack of interest in algorithms that are more difficult to integrate.
Analysis 9: The comparative analysis of the results between the customized method and WOS aimed to identify the differences in outcomes. WOS includes articles that mention terms even if they are not explicitly related to the authors’ keywords, while the customized method imposes strict filters. The customized method provides more rigorous results. A disadvantage is that it excludes articles that do not meet the strict criteria.
Analysis 10: The overall impact of the findings on future research highlights the importance of ML algorithms in diagnosing and classifying neurological diseases. The research identified gaps in the Neuro-ML field regarding the underrepresentation of rare diseases, the lack of addressing difficult-to-implement ML algorithms, and the absence of standardization in evaluating their performance. Exploring rare diseases and testing underutilized algorithms represent priority directions for future research.
The answers to the RQS established at the beginning of this research are as follows:
- RQ1: The most commonly used ML methods for diagnosing neurodegenerative diseases are SVM (597 articles), ANN (525 articles), RF (457 articles), CNN (251 articles), and LSTM (142 articles). Other models, such as XGBoost, SVR, and MLP, have been studied less. These methods are mainly applied in diagnosing Alzheimer’s, Parkinson’s, and MS.
- RQ2: ML models surpass traditional methods in diagnosing neurodegenerative diseases through their ability to process large volumes of heterogeneous data. ML models can identify hidden patterns. For example, RF and SVM have reported accuracies of over 90% in diagnosing Alzheimer’s and Parkinson’s, compared to traditional methods that rely on subjective clinical assessments. Performance varies depending on the size of the dataset and its quality.
- RQ3: The main challenges include unbalanced datasets, affecting the models’ generalization ability. Additionally, the lack of standardized protocols for evaluating the performance of ML models makes it difficult to compare results between studies. Computational resources represent another challenge. Models such as CNN and LSTM require significant resources for training, which is a limitation for researchers.
- RQ4: The major limitations arise from the challenges presented earlier. Thus, in the category of limitations, the dependence on datasets that need to be voluminous and correlated, the underrepresentation of rare diseases, the underrepresentation of a class of algorithms that present difficulties in implementation both computationally and algorithmically, as well as the lack of standardization of performance metrics in relation to the specifics of neural diseases are identified.
The results are discussed in detail below.
5.1. Distribution of Articles Based on ML Algorithms in Neurological Research
Analyses conducted using the WOS platform showed the involvement of the academic community in ML model research to be adapted to the Neuro-AI field. The results showed that the diseases most explored in the ML field were AD, PD, and MS. Intensive research focused on the three diseases, leaving other neurological diseases unexamined and which should be studied equally. From the category of ML algorithms, the most explored are SVMs. They were identified with 597 articles due to their ability to classify membership in a specific category using training datasets. The second ML algorithm identified in the literature is ANN, which has 525 articles. The large number of articles is explained by its ability to identify patterns associated with certain classes. This characteristic makes it suitable for addressing non-linear approaches. Most neurological conditions are non-linear in nature; consequently, ANN models are suitable for modeling the issues of neurodegenerative diseases. The third algorithm in the literature is RF, which is preferred due to its ability to explore heterogeneous data. Training sets prevent overfitting due to RF-specific mechanisms. LR is identified in the literature with 378 articles, and it is mentioned that it is suitable for ML classification problems based on symptomatology. The CNN model is identified with 251 articles, being less studied due to complex calculations and the demand for large datasets to generalize. The LSTM algorithm has 142 articles and analyzes sequential data such as EEG signals. The unequal distribution of ML algorithm usage shows researchers’ preference for certain models. This preference is associated with ease of implementation and lower requirements in terms of computational resources.
5.2. Distribution of ML Algorithms in the Context of Specific Neurological Diseases
The most studied neurological diseases in ML are AD, PD, and MS. For AD, the most suitable algorithm is SVM (37 articles), followed by RF (31 articles), CNN (34 articles), KNN (12 articles), and XGBoost (7 articles). Regarding PD, the most suitable model is again SVM (46 articles), followed by RF (48 articles), CNN (42 articles), KNN (23 articles), and XGBoost (18 articles). Regarding MS, the most studied model is SVM (9), but RF and CNN are hardly studied in this context. The results show that Alzheimer’s and PDs, which are common diseases, are better represented in the specialized literature. Rare diseases, such as ALS and prion diseases, are poorly studied in the literature. This observation indicates a gap in exploring these algorithms in the context of rare diseases.
The frequency of occurrence of ML algorithms in the authors’ keywords was analyzed through the WOS platform and a customized method proposed by the authors of the present article. The customized method showed that the preferred ML algorithm is ANN. It has 97 occurrences, SVM has 92, and RF has 63. Algorithms such as XGBoost and SVR are underrepresented in the authors’ keywords regarding frequency of occurrence. Regarding the comparative analysis of the results obtained through keyword identification in the customized method vs. WOS, the results show a series of differences that reveal a shortcoming in the literature. The WOS method counts articles that mention terms in a broader sense. Therefore, the authors’ keywords are not treated restrictively to the term algorithm. Instead, the customized method applies strict filters. Thus, only articles that mention the algorithm in the authors’ keywords are quantified. The proposal for quantification through the customized method provides a rigorous picture of the authors’ preferences in addressing certain ML models related to specific diseases.
A detailed analysis of metric performance in literature studies was reported on the two diseases considered the most studied in the literature, AD and PD. Regarding AD, the best accuracy identified in the literature was 97.46%, with an F1-Score of 93.19%, recall of 95.79%, and precision of 94.62% in ref. [149]. For Parkinson’s, the highest accuracy was 98.2%, as reported in ref. [170].
In addition to what has been presented, the authors emphasize that the clinical applicability of ML models in neurology has been discussed indirectly by focusing on diagnostic accuracy and disease progression prediction:
- ML models can shorten the diagnostic time and reduce the error rate in the early identification of neurodegenerative diseases (e.g., early diagnosis in Alzheimer’s with 97.46% accuracy using DBN-MOA).
- Algorithms allow personalized treatment by stratifying patients based on genetic or imaging biomarkers (e.g., in ALS or MS).
- The integration of ML into clinical practice remains dependent on standardized protocols and validation on large clinical datasets, which is recognized as a limitation and a priority direction for the future.
For an efficient clinical application, the authors recommend developing standardized validation procedures and expanding research to rare diseases where the impact of early diagnosis can be significant.
The ANN algorithm is the second most frequently used in the Neuro-ML field. Figure 11 shows that the total number of articles using a specific algorithm in the Neuro-ML field aims at the unified treatment of all neurological diseases. Figure 12 highlights the frequency of use of each algorithm based on the neurological disease. Therefore, although ANN is frequently mentioned in the general literature on Neuro-ML, it does not dominate in AD, PD, and PD disease studies. Figure 12 exclusively highlights the three neurodegenerative diseases. The difference between general use and applicability to specific conditions is not a contradiction but highlights an uneven distribution. Practically, ANN is used in general contexts, which makes it underrepresented in specific clinical applications for certain conditions.
5.3. Research Gaps Identification
These results lead to the identification of the following gaps in the literature:
- Most studies investigate common diseases such as AD and PD, leaving rare diseases like ALS and prion diseases insufficiently explored. This limitation reduces the applicability of ML models in diagnosing these conditions.
- Algorithms such as XGBoost, LR, and MLP have not been sufficiently studied in the context of neurological diseases. A possible explanation could be the researchers’ insufficient knowledge of these techniques. A second explanation could be related to the difficulties associated with their implementation.
- Models like CNN and LSTM require large computational resources. In addition to this limitation, these models also require large volumes of data for training. These requirements can pose an obstacle for researchers working with limited resources.
- The lack of standardized protocols for evaluating the performance of ML models in the context of neurological diseases makes it difficult to compare results between different studies.
5.4. Future Directions
These gaps in the literature have been identified based on the unique methodology proposed in this paper. The integration of ML algorithms to neurological diseases provides an overview of the current state of research in the field of Neuro-AI. The results highlight the elements that need to be improved in the field and emphasize the developments related to diagnosing these diseases. Based on the analysis conducted, the authors recommend the following aspects for future research:
- Investment in data collection for rare diseases like ALS and prion diseases. Researchers can develop ML models that allow for the most accurate diagnosis by creating datasets associated with these conditions.
- Researchers should explore the potential of XGBoost and LR algorithms in the context of neurological diseases. Their approach could lead to discoveries beyond the models extensively investigated in the literature.
- The development of standardized protocols for evaluating the performance of ML models concerning neurological diseases would make it possible to compare results between studies to more easily identify the best models and their best configurations for each type of neurological disease.
- Developing computational tools that prevent resource limitations allows research to be conducted without advanced hardware resources.
- Exploring the integration of multimodal data, such as medical images, genetic data, and clinical biomarkers, into the performance of ML models. This multidisciplinary approach will lead to improved identification of the mechanisms of neurological diseases, which can have direct implications for the personalization of treatments.
Integrating these recommendations into future research will improve the Neuro-AI field. The improvements aim at early diagnosis and personalized treatment of neurological diseases. These developments will positively impact patients’ quality of life suffering from various neurological diseases.
