Abstract
Seafood-borne pathogens, especially Listeria monocytogenes, pose a significant risk to global health, with the formation of biofilm on abiotic surfaces exacerbating contamination risks in the seafood industry. This investigation evaluates the biofilm inhibition efficacy of fucoidan against L. monocytogenes biofilms on commonly used processing surfaces. The minimum inhibitory concentration (MIC) of fucoidan was determined to be 150 µg/mL, and sub-MIC concentrations (1/8, 1/4, and 1/2 MIC) were assessed for their effects on inhibition of biofilm. This action resulted in a substantial, dose-dependent reduction in formation of biofilm, with maximum reductions of 2.91 log CFU/cm2 on hand gloves (HG), 2.46 log CFU/cm2 on silicone rubber (SR), and 2.11 log CFU/cm2 on stainless steel (SS). Gene expression analysis via real-time quantitative polymerase chain reaction (RT-qPCR) revealed the downregulation of quorum-sensing (QS) and virulence-associated genes (flaA, fbp, prfA, hlyA, and agrA), indicating fucoidan’s potential to inhibition of biofilm and bacterial pathogenicity. These results emphasize fucoidan as a promising environmental antimicrobial agent for mitigating L. monocytogenes biofilm in seafood handling environments, thus improving food safety and reducing contamination risks.
1. Introduction
Foodborne illnesses, triggered by the ingestion of contaminated food, remain a pressing public health issue due to their rising incidence [1,2]. Within the seafood industry, biofilms on abiotic (food-contact) surfaces undermine food safety and significantly increases the potential for cross-contamination during handling, posing a serious risk to consumer health [3,4]. Biofilms are complex, structured communities of microorganisms that attach firmly to food and processing surfaces. These bacterial cells are embedded in a protective matrix composed of extracellular polymeric substances (EPS), which they secrete, making them more resistant to conventional sanitation methods [5]. Among foodborne pathogens, Listeria monocytogenes is a key cause of foodborne illness, being one of the most prevalent serovars [6]. This bacterium is responsible for listeriosis, a severe foodborne illness known for having the highest mortality rate among zoonotic infections, resulting in substantial social, medical, and economic impacts. According to the Centers for Disease Control and Prevention (CDC), salmonellosis is responsible for approximately 1600 infections and 260 fatalities each year in the United States [7].
The persistence of L. monocytogenes in food handling surroundings is largely attributed to its ability to form biofilms on food products and food-contact surfaces [1]. Bacteria within biofilms exhibit enhanced resistance to environmental stressors and antimicrobial treatments, making them particularly difficult to eradicate compared with planktonic cells [8]. The development of biofilms in food processing environments presents serious challenges to hygiene and food safety, while also compromising product quality, damaging processing equipment, and reducing overall operational efficiency [3]. Notably, biofilms are liable for approximately 80% persistent infections of bacteria, further complicating efforts to eliminate these pathogens and underscoring the need for effective control measures [9].
Quorum sensing (QS) and EPS possess pivotal responsibilities in the biofilm formation against L. monocytogenes, a major foodborne pathogen known for its persistence in food processing environments. The bacterial communication (QS) mechanism regulates gene expression in response to population density, influencing behaviors such as virulence, motility, and biofilm development. In L. monocytogenes, QS systems—particularly those involving the autoinducer-2 (AI-2) signaling molecule mediated by the luxS gene—coordinate biofilm maturation and surface adherence, contributing to the pathogen’s resistance to environmental stresses and sanitizers [3,8].
EPS (consists of polysaccharides, nucleic acids, proteins, and lipids), forms the structural matrix of biofilms, enabling cell-to-cell adhesion and attachment to surfaces. This matrix provides physical protection and also blocks nutrients and facilitates horizontal gene transfer, further enhancing bacterial survival. The formation of EPS is closely linked to QS-regulated gene expression, with QS signals upregulating genes involved in EPS production and maintenance [4].
