Non-Invasive Food Authentication Using Vibrational Spectroscopy Techniques for Low-Resolution Food Fingerprinting
Abstract
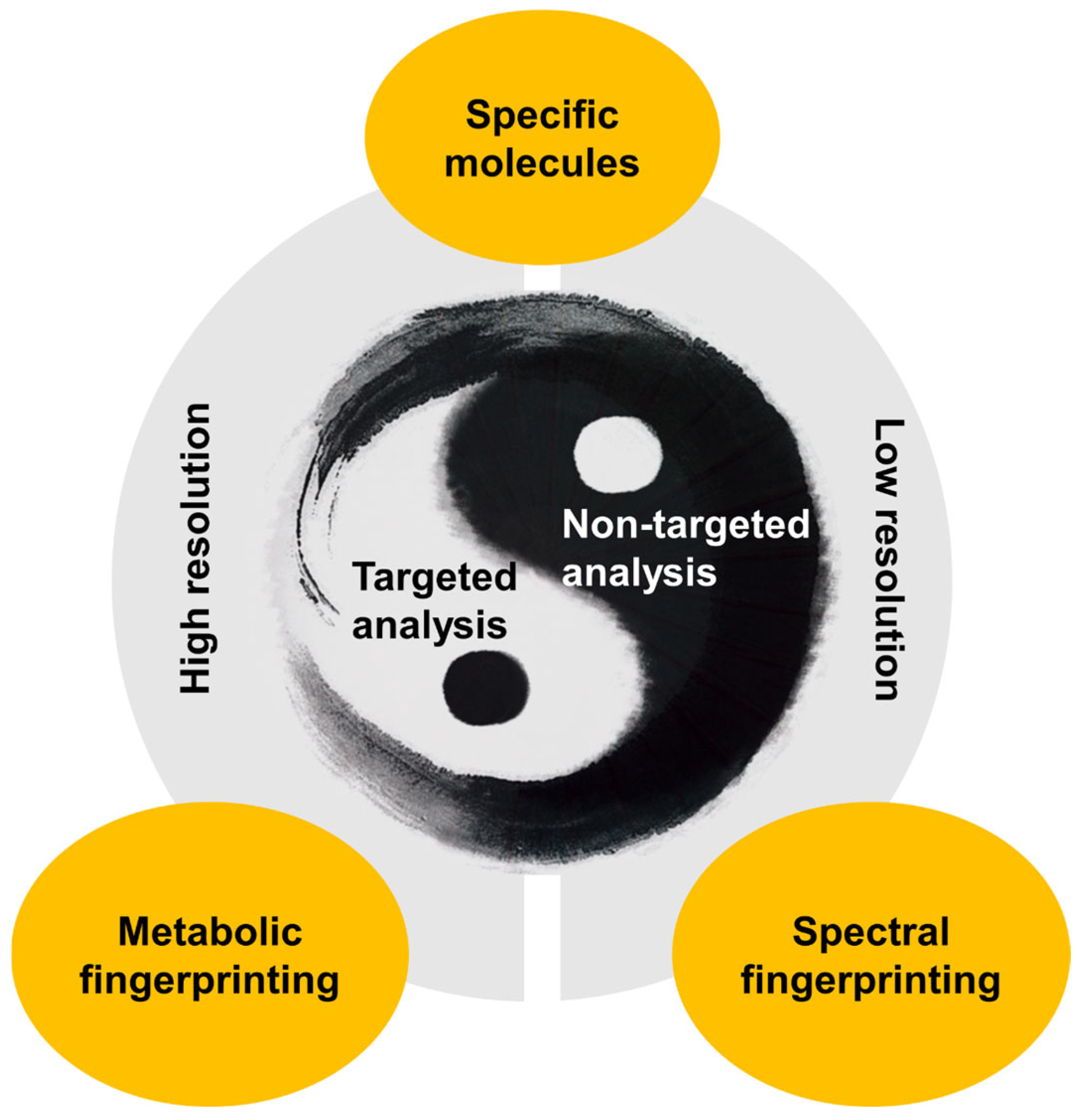
1. Introduction
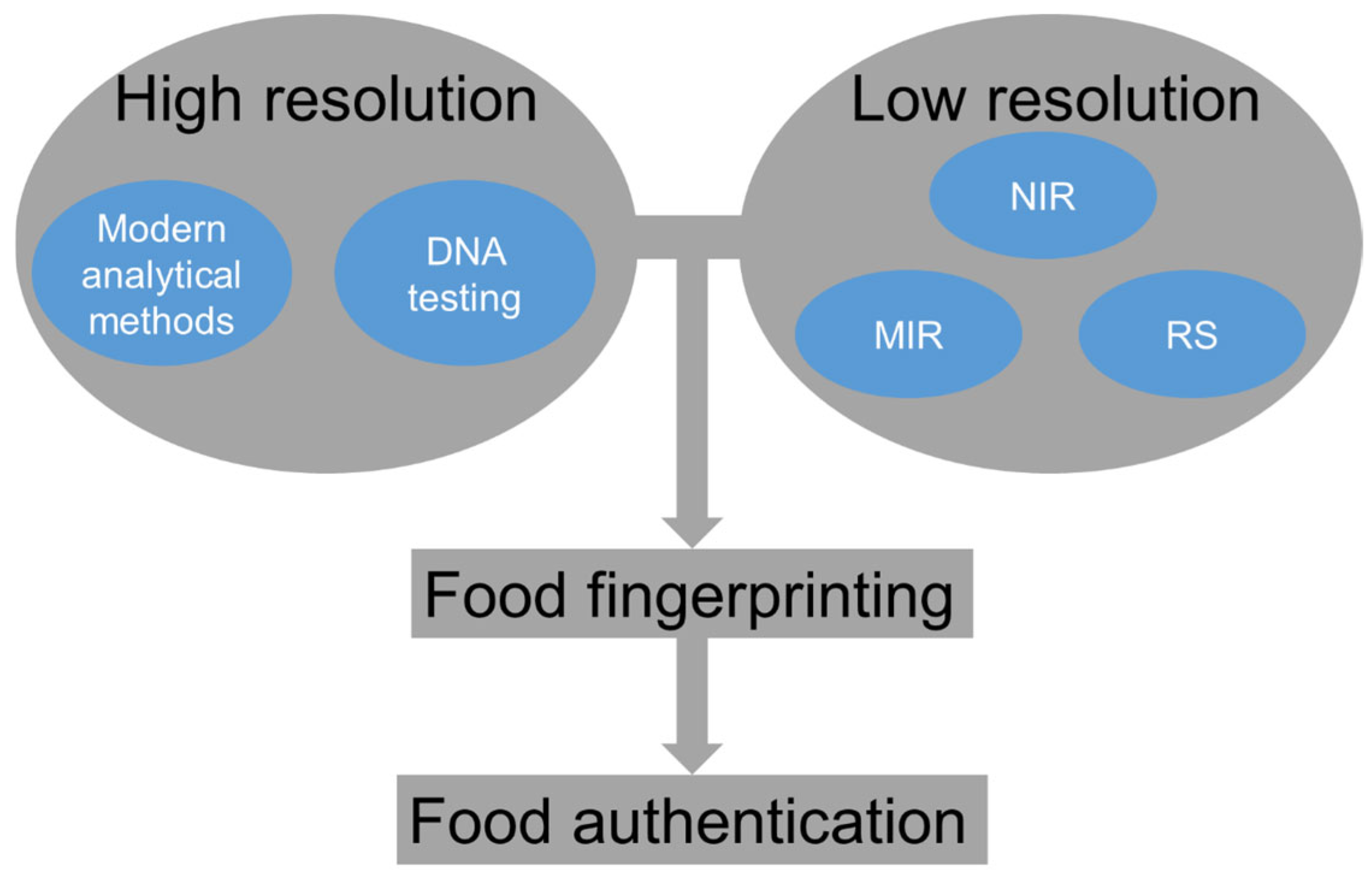
2. Food Fingerprinting and Vibrational Spectroscopy Techniques
3. Data Analysis, Experimental Design, and Method Validation
3.1. Chemometrics, Preprocessing, and Data Fusion
3.2. Experimental Design and Sampling Strategies
3.3. Validation and Method Monitoring
4. Vibrational Spectroscopic Techniques for Non-Invasive Food Authentication
4.1. NIR Spectroscopy for Non-Invasive Food Authentication
4.2. FTIR Spectroscopy for Non-Invasive Food Authentication
4.3. Raman Spectroscopy for Non-Invasive Food Authentication
5. Limitation of Vibrational Spectroscopy for Non-Invasive Food Authentication
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Echegaray, N.; Hassoun, A.; Jagtap, S.; Tetteh-Caesar, M.; Kumar, M.; Tomasevic, I.; Goksen, G.; Lorenzo, J.M. Meat 4.0: Principles and applications of industry 4.0 technologies in the meat industry. Appl. Sci. 2022, 12, 6986. [Google Scholar] [CrossRef]
- Hassoun, A.; Aït-Kaddour, A.; Abu-Mahfouz, A.M.; Rathod, N.B.; Bader, F.; Barba, F.J.; Biancolillo, A.; Cropotova, J.; Galanakis, C.M.; Jambrak, A.R.; et al. The fourth industrial revolution in the food industry-Part I: Industry 4.0 technologies. Crit. Rev. Food Sci. Nutr. 2023, 63, 6547–6563. [Google Scholar] [CrossRef]
- Chapman, J.; Power, A.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.E.; Truong, V.K.; Cozzolino, D. Challenges and opportunities of the fourth revolution: A brief insight into the future of food. Crit. Rev. Food Sci. Nutr. 2022, 62, 2845–2853. [Google Scholar] [CrossRef]
- Hitzmann, B.; Hauselmann, R.; Niemoeller, A.; Sangi, D.; Traenkle, J.; Glassey, J. Process analytical technologies in food industry-challenges and benefits: A status report and recommendations. Biotechnol. J. 2015, 10, 1095–1100. [Google Scholar] [CrossRef]
- Konur, S.; Lan, Y.; Thakker, D.; Morkyani, G.; Polovina, N.; Sharp, J. Towards design and implementation of Industry 4.0 for food manufacturing. Neural Comput. Appl. 2023, 35, 23753–23765. [Google Scholar] [CrossRef]
- Hassoun, A.; Siddiqui, S.A.; Smaoui, S.; Ucak, I.; Arshad, R.N.; Garcia-Oliveira, P.; Prieto, M.A.; Ait-Kaddour, A.; Perestrelo, R.; Câmara, J.S.; et al. Seafood processing, preservation, and analytical techniques in the age of Industry 4.0. Appl. Sci. 2022, 12, 1703. [Google Scholar] [CrossRef]
- Islam, S.; Cullen, J.M. Food traceability: A generic theoretical framework. Food Control 2021, 123, 107848. [Google Scholar] [CrossRef]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef]
- Kharbach, M.; Alaoui Mansouri, M.; Taabouz, M.; Yu, H. Current application of advancing spectroscopy techniques in food analysis: Data handling with chemometric approaches. Foods 2023, 12, 2753. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Fan, L.-Y.; Liu, Y.-F.; Zheng, X.-H. High-performance MoO3 nanosheets gas sensors for triethylamine detection: A rapid approach for assessing fish freshness. Ceram. Int. 2025, 51, 9912–9922. [Google Scholar] [CrossRef]
- Alvarado, V.L.S.; Diaz, F.J.; Parra, L.; Lloret, J.; Aldana, C.; Saavedra, Y. Proposal for a gas sensor device to classify hydrobiological species and estimate non-refrigeration time. IEEE Sens. J. 2025, 25, 18015–18022. [Google Scholar] [CrossRef]
