Antibiofilm Potential of Natural Essential Oils
Abstract
1. Introduction
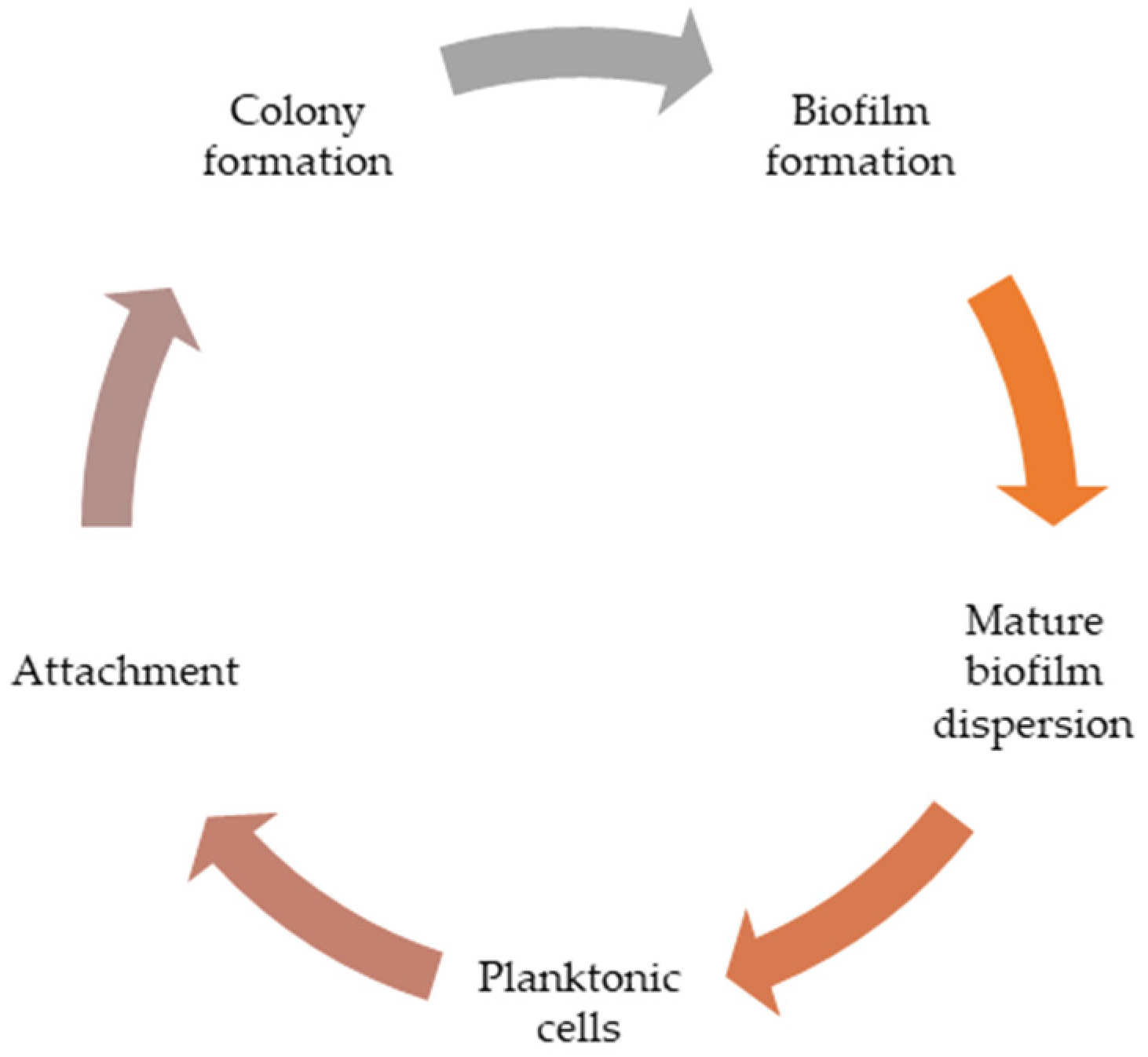
2. Biofilm: The Role in the Pathogenesis of the Disease
Biological Profile of Biofilm
3. New Natural AM Strategies
4. ABF Activity of EOs and Their Components
4.1. EOs as Antimicrobial Agents (Mechanisms of Action)
| Essential Oil Source | Pathogenic Microorganism | MIC | Reference |
|---|---|---|---|
| Croton conduplicatus Kunth (Euphorbiaceae) | S. aureus ATCC 25923 (MSSA) | 256 μg/mL | [33] |
| S. aureus ATCC 33591 (MRSA) | 512 μg/mL | [33] | |
| Foeniculum vulgare Mill. (Apiaceae) | S. aureus ATCC 29213 (MSSA) | 71.3 ±1.90 mg/mL | [34] |
| S. aureus ATCC 43300 (MRSA) | 60.30 ± 0.00 mg/mL | [34] | |
| S. aureus ATCC 6538 (MSSA) | 100.4 ± 34.80 mg/mL | [34] | |
| Hyssopus officinalis L. (Lamiaceae) | C. albicans | 0.9 ± 0.3 mg/mL | [32] |
| Origanum vulgare L. (Lamiaceae) | C. albicans * | 0.4 mg/mL | [32] |
| S. aureus * | 5 μL/mL | [35] | |
| S. aureus ** | 10 μL/mL | [35] | |
| Rosmarinus officinalis L. (Lamiaceae) | S. aureus * | 0.04% (v/v) | [36] |
| S. aureus ATCC 9144 | 1.25–2.5 μL/mL | [37] | |
| S. epidermidis S61 | 0.312–0.625 μL/mL | [37] | |
| Tagetes minuta L. (Asteraceae) | P. aeruginosa PAO1 | 312.5 μg/mL | [29] |
| Tetraclinis articulata (Vahl) Masters (Cupressaceae) | S. aureus ATCC 25923 | 0.38 nL/mL | [38] |
| Thymbra capitata L. (Lamiaceae) | P. aeruginosa (BL) | 1.11% | [39] |
| P. aeruginosa 10145 | 1.11% | ||
| Thymus vulgaris L. (Lamiaceae) | P. fluorescens KM121 | 20.0 μL/mL | [31] |
| S. aureus * | 0.02% (v/v) | [36] | |
| S. aureus ** | 0.63% (v/v) | [36] | |
| Candida albicans * | 0.4 mg/mL | [32] | |
| Haemophilus influenzae | 0.156 mg/mL | [30] | |
| H. parainfluenzae | 0.156 mg/mL | [30] | |
| P. aeruginosa | 1.50 mg/mL | [30] | |
| Zataria multiflora Boiss. (Lamiaceae) | P. aeruginosa ** | 4 μL/mL | [40] |
| Zingiber officinale Roscoe (Zingiberaceae) | S. mutans | 21.25 μL/mL | [41] |
4.2. EOs Compounds as Antimicrobial Agents
4.2.1. Phenolic Compounds
4.2.2. Terpene Compounds
5. Synergistic Effect of EO and Antibiotics
6. Toxic Activity of EOs
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarmah, P.; Dan, M.M.; Adapa, D.; Sarangi, T.K. A review on common pathogenic microorganisms and their impact on human health. Electronic. J. Biol. 2018, 14, 50–58. [Google Scholar]
- Boštíková, V.; Pasdiorová, M.; Marek, J.; Prášil, P.; Salavec, M.; Sleha, R.; Střtítecká, H.; Blažek, P.; Hanovcová, I.; Šošovičková, R.; et al. Biological factors influencing infectious diseases transmitted by invasive species of mosquitoes. Klin. Mikrobiol. Infekcni Lek. 2016, 22, 75–85. [Google Scholar]
