Unraveling the Immobilization Mechanisms of Biochar and Humic Acid on Heavy Metals: DOM Insights from EEMs-PARAFAC and 2D-COS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Pot Experiment and Characterization
2.3. DOM Extraction and Quenching Titration
2.4. Spectroscopic Determination
2.5. Parallel Factor Analysis and Complexation Modeling
2.6. Two-Dimensional Correlation Spectral Analysis
3. Results and Discussion
3.1. Effects of Biochar and Humic Acid on Heavy Metal Content in Crops and Soil
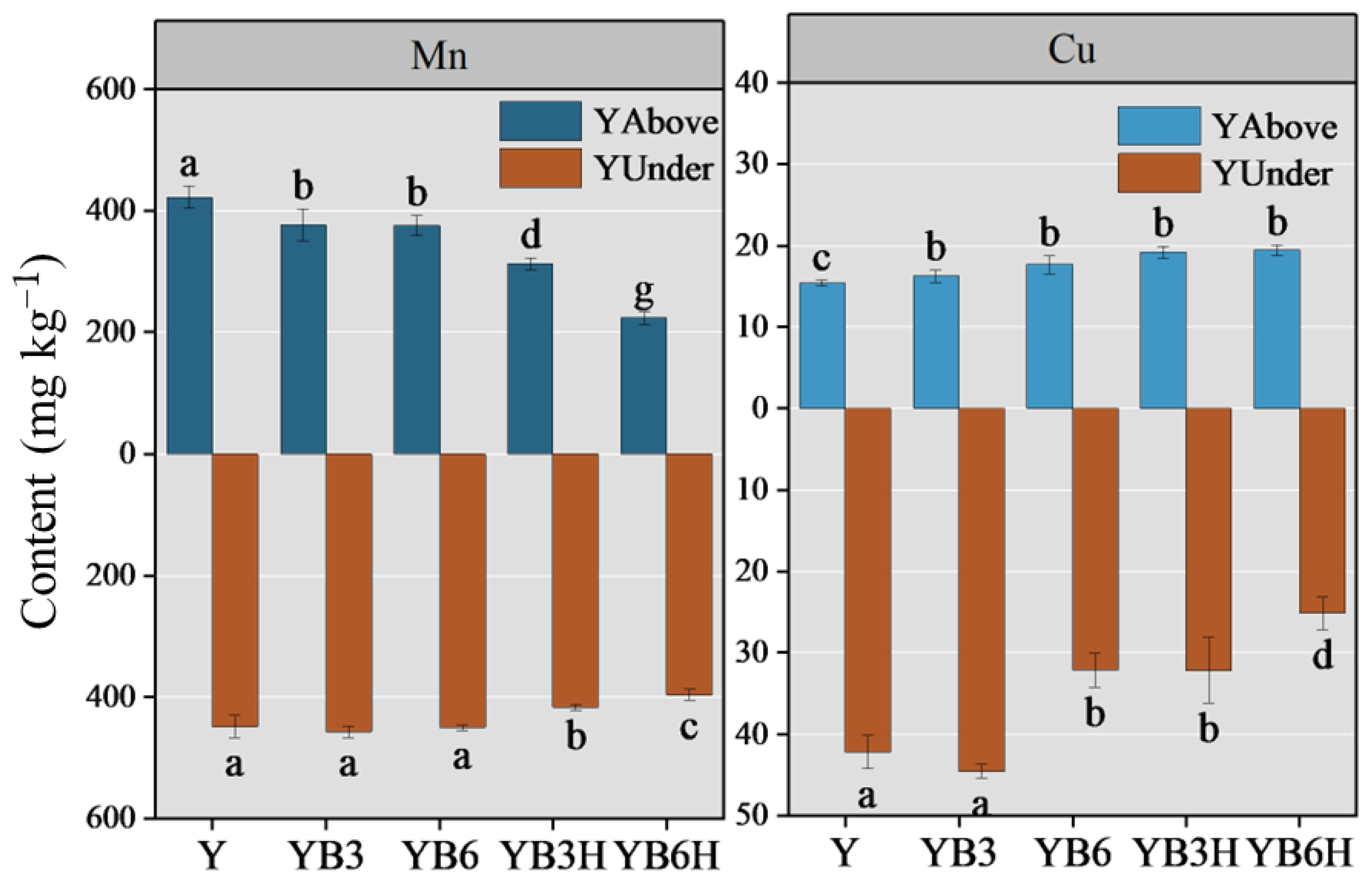
3.1.1. Heavy Metal Content in Crops
3.1.2. Heavy Metal Content and Morphology of Soils
3.2. Effect of Biochar and Humic Acid on the Content, Characterization and Component of DOM in Soil
3.2.1. Soil DOM Content
3.2.2. Soil DOM UV-Vis Absorption and Fluorescence Index
3.2.3. PARAFAC Analysis of Soil DOM
3.3. DOM and HM Bonding Capability and Its Characteristics
3.3.1. Quenching Curve of PARAFAC Component
3.3.2. Binding Capability of Humic Acid-like Substances to HM
3.3.3. DOM-HM Binding Order
3.4. Reflections on HM Remediation with Biochar/Biochar + HA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Ren, B.; Deng, X.; Yin, W.; Xie, Q.; Cai, Z.; Zou, H. Black Shale Bedrock Control of Soil Heavy Metal Typical High Geological Background in China Loushao Basin: Pollution Characteristics, Source and Influence Assessment Based on Spatial Analysis. J. Hazard. Mater. 2024, 477, 135072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Tian, B.; Li, J.; Luo, J.; Wang, X.; Ai, S.; Wang, X. Assessment of Soil Heavy Metal Pollution in Provinces of China Based on Different Soil Types: From Normalization to Soil Quality Criteria and Ecological Risk Assessment. J. Hazard. Mater. 2023, 441, 129891. [Google Scholar] [CrossRef]
- Yan, K.; Wang, H.; Lan, Z.; Zhou, J.; Fu, H.; Wu, L.; Xu, J. Heavy Metal Pollution in the Soil of Contaminated Sites in China: Research Status and Pollution Assessment over the Past Two Decades. J. Clean. Prod. 2022, 373, 133780. [Google Scholar] [CrossRef]
- Fei, X.; Lou, Z.; Xiao, R.; Ren, Z.; Lv, X. Source Analysis and Source-Oriented Risk Assessment of Heavy Metal Pollution in Agricultural Soils of Different Cultivated Land Qualities. J. Clean. Prod. 2022, 341, 130942. [Google Scholar] [CrossRef]
- Li, Z.; Shang, Q.; Zou, L.; Xing, Z.; Chen, G.; Chen, Z.; Zhou, J.; Liu, X. The Dynamic Response Mechanism of Crops to Manganese Uptake and Transfer Mediated by Different Intercropping Crop Attributes. J. Sci. Food Agric. 2024, 104, 9706–9718. [Google Scholar] [CrossRef] [PubMed]
- Szatyłowicz, E.; Krasowska, M. Assessment of Heavy Metals Leaching from Fly Ashes as an Indicator of Their Agricultural Use. Desalin. Water Treat. 2020, 199, 288–296. [Google Scholar] [CrossRef]