Targeting QS pathways and EPS synthesis has appeared as an encouraging approach for reducing biofilm formation in L. monocytogenes. Natural antimicrobial agents, such as plant-derived polyphenols and polysaccharides, have shown potential in disrupting QS signaling and degrading EPS, weakening biofilm integrity, and making the bacteria more susceptible to antimicrobial treatments [9]. Understanding the role of QS and EPS in L. monocytogenes biofilms is essential for acquiring efficient approaches to control biofilm-related contamination in biotic (food) processing environments.
Given the increasing challenges associated with biofilm-mediated contamination, research efforts have intensified to develop novel antimicrobial strategies with enhanced efficacy. L. monocytogenes is particularly notorious for its ability to establish resilient biofilms, facilitating its persistence in food handling surroundings and its resistance to conventional sanitization methods [10,11,12]. In seafood processing facilities, the manifestation of L. monocytogenes biofilms on abiotic surfaces present a critical challenge to food safety, necessitating the development of groundbreaking methods to mitigate contamination risks and protect public health.
Fucoidan is a sulfated polysaccharide produced in significant quantities by various algal species, and its diverse biological properties against biofilm have been previously investigated [13,14,15]. It is believed to exhibit antibacterial activity by altering bacterial cell wall permeability, disrupting nutrient exchange, and inhibiting bacterial growth [16,17,18]. The increasing resistance of microorganisms to common antibiotics has intensified the search for alternative antibiofilm agent [19,20]. Fucoidan (natural compound) has gained attention due to their reported minimal adverse effects and cost-effectiveness, positioning them as promising candidates for antimicrobial drug development [21,22,23].
Food additive plant-derived polyphenols, such as quercetin, have shown promising antimicrobial and antibiofilm properties. Quercetin, a flavonoid with known antioxidant activity, has been demonstrated to significantly decrease Salmonella biofilms on food and abiotic (food-contact) surfaces at sub-inhibitory concentrations (31.25–125 µg/mL), with log CFU/cm2 reductions ranging from 1.50 to 2.61 [8]. In addition to reducing microbial counts, quercetin interferes with key bacterial functions by downregulating genes accompanying with avrA, hilA (virulence), rpoS (stress response), and luxS (QS), thereby disrupting coordinated biofilm development. Specifically, increasing concentrations of quercetin markedly suppressed swarming and swimming motility and the expression of flagellar function (flaA, flgL), biofilm-related (vp0952, vp0962), virulence (VopQ, vp0450), and QS (aphA, luxS) (p < 0.05). The reduction in viable cell counts ranged from 0.10 to 2.17 and 0.26 to 2.31 log CFU/cm2, respectively, on stainless steel (SS) and hand gloves (HG), depending on the quercetin concentration [24].
Recent studies suggest that fucoidan not only exerts antibacterial effects but also interferes with biofilm formation and destabilizes preformed in several bacterial species biofilms, highlighting its prospective role in preventing biofilm-associated contaminations [22]. Additionally, effectiveness in reducing L. monocytogenes biofilms on seafood surfaces, particularly HG, silicon rubber (SR), and SS, persists mostly unknown.
Aims to assess the effects of fucoidan at sub-minimum inhibitory concentrations (sub-MIC) on the reduction in biofilm of L. monocytogenes on commonly used seafood contact surfaces, including HG, SR, and SS. Furthermore, it examines how fucoidan influences the relative genes associated with biofilm development and pathogenecity at the molecular level. The outcomes of this research will offer important insights into the potential of fucoidan as a natural antibacterial solution in the seafood processing surroundings, supporting enhanced food safety.
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
Listeria monocytogenes (ATCC19113) was used as the model organism in this study. The strain was kept at −70 °C in tryptic soy broth (TSB) containing 30% glycerol, following a previously established protocol [22]. For culturing, 100 μL of the frozen stock was inoculated in 10 mL TSB (BD Difco, Franklin Lakes, NJ, USA) and incubated at 30 °C in a shaking incubator (Vision Scientific, VS–8480, Daejeon, Republic of Korea) at 200 rpm. After overnight incubation, 100 μL of the resulting culture was transferred into a fresh 10 mL TSB medium and incubated for an additional 24 h under the same conditions.