- Bartlett, A.; Padfield, D.; Lear, L.; Bendall, R.; Vos, M. A comprehensive list of bacterial pathogens infecting humans. Microbiology 2022, 168, 001269. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, L.D.; Fonseca, Y.M.C.; Pérez, A.C.; Pérez, R.L. Current microbiological trends of microorganisms causing nosocomial infections. Microbes Infect. Dis. 2023, 4, 1150–1157. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Arampatzi, S.I.; Giannoglou, G.; Diza, E. Biofilm formation: A complicated microbiological process. Aristotle Univ. Med. J. 2011, 38, 21–29. [Google Scholar]
- Veena, B.R.; Shetty, K.V.; Saidutta, M.B. Characteristics of biofilms in bioreactors—A Review. In Proceedings of the 4th Nirma University International Conference on Engineering, Gujarat, India, 28–30 November 2013. [Google Scholar]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Szlauer, W.; Obłąk, E.; Paluch, E.; Baldy-Chudzik, K. Biofilm and methods of its eradication. Postep. Hig. Med. Dosw. 2019, 73, 397–413. [Google Scholar] [CrossRef]
- Zafer, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ghosh, S.; Bornman, C.; Elfaky, M.A. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Rep. Reg. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mangwani, N.; Dash, H.R.; Chauhan, A.; Das, S. Bacterial quorum sensing: Functional features and potential applications in biotechnology. J. Mol. Microbiol. Biotechnol. 2012, 22, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Juszczuk-Kubiak, E. Molecular aspects of the functioning of pathogenic bacteria biofilm based on quorum sensing (QS) signal-response system and innovative non-antibiotic strategies for their elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Poyrazoğlu Çoban, E.; Onur, M. Isolation and identification of biofilm-forming bacteria in foods supplied for consumption in open-air market stalls: A case study in Aydın province. Trak. Univ. J. Nat. Sci. 2023, 24, 77–84. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Mastoor, S.; Nazim, F.; Rizwan-Ul-Hasan, S.; Ahmed, K.; Khan, S.; Ali, S.N.; Abidi, S.H. Analysis of the antimicrobial and anti-biofilm activity of natural compounds and their analogues against Staphylococcus aureus isolates. Molecules 2022, 27, 6874. [Google Scholar] [CrossRef]
- Artini, M.; Papa, R.; Sapienza, F.; Božović, M.; Vrenna, G.; Assanti, V.T.G.; Sabatino, M.; Garzoli, S.; Fiscarelli, E.V.; Ragno, R.; et al. Essential oils biofilm modulation activity and machine learning analysis on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Microorganisms 2022, 10, 887. [Google Scholar] [CrossRef]
- Coelho, F.L.; Pereira, M.O. Exploring new treatment strategies for Pseudomonas aeruginosa biofilm infections based on plant essential oils. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex: Badajoz, Spain, 2013; pp. 83–89. [Google Scholar]