- Lupo, M.; Ajabshir, S.Z.; Sofia, D.; Barletta, D.; Poletto, M. Experimental Metrics of the Powder Layer Quality in the Selective Laser Sintering Process. Powder Technol. 2023, 419, 118346. [Google Scholar] [CrossRef]
- Lv, Y.; Kabanda, G.; Chen, Y.; Wu, C.; Li, W. Spatial Distribution and Ecological Risk Assessment of Heavy Metals in Manganese (Mn) Contaminated Site. Front. Environ. Sci. 2022, 10, 942544. [Google Scholar] [CrossRef]
- Ren, B.; Wang, Q.; Chen, Y.; Ding, W.; Zheng, X. Analysis of the Metals in Soil-Water Interface in a Manganese Mine. J. Anal. Methods Chem. 2015, 2015, 163163. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Zhang, Y.; Wang, J.; She, D. Synergistic Effects of Modified Biochar and Selenium on Reducing Heavy Metal Uptake and Improving Pakchoi Growth in Cd, Pb, Cu, and Zn-Contaminated Soil. J. Environ. Chem. Eng. 2024, 12, 113170. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Y.; Su, L.; Zou, W.; Wu, B.; Gao, W.; Hu, Z.; Li, A.; Zhou, Z.; Zhou, N. Oxygen-Limited Pyrolysis and Incineration Impact on Biochar Transport. Environ. Sci. Pollut. Res. Int. 2023, 30, 105247–105258. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Guo, G.; Zhao, L.; Niu, J.; Zhang, C. Soil Pollution Characteristics and Systemic Environmental Risk Assessment of a Large-Scale Arsenic Slag Contaminated Site. J. Clean. Prod. 2020, 251, 119721. [Google Scholar] [CrossRef]
- Lv, C.; Yang, S.; Chen, Y.; Xu, L.; Wang, A.; Zhang, Z.; Wang, S.; Yin, G.; Wei, Z.; Xia, Y.; et al. Biochar Derived from Tobacco Waste Significantly Reduces the Accumulations of Cadmium and Copper in Edible Parts of Two Vegetables: An in-Situ Field Study. Environ. Sci. Pollut. 2024, 31, 7533–7542. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Ding, X.; Khan, S.; Ayaz, T.; Fidel, R.; Khan, M.A. Biochar Efficacy for Reducing Heavy Metals Uptake by Cilantro (Coriandrum sativum) and Spinach (Spinaccia oleracea) to Minimize Human Health Risk. Chemosphere 2020, 244, 125543. [Google Scholar] [CrossRef]
- Li, Y.; Harir, M.; Uhl, J.; Kanawati, B.; Lucio, M.; Smirnov, K.S.; Koch, B.P.; Schmitt-Kopplin, P.; Hertkorn, N. How Representative Are Dissolved Organic Matter (Dom) Extracts? A Comprehensive Study of Sorbent Selectivity for Dom Isolation. Water Res. 2017, 116, 316–323. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Liu, X.; Gao, Y. Study on the Regulation Mechanism of Cadmium Adsorption System Mediated by Extraneous Dissolved Organic Matter. Ecotoxicol. Environ. Saf. 2021, 227, 112930. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Zhang, S.-B.; Zhao, M.-Y.; Zhao, Y.; Wei, Z.-M. Parallel Faction Analysis Combined with Two-Dimensional Correlation Spectroscopy Reveal the Characteristics of Mercury-Composting-Derived Dissolved Organic Matter Interactions. J. Hazard. Mater. 2020, 384, 121395. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xu, L.; Xu, Q.; Wu, D.; Ai, Y.; Li, D.; Liu, W.; Qu, J.; Wang, L.; et al. The Effect and Spectral Response Mechanism of Dissolved Organic Matter (Dom) in Pb(Ii) Adsorption onto Biochar. J. Environ. Chem. Eng. 2023, 11, 111115. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, P.; Guo, X. Oxidative Removal of Fluorescent Components from Soil Dom and Its Effect on Heavy Metals around Abandoned Mining Areas. Environ. Geochem. Health 2023, 46, 11. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Peng, Y.; Guo, X.; Li, Y.; Dai, B.; Huang, T. Study on the Complexation of Heavy Metals onto Biogas Slurry Dom Using Two-Dimensional Correlation Spectroscopy Combined with the Log-Transformed Synchronous Fluorescence Spectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 22878–22885. [Google Scholar] [CrossRef]
- Fan, T.; Yao, X.; Ren, H.; Ma, F.; Liu, L.; Huo, X.; Lin, T.; Zhu, H.; Zhang, Y. Multi-Spectroscopic Investigation of the Molecular Weight Distribution and Copper Binding Ability of Dissolved Organic Matter in Dongping Lake, China. Environ. Pollut. 2022, 300, 118931. [Google Scholar] [CrossRef] [PubMed]
- Feszterová, M.; Kowalska, M.; Hudec, M. Assessing the Impact of Soil Humic Substances, Textural Fractions on the Sorption of Heavy Metals (Cd, Pb). Appl. Sci. 2024, 14, 2806. [Google Scholar] [CrossRef]
- Tang, C.; Hou, J.; Liu, D.; Xi, B.; Li, J.; Yu, H. Applying Fluorescence Spectroscopy Coupled with Gaussian Band Fitting to Reveal Dynamic Variation Process of Humus Fractions from Riparian Soils Along an Urbanized River. Sci. Total Environ. 2024, 927, 172193. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, X.; Ablat, H.; Nurmamat, X.; Jia, H.; Wang, F.; Zhao, Z. Effect of the Molecular Weight of Humic Acids on the Adsorption of as(V) on Goethite. Water Air Soil Pollut. 2024, 235, 153. [Google Scholar] [CrossRef]
- Liu, M.; Han, X.; Guo, L.; Ding, H.; Lang, Y. Effects of Cu(Ii)-Dom Complexation on Dom Degradation: Insights from Spectroscopic Evidence. Sci. Total Environ. 2024, 921, 170928. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-L.; He, J.-Z.; Blaney, L.; Zhou, D.-M. Roxarsone Binding to Soil-Derived Dissolved Organic Matter: Insights from Multi-Spectroscopic Techniques. Chemosphere 2016, 155, 225–233. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Zhang, Z.-Y.; Gao, R.-T.; Tan, W.-B.; Cui, D.-Y. Insight into the Evolution, Redox, and Metal Binding Properties of Dissolved Organic Matter from Municipal Solid Wastes Using Two-Dimensional Correlation Spectroscopy. Chemosphere 2014, 117, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.-H.; An, Y.-C.; He, X.-S.; Yan, C.-L.; Jia, Y.-P.; Wang, H.-T.; He, L.