The authors acknowledge that the study predominantly focused on “classic” ML techniques (SVM, RF, ANN, and CNN), as revealed by the literature analysis from 2020 to 2025. Transformer models and federated learning techniques were not heavily represented in the data extracted from WOS for the Neuro-ML field up to March 2025, which reflects either the emerging nature of these technologies or the lack of widespread adoption in neurological applications documented in the analyzed journals. For transformer models, the search yielded 20 results, while for federated learning techniques, five results were obtained. However, explainable AI (XAI) is tangentially addressed by referring to works that use interpretable methods in SCA (e.g., the XAI model for SCA classification). Still, it has not been a primary focus. On another note, XAI is not a branch of ML, and the study focuses on specific ML algorithms. The authors will explore these directions in future approaches.
6. Conclusions
This paper conducted a literature review of the field of Neuro-AI. Subsequently, a WOS analysis was performed for the bibliometric analysis of the authors’ keywords and the countries of origin of the authors. Additionally, a growing interest among researchers in the field of Neuro-AI correlated with ML was identified. ML algorithms are investigated in the literature for diagnosing, classifying, and predicting neurological diseases. Most research focuses on Alzheimer’s, Parkinson’s, and MS.
The main findings identified in the literature are as follows:
- The extensive use of specific ML algorithms;
- High diagnostic performance for the most researched diseases;
- The unequal distribution of research on neurological diseases;
- Identification of training characteristics through biomarkers.
- The research gaps are mentioned below:
- The underrepresentation of rare diseases, such as ALS and prion diseases, which are insufficiently explored in the context of ML;
- Limiting underutilized algorithms, such as XGBoost, LR, and MLP, which are sporadically used in neurological research;
- The lack of standardization in evaluating ML model performance makes comparing results across different studies difficult.
- Unbalanced and limited datasets.
The relevance of the research is directed in two ways. The first aims to encourage researchers to tackle less-explored diseases, while the second refers to encouraging researchers to study less-explored models.
The research limitations include that the analysis focused exclusively on the WOS platform, which may have excluded relevant articles published in other databases or local journals. The focus on the 2020–2024 period may not reflect the complete evolution of research in Neuro-AI.
The lack of access to complete datasets for each study included in the analysis limited the thorough evaluation of the methodologies and reported outcomes.
This research evaluates researchers’ interest in ML models in the Neuro-AI field. The paper identified low interest in certain neurological diseases and some less popular ML models. The uneven distribution of research on diseases and algorithms suggests the need for future research directions. Exploring rare diseases, testing underutilized algorithms, and developing standardized evaluation protocols represent future research priorities. By identifying these gaps, the present research is a reference point for the Neuro-AI field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15105442/s1, NeuroAIStats.xlsx (includes sheets 1, 2, 3, 4 and 5), Author_keywords.xlsx.
Author Contributions
Conceptualization, C.-M.R.; methodology, C.-M.R. and A.S.; software, C.-M.R. and A.S.; validation, C.-M.R. and A.S.; formal analysis, C.-M.R. and A.S.; investigation, C.-M.R. and A.S.; resources, C.-M.R. and A.S.; data curation, C.-M.R. and A.S.; writing—original draft preparation, C.-M.R. and A.S.; writing—review and editing, C.-M.R. and A.S.; visualization, C.-M.R. and A.S.; supervision, C.-M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Petroleum-Gas University of Ploiesti, Romania.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| AdaBoost | Adaptive Boosting |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| AI | Artificial intelligence |
| ALS | Amyotrophic Lateral Sclerosis |
| ANN | Artificial Neural Network |
| APOE | Apolipoprotein E |
| APOE4 | Apolipoprotein E4 |
| AUC | Area under the curve |
| BAD | Brain age difference |
| BNB | Bernoulli Naive Bayes |
| BO-SVM | Bayesian Optimization–Support Vector Machine |
| CAD | Computer-aided diagnosis |
| CatBoost | Categorical Boosting |
| CJD | Creutzfeldt–Jakob Disease |
| CN | Capsule Network |
| CNN | Convolutional Neural Network |
| CogN | Cognitively normal |
| CSF | Cerebrospinal fluid |
| DAT-SPECT | Dopamine transporter single-photon emission computed tomography |
| DBN | Deep Belief Network |
| DBSCAN | Density-Based Spatial Clustering of Applications with Noise |
| dFBB | Delay-phase 18F-florbetaben |
| dMRI | Diffusion Magnetic Resonance Imaging |
| DL | Deep learning |
| DM-SamEn | Dense Multiscale Sample Entropy |
| dReHo | Dynamic regional homogeneity |
| DS | Decision Stump |
| DT | Decision Tree |
| EEG | Electroencephalogram |
| EMD | Empirical mode decomposition |
| EN | Elastic Net |
| ERT | Extremely Randomized Tree |
| FBB | 18F-florbetaben |
| FKNN | Fine K-Nearest Neighbor |
| F-RSF | Random Survival Forests for women |
| FTD | Frontotemporal Dementia |
| GAN | Generative Adversarial Network |
| GEO | Gene Expression Omnibus |
| GB | Gradient Boosting |
| GBDT | Gradient-Boosted Decision Tree |
| GBT | Gradient Boosting Tree |
| GNB | Gaussian Naive Bayes |
| GP | Gaussian Processes |
| GRU | Gated Recurrent Unit |
| HC | Healthy control |
| HD | Huntington’s Disease |
| HMM | Hidden Markov Model |
| HR | Huber Regressor |
| ICA | Independent Component Analysis |
| ID | Integrated difference |
| IDD | Intellectual and Developmental Disability |
| KMeans | K-Means Clustering |
| KNN | K-Nearest Neighbors |
| LassoR | Lasso Regression |
| LBD | Lewy Body Dementia |
| LDA | Linear Discriminant Analysis |
| LightGBM | Light Gradient Boosting Machine |
| LR | Logistic Regression |
| LSTM | Long Short-Term Memory |
| MARS | Multivariate Adaptive Regression Splines |
| MCI | Mild cognitive impairment |
| ML | Machine learning |
| MLP | Multilayer Perceptron |
| MNB | Multinomial Naive Bayes |
| MOA | Moonflower Optimization Algorithm |
| MRI | Magnetic Resonance Imaging |
| M-RSF | Random Survival Forests for men |
| MS | Multiple Sclerosis |
| NB | Naive Bayes |
| NC | Natural compounds |
| NDD | Neurodevelopmental disorder |
| Neuro-ML | Machine learning algorithms used in the field of neurological diseases |
| NI | Normal individual |
| NLP | Natural language processing |
| NMO | Neuromyelitis Optica |
| NTM | Neural Turing Machines |
| OASIS | Open Access Series of Imaging Studies |
| OCB | Oligoclonal bands |
| PCA | Principal Component Analysis |
| PD | Parkinson’s disease |
| PET | Positron Emission Tomography |
| P-MCI | Mild cognitive impairment patient proceeding to AD |
| PPMI | Parkinson’s Progression Markers Initiative |
| PRNP | Prion protein |
| QDA | Quadratic Discriminant Analysis |
| RBFN | Radial Basis Function Network |
| ReHo | Regional homogeneity |
| RF | Random Forest |
| RFECV | Recursive Feature Elimination with Cross-Validation |
| RFR | Random Forest Regression |
| RidgeR | Ridge Regression |
| RNN | Recurrent Neural Networks |
| ROC | Receiver operating characteristic |
| RQ | Research question |
| RSF | Random Survival Forests |
| rs-fMRI | Resting-state functional Magnetic Resonance Imaging |
| RT | Randomized Trees |
| SCA | Spinocerebellar Ataxia |
| SFFS | Sequential floating forward |
| SGD | Stochastic Gradient Descent |
| SHAP | Shapley Additive Explanations |
