Dietary Ethanolamine Plasmalogen from Ascidian Alleviates Chronic Hepatic Injury in Mice Treated with Continuous Acetaminophen
Abstract
1. Introduction
2. Materials and Methods
2.1. EtnGpl Preparation
2.2. Animals
2.3. Blood and Liver Preparation
2.4. Analysis of Liver Injury Parameters
2.5. Apoptosis Array Assay
2.6. Statistical Analysis
3. Results
3.1. Fatty Chain Composition in Experimental Diets
3.2. Parameters of Hepatic Injury and Oxidative Stress in Mice Fed APAP and EtnGpl
3.3. Hepatic Fatty Acid Contents in Mice Fed APAP and EtnGpl
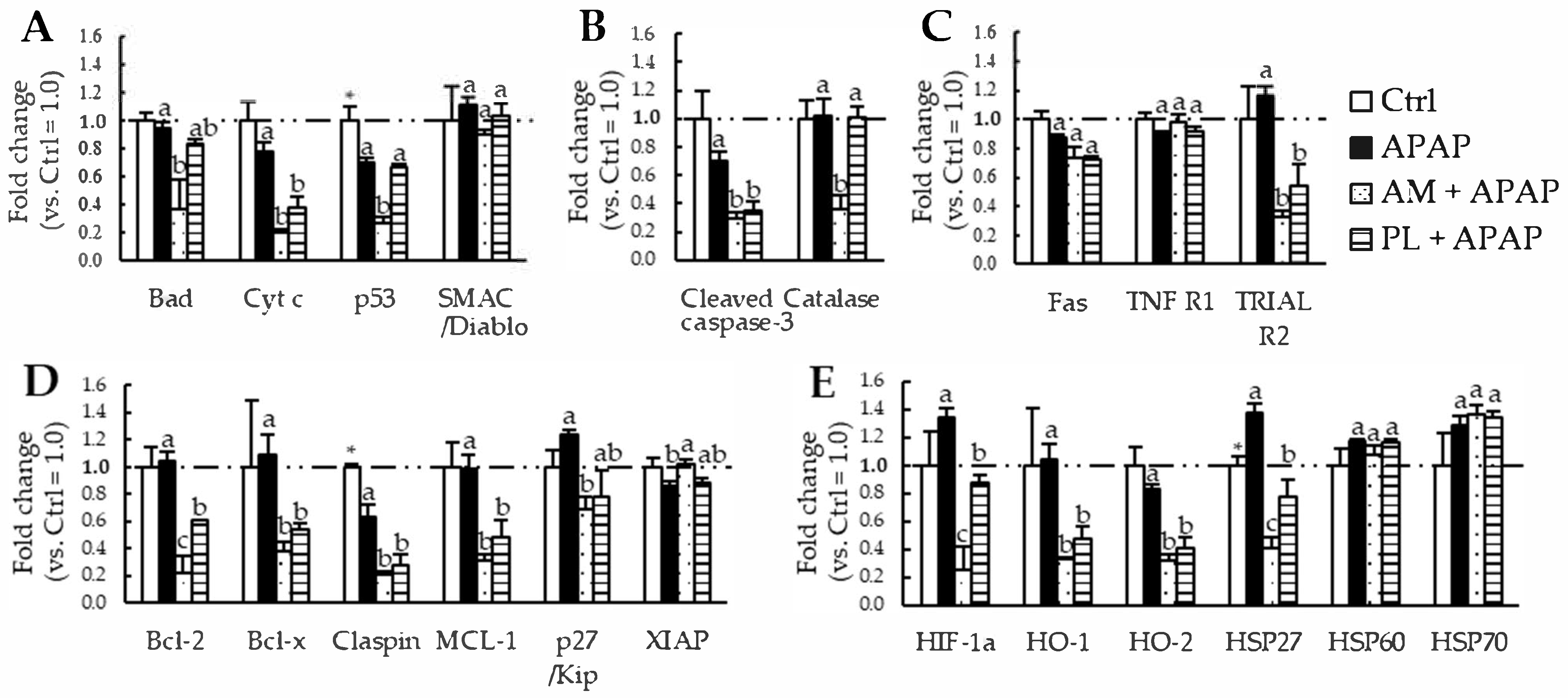
3.4. Expression of Apoptosis-Related Proteins in the Livers of Mice Fed APAP and EtnGpl
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the Levels of Amyloid-beta, Phospholipid Hydroperoxide, and Plasmalogen in the Blood of Patients with Alzheimer’s Disease: Possible Interactions between Amyloid-beta and These Lipids. J. Alzheimer’s Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef]
- Jang, J.E.; Park, H.S.; Yoo, H.J.; Baek, I.J.; Yoon, J.E.; Ko, M.S.; Kim, A.R.; Kim, H.S.; Park, H.S.; Lee, S.E.; et al. Protective role of endogenous plasmalogens against hepatic steatosis and steatohepatitis in mice. Hepatology 2017, 66, 416–431. [Google Scholar] [CrossRef]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and Detection of Plasmalogen in Marine Invertebrates by High-Performance Liquid Chromatography with Evaporative Light-Scattering Detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef]
- Hara, H.; Wakisaka, T.; Aoyama, Y. Lymphatic absorption of plasmalogen in rats. Br. J. Nutr. 2003, 90, 29–32. [Google Scholar] [CrossRef]
- Nishimukai, M.; Wakisaka, T.; Hara, H. Ingestion of plasmalogen markedly increased plasmalogen levels of blood plasma in rats. Lipids 2003, 38, 1227–1235. [Google Scholar] [CrossRef]
- Nishimukai, M.; Yamashita, M.; Watanabe, Y.; Yamazaki, Y.; Nezu, T.; Maeba, R.; Hara, H. Lymphatic absorption of choline plasmalogen is much higher than that of ethanolamine plasmalogen in rats. Eur. J. Nutr. 2011, 50, 427–436. [Google Scholar] [CrossRef]
- Nguma, E.; Yamashita, S.; Kumagai, K.; Otoki, Y.; Yamamoto, A.; Eitsuka, T.; Nakagawa, K.; Miyazawa, T.; Kinoshita, M. Ethanolamine Plasmalogen Suppresses Apoptosis in Human Intestinal Tract Cells in Vitro by Attenuating Induced Inflammatory Stress. ACS Omega 2021, 6, 3140–3148. [Google Scholar] [CrossRef]