- Choe, H.-S.; Son, S.-W.; Choi, H.-A. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am. J. Infect. Control 2012, 40, 249–254. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Landini, P.; Valle, J. Microbial biofilms: From molecular mechanisms and structure to antimicrobial therapy. Microorganisms 2022, 10, 1638. [Google Scholar] [CrossRef]
- Song, X.; Xia, Y.-X.; He, Z.-D.; Zhang, H.-J. A Review of natural products with anti-biofilm activity. Curr. Org. Chem. 2018, 22, 788–816. [Google Scholar] [CrossRef]
- uzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’abrosca, B. Plant derived natural products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm activity and molecular mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.; Jesudhasan, P.; Pillai, S.; Patil, B. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Waheed, A.; Azeem, M.; Parveen, A.; Yameen, M.A.; Iqbal, J.; Ali, M.; Wang, S.; Qayyum, S.; Noor, A.; et al. Essential oil from Tagetes minuta has antiquorum sensing and antibiofilm potential against Pseudomonas aeruginosa strain PAO1. ACS Omega 2023, 8, 35866–35873. [Google Scholar] [CrossRef]
- Bakó, C.; Balázs, V.L.; Kerekes, E.; Kocsis, B.; Nagy, D.U.; Szabó, P.; Micalizzi, G.; Mondello, L.; Krisch, J.; Pethő, D.; et al. Flowering phenophases influence the antibacterial and anti-biofilm effects of Thymus vulgaris L. essential oil. BMC Complement. Med. Ther. 2023, 23, 168. [Google Scholar] [CrossRef]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Olkowicz, M.; Czaczyk, K. Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int. Biodeterior. Biodegrad. 2016, 114, 252–259. [Google Scholar] [CrossRef]
- Proškovcová, M.; Čonková, E.; Váczi, P.; Harčárová, M.; Malinovská, Z. Antibiofilm activity of selected plant essential oils from the Lamiaceae family against Candida albicans clinical isolates. Ann. Agric. Environ. Med. 2021, 28, 260–266. [Google Scholar] [CrossRef]
- de Oliveira, G.D.; da Rocha, W.R.V.; Rodrigues, J.F.B.; Alves, H.d.S. Synergistic and antibiofilm effects of the essential oil from Croton conduplicatus (Euphorbiaceae) against methicillin-resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Grygorcewicz, B.; Pruss, A.; Wojciuk, B.; Giedrys-Kalemba, S.; Dołęgowska, B.; Zielińska-Bliźniewska, H.; Olszewski, J.; Sienkiewicz, M.; Kochan, E. Synergistic effect of fennel essential oil and hydrogen peroxide on bacterial biofilm. Adv. Dermatol. Allergol. 2020, XXXVII, 690–694. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Brożyna, M.; Dudek, B.; Kozłowska, W.; Malec, K.; Paleczny, J.; Detyna, J.; Fabianowska-Majewska, K.; Junka, A. The chronic wound milieu changes essential oils’ antibiofilm activity—An in vitro and larval model study. Sci. Rep. 2024, 14, 2218. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Achmit, M.; Aoussar, N.; Mellouki, F.; Mhand, R.A.; Ibáñez, M.D.; Blázquez, M.A.; Akssira, M.; Zerouali, K.; Rhallabi, N. In vitro antibacterial and biofilm inhibitory activity of the sawdust essential oil of Tetraclinis articulata (Vahl) against catheter-associated Staphylococcus aureus clinical isolates. Curr. Res. Biotechnol. 2021, 3, 1–5. [Google Scholar] [CrossRef]
- Qaralleh, H. Thymol rich Thymbra capitata essential oil inhibits Quorum Sensing, virulence and biofilm formation of beta lactamase producing Pseudomonas aeruginosa. Nat. Prod. Sci. 2019, 25, 172–180. [Google Scholar] [CrossRef]
- Varposhti, M.; Abdi Ali, A.; Mohammadi, P.; Saboora, A. Effects of extracts and an essential oil from some medicinal plants against biofilm formation of Pseudomonas aeruginosa. J. Med. Microbiol. Infect. Dis. 2013, 1, 36–40. [Google Scholar]
- Kıvanç, A.K.; Kıvanç, M. Ginger essential oil inhibits biofilm formation of Streptococcus mutans on stainless steel placeholder. Med. Health Jun. 2023, 18, 159–169. [Google Scholar] [CrossRef]
- Abdullah; Asghar, A.; Algburi, A.; Huang, Q.; Ahmad, T.; Zhong, H.; Javed, H.U.; Ermakov, A.M.; Chikindas, M.L. Anti-biofilm potential of Elletaria cardamomum essential oil against Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748. Front. Microbiol. 2021, 12, 620227. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic compounds from new natural sources—Plant genotype and ontogenetic variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, H.; Nabilah, T.U.; Yudhawan, I.; Siregar, K.A.A.K.; Sammulia, S.F.; Fitriani. Investigation and development of anti polymicrobial biofilm from several essential oils: A Review. Biointerface Res. Appl. Chem. 2023, 13, 103. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.W.; Park, S.H.; Ha, S.D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Šegvić Klarić, M. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Karuppiah, V.; Thirunanasambandham, R.; Thangaraj, G. Anti-quorum sensing and antibiofilm potential of 1,8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J. Microbiol. Biotechnol. 2021, 37, 66. [Google Scholar] [CrossRef]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-cineol (eucalyptol) disrupts membrane integrity and induces oxidative stress in methicillin-resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef]