| S-MCI | Stable Mild cognitive impairment patient |
| SMOTE | Synthetic minority over-sampling technique |
| SUVR | Standardized uptake value ratio |
| SVD | Singular Value Decomposition |
| SVM | Support Vector Machine |
| SVR | Support Vector Regression |
| T-SNE | t-Distributed Stochastic Neighbor Embedding |
| VGRF | Vertical ground reaction force |
| WOS | Web of Science |
| XAI | Explainable artificial intelligence |
| XGBoost | Extreme Gradient Boosting |
References
- Rosca, C.-M.; Stancu, A.; Tănase, M.R. A Comparative Study of Azure Custom Vision Versus Google Vision API Integrated into AI Custom Models Using Object Classification for Residential Waste. Appl. Sci. 2025, 15, 3869. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A.; Neculaiu, C.-F.; Gortoescu, I.-A. Designing and Implementing a Public Urban Transport Scheduling System Based on Artificial Intelligence for Smart Cities. Appl. Sci. 2024, 14, 8861. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. Earthquake Prediction and Alert System Using IoT Infrastructure and Cloud-Based Environmental Data Analysis. Appl. Sci. 2024, 14, 10169. [Google Scholar] [CrossRef]
- Popescu, C.; Dissanayake, H.; Mansi, E.; Stancu, A. Eco Breakthroughs: Sustainable Materials Transforming the Future of Our Planet. Sustainability 2024, 16, 10790. [Google Scholar] [CrossRef]
- Alshehri, S.; Alahmari, K.A.; Alasiry, A. A Comprehensive Evaluation of AI-Assisted Diagnostic Tools in ENT Medicine: Insights and Perspectives from Healthcare Professionals. J. Pers. Med. 2024, 14, 354. [Google Scholar] [CrossRef]
- Kwok, T.N.C.; Henry, C.; Saffaran, S.; Meeus, M.; Bates, D.; Van Laere, D.; Boylan, G.; Boardman, J.P.; Sharkey, D. Application and potential of artificial intelligence in neonatal medicine. Semin. Fetal Neonatal Med. 2022, 27, 101346. [Google Scholar] [CrossRef]
- Joshi, G.; Jain, A.; Araveeti, S.R.; Adhikari, S.; Garg, H.; Bhandari, M. FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics 2024, 13, 498. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Passaro, A.P.; Lebos, A.L.; Yao, Y.; Stice, S.L. Immune Response in Neurological Pathology: Emerging Role of Central and Peripheral Immune Crosstalk. Front. Immunol. 2021, 12, 676621. [Google Scholar] [CrossRef]
- Needham, E.J.; Chou, S.H.Y.; Coles, A.J.; Menon, D.K. Neurological Implications of COVID-19 Infections. Neurocrit. Care 2020, 32, 667–671. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. Fusing Machine Learning and AI to Create a Framework for Employee Well-Being in the Era of Industry 5.0. Appl. Sci. 2024, 14, 10835. [Google Scholar] [CrossRef]
- Wang, T.V.; Song, P.C. Neurological Voice Disorders: A Review. Int. J. Head Neck Surg. 2022, 13, 32–40. [Google Scholar] [CrossRef]
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of Various Types of Nanomaterials for the Treatment of Neurological Disorders. Nanomaterials 2022, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Solla, P.; Sechi, L.A. Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases. Pharmaceuticals 2022, 15, 1077. [Google Scholar] [CrossRef]
- Wang, P.; Lynn, A.; Miskimen, K.; Song, Y.E.; Wisniewski, T.; Cohen, M.; Appleby, B.S.; Safar, J.G.; Haines, J.L. Genome-wide association studies identify novel loci in rapidly progressive Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 2034–2046. [Google Scholar] [CrossRef]
- Saleem, T.J.; Zahra, S.R.; Wu, F.; Alwakeel, A.; Alwakeel, M.; Jeribi, F.; Hijji, M. Deep Learning-Based Diagnosis of Alzheimer’s Disease. J. Pers. Med. 2022, 12, 815. [Google Scholar] [CrossRef]
- Rahman, S.; Hasan, M.; Sarkar, A.K.; Khan, F. Classification of Parkinson’s Disease using Speech Signal with Machine Learning and Deep Learning Approaches. Eur. J. Electr. Eng. Comput. Sci. 2023, 7, 20–27. [Google Scholar] [CrossRef]
- Zeng, Q.; Shen, J.; Chen, K.; Zhou, J.; Liao, Q.; Lu, K.; Yuan, J.; Bi, F. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 2020, 10, 12998. [Google Scholar] [CrossRef]
- Wang, H. Phylogenetic analysis of microRNA biomarkers for amyotrophic lateral sclerosis. BIOCELL 2021, 45, 547–561. [Google Scholar] [CrossRef]
- Stefanova, E.; Marjanović, A.; Dobričić, V.; Mandić-Stojmenović, G.; Stojković, T.; Branković, M.; Šarčević, M.; Novaković, I.; Kostić, V.S. Frequency of C9orf72, GRN, and MAPT pathogenic variants in patients recruited at the Belgrade Memory Center. Neurogenetics 2024, 25, 193–200. [Google Scholar] [CrossRef]
- Tellone, E.; Galtieri, A.; Ficarra, S. Reviewing Biochemical Implications of Normal and Mutated Huntingtin in Huntington’s Disease. Curr. Med. Chem. 2020, 27, 5137–5158. [Google Scholar] [CrossRef] [PubMed]
- Rosser, A.E.; Busse, M.E.; Gray, W.P.; Badin, R.A.; Perrier, A.L.; Wheelock, V.; Cozzi, E.; Martin, U.P.; Salado-Manzano, C.; Mills, L.J.; et al. Translating cell therapies for neurodegenerative diseases: Huntington’s disease as a model disorder. Brain 2022, 145, 1584–1597. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.H.; Rutchik, J.S. A rare case of early onset lewy body dementia with parkinsonism associated with chronic exposure to copper contaminated drinking water. Front. Toxicol. 2024, 6, 1451235. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Villar-Piqué, A.; Hermann, P.; Escaramís, G.; Calero, M.; Chen, C.; Kruse, N.; Cramm, M.; Golanska, E.; Sikorska, B.; et al. Diagnostic accuracy of cerebrospinal fluid biomarkers in genetic prion diseases. Brain 2022, 145, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Kothekar, H.; Chaudhary, K. Kuru Disease: Bridging the Gap Between Prion Biology and Human Health. Cureus 2024, 16, e51708. [Google Scholar] [CrossRef]
- Ouchi, H.; Ishiguro, H.; Shibano, K.; Hara, K.; Sugawara, M.; Enomoto, K.; Miyata, H. Primary degeneration of oculomotor, motor, and somatosensory systems and auditory and visual pathways in spinocerebellar ataxia type 7: A clinicopathological study in a Japanese autopsy case. Neuropathology 2023, 43, 164–175. [Google Scholar] [CrossRef]
- Palvadeau, R.; Kaya-Güleç, Z.E.; Şimşir, G.; Vural, A.; Öztop-Çakmak, Ö.; Genç, G.; Aygün, M.S.; Falay, O.; Başak, A.N.; Ertan, S. Cerebellar cognitive-affective syndrome preceding ataxia associated with complex extrapyramidal features in a Turkish SCA48 family. Neurogenetics 2020, 21, 51–58. [Google Scholar] [CrossRef]
- Duggirala, N.; Ngo, K.J.; Pagnoni, S.M.; Rosa, A.L.; Fogel, B.L. Spinocerebellar ataxia type 14 (SCA14) in an Argentinian family: A case report. J. Med. Case Rep. 2023, 17, 168. [Google Scholar] [CrossRef]
- Podbielska, M.; Szulc, Z.M.; Ariga, T.; Pokryszko-Dragan, A.; Fortuna, W.; Bilinska, M.; Podemski, R.; Jaskiewicz, E.; Kurowska, E.; Yu, R.K.; et al. Distinctive sphingolipid patterns in chronic multiple sclerosis lesions. J. Lipid Res. 2020, 61, 1464–1479. [Google Scholar] [CrossRef]
- Oraby, M.I.; El Masry, H.A.; Abd El Shafy, S.S.; Abdul Galil, E.M. Serum level of brain-derived neurotrophic factor in patients with relapsing–remitting multiple sclerosis: A potential biomarker for disease activity. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 40. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Cărbureanu, M. A Comparative Analysis of Sorting Algorithms for Large-Scale Data: Performance Metrics and Language Efficiency. In Emerging Trends and Technologies on Intelligent Systems: Proceedings of the ETTIS 2024, Noida, India, 27–28 March 2024; Lecture Notes in Networks and Systems; Springer Nature: Berlin/Heidelberg, Germany, 2025; Volume 1073, pp. 99–113. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. A Comprehensive Review of Machine Learning Models for Optimizing Wind Power Processes. Appl. Sci. 2025, 15, 3758. [Google Scholar] [CrossRef]
- Liu, J. SSH Application Classification Based on Machine Learning. Mach. Learn. Theory Pract. 2021, 2, 20–27. [Google Scholar] [CrossRef]