- Nguma, E.; Yamashita, S.; Han, K.H.; Otoki, Y.; Yamamoto, A.; Nakagawa, K.; Fukushima, M.; Miyazawa, T.; Kinoshita, M. Dietary Ethanolamine Plasmalogen Alleviates DSS-Induced Colitis by Enhancing Colon Mucosa Integrity, Antioxidative Stress, and Anti-inflammatory Responses via Increased Ethanolamine Plasmalogen Molecular Species: Protective Role of Vinyl Ether Linkages. J. Agric. Food Chem. 2021, 69, 13034–13044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, C.; Zhang, T.; Li, X.; Li, P.; Lu, M.; Ye, Z.; Du, Y.; Du, R.; Zhang, W.; et al. Early-Life Gut Microbiota Governs Susceptibility to Colitis via Microbial-Derived Ether Lipids. Research 2023, 6, 0037. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Darwish, W.S.; Terada, K.; Chiba, H.; Hui, S.P. Choline and Ethanolamine Plasmalogens Prevent Lead-Induced Cytotoxicity and Lipid Oxidation in HepG2 Cells. J. Agric. Food Chem. 2019, 67, 7716–7725. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.-Y.; Shi, H.-H.; Wang, C.-C.; Jiang, X.-M.; Xue, C.-H.; Yanagita, T.; Zhang, T.-T.; Wang, Y.-M. Eicosapentaenoic Acid-Enriched Phosphoethanolamine Plasmalogens Alleviated Atherosclerosis by Remodeling Gut Microbiota to Regulate Bile Acid Metabolism in LDLR–/– Mice. J. Agric. Food Chem. 2020, 68, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Lipidomics Approach in High-Fat-Diet-Induced Atherosclerosis Dyslipidemia Hamsters: Alleviation Using Ether-Phospholipids in Sea Urchin. J. Agric. Food Chem. 2021, 69, 9167–9177. [Google Scholar] [CrossRef]
- Tolman, K.G. Defining patient risks from expanded preventive therapies. Am. J. Cardiol. 2000, 85, 15E–19E. [Google Scholar] [CrossRef]
- Lancaster, E.M.; Hiatt, J.R.; Zarrinpar, A. Acetaminophen hepatotoxicity: An updated review. Arch. Toxicol. 2015, 89, 193–199. [Google Scholar] [CrossRef]
- Dahlin, D.C.; Miwa, G.T.; Lu, A.Y.; Nelson, S.D. N-acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA 1984, 81, 1327–1331. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Lorincz, T.; Jemnitz, K.; Kardon, T.; Mandl, J.; Szarka, A. Ferroptosis is Involved in Acetaminophen Induced Cell Death. Pathol. Oncol. Res. 2015, 21, 1115–1121. [Google Scholar] [CrossRef]
- Han, K.H.; Fukushima, M.; Ohba, K.; Shimada, K.; Sekikawa, M.; Chiji, H.; Lee, C.H.; Nakano, M. Hepatoprotective effects of the water extract from adzuki bean hulls on acetaminophen-induced damage in rat liver. J. Nutr. Sci. Vitaminol. 2004, 50, 380–383. [Google Scholar] [CrossRef]
- Han, K.H.; Ohba, K.; Lee, C.H.; Shimada, K.; Sekikawa, M.; Fukushima, M. Lipid metabolism in rats fed acetaminophen with coadministration of adzuki bean extract. Food Sci. Biotechnol. 2007, 16, 584–589. [Google Scholar]
- Yamashita, S.; Honjo, A.; Aruga, M.; Nakagawa, K.; Miyazawa, T. Preparation of marine plasmalogen and selective identification of molecular species by LC-MS/MS. J Oleo Sci. 2014, 63, 423–430. [Google Scholar] [CrossRef]