- Cheruvanachari, P.; Pattnaik, S.; Mishra, M.; Pragyandipta, P.; Naik, P.K. Terpinen-4-ol, an active constituent of kewda essential oil, mitigates biofilm forming ability of multidrug resistant Staphylococcus aureus and Klebsiella pneumoniae. J. Biol. Act. Prod. Nat. 2022, 12, 406–420. [Google Scholar] [CrossRef]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Ahamed, M.J.N.; Ibrahim, F.B.; Srinivasan, H. Synergistic interactions of antimicrobials to counteract the drug-resistant microorganisms. Biointerface Res. Appl. Chem. 2022, 12, 861–872. [Google Scholar] [CrossRef]
- Sharif, S.A.; Ismaeil, A.S.; Ahmad, A.A. Synergistic effect of different plant extracts and antibiotics on some pathogenic bacteria. Sci. J. Univ. Zakho 2020, 8, 7–11. [Google Scholar] [CrossRef]
- Simões, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and anti-biofilm properties of carvacrol alone and in combination with cefixime against Escherichia coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. These preliminary results suggest the formulation of a new generation of antimicrobial agents based on a combination of antimicrobial compounds with different origin. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef]
- Salvagno, L.; Sblano, S.; Fracchiolla, G.; Corbo, F.; Clodoveo, M.L.; Rosato, A. Antibiotics–Mentha piperita essential oil synergism inhibits mature bacterial biofilm. Chim. Oggi-Chem. Today 2020, 38, 14–17. [Google Scholar]
- Singh, S.; Chaurasia, P.K.; Bharati, S.L.; Golla, U. A mini-review on the safety profile of essential oils. MOJ Biol. Med. 2022, 7, 33–36. [Google Scholar] [CrossRef]
- Judzentiene, A.; Garjonyte, R. Compositional variability and toxic activity of mugwort (Artemisia vulgaris) essential oils. Nat. Prod. Comm. 2016, 11, 1353–1356. [Google Scholar] [CrossRef]
- Burlec, A.F.; Macovei, I.; Sacarescu, A.; Corciova, A.; Mircea, C.; Iancu, C.E.; Cioanca, O.; Hancianu, M. Essential oils in wellness centers: Overview on European Union legislation, potential therapeutic effects and toxicity. Farmacia 2020, 68, 992–998. [Google Scholar] [CrossRef]
- Tamburlin, I.S.; Roux, E.; Feuillée, M.; Labbé, J.; Aussaguès, Y.; El Fadle, F.E.; Fraboul, F.; Bouvier, G. Toxicological safety assessment of essential oils used as food supplements to establish safe oral recommended doses. Food Chem. Toxicol. 2021, 157, 112603. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurzyńska-Wierdak, R. Antibiofilm Potential of Natural Essential Oils. Appl. Sci. 2025, 15, 5847. https://doi.org/10.3390/app15115847
Nurzyńska-Wierdak R. Antibiofilm Potential of Natural Essential Oils. Applied Sciences. 2025; 15(11):5847. https://doi.org/10.3390/app15115847
Chicago/Turabian StyleNurzyńska-Wierdak, Renata. 2025. "Antibiofilm Potential of Natural Essential Oils" Applied Sciences 15, no. 11: 5847. https://doi.org/10.3390/app15115847
APA StyleNurzyńska-Wierdak, R. (2025). Antibiofilm Potential of Natural Essential Oils. Applied Sciences, 15(11), 5847. https://doi.org/10.3390/app15115847