- Sharp, J. Exam Ref AI-900 Microsoft Azure AI Fundamentals; Pearson Education: London, UK, 2022. [Google Scholar]
- Rosca, C.-M.; Gortoescu, I.A.; Tanase, M.R. Artificial Intelligence—Powered Video Content Generation Tools. Rom. J. Pet. Gas Technol. 2024, 5, 131–144. [Google Scholar] [CrossRef]
- Endut, N.; Hamzah, W.M.A.F.W.; Ismail, I.; Yusof, M.K.; Baker, Y.A.; Yusoff, H. A Systematic Literature Review on Multi-Label Classification based on Machine Learning Algorithms. TEM J. 2022, 11, 658–666. [Google Scholar] [CrossRef]
- Ling, Q. Machine learning algorithms review. Appl. Comput. Eng. 2023, 4, 91–98. [Google Scholar] [CrossRef]
- Liao, M.; Duan, H.; Wang, G. Application of Machine Learning Techniques to Detect the Children with Autism Spectrum Disorder. J. Healthc. Eng. 2022, 2022, 9340027. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A.; Ariciu, A.V. Algorithm for child adoption process using artificial intelligence and monitoring system for children. Internet Things 2024, 26, 101170. [Google Scholar] [CrossRef]
- Ramadurgam, S.; Perera, D.G. An Efficient FPGA-Based Hardware Accelerator for Convex Optimization-Based SVM Classifier for Machine Learning on Embedded Platforms. Electronics 2021, 10, 1323. [Google Scholar] [CrossRef]
- Huang, Z. A Research on Image Recognition and Classification Based on Traditional Machine Learning and Deep Learning. Trans. Comput. Sci. Intell. Syst. Res. 2024, 5, 766–773. [Google Scholar] [CrossRef]
- Ye, S. Blended Learning for Machine Learning-based Image Classification. EAI Endorsed Trans. E-Learn. 2023, 9, 1–7. [Google Scholar] [CrossRef]
- Sinra, A.; Waluyo Poetro, B.S.; Angriani, H.; Zein, H.; Musdar, I.A.; Taruk, M. Optimizing Neurodegenerative Disease Classification with Canny Segmentation and Voting Classifier: An Imbalanced Dataset Study. Int. J. Artif. Intell. Med. Issues 2023, 1, 95–105. [Google Scholar] [CrossRef]
- Escamilla-Luna, O.; Wister, M.A.; Hernandez-Torruco, J. Machine Learning Algorithms for Classification Patients with Parkinson’s Disease and Hereditary Ataxias. J. Commun. Softw. Syst. 2023, 19, 9–18. [Google Scholar] [CrossRef]
- Tian, J.; Smith, G.; Guo, H.; Liu, B.; Pan, Z.; Wang, Z.; Xiong, S.; Fang, R. Modular machine learning for Alzheimer’s disease classification from retinal vasculature. Sci. Rep. 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valbuena, I. Phenotype parallels protein seeding capacity in neurodegenerative diseases. Brain Pathol. 2024, 34, e13238. [Google Scholar] [CrossRef]
- Payares-Garcia, D.; Mateu, J.; Schick, W. Spatially informed Bayesian neural network for neurodegenerative diseases classification. Stat. Med. 2023, 42, 105–121. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ran, A.R.; Wang, S.; Chan, V.T.T.; Sham, K.; Hilal, S.; Venketasubramanian, N.; Cheng, C.-Y.; Sabanayagam, C.; Tham, Y.C.; et al. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: A retrospective, multicentre case-control study. Lancet Digit. Health 2022, 4, e806–e815. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; Debuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimer’s Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef]
- Roșca, C.-M.; Bold, R.-A.; Gerea, A.-E. A Comprehensive Patient Triage Algorithm Incorporating ChatGPT API for Symptom-Based Healthcare Decision-Making. In Emerging Trends and Technologies on Intelligent Systems: Proceedings of the ETTIS 2024, Noida, India, 27–28 March 2024; Lecture Notes in Networks and Systems; Springer Nature: Berlin/Heidelberg, Germany, 2025; Volume 1073, pp. 167–178. [Google Scholar] [CrossRef]
- Sun, M.; Yan, T.; Liu, R.; Zhao, X.; Zhou, X.; Ma, Y.; Jia, J. Predictive value of machine learning in diagnosing cognitive impairment in patients with Parkinson’s disease: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 3775–3784. [Google Scholar] [CrossRef]
- Rosca, C.-M. Comparative Analysis of Object Classification Algorithms: Traditional Image Processing Versus Artificial Intelligence-Based Approach. Rom. J. Pet. Gas Technol. 2023, 4, 169–180. [Google Scholar] [CrossRef]
- Quazi, S. Artificial intelligence and machine learning in precision and genomic medicine. Med. Oncol. 2022, 39, 120. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. Machine Learning and Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2761. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Yang, H.; Chen, D.; Qin, Y.; Han, H.; Cui, J.; Bai, W.; Ma, Y.; Zhang, R.; Yu, H. XGBoost-SHAP-based interpretable diagnostic framework for alzheimer’s disease. BMC Med. Inform. Decis. Mak. 2023, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Helaly, H.A.; Badawy, M.; Haikal, A.Y. Deep Learning Approach for Early Detection of Alzheimer’s Disease. Cogn. Comput. 2022, 14, 1711–1727. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, D.; Caramelo, F.; Moreira, A.P.; Santana, I.; Abrunhosa, A.; Castelo-Branco, M. Combined Structural MR and Diffusion Tensor Imaging Classify the Presence of Alzheimer’s Disease With the Same Performance as MR Combined With Amyloid Positron Emission Tomography: A Data Integration Approach. Front. Neurosci. 2022, 15, 638175. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Vrahatis, A.G.; Lazaros, K.; Skolariki, K.; Exarchos, T.P.; Vlamos, P. Machine Learning Analysis of Alzheimer’s Disease Single-Cell RNA-Sequencing Data across Cortex and Hippocampus Regions. Curr. Issues Mol. Biol. 2023, 45, 8652–8669. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, J.; Zhang, Y.; Liu, Y.; Shan, S.; Ye, S.; Li, B.; Fan, D.; Luo, Y. Different observation period of exercise training in amyotrophic lateral sclerosis patients: A meta-analysis. Front. Neurol. 2022, 13, 986882. [Google Scholar] [CrossRef]
- Wong, J.K.; Roselle, A.K.; Shue, T.M.; Shimshak, S.J.E.; Beaty, J.M.; Celestin, N.M.; Gao, I.; Griffin, R.P.; Cudkowicz, M.E.; Sadiq, S.A. Apolipoprotein B-100-mediated motor neuron degeneration in sporadic amyotrophic lateral sclerosis. Brain Commun. 2022, 4, fcac207. [Google Scholar] [CrossRef]
- Ingre, C.; Chen, L.; Zhan, Y.; Termorshuizen, J.; Yin, L.; Fang, F. Lipids, apolipoproteins, and prognosis of amyotrophic lateral sclerosis. Neurology 2020, 94, e1835–e1844. [Google Scholar] [CrossRef]
- Van Bergeijk, K.H.; Voors, A.A.; Wykrzykowska, J.J. Prime time for machine learning to predict clinical outcomes in valvular heart disease? Eur. J. Heart Fail. 2021, 23, 2033–2034. [Google Scholar] [CrossRef]
- Torres, J.; Malla, S.D.; Silveira, V.; Mainero, L.; Czeisler, C.; Díaz-Rossello, J.L.; Maccarrone, A.; Medoro, A.; Sanchez, P.; Blasina, F.; et al. Local clinical informatics investments are required for in silico biomarker generation across the globe: Lessons learned from a secondary analysis of the PROP trial. J. Glob. Health Rep. 2022, 6, e2022038. [Google Scholar] [CrossRef]
- Jaramillo, J.; Solano, J.M.; Aristizábal, A.; Martínez, J. Analysis of SOD1 and C9orf72 mutations in patients with amyotrophic lateral sclerosis in Antioquia, Colombia. Biomédica 2022, 42, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, M.; Lapp, H.S.; Weber, C.; Plitzko, L.; Seifert, M.; Steinacker, P.; Otto, M.; Hermann, A.; Günther, R. Comparative analysis of neurofilaments and biomarkers of muscular damage in amyotrophic lateral sclerosis. Brain Commun. 2024, 6, fcae288. [Google Scholar] [CrossRef]
- Tay, Y.T.; Chai, J.Y.; Jabin, K.B.S.; Ang, K. Staging model for amyotrophic lateral sclerosis in Singapore. Singap. Med. J. 2022, 63, 371–375. [Google Scholar] [CrossRef]
- Thakore, N.J.; Lapin, B.R.; Mitsumoto, H.; Pooled Resource Open-Access Als Clinical Trials, C. Early initiation of riluzole may improve absolute survival in amyotrophic lateral sclerosis. Muscle Nerve 2022, 66, 702–708. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Ariciu, A.V. Unlocking Customer Sentiment Insights with Azure Sentiment Analysis: A Comprehensive Review and Analysis. Rom. J. Pet. Gas Technol. 2023, 4, 173–182. [Google Scholar] [CrossRef]