The suspension of bacteria was centrifuged at 5400 rpm for 10 min at 4 °C after 18 h of incubation. It was then twice rinsed with sterile phosphate-buffered saline (PBS, pH 7.4) and resuspended in the same buffer. Peptone water (PW; Oxoid, Basingstoke, England) was then used to dilute the final suspension to reach a standardized inoculum concentration of 105 Log CFU/mL. For additional research, this generated inoculum was then utilized to promote the creation of biofilms on abiotic surfaces.
2.2. Food-Contact Surfaces Preparation
Food-contact surface preparation was carried out with slight modifications based on previously reported protocols [24]. Silicone rubber (SR) (Komax Industrial Co., Ltd., Goyang-ro, Republic of Korea) was chosen due to its common use in food processing environments. Hand gloves (HG), silicone rubber (SR), and stainless steel (SS) coupons (dimensions: 2 cm × 2 cm × 0.1 cm) were prepared according to earlier studies [24]. To remove any residual organic material, the surfaces were thoroughly cleaned using sterile distilled water (DW). To eliminate any background microorganisms, each side of the coupons was exposed to UV-C light (wavelength and intensity were 254 nm and 120 mJ/cm2, respectively) for 15 min. Following sterilization, the sanitized coupons were dipped in 10 mL of TSB medium, which was inoculated with L. monocytogenes at an initial concentration of 105 log CFU/mL. The inoculated samples were incubated under static conditions at 30 °C for 24 h to allow for biofilm development prior to subsequent experimental procedures.
2.3. Fucoidan Preparation and MIC Determination
Fucoidan obtained from Sigma-Aldrich (St. Louis, MO, USA) and stock solution was prepared at a concentration of 1 mg/mL dissolved in sterile DW. A conventional microdilution technique, with previous reported methods, was used to determine the MIC of fucoidan against L. monocytogenes [25]. A variety of fucoidan concentrations were obtained in TSB by performing a two-fold serial dilution. A sterile 96-well microplate (Corning Incorporated, Corning, NY, USA) was filled with 100 µL of bacterial suspension that had been adjusted to 105 log CFU/mL and 100 µL of each diluted fucoidan solution (final concentrations: 0–300 µg/mL). For the MIC experiment, this led to a final amount of 200 µL/well.
Microbial growth was measured using a microplate reader (Spectra Max 190, Sunnyvale, CA, USA) for measuring absorbance at 600 nm after the plate was incubated for 24 h at 30 °C. In order to verify the MIC, 100 µL aliquots that showed no discernible microbial growth were cultured on Tryptic Soy Agar (TSA) for colony enumeration (Thermo Scientific, Oxoid, Basingstoke, UK).
Three duplicates of each experiment were carried out. A MIC of 150 µg/mL was found for fucoidan. To assess the impact of fucoidan on the production of L. monocytogenes biofilms at 0, 1/8, 1/4, and 1/2 MIC were used in the following tests.
2.4. Biofilm Inhibitionand the Detachment of Biofilm Process
The biofilm inhibition test was performed using a previous published methodology [26]. The MIC of fucoidan against L. monocytogenes was found to be 150 µg/mL in this investigation. Although not directly bactericidal, inhibition of biofilm was assessed at sub-MIC levels, which could include affected bacterial virulence characteristics. A control group devoid of fucoidan and test groups, which were administered fucoidan at 1/8, 1/4, and 1/2 MIC, were part of the experimental design.
In 50 mL Falcon tubes containing food-contact surface samples, 10 mL of TSB was mixed with fucoidan at the specified doses (0, 1/8, 1/4, and 1/2 MIC) to promote the development of biofilms. A vortex mixer (Scientific Industries, SI-0256, Bohemia, NY, USA) was used to homogenize the liquid after a 100 µL aliquot of microbial suspension (105 Log CFU/mL) was added. To enable the formation of biofilm, the samples were subsequently incubated for 24 h at 30 °C under static conditions.