- Maulik, N.; Bagchi, D.; Jones, R.; Cordis, G.; Das, D.K. Identification and characterization of plasmalogen fatty acids in swine heart. J. Pharm. Biomed. Anal. 1993, 11, 1151–1156. [Google Scholar] [CrossRef]
- Jackson, D.R.; Cassilly, C.D.; Plichta, D.R.; Vlamakis, H.; Liu, H.; Melville, S.B.; Xavier, R.J.; Clardy, J. Plasmalogen Biosynthesis by Anaerobic Bacteria: Identification of a Two-Gene Operon Responsible for Plasmalogen Production in Clostridium perfringens. ACS Chem. Biol. 2020, 16, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, D.L.; Webb, S.K.; Schmelz, E.-M.; Merrill, A.H. Dietary Sphingomyelin Inhibits 1,2-Dimethylhydrazine–Induced Colon Cancer in CF1 Mice. J. Nutr. 1994, 124, 615–620. [Google Scholar] [CrossRef] [PubMed]
- de Meijer, V.E.; Kalish, B.T.; Meisel, J.A.; Le, H.D.; Puder, M. Dietary fish oil aggravates paracetamol-induced liver injury in mice. JPEN J. Parenter. Enteral. Nutr. 2013, 37, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Xue, C.; Wang, J.; Wang, Y.; Xu, J.; Li, Z. The exogenous natural phospholipids, EPA-PC and EPA-PE, contribute to ameliorate inflammation and promote macrophage polarization. Food Funct. 2020, 11, 6542–6551. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Xue, C.; Wang, J.; Wang, Y.; Xu, J.; Li, Z. Exogenous natural EPA-enriched phosphatidylcholine and phosphatidylethanolamine ameliorate lipid accumulation and insulin resistance via activation of PPARα/γ in mice. Food Funct. 2020, 11, 8248–8258. [Google Scholar] [CrossRef]
- Anari, M.; Montgomery, M.K. Phospholipid metabolism in the liver—Implications for phosphatidylserine in non-alcoholic fatty liver disease. Biochem. Pharmacol. 2023, 213, 115621. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tanabe, Y.; Fujii, Y.; Kikuta, T.; Shibata, H.; Shido, O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 2005, 135, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, A.S.; Schebb, N.H.; Steinhilber, D. Formation of lipoxins and resolvins in human leukocytes. Prostaglandins Other Lipid Mediat. 2023, 166, 106726. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Smyl, C.; Dogan, I.; Rothe, M.; Weylandt, K.-H. Quantitative Profiling of Hydroxy Lipid Metabolites in Mouse Organs Reveals Distinct Lipidomic Profiles and Modifications Due to Elevated n-3 Fatty Acid Levels. Biology 2017, 6, 9. [Google Scholar] [CrossRef]
- Nilsson, A.; Landin, B.; Jensen, E.; Akesson, B. Absorption and lymphatic transport of exogenous and endogenous arachidonic and linoleic acid in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1987, 252, G817–G824. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef]