- Salvi, M.; Molinari, F.; Ciccarelli, M.; Testi, R.; Taraglio, S.; Imperiale, D. Quantitative analysis of prion disease using an AI-powered digital pathology framework. Sci. Rep. 2023, 13, 17759. [Google Scholar] [CrossRef]
- Bizzi, A.; Pascuzzo, R.; Blevins, J.; Grisoli, M.; Lodi, R.; Moscatelli, M.E.M.; Castelli, G.; Cohen, M.L.; Schonberger, L.B.; Foutz, A.; et al. Evaluation of a New Criterion for Detecting Prion Disease With Diffusion Magnetic Resonance Imaging. JAMA Neurol. 2020, 77, 1141. [Google Scholar] [CrossRef]
- Bradford, B.M.; McGuire, L.I.; Hume, D.A.; Pridans, C.; Mabbott, N.A. Microglia deficiency accelerates prion disease but does not enhance prion accumulation in the brain. Glia 2022, 70, 2169–2187. [Google Scholar] [CrossRef]
- Katzeff, J.S.; Bright, F.; Phan, K.; Kril, J.J.; Ittner, L.M.; Kassiou, M.; Hodges, J.R.; Piguet, O.; Kiernan, M.C.; Halliday, G.M.; et al. Biomarker discovery and development for frontotemporal dementia and amyotrophic lateral sclerosis. Brain 2022, 145, 1598–1609. [Google Scholar] [CrossRef]
- Medina-Rioja, R.; Gonzalez-Calderon, G.; Masellis, M. Frontotemporal dementia. Can. Med. Assoc. J. 2023, 195, E1660. [Google Scholar] [CrossRef] [PubMed]
- Van Engelen, M.-P.E.; Heijst, H.; Willemse, E.A.J.; Oudega, M.L.; Vermunt, L.; Scheltens, P.; Vijverberg, E.G.B.; Pijnenburg, Y.A.L.; Teunissen, C.E. Urine as matrix for analysis of neurofilament light chain is not suitable to distinguish frontotemporal dementia from psychiatric diseases. Brain Commun. 2023, 5, fcad120. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Chintalapudi, N.; Hossain, M.A.; Losco, G.; Ruocco, C.; Sagaro, G.G.; Traini, E.; Nittari, G.; Amenta, F. Artificial Intelligence Models in the Diagnosis of Adult-Onset Dementia Disorders: A Review. Bioengineering 2022, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.A.; Senan, E.M.; Rassem, T.H.; Makbol, N.M.; Alanazi, A.A.; Al-Mekhlafi, Z.G.; Almurayziq, T.S.; Ghaleb, F.A. Multi-Method Analysis of Medical Records and MRI Images for Early Diagnosis of Dementia and Alzheimer’s Disease Based on Deep Learning and Hybrid Methods. Electronics 2021, 10, 2860. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, F.; Delgado-Alvarez, A.; Delgado-Alonso, C.; Díaz-Álvarez, J.; Pytel, V.; Valles-Salgado, M.; Gil, M.J.; Hernández-Lorenzo, L.; Matías-Guiu, J.; Ayala, J.L.; et al. Diagnosis of Alzheimer’s disease and behavioural variant frontotemporal dementia with machine learning-aided neuropsychological assessment using feature engineering and genetic algorithms. Int. J. Geriatr. Psychiatry 2022, 37, 1–13. [Google Scholar] [CrossRef]
- Rosca, C.-M. Convergence Catalysts: Exploring the Fusion of Embedded Systems, IoT, and Artificial Intelligence. In Engineering Applications of AI and Swarm Intelligence; Yang, X.-S., Ed.; Springer Nature: Singapore, 2025; pp. 69–87. [Google Scholar] [CrossRef]
- Das, S.; Panigrahi, P.; Chakrabarti, S. Corpus Callosum Atrophy in Detection of Mild and Moderate Alzheimer’s Disease Using Brain Magnetic Resonance Image Processing and Machine Learning Techniques. J. Alzheimer’s Dis. Rep. 2021, 5, 771–788. [Google Scholar] [CrossRef]
- Zadgaonkar, A.; Keskar, R.; Kakde, O. Towards a Machine Learning Model for Detection of Dementia Using Lifestyle Parameters. Appl. Sci. 2023, 13, 10630. [Google Scholar] [CrossRef]
- Miltiadous, A.; Tzimourta, K.D.; Afrantou, T.; Ioannidis, P.; Grigoriadis, N.; Tsalikakis, D.G.; Angelidis, P.; Tsipouras, M.G.; Glavas, E.; Giannakeas, N.; et al. A Dataset of Scalp EEG Recordings of Alzheimer’s Disease, Frontotemporal Dementia and Healthy Subjects from Routine EEG. Data 2023, 8, 95. [Google Scholar] [CrossRef]
- Lau, A.; Beheshti, I.; Modirrousta, M.; Kolesar, T.A.; Goertzen, A.L.; Ko, J.H. Alzheimer’s Disease-Related Metabolic Pattern in Diverse Forms of Neurodegenerative Diseases. Diagnostics 2021, 11, 2023. [Google Scholar] [CrossRef]
- Storelli, L.; Azzimonti, M.; Gueye, M.; Vizzino, C.; Preziosa, P.; Tedeschi, G.; De Stefano, N.; Pantano, P.; Filippi, M.; Rocca, M.A. A Deep Learning Approach to Predicting Disease Progression in Multiple Sclerosis Using Magnetic Resonance Imaging. Investig. Radiol. 2022, 57, 423–432. [Google Scholar] [CrossRef]
- Arrambide, G.; Comabella, M.; Tur, C. Big data and artificial intelligence applied to blood and CSF fluid biomarkers in multiple sclerosis. Front. Immunol. 2024, 15, 1459502. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Khan, I.U.; Bashamakh, A.; Alghool, F.A.; Aboulnour, M.; Alsuwayan, N.M.; Alturaif, R.A.K.; Brahimi, S.; Aljameel, S.S.; Al Ghamdi, K. Multiple Sclerosis Diagnosis Using Machine Learning and Deep Learning: Challenges and Opportunities. Sensors 2022, 22, 7856. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Goyal, M.; Johri, S.; Kumar, V.; Khaibullin, T.; Rizvanov, A.A.; Verma, S.; Khaiboullina, S.F.; Baranwal, M. Serum and Cerebrospinal Fluid Cytokine Biomarkers for Diagnosis of Multiple Sclerosis. Mediat. Inflamm. 2020, 2020, 2727042. [Google Scholar] [CrossRef]
- Saba, T.; Mujahid, M.; Rehman, A.; Alamri, F.S.; Ayesha, N. Enhancing trustworthiness and reliability: Advance explainable artificial intelligence framework for real world Sclerosis detection. Phys. Scr. 2024, 99, 105017. [Google Scholar] [CrossRef]
- Hagiwara, A.; Otsuka, Y.; Andica, C.; Kato, S.; Yokoyama, K.; Hori, M.; Fujita, S.; Kamagata, K.; Hattori, N.; Aoki, S. Differentiation between multiple sclerosis and neuromyelitis optica spectrum disorders by multiparametric quantitative MRI using convolutional neural network. J. Clin. Neurosci. 2021, 87, 55–58. [Google Scholar] [CrossRef]
- Sun, X.; Ren, X.; Zhang, J.; Nie, Y.; Hu, S.; Yang, X.; Jiang, S. Discovering miRNAs Associated With Multiple Sclerosis Based on Network Representation Learning and Deep Learning Methods. Front. Genet. 2022, 13, 899340. [Google Scholar] [CrossRef]
- Rosca, C.-M. New Algorithm to Prevent Online Test Fraud Based on Cognitive Services and Input Devices Events. In Proceedings of the Third Emerging Trends and Technologies on Intelligent Systems—ETTIS 2023. Lecture Notes in Networks and Systems, Noida, India, 23–24 February 2023; Noor, A., Saroha, K., Pricop, E., Sen, A., Trivedi, G., Eds.; Springer Nature: Singapore, 2023; Volume 730, pp. 207–219. [Google Scholar] [CrossRef]
- Wang, W.; Lee, J.; Harrou, F.; Sun, Y. Early Detection of Parkinson’s Disease Using Deep Learning and Machine Learning. IEEE Access 2020, 8, 147635–147646. [Google Scholar] [CrossRef]
- Makarious, M.B.; Leonard, H.L.; Vitale, D.; Iwaki, H.; Sargent, L.; Dadu, A.; Violich, I.; Hutchins, E.; Saffo, D.; Bandres-Ciga, S.; et al. Multi-modality machine learning predicting Parkinson’s disease. NPJ Park. Dis. 2022, 8, 35. [Google Scholar] [CrossRef]
- Gong, Z.; Lei, L. Spinocerebellar ataxia type 11 (SCA11): An update. Eur. J. Neurosci. 2023, 58, 2623–2640. [Google Scholar] [CrossRef]
- Shivakoti, M.; Medaramatla, S.C.; Godavarthi, D.; Shivakoti, N. Prognoza: Parkinson’s Disease Prediction Using Classification Algorithms. EAI Endorsed Trans. Pervasive Health Technol. 2023, 9, 1–8. [Google Scholar] [CrossRef]
- Banou, E.; Vrahatis, A.G.; Krokidis, M.G.; Vlamos, P. Machine Learning Analysis of Genomic Factors Influencing Hyperbaric Oxygen Therapy in Parkinson’s Disease. BioMedInformatics 2024, 4, 127–138. [Google Scholar] [CrossRef]
- Giannakopoulou, K.-M.; Roussaki, I.; Demestichas, K. Internet of Things Technologies and Machine Learning Methods for Parkinson’s Disease Diagnosis, Monitoring and Management: A Systematic Review. Sensors 2022, 22, 1799. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Shen, K.; Liu, K.; Ashok, A.; Ramirez-Zamora, A.; Chen, J.; Li, Y.; Fang, R. Deep learning predicts prevalent and incident Parkinson’s disease from UK Biobank fundus imaging. Sci. Rep. 2024, 14, 3637. [Google Scholar] [CrossRef] [PubMed]