After incubation, they were washed two times with sterile DW to eliminate any loosely attached bacteria. To remove the adhering bacteria, the surfaces were vortexed for 2 min after being submerged in 10 mL of peptone water (PW), including ten sterile glass beads. To count the colony, the recovered microbial solution was serially diluted and cultured on TSA. The colony-forming units (CFU) were counted following a 24 h incubation at 30 °C.
The antibiofilm effectiveness of fucoidan was measured by calculating the decrease in microbial populations (Log CFU/cm2) in comparison to the control.
2.5. RNA Extraction, Complementary DNA (cDNA) Synthesis, and Real-Time Quantitative PCR (RT–qPCR) Analysis
This study was carried out with slight modifications from established methods [27,28,29] to assess the influence of fucoidan on QS and virulence-related gene expression in L. monocytogenes. Bacterial suspensions treated with fucoidan (105 log CFU/mL) were inoculated in 10 mL of TSB within Falcon tubes and incubated at 30 °C for 24 h to promote biofilm development. Following incubation, the bacterial cells were harvested by centrifugation, and total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany).
A NanoDrop spectrophotometer (BioTek Instruments, Chicago, IL, USA) was used for measuring absorbance at 260/280 nm and 260/230 nm ratios to evaluate the concentration and purity of RNA. The Maxime RT PreMix (Ran-Damon Primer) kit (iNtRON Biotechnology Co., Ltd., Seoul, Republic of Korea) was used to synthesize cDNA. Table 1 lists the primer sequences for RT-qPCR, using 16S rRNA as the housekeeping reference gene.

Table 1.
PCR primer used for Real-Time PCR.
A 20 µL RT-qPCR reaction was performed using 1 µL of cDNA template, gene-specific primers, and Power SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Warrington, UK). Amplification was performed on a CFX Real-Time PCR System (Bio-Rad, Hercules, CA, USA) under the following cycle conditions: initial denaturation at 95 °C for 20 s, annealing at 50 °C for 20 s, and extension at 72 °C for 20 sec. A melting curve analysis was used to establish product specificity. The 2−ΔΔCt technique was used to determine relative gene expression levels.
2.6. Statistical Analysis
Each experiment was carried out in triplicate, and the results are described as mean and the standard error of the mean (SEM). To evaluate statistical significance, one-way analysis of variance (ANOVA) was employed and Duncan’s multiple range test for post hoc comparison was used. Differences were considered statistically significant at p < 0.05. All statistical analyses were carried out using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. MIC of Fucoidan
Fucoidan’s inhibitory impact was dose-dependent and varied among bacterial species. The MIC was regarded as the lowest possible concentration of fucoidan that resulted in no apparent bacterial growth. Additional studies were carried out to evaluate the MIC for antibacterial activity against L. monocytogenes. Fucoidan doses of 0 to 150 µg/mL were utilized and Fucoidan’s inhibitory impact was dose-dependent and varied among bacterial species. The MIC was regarded as the lowest possible concentration of fucoidan that resulted in no apparent bacterial growth. Additional studies were carried out to evaluate the MIC for antibacterial activity against L. monocytogenes.
Fucoidan doses of 0 to 150 µg/mL showed no significant influence on bacterial growth (p ≥ 0.05). Based on these findings, the sub-MIC range was established as 31.25–125 µg/mL. The MIC of fucoidan hostile to L. monocytogenes was determined to be 150 µg/mL. For further investigations, sub-MIC corresponding to 1/8, 1/4, and 1/2 MIC were employed to evaluate the impact of fucoidan on bacterial biofilm formation.
3.2. Anti-Biofilm Activity of Fucoidan on Food-Contact Surfaces Against L. monocytogenes
Fucoidan demonstrated significant anti-biofilm activity hostile to L. monocytogenes on HG, SR, and SS, as illustrated in Figure 1, Figure 2 and Figure 3. A concentration-dependent reduction in biofilm formation was observed, with higher fucoidan concentrations yielding greater inhibitory effects. On HG surfaces, biofilm formation was reduced by 0.50, 1.07, and 2.91 log CFU/cm2 at different concentrations corresponding to 1/8, 1/4, and 1/2 of the MIC, respectively (Figure 1). Likewise, biofilm inhibition on SR surfaces reached 0.24, 1.16, and 2.45 log CFU/cm2 at the same respective concentrations (Figure 2). On SS surfaces, biofilm inhibition values of 0.53, 1.18, and 2.11 log CFU/cm2 were recorded at concentrations of 1/8, 1/4, and 1/2 MIC, respectively (Figure 3).