- Dorninger, F.; Brodde, A.; Braverman, N.E.; Moser, A.B.; Just, W.W.; Forss-Petter, S.; Brügger, B.; Berger, J. Homeostasis of phospholipids—The level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 117–128. [Google Scholar] [CrossRef]
- Werner, E.R.; Swinkels, D.; Juric, V.; Dorninger, F.; Baes, M.; Keller, M.A.; Berger, J.; Watschinger, K. Normal plasmalogen levels are maintained in tissues from mice with hepatocyte-specific deletion in peroxin 5. Brain Res. Bull. 2023, 193, 158–165. [Google Scholar] [CrossRef]
- Fu, S.-S.; Wen, M.; Zhao, Y.-C.; Shi, H.-H.; Wang, Y.-M.; Xue, C.-H.; Wei, Z.-H.; Zhang, T.-T. Short-term supplementation of EPA-enriched ethanolamine plasmalogen increases the level of DHA in the brain and liver of n-3 PUFA deficient mice in early life after weaning. Food Funct. 2022, 13, 1906–1920. [Google Scholar] [CrossRef]
- Xanthoudakis, S.; Nicholson, D.W. Heat-shock proteins as death determinants. Nat. Cell Biol. 2000, 2, E163–E165. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Mnich, K.; Samali, A. Heat shock preconditioning protects against ER stress-induced apoptosis through the regulation of the BH3-only protein BIM. FEBS Open Bio 2014, 4, 813–821. [Google Scholar] [CrossRef]
- Kennedy, D.; Mnich, K.; Oommen, D.; Chakravarthy, R.; Almeida-Souza, L.; Krols, M.; Saveljeva, S.; Doyle, K.; Gupta, S.; Timmerman, V.; et al. HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2017, 8, e3026. [Google Scholar] [CrossRef]
- Eraky, S.M.; Abo El-Magd, N.F. Omega-3 fatty acids protect against acetaminophen-induced hepatic and renal toxicity in rats through HO-1-Nrf2-BACH1 pathway. Arch. Biochem. Biophys. 2020, 687, 108387. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Colletti, L.M. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology 2010, 52, 691–702. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, D.; Zhang, Q.; Zhang, G.; Xia, M.; Li, J.; Zhan, W.; Shen, E.; Li, Z.; Lin, L.; et al. Treatment of acetaminophen-induced liver failure by blocking the death checkpoint protein TRAIL. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165583. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Pfeiffer, D.R.; Calhoon, E.A.; Madiai, F.; Marcucci, G.; Liu, S.; Jurkowitz, M.S. Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J. Biol. Chem. 2011, 286, 24916–24930. [Google Scholar] [CrossRef]
- Miyazawa, T.; Yamaguchi, M.; Lee, J.-H.; Fujimoto, K.; Kaneda, T. Decomposition of Lipid Hydroperoxide by Choline and Ethanolamine. Agric. Biol. Chem. 1984, 48, 1375–1377. [Google Scholar] [CrossRef][Green Version]