- Gelvez-Almeida, E.; Váasquez-Coronel, A.; Guatelli, R.; Aubin, V.; Mora, M. Classification of Parkinson’s disease patients based on spectrogram using local binary pattern descriptors. J. Phys. Conf. Ser. 2022, 2153, 012014. [Google Scholar] [CrossRef]
- Vyas, T.; Yadav, R.; Solanki, C.; Darji, R.; Desai, S.; Tanwar, S. Deep learning-based scheme to diagnose Parkinson’s disease. Expert Syst. 2022, 39, e12739. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Y.; Wu, J.; Brendel, M.; Lu, J.; Ge, J.; Bernhardt, A.; Li, L.; Alberts, I.; Katzdobler, S.; et al. Differential diagnosis of parkinsonism based on deep metabolic imaging indices. J. Nucl. Med. 2022, 63, 1741–1747. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, P.; Wu, J.; Brendel, M.; Lu, J.; Ge, J.; Tang, C.; Hong, J.; Xu, Q.; Liu, F.; et al. Decoding the dopamine transporter imaging for the differential diagnosis of parkinsonism using deep learning. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2798–2811. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, Y.; An, M.; Xiao, W. A boosting-based prediction model for disease progression. J. Comput. Electron. Inf. Manag. 2024, 12, 15–19. [Google Scholar] [CrossRef]
- Ahn, S.; Shin, J.; Song, S.J.; Yoon, W.T.; Sagong, M.; Jeong, A.; Kim, J.H.; Yu, H.G. Neurologic Dysfunction Assessment in Parkinson Disease Based on Fundus Photographs Using Deep Learning. JAMA Ophthalmol. 2023, 141, 234. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Popescu, M.; Patrascioiu, C.; Stancu, A. Comparative Analysis of pH Level Between Pasteurized and UTH Milk Using Dedicated Developed Application. Rev. Chim. 2019, 70, 3917–3920. [Google Scholar] [CrossRef]
- Nath, S.; Caron, N.S.; May, L.; Gluscencova, O.B.; Kolesar, J.; Brady, L.; Kaufman, B.A.; Boulianne, G.L.; Rodriguez, A.R.; Tarnopolsky, M.A.; et al. Functional characterization of variants of unknown significance in a spinocerebellar ataxia patient using an unsupervised machine learning pipeline. Hum. Genome Var. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Ma, J.; Tsakadze, N.; Benes-Lima, L.; Gonzalez, J.A.; Hoffmann, M. Genetic rhabdomyolysis within the spectrum of the Spinocerebellar Ataxia type 2 responsive to pregabalin. Cerebellum Ataxias 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Flores, M.; Corral-Juan, M.; Gasch-Navalón, E.; Cirillo, D.; Sanchez, I.; Matilla-Dueñas, A. Novel genotype–phenotype correlations, differential cerebellar allele-specific methylation, and a common origin of the (ATTTC)n insertion in spinocerebellar ataxia type 37. Hum. Genet. 2024, 143, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Ru, D.; Li, J.; Xie, O.; Peng, L.; Jiang, H.; Qiu, R. Explainable artificial intelligence based on feature optimization for age at onset prediction of spinocerebellar ataxia type 3. Front. Neuroinform. 2022, 16, 978630. [Google Scholar] [CrossRef]
- Ngo, T.; Nguyen, D.C.; Pathirana, P.N.; Corben, L.A.; Delatycki, M.B.; Horne, M.; Szmulewicz, D.J.; Roberts, M. Federated Deep Learning for the Diagnosis of Cerebellar Ataxia: Privacy Preservation and Auto-Crafted Feature Extractor. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 803–811. [Google Scholar] [CrossRef]
- Bilek, F.; Balgetir, F.; Demir, C.F.; Alkan, G.; Arslan-Tuncer, S. Quantitative Assessment of Ataxia in Multiple Sclerosis Patients using Spatiotemporal Parameters: A Relief-Based Machine Learning Analysis. Phys. Med. Rehabil. Kurortmed. 2021, 31, 367–376. [Google Scholar] [CrossRef]
- Ganesh, S.; Chithambaram, T.; Krishnan, N.R.; Vincent, D.R.; Kaliappan, J.; Srinivasan, K. Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review. Diagnostics 2023, 13, 3592. [Google Scholar] [CrossRef]
- Joseph, A.; Chandra, J. Machine Learning Approaches for Efficient Analysis of Neuroimaging Techniques. In Proceedings of the 4th ETLTC International Conference on ICT Integration in Technical Education, Aizuwakamatsu, Japan, 25–28 January 2022; p. 03027. [Google Scholar] [CrossRef]
- Riad, R.; Lunven, M.; Titeux, H.; Cao, X.-N.; Hamet Bagnou, J.; Lemoine, L.; Montillot, J.; Sliwinski, A.; Youssov, K.; Cleret De Langavant, L.; et al. Predicting clinical scores in Huntington’s disease: A lightweight speech test. J. Neurol. 2022, 269, 5008–5021. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, H.-P.; Lin, W.-Y.; Tsai, F.-J. Identification of contributing genes of Huntington’s disease by machine learning. BMC Med. Genom. 2020, 13, 176. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Rădulescu, G.; Stancu, A. Artificial Intelligence of Things Infrastructure for Quality Control in Cast Manufacturing Environments Shedding Light on Industry Changes. Appl. Sci. 2025, 15, 2068. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A.; Popescu, M. The Impact of Cloud Versus Local Infrastructure on Automatic IoT-Driven Hydroponic Systems. Appl. Sci. 2025, 15, 4016. [Google Scholar] [CrossRef]
- Horigome, T.; Hino, K.; Toyoshiba, H.; Shindo, N.; Funaki, K.; Eguchi, Y.; Kitazawa, M.; Fujita, T.; Mimura, M.; Kishimoto, T. Identifying neurocognitive disorder using vector representation of free conversation. Sci. Rep. 2022, 12, 12461. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zou, K.; Deng, Z.A.; Zhou, J.; Dang, X.; Zhu, S.; Liu, L.; Fang, C. A Study of Dementia Prediction Models Based on Machine Learning with Survey Data of Community-Dwelling Elderly People in China. J. Alzheimer’s Dis. 2022, 89, 669–679. [Google Scholar] [CrossRef]
- Shehzad, A.; Rockwood, K.; Stanley, J.; Dunn, T.; Howlett, S.E. Use of Patient-Reported Symptoms from an Online Symptom Tracking Tool for Dementia Severity Staging: Development and Validation of a Machine Learning Approach. J. Med. Internet Res. 2020, 22, e20840. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.-R.; Wang, H.-F.; Yang, M.; Feng, J.-F.; Yu, J.-T.; Cheng, W. Development of a novel dementia risk prediction model in the general population: A large, longitudinal, population-based machine-learning study. eClinicalMedicine 2022, 53, 101665. [Google Scholar] [CrossRef]
- Twait, E.L.; Andaur Navarro, C.L.; Gudnason, V.; Hu, Y.-H.; Launer, L.J.; Geerlings, M.I. Dementia prediction in the general population using clinically accessible variables: A proof-of-concept study using machine learning. The AGES-Reykjavik study. BMC Med. Inform. Decis. Mak. 2023, 23, 168. [Google Scholar] [CrossRef]
- Rennie, A.; Ekman, U.; Shams, S.; Rydén, L.; Samuelsson, J.; Zettergren, A.; Kern, S.; Oppedal, K.; Blanc, F.; Hort, J.; et al. Cerebrovascular and Alzheimer’s disease biomarkers in dementia with Lewy bodies and other dementias. Brain Commun. 2024, 6, fcae290. [Google Scholar] [CrossRef]
- Hijazi, Z.; Yassi, N.; O’Brien, J.T.; Watson, R. The influence of cerebrovascular disease in dementia with Lewy bodies and Parkinson’s disease dementia. Eur. J. Neurol. 2022, 29, 1254–1265. [Google Scholar] [CrossRef]
- Bit, S.; Dey, P.; Maji, A.; Khan, T.K. MRI-based mild cognitive impairment and Alzheimer’s disease classification using an algorithm of combination of variational autoencoder and other machine learning classifiers. J. Alzheimer’s Dis. Rep. 2024, 8, 1434–1452. [Google Scholar] [CrossRef]
- Sarma, M.; Chatterjee, S. Machine Learning-Based Alzheimer’s Disease Stage Diagnosis Utilizing Blood Gene Expression and Clinical Data: A Comparative Investigation. Diagnostics 2025, 15, 211. [Google Scholar] [CrossRef]
- Abbas, Q.; Hussain, A.; Baig, A.R. CAD-ALZ: A Blockwise Fine-Tuning Strategy on Convolutional Model and Random Forest Classifier for Recognition of Multistage Alzheimer’s Disease. Diagnostics 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Q.; Li, H.; Chen, M. Enhancing Feature Selection for Imbalanced Alzheimer’s Disease Brain MRI Images by Random Forest. Appl. Sci. 2023, 13, 7253. [Google Scholar] [CrossRef]