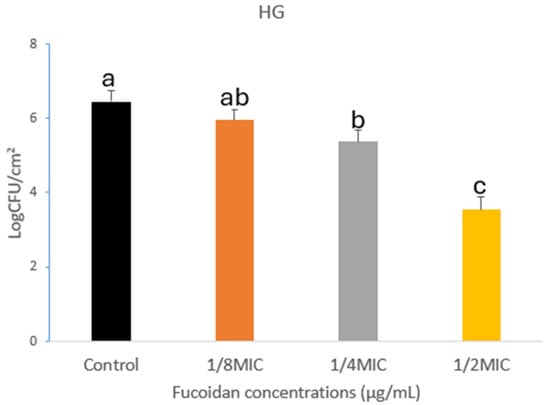
Figure 1.
Biofilm development of Listeria monocytogenes on HG surfaces. Data are shown as mean ± standard error of the mean (SEM) based on replicates (n = 3). Distinct letters (a–c) denote statistically significant differences at (p < 0.05).
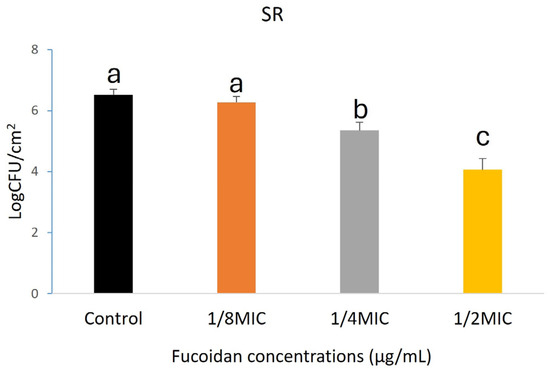
Figure 2.
Formation of Listeria monocytogenes biofilm on SR surfaces. Values represent the mean ± standard error of the mean (SEM) from three independent replicates (n = 3). Different lowercase letters (a–c) indicate significant differences (p < 0.05).
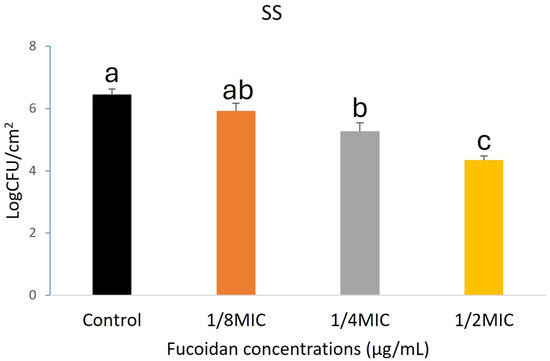
Figure 3.
The data shows the dose-dependent effect of fucoidan in reducing the biofilm of Listeria monocytogenes on the SS surface. Results are stated as mean ± SEM (n = 3), with statistically significant differences indicated by different letters (a–c) at p < 0.05.
3.3. Relative Expression Levels
Figure 4 illustrates the downregulation levels of virulence and stress response genes (flaA, fbp, prfA, hlyA, and agrA), measured by RT-qPCR, in response to sub-inhibitory concentrations of fucoidan (ranging from 0 to 125 µg/mL). Gene expression was significantly downregulated at different concentrations of fucoidan (p < 0.05). The fold changes in gene expression for flaA, fbp, prfA, hlyA, and agrA at control, 1/8, 1/4, and 1/2 MIC were as follows: flaA—1.00, 0.864, 0.537, 0.308; fbp—1.00, 0.850, 0.475, 0.321; prfA—1.00, 0.825, 0.546, 0.364; hlyA—1.00, 0.824, 0.524, 0.364; agrA—1.00, 0.856, 0.570, 0.351. These results indicate that fucoidan treatment affects the demonstration of key genes associated with bacterial pathogenocity and QS in L. monocytogenes.