- Noto, G.; Coletta, L.; Vainieri, M. Measuring the performance of collaborative governance in food safety management: An Italian case study. Public Money Manag. 2022, 42, 627–636. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Y.; Zhang, J.; Yin, C.; Chen, Y.; Men, H. Origin traceability of agricultural products: A lightweight collaborative neural network for spectral information processing. Food Res. Int. 2025, 208, 116131. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M.; Brereton, P.; Hsu, R.; Kelly, S.; Marmiroli, N.; Melini, F.; Soukoulis, C.; Ding, W.P. Food authenticity assessment: Ensuring compliance with food legislation and traceability requirements. Qual. Assur. Saf. Crops Foods 2009, 1, 93–100. [Google Scholar] [CrossRef]
- Fritsche, J. Recent developments and digital perspectives in food safety and authenticity. J. Agric. Food Chem. 2018, 66, 7562–7567. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferranti, P. The evolution of analytical chemistry methods in foodomics. J. Chromatogr. A 2016, 1428, 3–15. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; Valverde-Som, L.; Pérez-Castaño, E.; González-Casado, A. Chromatographic fingerprinting: An innovative approach for food ‘identitation’ and food authentication—A tutorial. Anal. Chim. Acta 2016, 909, 9–23. [Google Scholar] [CrossRef]
- Mialon, N.; Roig, B.; Capodanno, E.; Cadiere, A. Untargeted metabolomic approaches in food authenticity: A review that showcases biomarkers. Food Chem. 2023, 398, 133856. [Google Scholar] [CrossRef]
- Karoui, R. Chapter 2—Spectroscopic technique: Mid-infrared (MIR) and Fourier transform mid-infrared (FT-MIR) spectroscopies. In Modern Techniques for Food Authentication, 2nd ed.; Sun, D.-W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 23–50. [Google Scholar]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Cozzolino, D. Foodomics and infrared spectroscopy: From compounds to functionality. Curr. Opin. Food Sci. 2015, 4, 39–43. [Google Scholar] [CrossRef]
- Arendse, E.; Nieuwoudt, H.; Magwaza, L.S.; Nturambirwe, J.F.I.; Fawole, O.A.; Opara, U.L. Recent advancements on vibrational spectroscopic techniques for the detection of authenticity and adulteration in horticultural products with a specific focus on oils, juices and powders. Food Bioprocess Technol. 2021, 14, 1–22. [Google Scholar] [CrossRef]
- Cozzolino, D. Low resolution food fingerprinting: Vibrational spectroscopic methods for nondestructive food authentication. Curr. Opin. Food Sci. 2024, 60, 101229. [Google Scholar] [CrossRef]
- Bec, K.B.; Huck, C.W. Breakthrough potential in near-infrared spectroscopy: Spectra simulation. A review of recent developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Løkke, M.M.; Micklander, E.; Engelsen, S.B. Vibrational microspectroscopy of food. Raman vs. FT-IR. Trends Food Sci. Technol. 2003, 14, 50–57. [Google Scholar] [CrossRef]
- Su, W.H.; He, H.J.; Sun, D.W. Non-destructive and rapid evaluation of staple foods quality by using spectroscopic techniques: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1039–1051. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Kim, H.; Peng, X.; Yoon, J. Activatable near-infrared versatile fluorescent and chemiluminescent dyes based on the dicyanomethylene-4H-pyran scaffold: From design to imaging and theranostics. Angew. Chem. Int. Ed. 2024, 63, e202311764. [Google Scholar] [CrossRef]
- Wu, L.M.; Xu, D.G.; Wang, Y.Y.; Ge, M.L.; Li, H.B.; Wang, Z.L.; Yao, J.Q. Common path continuous terahertz reflection and attenuated total reflection imaging. Acta Phys. Sin. 2021, 70, 118701. [Google Scholar] [CrossRef]