-S. Fluorescent Characteristic and Compositional Change of Dissolved Organic Matter and Its Effect on Heavy Metal Distribution in Composting Leachates. Environ. Sci. Pollut. Res. 2018, 25, 18866–18878. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Qu, J. Effect of Dom Derived from Composting on the Changes of Pb Bioactivity in Black Soil. J. Environ. Chem. Eng. 2024, 12, 112232. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.-R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.-Y. Biochars Induced Modification of Dissolved Organic Matter (Dom) in Soil and Its Impact on Mobility and Bioaccumulation of Arsenic and Cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Xie, F.; Li, H.; Liu, Q.; Lai, L. Study on the Mechanism of Co-Pyrolysed Biochar on Soil Dom Evolution in Short-Term Cabbage Waste Decomposition. Chemosphere 2023, 344, 140291. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Valev. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Fan, C.-H.; Zhang, Y.-C.; Wang, J.-H. Effect of Temperatures and Lead Ions on 3d-Eems of Dissolved Organic Matter (Dom) Derived from Straw Humification. Spectrosc. Spect. Anal. 2015, 35, 3117–3122. [Google Scholar] [CrossRef]
- Jung, Y.M.; Noda, I. New Approaches to Generalized Two-Dimensional Correlation Spectroscopy and Its Applications. Appl. Spectrosc. Rev. 2006, 41, 515–547. [Google Scholar] [CrossRef]
- Du, F.; Yang, Z.; Liu, P.; Wang, L. Accumulation, Translocation, and Assessment of Heavy Metals in the Soil-Rice Systems near a Mine-Impacted Region. Environ. Sci. Pollut. Res. 2018, 25, 32221–32230. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks and Applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wu, J.; Huang, S.; Xin, L.; Zhao, Q. Competitive Adsorption Behaviors and Mechanisms of Cd, Ni, and Cu by Biochar When Coexisting with Microplastics under Single, Binary, and Ternary Systems. Sci. Total Environ. 2024, 913, 169524. [Google Scholar] [CrossRef]
- Alozie, N.; Heaney, N.; Lin, C. Biochar Immobilizes Soil-Borne Arsenic but Not Cationic Metals in the Presence of Low-Molecular-Weight Organic Acids. Sci. Total Environ. 2018, 630, 1188–1194. [Google Scholar] [CrossRef]
- Lv, X.; Yu, L.; Li, F.; Gong, J.; He, Y.; Chen, Z. Penta-Ms 2 (M = Mn, Ni, Cu/Ag and Zn/Cd) Monolayers with Negative Poisson’s Ratios and Tunable Bandgaps as Water-Splitting Photocatalysts. J. Mater. Chem. 2021, 9, 6993–7004. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Lei, M.; Alam, M.; Khan, M.A.; Khan, A. Biochar Characteristics, Applications and Importance in Health Risk Reduction through Metal Immobilization. Environ. Technol. Innov. 2020, 20, 101121. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, S.; Wu, J.; Yi, X.; Dai, G.; Shu, Y. Three-Year Field Experiments Revealed the Immobilization Effect of Natural Aging Biochar on Typical Heavy Metals (Pb, Cu, Cd). Sci. Total Environ. 