- Alwayn, I.P.; Gura, K.; Nose, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C.; et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Pan, P.H.; Wang, J.D.; Yang, C.P.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Lo, Y.L.; Chang, Y.H.; et al. DHA alleviated hepatic and adipose inflammation with increased adipocyte browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2023, 122, 109457. [Google Scholar] [CrossRef]
- Speck, R.F.; Lauterburg, B.H. Fish oil protects mice against acetaminophen hepatotoxicity in vivo. Hepatology 1991, 13, 557–561. [Google Scholar] [CrossRef]
- Sesink, A.L.; Termont, D.S.; Kleibeuker, J.H.; Van der Meer, R. Red meat and colon cancer: The cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999, 59, 5704–5709. [Google Scholar]
- Takahashi, T.; Kato, S.; Ito, J.; Shimizu, N.; Parida, I.S.; Itaya-Takahashi, M.; Sakaino, M.; Imagi, J.; Yoshinaga, K.; Yoshinaga-Kiriake, A.; et al. Dietary triacylglycerol hydroperoxide is not absorbed, yet it induces the formation of other triacylglycerol hydroperoxides in the gastrointestinal tract. Redox Biol. 2022, 57, 102471. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Slaughter, M.R.; Swain, A.; Birmingham, J.M.; Greenhill, R.W.; Elcock, F.; Bugelski, P.J. Repeated acetaminophen dosing in rats: Adaptation of hepatic antioxidant system. Hum. Exp. Toxicol. 2000, 19, 277–283. [Google Scholar] [CrossRef]
- Schafer, C.; Schroder, K.R.; Hoglinger, O.; Tollabimazraehno, S.; Lornejad-Schafer, M.R. Acetaminophen changes intestinal epithelial cell membrane properties, subsequently affecting absorption processes. Cell Physiol. Biochem. 2013, 32, 431–447. [Google Scholar] [CrossRef]
- Gong, S.; Lan, T.; Zeng, L.; Luo, H.; Yang, X.; Li, N.; Chen, X.; Liu, Z.; Li, R.; Win, S.; et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 2018, 69, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yang, X.; Jin, P.-R.; Won, K.-J.; Kim, C.H.; Jeong, H. The Discovery of Gut Microbial Metabolites as Modulators of Host Susceptibility to Acetaminophen-Induced Hepatotoxicity. Drug Metab. Dispos. 2024, 52, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, P. Targeting gut microbiota to counteract acetaminophen-induced acute liver injury. Trends Microbiol. 2024, 32, 419–421. [Google Scholar] [CrossRef]
- Gao, W.; Wang, G.; Yuan, H.; Chen, Y.; Che, J.; Cheng, Z.; Chen, L.; Zhang, L.; Zhu, Y.; Liu, X.; et al. Gram-positive probiotics improves acetaminophen-induced hepatotoxicity by inhibiting leucine and Hippo-YAP pathway. Cell Biosci. 2025, 15, 32. [Google Scholar] [CrossRef] [PubMed]
| Acyl | AM EtnGpl | PL EtnGpl | Alkenyl | AM EtnGpl | PL EtnGpl |
|---|---|---|---|---|---|
| mol% in Total Fatty Chains | mol% in Total Fatty Chains | ||||
| 16:0 | 1.3 | 7.4 | 16:0ol | 4.5 | 1.5 |
| 16:1n-7 | 1.6 | 0.1 | 18:0ol | 34.4 | 2.1 |
| 18:0 | 3.9 | 36.3 | 18:1ol | 3.2 | 0.3 |