- Arjunan, V.; Sakiladevi, S.; Marchewka, M.K.; Mohan, S. FTIR, FT-Raman, FT-NMR and quantum chemical investigations of 3-acetylcoumarin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 79–89. [Google Scholar] [CrossRef]
- Gullifa, G.; Barone, L.; Papa, E.; Giuffrida, A.; Materazzi, S.; Risoluti, R. Portable NIR spectroscopy: The route to green analytical chemistry. Front. Chem. 2023, 11, 1214825. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.R.; Zhang, P.; Daengngam, C.; Peng, S.L.; Chongcheawchamnan, M. A review of artificial intelligence methods combined with Raman spectroscopy to identify the composition of substances. J. Raman Spectrosc. 2022, 53, 6–19. [Google Scholar] [CrossRef]
- Batista, A.M.; Foiani, L.; Champeau, M.; Martinho, H. Advances in tissues and cells characterization by Raman micro-spectroscopy, atomic force microscopy, and tip-enhanced Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 1848–1860. [Google Scholar] [CrossRef]
- Liu, G.K.; Zheng, H.; Lu, J.L. Recent progress and perspective of trace antibiotics detection in aquatic environment by surface-enhanced Raman spectroscopy. Trends Environ. Anal. Chem. 2017, 16, 16–23. [Google Scholar] [CrossRef]
- Sendin, K.; Williams, P.J.; Manley, M. Near infrared hyperspectral imaging in quality and safety evaluation of cereals. Crit. Rev. Food Sci. Nutr. 2018, 58, 575–590. [Google Scholar] [CrossRef]
- Amodio, M.L.; Chaudhry, M.M.A.; Colelli, G. Spectral and hyperspectral technologies as an additional tool to increase information on quality and origin of horticultural crops. Agronomy 2020, 10, 7. [Google Scholar] [CrossRef]
- Ahmad, M.; Vitale, R.; Silva, C.S.; Ruckebusch, C.; Cocchi, M. A novel proposal to investigate the interplay between the spatial and spectral domains in near-infrared spectral imaging data by means of image decomposition, encoding and localization (IDEL). Anal. Chim. Acta 2022, 1191, 339285. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Zheng, B.Y.; He, Y. Applications of a hyperspectral imaging system used to estimate wheat grain protein: A review. Front. Plant Sci. 2022, 13, 837200. [Google Scholar] [CrossRef]
- Wolf, S.; Popp, J.; Frosch, T. Multifocal hyperspectral Raman imaging setup for multi-well plates. Sens. Actuators B Chem. 2023, 375, 132949. [Google Scholar] [CrossRef]
- Wang, Z.X.; Xu, P.; Liu, B.H.; Cao, Y.K.; Liu, Z.; Liu, Z.J. Hyperspectral imaging for underwater object detection. Sens. Rev. 2021, 41, 176–191. [Google Scholar] [CrossRef]
- Bai, H.X.; Xue, Q.S.; Hao, X.J.; Li, H.; Huang, L.Y.; Li, C.; Yang, J.Y. Underwater hyperspectral imaging system with dual-scanning mode. Appl. Opt. 2022, 61, 4226–4237. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Qu, F.; Wang, X.; Zhai, X.; Li, J.; Yu, K.; Zhao, Y. Terahertz spectroscopy and imaging techniques for herbal medicinal plants detection: A comprehensive review. Crit. Rev. Anal. Chem. 2024, 54, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Saeys, W.; Do Trong, N.N.; Van Beers, R.; Nicolai, B.M. Multivariate calibration of spectroscopic sensors for postharvest quality evaluation: A review. Postharvest Biol. Technol. 2019, 158, 110981. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R. A Tutorial on Near Infrared Spectroscopy and Its Calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- McGrath, T.F.; Haughey, S.A.; Patterson, J.; Fauhl-Hassek, C.; Donarski, J.; Alewijn, M.; van Ruth, S.; Elliott, C.T. What are the scientific challenges in moving from targeted to non-targeted methods for food fraud testing and how can they be addressed?—Spectroscopy case study. Trends Food Sci. Technol. 2018, 76, 38–55. [Google Scholar] [CrossRef]