2024, 912, 169384. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, B.; Wang, D.; Pu, Y.; Zhan, M.; Xu, X.; Wang, J.; Jiao, W. A Review on the Chemical Speciation and Influencing Factors of Heavy Metals in Municipal Solid Waste Landfill Humus. Waste Dispos. Sustain. Energy 2024, 6, 209–218. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, C.; Yu, Z.; Rong, H.; Zhang, C.; Zhou, S. Effect of Biochar on Transformation of Dissolved Organic Matter and Dtpa-Extractable Cu and Cd during Sediment Composting. Environ. Sci. Pollut. Res. 2022, 29, 27977–27987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, X.; Wang, Z.; Yang, X.; Li, S.; Liang, G.; Xie, X. Insight into Metal Binding Properties of Biochar-Derived Dom Using Eem-Parafac and Differential Absorption Spectra Combined with Two-Dimensional Correlation Spectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 13375–13393. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, X.; Li, J.; Luo, Y.; Li, J.; Pei, Y.; Jiang, J. The Spectral Characteristics of Biochar-Derived Dissolved Organic Matter at Different Pyrolysis Temperatures. J. Environ. Chem. Eng. 2021, 9, 106075. [Google Scholar] [CrossRef]
- Hua, H.; Liu, M.; Liu, C.-Q.; Lang, Y.; Xue, H.; Li, S.; La, W.; Han, X.; Ding, H. Differences in the Spectral Characteristics of Dissolved Organic Matter Binding to Cu(Ii) in Wetland Soils with Moisture Gradients. Sci. Total Environ. 2023, 874, 162509. [Google Scholar] [CrossRef]
- Wu, D.; Lu, Y.; Liang, H.; Ma, L.; Huang, T.; Li, R. Differences in Humic Acid Structure Extracted from Different Types of Peat. Int. J. Coal Prep. Util. 2024, 1–16. [Google Scholar] [CrossRef]
- Yue, Y.; Xu, L.; Li, G.; Gao, X.; Ma, H. Characterization of Dissolved Organic Matter Released from Aged Biochar: A Comparative Study of Two Feedstocks and Multiple Aging Approaches. Molecules 2023, 28, 4558. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, Z.; Song, H.; Li, K.; Lu, H.; Liu, Y.; Cheng, F. Biochar Induced Changes of Soil Dissolved Organic Matter: The Release and Adsorption of Dissolved Organic Matter by Biochar and Soil. Sci. Total Environ. 2021, 783, 147091. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Han, C.; Gao, L.; Wu, H.; Li, M. A New View into Three-Dimensional Excitation-Emission Matrix Fluorescence Spectroscopy for Dissolved Organic Matter. Sci. Total Environ. 2023, 855, 158963. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating Binding Characteristics of Cadmium and Copper to Dom Derived from Compost and Rice Straw Using Eem-Parafac Combined with Two-Dimensional Ftir Correlation Analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Liu, X.; Liu, Z.; Gu, P.; Deng, M.; Liu, Q. Periodical Changes of Dissolved Organic Matter (Dom) Properties Induced by Biochar Application and Its Impact on Downward Migration of Heavy Metals under Flood Conditions. J. Clean. Prod. 2020, 275, 123787. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Tian, Y.; Hou, Z.; He, K.; Fu, L.; Xu, H. Impact of Land Use on the Dom Composition in Different Seasons in a Subtropical River Flowing through a Region Undergoing Rapid Urbanization. J. Clean. Prod. 2019, 212, 1224–1231. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Ren, D.; Li, Y. Spatial Distribution of Sediment Dissolved Organic Matter in Oligotrophic Lakes and Its Binding Characteristics with Pb(Ii) and Cu(Ii). Environ. Sci. Pollut. Res. 2024, 31, 43369–43380. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Feng, S. Optical Property of Dissolved Organic Matter and Its Correlation with Heavy Metals in Surface Water around Metal Mines to Be Exploited in Southern Anhui Province, China. Water Supply 2022, 22, 6765–6776. [Google Scholar] [CrossRef]
- Chua, M.H.; Hui, B.Y.; Chin, K.L.; Zhu, Q.; Liu, X.; Xu, J. Recent Advances in Aggregation-Induced Emission (Aie)-Based Chemosensors for the Detection of Organic Small Molecules. Mater. Chem. Front. 2023, 7, 5561–5660. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (Aie): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.Y.; Yoon, S.; Kim, J.S. Fret-Derived Ratiometric Fluorescence Sensor for Cu2+. Tetrahedron 2008, 64, 1294–1300. [Google Scholar] [CrossRef]
- Susetyo, W.; Carreira, L.A.; Azarraga, L.V.; Grimm, D.M. Fluorescence Techniques for Metal-Humic Interactions. Fresenius’ J. Anal. Chem. 1991, 339, 624–635. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Y.; Liu, X.; Miao, T.; Guan, Q.; Yang, R.; Qu, J. Insight into Interactions of Heavy Metals with Livestock Manure Compost-Derived Dissolved Organic Matter Using Eem-Parafac and 2d-Ftir-Cos Analyses. J. Hazard. Mater. 2021, 420, 126532. [Google Scholar] [CrossRef]
- Rikta, S.Y.; Tareq, S.M.; Uddin, M.K. Toxic Metals (Ni2+, Pb2+, Hg2+) Binding Affinity of Dissolved Organic Matter (Dom) Derived from Different Ages Municipal Landfill Leachate. Appl. Water Sci. 2018, 8, 5. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Luo, N.; Yang, R.; Wen, J.; Huang, B.; Zeng, G. Application Potential of Biochar in Environment: Insight from Degradation of Biochar-Derived Dom and Complexation of Dom with Heavy Metals. Sci. Total Environ. 2019, 646, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Noor, A. Recent Developments in Two Coordinate Transition Metal Chemistry. Coord. Chem. Rev. 2023, 476, 214941. [Google Scholar] [CrossRef]
- He, C.; Liu, N.; Meng, W.; Li, Z. Characterization of Metal-Binding Behavior of Dom Component Structure in Biochar: Analysis of Binding Sites, Binding Sequences and Impact Pathways. J. Environ. Chem. Eng. 2024, 12, 113861. [Google Scholar] [CrossRef]