| 18:1n-9 | 2.0 | 6.6 | Total | 42.1 | 3.9 |
| 18:2n-6 | 0.3 | 8.1 | |||
| 18:3n-3 | 0.8 | 0.0 | |||
| 20:4n-6 | 1.8 | 28.6 | |||
| 20:5n-3 | 32.9 | 0.5 | |||
| 22:4n-6 | nd | 1.3 | |||
| 22:5n-3 | 0.4 | 2.0 | |||
| 22:6n-3 | 9.4 | 3.7 | |||
| Others | 0.8 | 0.9 | |||
| (A) | (B) | ||||||
|---|---|---|---|---|---|---|---|
| Acyl | Basal Diet | AM Diet | PL Diet | Acyl | Basal Diet | AM Diet | PL Diet |
| μmol/100 g Diet | μmol/100 g Diet | ||||||
| 14:0 | 199.4 | 200.6 | 199.4 | 14:0 | 209.5 | 210.7 | 209.5 |
| 16:0 | 3141.0 | 3146.1 | 3159.4 | 16:0 | 3352.8 | 3357.9 | 3371.3 |
| 16:1n-7 | 254.9 | 259.0 | 254.9 | 16:1n-7 | 216.2 | 220.2 | 216.2 |
| 18:0 | 936.0 | 948.5 | 1046.4 | 18:0 | 1086.3 | 1098.7 | 1196.6 |
| 18:1n-9 | 4853.5 | 4858.7 | 4866.8 | 18:1n-9 | 5053.5 | 5058.7 | 5066.8 |
| 18:1n-7 | 374.2 | 376.5 | 375.8 | 18:1n-7 | 384.4 | 386.7 | 386.0 |
| 18:2n-6 | 10,428.8 | 10,429.9 | 10,455.3 | 18:2n-6 | 10,464.5 | 10,465.6 | 10,490.9 |
| 18:3n-3 | 1220.0 | 1222.2 | 1220.3 | 18:3n-3 | 1271.5 | 1273.8 | 1271.8 |
| 20:4n-6 | 83.0 | 87.4 | 145.4 | 20:4n-6 | 134.3 | 138.7 | 196.7 |
| 20:5n-3 | 243.3 | 327.7 | 244.2 | 20:5n-3 | 255.7 | 340.0 | 256.5 |
| 22:4n-6 | 6.7 | 6.7 | 10.5 | 22:4n-6 | nd | nd | 3.7 |
| 22:5n-3 | 36.7 | 37.8 | 42.5 | 22:5n-3 | 45.4 | 46.5 | 51.2 |
| 22:6n-3 | 1142.4 | 1167.0 | 1147.1 | 22:6n-3 | 796.1 | 820.7 | 800.8 |
| n-3/n-6 ratio | 0.25 | 0.26 | 0.25 | n-3/n-6 ratio | 0.22 | 0.23 | 0.22 |
| Ctrl | APAP | AM + APAP | PL + APAP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 24.4 | ± | 0.4 | 24.3 | ± | 0.4 a | 24.4 | ± | 0.4 a | 24.3 | ± | 0.3 a |
| Final body weight (g) | 44.7 | ± | 1.5 | 40.5 | ± | 0.8 b | 46.5 | ± | 1.1 a | 41.0 | ± | 1.1 b |
| Body weight gain | 9.4 | ± | 0.8 * | 5.9 | ± | 0.4 b | 9.7 | ± | 0.6 a | 6.6 | ± | 0.7 b |
| during APAP treatment (g) | ||||||||||||
| Feed intake | 261.6 | ± | 0.8 | 243.9 | ± | 7.6 a | 295.6 | ± | 19.8 a | 269.5 | ± | 6.8 a |
| during APAP treatment (g) | ||||||||||||
| Plasma AST (IU/L) | 54.5 | ± | 6.1 | 59.3 | ± | 6.1 a | 48.4 | ± | 5.5 a | 57.8 | ± | 6.5 a |
| Plasma ALT (IU/L) | 14.4 | ± | 3.1 | 10.5 | ± | 1.0 a | 8.6 | ± | 1.0 a | 11.6 | ± | 1.7 a |
| Plasma TNF-α (pg/mL) | 19.3 | ± | 0.6 | 20.8 | ± | 0.6 a | 22.6 | ± | 1.2 a | 21.0 | ± | 1.4 a |
| Liver weight (g) | 2.2 | ± | 0.2 * | 1.8 | ± | 0.1 b | 2.2 | ± | 0.1 a | 1.9 | ± | 0.1 b |
| Liver GSH (µmol/mg protein) | 18.4 | ± | 3.8 | 16.4 | ± | 3.2 a | 19.9 | ± | 2.0 a | 16.0 | ± | 2.8 a |
| Liver MDA (nmol/mg protein) | 11.6 | ± | 0.9 * | 16.2 | ± | 1.5 a | 10.2 | ± | 0.7 b | 10.6 | ± | 1.1 b |
| Spleen weight (mg) | 140.0 | ± | 10.7 | 113.3 | ± | 9.4 a | 120.8 | ± | 6.2 a | 125.8 | ± | 8.4 a |