- Safi, K.; Aly, W.H.F.; Alakkoumi, M.; Kanj, H.; Ghedira, M.; Hutin, E. EMD-Based Method for Supervised Classification of Parkinson’s Disease Patients Using Balance Control Data. Bioengineering 2022, 9, 283. [Google Scholar] [CrossRef]
- Gupta, C.; Chandrashekar, P.; Jin, T.; He, C.; Khullar, S.; Chang, Q.; Wang, D. Bringing machine learning to research on intellectual and developmental disabilities: Taking inspiration from neurological diseases. J. Neurodev. Disord. 2022, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Jiang, Z.-Q.; Liu, D.; Wu, L.-L. Application and research progress of machine learning in the diagnosis and treatment of neurodevelopmental disorders in children. Front. Psychiatry 2022, 13, 960672. [Google Scholar] [CrossRef]
- Mohammadi-Ghazi, R.; Nguyen, H.; Mishra, R.K.; Enriquez, A.; Najafi, B.; Stephen, C.D.; Gupta, A.S.; Schmahmann, J.D.; Vaziri, A. Objective Assessment of Upper-Extremity Motor Functions in Spinocerebellar Ataxia Using Wearable Sensors. Sensors 2022, 22, 7993. [Google Scholar] [CrossRef]
- Yang, J.H.; Park, J.H.; Jang, S.-H.; Cho, J. Novel Method of Classification in Knee Osteoarthritis: Machine Learning Application Versus Logistic Regression Model. Ann. Rehabil. Med. 2020, 44, 415–427. [Google Scholar] [CrossRef]
- Ceyhan, B.; Bek, S.; Onal-Suzek, T. Machine Learning-Based Prediction Models for Cognitive Decline Progression: A Comparative Study in Multilingual Settings Using Speech Analysis. J. Aging Res. Amp; Lifestyle 2024, 13, 43–50. [Google Scholar] [CrossRef]
- Ma, Y.; Bland, J.K.S.; Fujinami, T. Classification of Alzheimer’s Disease and Frontotemporal Dementia Using Electroencephalography to Quantify Communication between Electrode Pairs. Diagnostics 2024, 14, 2189. [Google Scholar] [CrossRef]
- Sharma, V.; Midhunchakkaravarthy, D. Local post-hoc interpretable machine learning model for prediction of dementia in young adults. Indones. J. Electr. Eng. Comput. Sci. 2023, 32, 1569. [Google Scholar] [CrossRef]
- De La Fuente Garcia, S.; Ritchie, C.W.; Luz, S. Artificial Intelligence, Speech, and Language Processing Approaches to Monitoring Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 78, 1547–1574. [Google Scholar] [CrossRef] [PubMed]
- Gunata, M.; Arslan, A.K.; Çolak, C.; Parlakpinar, H. Estimation of Risk Factors Related to Heart Diseases with Multilayer Perceptron Model. Med. Rec. 2022, 4, 171–178. [Google Scholar] [CrossRef]
- Erdaş, Ç.B.; Sümer, E. CNN-Based Neurodegenerative Disease Classification Using QR-Represented Gait Data. Brain Behav. 2024, 14, e70100. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Georgieva, M.; Desai, U.; Kirson, N.; Lane, H.; Cheung, H.C.; Westermeyer, B.; Biglan, K. Disease Progression and Longitudinal Clinical Outcomes of Lewy Body Dementia in the NACC Database. Neurol. Ther. 2023, 12, 177–195. [Google Scholar] [CrossRef]
- Saini, D.; Mukherjee, A.; Roy, A.; Biswas, A. A Comparative Study of the Behavioral Profile of the Behavioral Variant of Frontotemporal Dementia and Parkinson’s Disease Dementia. Dement. Geriatr. Cogn. Disord. Extra 2020, 10, 182–194. [Google Scholar] [CrossRef]
- Thanoun, M.Y.; Yaseen, M.T.; Aleesa, A.M. Development of Intelligent Parkinson Disease Detection System Based on Machine Learning Techniques Using Speech Signal. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 388–392. [Google Scholar] [CrossRef]
- Ullah, M.A.; Afrin, S.H.; Nazib, K.M.; Roy, R.; Ali, L.E. Unravelling Parkinson’s Disease Prediction: An Evaluation of Feature Selection Techniques with a Focus on PCA and KNN Performance. Rev. Comput. Eng. Stud. 2023, 10, 20–27. [Google Scholar] [CrossRef]
- Merkin, A.; Krishnamurthi, R.; Medvedev, O.N. Machine learning, artificial intelligence and the prediction of dementia. Curr. Opin. Psychiatry 2022, 35, 123–129. [Google Scholar] [CrossRef]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Kim, Y.K.; Park, S. Bioinformatics and Deep Learning Approach to Discover Food-Derived Active Ingredients for Alzheimer’s Disease Therapy. Foods 2025, 14, 127. [Google Scholar] [CrossRef]
- Woods, C.; Xing, X.; Khanal, S.; Lin, A.-L. Machine Learning-Driven Prediction of Brain Age for Alzheimer’s Risk: APOE4 Genotype and Gender Effects. Bioengineering 2024, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Sarica, A.; Pelagi, A.; Aracri, F.; Arcuri, F.; Quattrone, A.; Quattrone, A. Sex Differences in Conversion Risk from Mild Cognitive Impairment to Alzheimer’s Disease: An Explainable Machine Learning Study with Random Survival Forests and SHAP. Brain Sci. 2024, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, N.; Alam, S.; Aqeel, I.; Shuaib, M.; Mohsen Khormi, I.; Khan, S.B.; Malibari, A.A. Deep Belief Networks (DBN) with IoT-Based Alzheimer’s Disease Detection and Classification. Appl. Sci. 2023, 13, 7833. [Google Scholar] [CrossRef]
- Shukla, A.; Tiwari, R.; Tiwari, S. Alzheimer’s Disease Detection from Fused PET and MRI Modalities Using an Ensemble Classifier. Mach. Learn. Knowl. Extr. 2023, 5, 512–538. [Google Scholar] [CrossRef]
- Shin, H.-J.; Yoon, H.; Kim, S.; Kang, D.-Y. Classification of Alzheimer’s Disease Using Dual-Phase 18F-Florbetaben Image with Rank-Based Feature Selection and Machine Learning. Appl. Sci. 2022, 12, 7355. [Google Scholar] [CrossRef]
- Bangyal, W.H.; Rehman, N.U.; Nawaz, A.; Nisar, K.; Ibrahim, A.A.A.; Shakir, R.; Rawat, D.B. Constructing Domain Ontology for Alzheimer Disease Using Deep Learning Based Approach. Electronics 2022, 11, 1890. [Google Scholar] [CrossRef]
- Hooshmand, K.; Halliday, G.M.; Pineda, S.S.; Sutherland, G.T.; Guennewig, B. Overlap between Central and Peripheral Transcriptomes in Parkinson’s Disease but Not Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 5200. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Lin, C.-H.; Tsai, T.-H.; Huang, C.-H.; Li, J.-L.; Chen, L.-K.; Li, C.-H.; Tsai, T.-F.; Wang, P.-N. Discovery of a Metabolic Signature Predisposing High Risk Patients with Mild Cognitive Impairment to Converting to Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10903. [Google Scholar] [CrossRef]
- Song, M.; Jung, H.; Lee, S.; Kim, D.; Ahn, M. Diagnostic Classification and Biomarker Identification of Alzheimer’s Disease with Random Forest Algorithm. Brain Sci. 2021, 11, 453. [Google Scholar] [CrossRef]
- Nguyen, M.T.P.; Tran, M.K.P.; Nakano, T.; Tran, T.H.; Nguyen, Q.D.N. An Approach for Detecting Parkinson’s Disease by Integrating Optimal Feature Selection Strategies with Dense Multiscale Sample Entropy. Information 2024, 16, 1. [Google Scholar] [CrossRef]
- Khedimi, M.; Zhang, T.; Merzougui, H.; Zhao, X.; Geng, Y.; Djaroudib, K.; Lorenz, P. Architecture-Aware Augmentation: A Hybrid Deep Learning and Machine Learning Approach for Enhanced Parkinson’s Disease Detection. Bioengineering 2024, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Majda-Zdancewicz, E.; Potulska-Chromik, A.; Nojszewska, M.; Kostera-Pruszczyk, A. Speech Signal Analysis in Patients with Parkinson’s Disease, Taking into Account Phonation, Articulation, and Prosody of Speech. Appl. Sci. 2024, 14, 11085. [Google Scholar] [CrossRef]
- Hussain, S.S.; Degang, X.; Shah, P.M.; Islam, S.U.; Alam, M.; Khan, I.A.; Awwad, F.A.; Ismail, E.A.A. Classification of Parkinson’s Disease in Patch-Based MRI of Substantia Nigra. Diagnostics 2023, 13, 2827. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, R.; Kumar, M.; Arya, S.; Mendirtta, N.; Kumar, S.; Towfek, S.K.; Khafaga, D.S.; Alkahtani, H.K.; Abdelhamid, A.A. A Novel Artificial-Intelligence-Based Approach for Classification of Parkinson’s Disease Using Complex and Large Vocal Features. Biomimetics 2023, 8, 351. [Google Scholar] [CrossRef]