- Tang, J.; Zhuang, L.; Yu, Z.; Liu, X.; Wang, Y.; Wen, P.; Zhou, S. Insight into Complexation of Cu(Ii) to Hyperthermophilic Compost-Derived Humic Acids by Eem-Parafac Combined with Heterospectral Two Dimensional Correlation Analyses. Sci. Total Environ. 2019, 656, 29–38. [Google Scholar] [CrossRef]
- Verma, C.; Singh, A.; Singh, P.; Rhee, K.Y.; Alfantazi, A. Regioisomeric Effect of Heteroatoms and Functional Groups of Organic Ligands: Impacts on Coordination Bonding and Corrosion Protection Performance. Coord. Chem. Rev. 2024, 515, 215966. [Google Scholar] [CrossRef]









| SUVA254 | SUVA260 | FI | BIX | HIX | |
|---|---|---|---|---|---|
| Y | 9.96 ± 1.05 c | 8.80 ± 0.67 c | 2.31 | 0.81 | 3.64 |
| YB3 | 11.49 ± 0.46 c | 10.65 ± 0.44 c | 2.46 | 0.80 | 4.50 |
| YB6 | 12.28 ± 0.55 c | 11.35 ± 0.5 c | 2.31 | 0.76 | 4.75 |
| YB3H | 86.11 ± 2.51 a | 82.3 ± 2.5 a | 2.21 | 0.67 | 10.25 |
| YB6H | 28.71 ± 3.88 b | 28.48 ± 1.97 b | 2.06 | 0.68 | 11.25 |
| Log KM | R2 | f | ||
|---|---|---|---|---|
| Mn | BC-C1 | 2.01 | 0.88 | 0.61 |
| BC-C2 | 2.56 | 0.93 | 1.31 | |
| BC-C3 | 3.21 | 0.89 | 2.79 | |
| BC + H-C1 | 3.36 | 0.92 | 8.23 | |
| BC + H-C2 | 3.68 | 0.99 | 5.42 | |
| BC + H-C3 | 3.56 | 0.98 | 1.07 | |
| Cu | BC-C1 | 1.25 | 0.94 | 0.39 |
| BC-C2 | 0.87 | 0.84 | 0.48 | |
| BC-C3 | 1.30 | 0.97 | 0.54 | |
| BC + H-C1 | 0.71 | 0.82 | 0.69 | |
| BC + H-C2 | 0.73 | 0.82 | 0.54 | |
| BC + H-C3 | 0.86 | 0.83 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Q.; Li, Z.; Wang, J.; Zou, L.; Xing, Z.; Ni, J.; Liu, X.; Chen, G.; Chen, Z.; Jiang, Z. Unraveling the Immobilization Mechanisms of Biochar and Humic Acid on Heavy Metals: DOM Insights from EEMs-PARAFAC and 2D-COS Analysis. Appl. Sci. 2025, 15, 5803. https://doi.org/10.3390/app15115803
Shang Q, Li Z, Wang J, Zou L, Xing Z, Ni J, Liu X, Chen G, Chen Z, Jiang Z. Unraveling the Immobilization Mechanisms of Biochar and Humic Acid on Heavy Metals: DOM Insights from EEMs-PARAFAC and 2D-COS Analysis. Applied Sciences. 2025; 15(11):5803. https://doi.org/10.3390/app15115803
Chicago/Turabian StyleShang, Qiuyao, Zhixian Li, Jianwu Wang, Li Zou, Zhenan Xing, Jiaqi Ni, Xiling Liu, Guoliang Chen, Zhang Chen, and Zhichao Jiang. 2025. "Unraveling the Immobilization Mechanisms of Biochar and Humic Acid on Heavy Metals: DOM Insights from EEMs-PARAFAC and 2D-COS Analysis" Applied Sciences 15, no. 11: 5803. https://doi.org/10.3390/app15115803
APA StyleShang, Q., Li, Z., Wang, J., Zou, L., Xing, Z., Ni, J., Liu, X., Chen, G., Chen, Z., & Jiang, Z. (2025). Unraveling the Immobilization Mechanisms of Biochar and Humic Acid on Heavy Metals: DOM Insights from EEMs-PARAFAC and 2D-COS Analysis. Applied Sciences, 15(11), 5803. https://doi.org/10.3390/app15115803