- Musio, B.; Ragone, R.; Todisco, S.; Rizzuti, A.; Iorio, E.; Chirico, M.; Pisanu, M.E.; Meloni, N.; Mastrorilli, P.; Gallo, V. Non-targeted nuclear magnetic resonance analysis for food authenticity: A comparative study on tomato samples. Molecules 2024, 29, 4441. [Google Scholar] [CrossRef]
- Szymanska, E.; Gerretzen, J.; Engel, J.; Geurts, B.; Blanchet, L.; Buydens, L.M.C. Chemometrics and qualitative analysis have a vibrant relationship. TrAC Trends Anal. Chem. 2015, 69, 34–51. [Google Scholar] [CrossRef]
- Skov, T.; Honoré, A.H.; Jensen, H.M.; Næs, T.; Engelsen, S.B. Chemometrics in foodomics: Handling data structures from multiple analytical platforms. TrAC Trends Anal. Chem. 2014, 60, 71–79. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Marins-Goncalves, L.; De Souza, D. An integrative review of analytical techniques used in food authentication: A detailed description for milk and dairy products. Food Chem. 2024, 457, 140206. [Google Scholar] [CrossRef]
- Cozzolino, D.; Williams, P.J.; Hoffman, L.C. An overview of pre-processing methods available for hyperspectral imaging applications. Microchem. J. 2023, 193, 109129. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Simonetti, R.; Casale, M. The impact of signal pre-processing on the final interpretation of analytical outcomes—A tutorial. Anal. Chim. Acta 2019, 1058, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, B.; Owen, S.; Kolobaric, A.; Chapman, J.; Cozzolino, D. Pre-processing applied to instrumental data in analytical chemistry: A brief review of the methods and examples. Crit. Rev. Anal. Chem. 2023, 54, 2745–2753. [Google Scholar] [CrossRef]
- Wang, L.; Meng, Q.X.; Ren, L.P.; Yang, J.S. Near infrared reflectance spectroscopy (NIRS) and its application in the determination for the quality of animal feed and products. Spectrosc. Spectr. Anal. 2010, 30, 1482–1487. [Google Scholar] [CrossRef]
- Cozzolino, D. The sample, the spectra and the maths-The critical pillars in the development of robust and sound applications of vibrational spectroscopy. Molecules 2020, 25, 3674. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistry-part I: History, experimental design and data analysis tools. Anal. Bioanal. Chem. 2017, 409, 5891–5899. [Google Scholar] [CrossRef]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistrypart II: Modeling, validation, and applications. Anal. Bioanal. Chem. 2018, 410, 6691–6704. [Google Scholar] [CrossRef]
- Lohumi, S.; Mo, C.; Kang, J.-S.; Hong, S.-J.; Cho, B.-K. Nondestructive evaluation for the viability of watermelon (citrullus lanatus) seeds using Fourier transform near infrared spectroscopy. J. Biosyst. Eng. 2013, 38, 312–317. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.-K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I. Identification of animal meat muscles by visible and near infrared reflectance spectroscopy. LWT-Food Sci. Technol. 2004, 37, 447–452. [Google Scholar] [CrossRef]
- Ding, H.B.; Xu, R.J. Near-infrared spectroscopic technique for detection of beef hamburger adulteration. J. Agric. Food Chem. 2000, 48, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Gayo, J.; Hale, S.A.; Blanchard, S.M. Quantitative analysis and detection of adulteration in crab meat using visible and near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 1130–1136. [Google Scholar] [CrossRef]