- Li, K.; Tian, Y.; Chen, H.; Ma, X.; Li, S.; Li, C.; Wu, S.; Liu, F.; Du, Y.; Su, W. Temporal Dynamic Alterations of Regional Homogeneity in Parkinson’s Disease: A Resting-State fMRI Study. Biomolecules 2023, 13, 888. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.-J.; Yoon, Y. RNA Sequences-Based Diagnosis of Parkinson’s Disease Using Various Feature Selection Methods and Machine Learning. Appl. Sci. 2023, 13, 2698. [Google Scholar] [CrossRef]
- Elshewey, A.M.; Shams, M.Y.; El-Rashidy, N.; Elhady, A.M.; Shohieb, S.M.; Tarek, Z. Bayesian Optimization with Support Vector Machine Model for Parkinson Disease Classification. Sensors 2023, 23, 2085. [Google Scholar] [CrossRef]
- Castelli Gattinara Di Zubiena, F.; Menna, G.; Mileti, I.; Zampogna, A.; Asci, F.; Paoloni, M.; Suppa, A.; Del Prete, Z.; Palermo, E. Machine Learning and Wearable Sensors for the Early Detection of Balance Disorders in Parkinson’s Disease. Sensors 2022, 22, 9903. [Google Scholar] [CrossRef]
- Li, A.; Li, C. Detecting Parkinson’s Disease through Gait Measures Using Machine Learning. Diagnostics 2022, 12, 2404. [Google Scholar] [CrossRef]
- Chen, P.-H.; Hou, T.-Y.; Cheng, F.-Y.; Shaw, J.-S. Prediction of Cognitive Degeneration in Parkinson’s Disease Patients Using a Machine Learning Method. Brain Sci. 2022, 12, 1048. [Google Scholar] [CrossRef]
- Mian, T.S. An Unsupervised Neural Network Feature Selection and 1D Convolution Neural Network Classification for Screening of Parkinsonism. Diagnostics 2022, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Rosca, C.-M.; Stancu, A.; Iovanovici, E.M. The New Paradigm of Deepfake Detection at the Text Level. Appl. Sci. 2025, 15, 2560. [Google Scholar] [CrossRef]
- Demir, F.; Siddique, K.; Alswaitti, M.; Demir, K.; Sengur, A. A Simple and Effective Approach Based on a Multi-Level Feature Selection for Automated Parkinson’s Disease Detection. J. Pers. Med. 2022, 12, 55. [Google Scholar] [CrossRef]
- Qasim, H.M.; Ata, O.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Almehmadi, M. Hybrid Feature Selection Framework for the Parkinson Imbalanced Dataset Prediction Problem. Medicina 2021, 57, 1217. [Google Scholar] [CrossRef]
- Arora, S.; Tsanas, A. Assessing Parkinson’s Disease at Scale Using Telephone-Recorded Speech: Insights from the Parkinson’s Voice Initiative. Diagnostics 2021, 11, 1892. [Google Scholar] [CrossRef]
- Skaramagkas, V.; Andrikopoulos, G.; Kefalopoulou, Z.; Polychronopoulos, P. A Study on the Essential and Parkinson’s Arm Tremor Classification. Signals 2021, 2, 201–224. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Hsu, S.-W.; Lee, T.-L.; Sung, P.-S.; Lin, C.-C. Using Artificial Neural Network to Discriminate Parkinson’s Disease from Other Parkinsonisms by Focusing on Putamen of Dopamine Transporter SPECT Images. Biomedicines 2020, 9, 12. [Google Scholar] [CrossRef]
- Aich, S.; Youn, J.; Chakraborty, S.; Pradhan, P.M.; Park, J.-H.; Park, S.; Park, J. A Supervised Machine Learning Approach to Detect the On/Off State in Parkinson’s Disease Using Wearable Based Gait Signals. Diagnostics 2020, 10, 421. [Google Scholar] [CrossRef]
- Borzì, L.; Fornara, S.; Amato, F.; Olmo, G.; Artusi, C.A.; Lopiano, L. Smartphone-Based Evaluation of Postural Stability in Parkinson’s Disease Patients During Quiet Stance. Electronics 2020, 9, 919. [Google Scholar] [CrossRef]
- Blanck-Lubarsch, M.; Dirksen, D.; Feldmann, R.; Bormann, E.; Hohoff, A. Simplifying Diagnosis of Fetal Alcohol Syndrome Using Machine Learning Methods. Front. Pediatr. 2022, 9, 707566. [Google Scholar] [CrossRef]
- Tur, C. Machine and deep learning in MS research are just powerful statistics—Yes. Mult. Scler. J. 2021, 27, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Munger, E.; Hickey, J.W.; Dey, A.K.; Jafri, M.S.; Kinser, J.M.; Mehta, N.N. Application of machine learning in understanding atherosclerosis: Emerging insights. APL Bioeng. 2021, 5, 011505. [Google Scholar] [CrossRef] [PubMed]
- Ekmekyapar, T.; Taşcı, B. Exemplar MobileNetV2-Based Artificial Intelligence for Robust and Accurate Diagnosis of Multiple Sclerosis. Diagnostics 2023, 13, 3030. [Google Scholar] [CrossRef] [PubMed]
- Norris, C. Annals of Biomedical Engineering 2022 Year in Review. Ann. Biomed. Eng. 2023, 51, 865–867. [Google Scholar] [CrossRef]
- Pontillo, G.; Penna, S.; Cocozza, S.; Quarantelli, M.; Gravina, M.; Lanzillo, R.; Marrone, S.; Costabile, T.; Inglese, M.; Morra, V.B.; et al. Stratification of multiple sclerosis patients using unsupervised machine learning: A single-visit MRI-driven approach. Eur. Radiol. 2022, 32, 5382–5391. [Google Scholar] [CrossRef]
- Karim, F.K.; Ghorashi, S.; Ishak, A.B.; Elhag, A.A.; Mohamed, N. Innovative Mathematical Modelling Approaches to Diagnose Chronic Neurological Disorders with Deep Learning. Therm. Sci. 2024, 28, 5217–5229. [Google Scholar] [CrossRef]
- Afifi, N.; Abdel-Hamid, A.T.; Abdullah, B.A. Detection of Multiple Sclerosis Using Convolutional Neural Networks: A Comparative Study. In Proceedings of the 10th International Conference on Soft Computing & Machine Intelligence, Mexico City, Mexico, 25–26 November 2023; pp. 77–80. [Google Scholar] [CrossRef]
- Omrani, M.; Chiarelli, R.R.; Acquaviva, M.; Bassani, C.; Dalla Costa, G.; Montini, F.; Preziosa, P.; Pagani, L.; Grassivaro, F.; Guerrieri, S.; et al. Machine learning-driven diagnosis of multiple sclerosis from whole blood transcriptomics. Brain Behav. Immun. 2024, 121, 269–277. [Google Scholar] [CrossRef]
- Ata, N.; Zahoor, I.; Hoda, N.; Adnan, S.M.; Vijayakumar, S.; Louis, F.; Poisson, L.; Rattan, R.; Kumar, N.; Cerghet, M.; et al. Artificial neural network-based prediction of multiple sclerosis using blood-based metabolomics data. Mult. Scler. Relat. Disord. 2024, 92, 105942. [Google Scholar] [CrossRef]
- Kaur, R.; Levy, J.; Motl, R.W.; Sowers, R.; Hernandez, M.E. Deep Learning for Multiple Sclerosis Differentiation Using Multi-Stride Dynamics in Gait. IEEE Trans. Biomed. Eng. 2023, 70, 2181–2192. [Google Scholar] [CrossRef]
- Banijamali, S.M.A.; Versek, C.; Babinski, K.; Kamarthi, S.; Green-Laroche, D.; Sridhar, S. Portable multi-focal visual evoked potential diagnostics for multiple sclerosis/optic neuritis patients. Doc. Ophthalmol. 2024, 149, 23–45. [Google Scholar] [CrossRef]
- Maggi, P.; Fartaria, M.J.; Jorge, J.; La Rosa, F.; Absinta, M.; Sati, P.; Meuli, R.; Du Pasquier, R.; Reich, D.S.; Cuadra, M.B.; et al. CVSnet: A machine learning approach for automated central vein sign assessment in multiple sclerosis. NMR Biomed. 2020, 33, e4283. [Google Scholar] [CrossRef] [PubMed]
- Spieker, E.L.; Dvorani, A.; Salchow-Hömmen, C.; Otto, C.; Ruprecht, K.; Wenger, N.; Schauer, T. Targeting Transcutaneous Spinal Cord Stimulation Using a Supervised Machine Learning Approach Based on Mechanomyography. Sensors 2024, 24, 634. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Bellomo, G.; Di Sabatino, E.; Sperandei, S.; Mancini, A.; Blennow, K.; Zetterberg, H.; Parnetti, L.; Di Filippo, M. The Immune Signature of CSF in Multiple Sclerosis with and without Oligoclonal Bands: A Machine Learning Approach to Proximity Extension Assay Analysis. Int. J. Mol. Sci. 2023, 25, 139. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, M.; Abedalaziz, W.; Alawad, N.A.; Gharaibeh, H.; Nasayreh, A.; El-Heis, M.; Altalhi, M.; Forestiero, A.; Abualigah, L. Optimal Integration of Machine Learning for Distinct Classification and Activity State Determination in Multiple Sclerosis and Neuromyelitis Optica. Technologies 2023, 11, 131. [Google Scholar] [CrossRef]
- Ponce De Leon-Sanchez, E.R.; Dominguez-Ramirez, O.A.; Herrera-Navarro, A.M.; Rodriguez-Resendiz, J.; Paredes-Orta, C.; Mendiola-Santibañez, J.D. A Deep Learning Approach for Predicting Multiple Sclerosis. Micromachines 2023, 14, 749. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).