| Ctrl | APAP | AM + APAP | PL + APAP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nmol/mg protein | ||||||||||||
| 16:0 | 111.9 | ± | 11.0 * | 61.5 | ± | 4.2 b | 81.3 | ± | 2.1 a | 79.3 | ± | 7.1 a |
| 16:1n-7 | 9.4 | ± | 1.3 * | 4.3 | ± | 0.6 a | 5.9 | ± | 0.6 a | 4.9 | ± | 0.9 a |
| 18:0 | 21.9 | ± | 1.3 * | 17.9 | ± | 1.2 a | 18.9 | ± | 0.6 a | 18.9 | ± | 0.8 a |
| 18:1n-9 | 96.9 | ± | 13.7 * | 38.5 | ± | 4.5 a | 53.3 | ± | 2.6 a | 40.1 | ± | 5.1 a |
| 18:1n-7 | 10.0 | ± | 1.3 * | 3.8 | ± | 0.4 ab | 5.1 | ± | 0.5 a | 3.5 | ± | 0.4 b |
| 18:2n-6 | 61.9 | ± | 3.9 * | 50.0 | ± | 2.3 a | 59.1 | ± | 2.6 a | 62.1 | ± | 6.1 a |
| 18:3n-3 | 3.5 | ± | 0.4 | 2.6 | ± | 0.2 a | 4.9 | ± | 1.9 a | 3.0 | ± | 0.4 a |
| 20:3n-6 | 3.0 | ± | 0.1 * | 2.3 | ± | 0.1 ab | 2.7 | ± | 0.2 a | 1.9 | ± | 0.2 b |
| 20:4n-6 | 11.7 | ± | 0.3 | 10.7 | ± | 0.5 a | 10.1 | ± | 0.4 a | 10.9 | ± | 0.6 a |
| 20:5n-3 | 3.5 | ± | 0.1 * | 2.7 | ± | 0.3 a | 3.3 | ± | 0.2 a | 2.9 | ± | 0.4 a |
| 22:5n-3 | 1.6 | ± | 0.3 | 1.3 | ± | 0.1 b | 2.1 | ± | 0.2 a | 1.8 | ± | 0.3 ab |
| 22:6n-3 | 19.7 | ± | 1.2 | 17.9 | ± | 0.6 b | 22.6 | ± | 0.7 a | 20.1 | ± | 1.7 ab |
| Total | 354.7 | ± | 29.3 * | 213.3 | ± | 12.7 b | 269.2 | ± | 7.8 a | 249.3 | ± | 22.7 ab |
| DHA/ARA ratio | 1.7 | ± | 0.1 | 1.7 | ± | 0.1 b | 2.3 | ± | 0.1 a | 1.8 | ± | 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogame, R.; Tominaga, Y.; Echigoya, M.; Nakagawa, K.; Fukushima, M.; Miyazawa, T.; Kinoshita, M.; Yamashita, S. Dietary Ethanolamine Plasmalogen from Ascidian Alleviates Chronic Hepatic Injury in Mice Treated with Continuous Acetaminophen. Appl. Sci. 2025, 15, 5968. https://doi.org/10.3390/app15115968
Sogame R, Tominaga Y, Echigoya M, Nakagawa K, Fukushima M, Miyazawa T, Kinoshita M, Yamashita S. Dietary Ethanolamine Plasmalogen from Ascidian Alleviates Chronic Hepatic Injury in Mice Treated with Continuous Acetaminophen. Applied Sciences. 2025; 15(11):5968. https://doi.org/10.3390/app15115968
Chicago/Turabian StyleSogame, Ryosuke, Yuki Tominaga, Momoka Echigoya, Kiyotaka Nakagawa, Michihiro Fukushima, Teruo Miyazawa, Mikio Kinoshita, and Shinji Yamashita. 2025. "Dietary Ethanolamine Plasmalogen from Ascidian Alleviates Chronic Hepatic Injury in Mice Treated with Continuous Acetaminophen" Applied Sciences 15, no. 11: 5968. https://doi.org/10.3390/app15115968
APA StyleSogame, R., Tominaga, Y., Echigoya, M., Nakagawa, K., Fukushima, M., Miyazawa, T., Kinoshita, M., & Yamashita, S. (2025). Dietary Ethanolamine Plasmalogen from Ascidian Alleviates Chronic Hepatic Injury in Mice Treated with Continuous Acetaminophen. Applied Sciences, 15(11), 5968. https://doi.org/10.3390/app15115968