- Gayo, J.; Hale, S.A. Detection and quantification of species authenticity and adulteration in crabmeat using visible and near-infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Bochko, V.A.; Martinkauppi, B.; Saranwong, S.; Mantere, T. A review of optical nondestructive visual and near-infrared methods for food quality and safety. Int. J. Spectrosc. 2013, 2013, 341402. [Google Scholar] [CrossRef]
- Haughey, S.A.; Graham, S.F.; Cancouët, E.; Elliott, C.T. The application of near-infrared reflectance spectroscopy (NIRS) to detect melamine adulteration of soya bean meal. Food Chem. 2013, 136, 1557–1561. [Google Scholar] [CrossRef]
- Lu, C.H.; Xiang, B.R.; Hao, G.; Xu, J.P.; Wang, Z.W.; Chen, C.Y. Rapid detection of melamine in milk powder by near infrared spectroscopy. J. Near Infrared Spectrosc. 2009, 17, 59–67. [Google Scholar] [CrossRef]
- Xu, L.; Yan, S.-M.; Cai, C.-B.; Wang, Z.-J.; Yu, X.-P. The feasibility of using near-infrared spectroscopy and chemometrics for untargeted detection of protein adulteration in yogurt: Removing unwanted variations in pure yogurt. J. Anal. Methods Chem. 2013, 2013, 201873. [Google Scholar] [CrossRef]
- Twomey, M.; Downey, G.; McNulty, P.B. The potential of NIR spectroscopy for the detection of the adulteration of orange juice. J. Sci. Food Agric. 1995, 67, 77–84. [Google Scholar] [CrossRef]
- Contal, L.; León, V.; Downey, G. Detection and quantification of apple adulteration in strawberry and raspberry purées using visible and near infrared spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 289–299. [Google Scholar] [CrossRef]
- Sinelli, N.; Casale, M.; Di Egidio, V.; Oliveri, P.; Bassi, D.; Tura, D.; Casiraghi, E. Varietal discrimination of extra virgin olive oils by near and mid infrared spectroscopy. Food Res. Int. 2010, 43, 2126–2131. [Google Scholar] [CrossRef]
- Xie, L.-J.; Ye, X.-Q.; Liu, D.-H.; Ying, Y.-B. Application of principal component-radial basis function neural networks (PC-RBFNN) for the detection of water-adulterated bayberry juice by near-infrared spectroscopy. J. Zhejiang Univ. Sci. B 2008, 9, 982–989. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Y.; Liu, C.; Li, Z.; Xu, Z. Dual NIR-channel fluorescent probe for detecting ONOO− in vitro and vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 320, 124665. [Google Scholar] [CrossRef] [PubMed]
- Veloso Tropia, N.; Reis Vilela, R.S.; de Sales Silva, F.A.; Andrade, D.R.; Costa, A.C.; Cidrini, F.A.A.; de Souza Pinheiro, J.; Pucetti, P.; Chizzotti, M.L.; de Campos Valadares Filho, S. Regression models from portable NIR spectra for predicting the carcass traits and meat quality of beef cattle. PLoS ONE 2024, 19, e0303946. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; He, H.-J.; Zhang, J.; Chew, K.W.; Ou, X. NIR sensors combined with chemometric algorithms in intelligent quality evaluation of sweetpotato roots from ‘Farm’ to ‘Table’: Progresses, challenges, trends, and prospects. Food Chem. X 2024, 22, 101449. [Google Scholar] [CrossRef]
- Cozzolino, D. The ability of near infrared (NIR) spectroscopy to predict functional properties in foods: Challenges and opportunities. Molecules 2021, 26, 6981. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, D.; Wang, Y.; Xi, Q.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Rapid identification of cod authenticity based on hyperspectral imaging technology. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 326, 125258. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Y.; Shi, Q.; Wei, L.N.; Xu, L.; Guo, X.M.; Hu, O.; Lan, W.; Xie, S.P.; Yang, T.M. Rapid recognition of geoherbalism and authenticity of a Chinese herb by data fusion of near-infrared spectroscopy (NIR) and mid-infrared (MIR) spectroscopy combined with chemometrics. J. Spectrosc. 2019, 2019, 2467185. [Google Scholar] [CrossRef]
- Parastar, H.; van Kollenburg, G.; Weesepoel, Y.; van den Doel, A.; Buydens, L.; Jansen, J. Integration of handheld NIR and machine learning to “Measure & Monitor” chicken meat authenticity. Food Control 2020, 112, 107149. [Google Scholar]
- Zhao, L.L.; Zhang, M.; Wang, H.X.; Mujumdar, A.S. Monitoring of free fatty acid content in mixed frying oils by means of LF-NMR and NIR combined with BP-ANN. Food Control 2022, 133, 108599. [Google Scholar] [CrossRef]
- Peng, D.; Xu, R.; Zhou, Q.; Yue, J.; Su, M.; Zheng, S.; Li, J. Discrimination of milk freshness based on synchronous two-dimensional visible/near-infrared correlation spectroscopy coupled with chemometrics. Molecules 2023, 28, 5728. [Google Scholar] [CrossRef]
- Cozzolino, D.; Bures, D.; Hoffman, L.C. Evaluating the use of a similarity index (SI) combined with near infrared (NIR) spectroscopy as method in meat species authenticity. Foods 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Martelo-Vidal, M.J.; Vázquez, M. Classification of red wines from controlled designation of origin by ultraviolet-visible and near-infrared spectral analysis. Cienc. Tec. Vitivinic. 2014, 29, 35–43. [Google Scholar] [CrossRef]
- Ma, J.; Qi, Y.; Lei, M.; Xuan, H.; Li, X.; Lu, W.; Guo, J.; Chen, H. Analysis and discrimination of adhesive species using ATR-FTIR combined with Raman, and HS-GC-IMS together with multivariate statistical analysis. J. Chromatogr. A 2024, 1736, 465402. [Google Scholar] [CrossRef]
- Nittari, G.; Roy, P.; Martinelli, I.; Bellitto, V.; Tomassoni, D.; Traini, E.; Tayebati, S.K.; Amenta, F. Rodent models of Huntington’s disease: An overview. Biomedicines 2023, 11, 3331. [Google Scholar] [CrossRef] [PubMed]
- Soureshjani, H.K.; Bahador, M.; Tadayon, M.R.; Dehkordi, A.G. Modeling seed germination of quinoa (Chenopodium quinoa Willd.) at different temperatures and water potentials. Acta Physiol. Plant. 2022, 44, 102. [Google Scholar] [CrossRef]
- Nicolaou, N.; Goodacre, R. Rapid and quantitative detection of the microbial spoilage in milk using Fourier transform infrared spectroscopy and chemometrics. Analyst 2008, 133, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Polshin, E.; Aernouts, B.; Saeys, W.; Delvaux, F.; Delvaux, F.R.; Saison, D.; Hertog, M.; Nicolaï, B.M.; Lammertyn, J. Beer quality screening by FT-IR spectrometry: Impact of measurement strategies, data pre-processings and variable selection algorithms. J. Food Eng. 2011, 106, 188–198. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J.M.K. Detection of inverted beet sugar adulteration of honey by FTIR spectroscopy. J. Sci. Food Agric. 2001, 81, 683–690. [Google Scholar] [CrossRef]
- Kelly, J.D.; Downey, G. Detection of sugar adulterants in apple juice using fourier transform infrared spectroscopy and chemometrics. J. Agric. Food Chem. 2005, 53, 3281–3286. [Google Scholar] [CrossRef]
- Yajima, Y.; Wakabayashi, H.; Suehara, K.-I.; Kameoka, T.; Hashimoto, A. Simultaneous content determination of mono-, di-, and fructo-oligosaccharides in citrus fruit juices using an FTIR-PLS method based on selected absorption bands. Int. J. Food Sci. 2024, 2024, 9265590. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Analysis of red wines using an electronic tongue and infrared spectroscopy, correlations with phenolic content and color parameters. LWT 2020, 118, 108785. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Troianou, V.E.; Pappas, C.S.; Kotseridis, Y.S.; Polissiou, M.G. Differentiation of Greek red wines on the basis of grape variety using attenuated total reflectance Fourier transform infrared spectroscopy. Food Chem. 2008, 111, 192–196. [Google Scholar] [CrossRef]
- Alkurd, R.A.; Al-Akayleh, F.T.; Al-Remawi, M.M.; Hasan, M.R.; Maqousi, A.; Ramadan, K.; Arafat, T.A. Evaluation of a Jordanian commercial chocolate brand fortified with micronutrients of vitamins B12 and D3, iron and zinc. Food Sci. Technol. 2023, 43, e105122. [Google Scholar] [CrossRef]
- Dashti, A.; Weesepoel, Y.; Muller-Maatsch, J.; Parastar, H.; Kobarfard, F.; Daraei, B.; Yazdanpanah, H. Assessment of meat authenticity using portable Fourier transform infrared spectroscopy combined with multivariate classification techniques. Microchem. J. 2022, 181, 107735. [Google Scholar] [CrossRef]
- Kurz, C.; Leitenberger, M.; Carle, R.; Schieber, A. Evaluation of fruit authenticity and determination of the fruit content of fruit products using FT-NIR spectroscopy of cell wall components. Food Chem. 2010, 119, 806–812. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, X.L.; Liu, Z.X.; Zhang, X.X.; Hu, J.; Lü, J.H. Discrimination of foodborne pathogenic bacteria using synchrotron FTIR microspectroscopy. Nucl. Sci. Tech. 2017, 28, 49. [Google Scholar] [CrossRef]
- Teng, A.S.J.; Habermehl, P.E.; van Houdt, R.; de Jong, M.D.; van Mansfeld, R.; Matamoros, S.P.F.; Spijkerman, I.J.B.; van Meer, M.P.A.; Visser, C.E. Comparison of fast Fourier transform infrared spectroscopy biotyping with whole genome sequencing-based genotyping in common nosocomial pathogens. Anal. Bioanal. Chem. 2022, 414, 7179–7189. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, P.; Fengou, L.-C.; Nychas, G.-J.; Fowler, S.M.; Mao, Y.; Luo, X.; Zhang, Y. Preliminary investigation into the prediction of indicators of beef spoilage using Raman and Fourier transform infrared spectroscopy. Meat Sci. 2023, 200, 109168. [Google Scholar] [CrossRef]
- Song, S.W.; Jang, J.I.; Park, C.R.; Kim, H.M. Optically pumped and matrix-assisted anti-Stokes Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 924–933. [Google Scholar] [CrossRef]
- Xu, Z.C.; Oguchi, K.; Taguchi, Y.; Sano, Y.; Miyawaki, Y.; Cheon, D.; Katoh, K.; Ozeki, Y. Stimulated Raman scattering spectroscopy with quantum-enhanced balanced detection. Opt. Express 2022, 30, 18589–18598. [Google Scholar] [CrossRef]
- Calisan Kinter, R.; Ozbaran, B.; Inal Kaleli, I.; Kose, S.; Bildik, T.; Ghaziuddin, M. The sensory profiles, eating behaviors, and quality of life of children with autism spectrum disorder and avoidant/restrictive food intake disorder. Psychiatr. Q. 2024, 95, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.A.; Moores, R.R., Jr.; Hendricks-Munoz, K.D.; Romfh, P.; Rozycki, H.J. Resonance Raman spectroscopy tissue oxygenation measurements in neonates. Neonatology 2023, 120, 363–370. [Google Scholar] [CrossRef]
- Hwang, J.-E.; Park, J.-Y.; Jung, M.H.; Eom, K.; Moon, H.S.; Joung, H.; Kim, Y.J. Evaluation of a commercial device based on reflection spectroscopy as an alternative to resonance Raman spectroscopy in measuring skin carotenoid levels: Randomized controlled trial. Sensors 2023, 23, 7654. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.H.; Sheng, R.; Chen, Q.S. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Chuesiang, P.; Ryu, V.; Siripatrawan, U.; He, L.L.; McLandsborough, L. Aptamer-based surface enhanced Raman spectroscopy (SERS) for the rapid detection of Salmonella Enteritidis contaminated in ground beef. LWT 2021, 150, 111937. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; He, L.L. Development of a portable SERS method for testing the antibiotic sensitivity of foodborne bacteria. J. Microbiol. Methods 2022, 198, 106496. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, Y.; Jiang, S.; Wang, Z.; Yu, Q.; Ji, C.; Guo, J.; Kong, X. Quantitative monitoring ofloxacin in beef by TLC-SERS combined with machine learning analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123790. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.Y.; Zhang, Y.Y.; Fan, Y.X.; Lai, K.Q. Ultra sensitive detection of malachite green in fish muscle with gold nanoparticles and graphene oxide hybrid as a substrate for surface enhanced Raman scattering. J. Food Meas. Charact. 2020, 14, 658–667. [Google Scholar] [CrossRef]
- Pan, C.Y.; Zhang, S.Y.; Xiong, X.L.; Li, Z.; Ai, B.W.; Qian, W.P.; Dong, J. Dynamically monitoring pH in living organisms based on a SERS-active optical fiber. Adv. Mater. Interfaces 2022, 9, 2200328. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Karina, M.; Hsu, C.H.; Srikandace, Y.; Prabowo, B.A.; Lai, C.S. Facile bacterial cellulose nanofibrillation for the development of a plasmonic paper sensor. ACS Biomater. Sci. Eng. 2020, 6, 3122–3131. [Google Scholar] [CrossRef]
- Guo, Q.; Peng, Y.; Qin, J.; Chao, K.; Zhao, X.; Yin, T. Advance in detection technique of lean meat powder residues in meat using SERS: A review. Molecules 2023, 28, 7504. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Gangadoo, S.; Truong, V.K.; Cozzolino, D. Spectroscopic approaches for rapid beer and wine analysis. Curr. Opin. Food Sci. 2019, 28, 67–73. [Google Scholar] [CrossRef]
- He, H.J.; Sun, D.W. Hyperspectral imaging technology for rapid detection of various microbial contaminants in agricultural and food products. Trends Food Sci. Technol. 2015, 46, 99–109. [Google Scholar] [CrossRef]
- Walsh, K.B.; McGlone, V.A.; Han, D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]

| Technique | Enhancement Factor | Food Application | Key Limitation |
|---|---|---|---|
| SERS | 105−106 | Pesticides in fruits | Substrate reproducibility |
| CARS | 103−104 | Lipid oxidation in meat | Nonresonant background |
| Resonance Raman | 102−103 | Carotenoids in seafood | UV-induced fluorescence |
| Feature | NIR | FTIR | Raman |
|---|---|---|---|
| Water Interference | Moderate | High | Low |
| Sample Prep | Minimal | Minimal–Moderate | Minimal |
| Sensitivity | Medium | High | Very High (esp. SERS) |
| Instrument Cost | Medium | Medium | High |
| Portability | High | Medium | Increasing |
| Major Applications | Bulk analysis and adulteration | Molecular fingerprinting and sugar detection | Protein/lipid analysis and contaminant detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Zeng, Q. Non-Invasive Food Authentication Using Vibrational Spectroscopy Techniques for Low-Resolution Food Fingerprinting. Appl. Sci. 2025, 15, 5948. https://doi.org/10.3390/app15115948
He W, Zeng Q. Non-Invasive Food Authentication Using Vibrational Spectroscopy Techniques for Low-Resolution Food Fingerprinting. Applied Sciences. 2025; 15(11):5948. https://doi.org/10.3390/app15115948
Chicago/Turabian StyleHe, Wanchong, and Qinghua Zeng. 2025. "Non-Invasive Food Authentication Using Vibrational Spectroscopy Techniques for Low-Resolution Food Fingerprinting" Applied Sciences 15, no. 11: 5948. https://doi.org/10.3390/app15115948
APA StyleHe, W., & Zeng, Q. (2025). Non-Invasive Food Authentication Using Vibrational Spectroscopy Techniques for Low-Resolution Food Fingerprinting. Applied Sciences, 15(11), 5948. https://doi.org/10.3390/app15115948