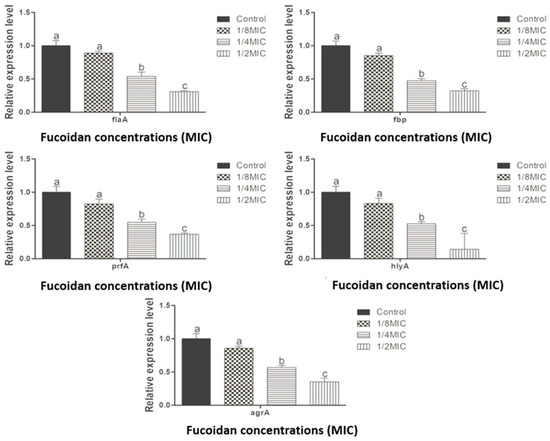
Figure 4.
Downregulation levels of agrA, prfA, fbp, flaA, and hylA genes in Listeria monocytogenes culture under control, 1/8 MIC, 1/4 MIC, and 1/2 MIC quercetin treatments. Data represent the mean of three replicates (n = 3). Different letters (a–c) indicate significant differences (p < 0.05).
4. Discussion
The effectiveness of fucoidan in preventing L. monocytogenes inhibition of biofilm on abiotic surfaces and examined its possible modes of action. The findings showed that fucoidan significantly inhibited biofilm development and disrupted already-formed biofilms, even at concentrations below the MIC. Fucoidan, a sulfated polysaccharide extracted from Fucus vesiculosus, is known for its antimicrobial activity against various foodborne pathogens and dental plaque bacteria [30,31]. Earlier research has reported MIC values for fucoidan between 125 and 1000 µg/mL [32]. Regarding its antibiofilm effects, 250 µg/mL were particularly efficient in suppressing of Streptococcus mutans and S. sobrinus biofilm formation and planktonic cells [32]. Data highlight the prospect of fucoidan as an environmental antibacterial negotiator suitable for applications in the seafood processing industry.
Biofilm formation on abiotic surfaces tips to persistent contamination and resistance to conventional sanitation methods, presenting ongoing challenges in seafood production [33]. In the current study, fucoidan significantly reduced L. monocytogenes biofilm formation on HG, SR, and SS surfaces, with the most pronounced effects monitored at 1/2 MIC and MIC levels (see Figure 1, Figure 2 and Figure 3). Comparable antibiofilm effects of fucoidan have also been reported against Staphylococcus aureus and Escherichia coli [34]. The MIC of 150 µg/mL for L. monocytogenes identified here aligns with previous findings for Gram-negative bacteria like Pseudomonas aeruginosa and E. coli, which reported MIC values between 100 and 200 µg/mL [35]. These observations support the broad-spectrum and concentration-dependent antibacterial activity of fucoidan. Notably, the biofilm-associated bacterial counts were reduced by up to 2.91 log CFU/cm2 on HG, 2.46 log CFU/cm2 on SR, and 2.11 log CFU/cm2 on SS surfaces.
In addition, the antimicrobial efficacy of fucoidan F85 was evaluated against Streptococcus mutans in Brain Heart Infusion (BHI) broth at 0 to 250 µg/mL. The untreated group, which served as a baseline for bacterial growth, demonstrated a substantial proliferation—rising from an initial inoculum of approximately 5.2 log CFU/mL to around 8.6 log CFU/mL within 18 h. This elevated bacterial load remained stable over the next 30 h, with only a modest decline of about 1 log unit observed by the 48 h mark, likely due to nutrient depletion or accumulation of toxic metabolic by-products.
Conversely, treatment with fucoidan at 125 µg/mL presented a strong bacteriostatic effect, completely inhibiting the growth of S. mutans throughout the 48 h incubation period. Notably, the highest tested concentration (250 µg/mL) exerted a pronounced bactericidal action, resulting in a sustained and progressive decline in viable cell counts. By the end of the 48 h period, the bacterial population had dropped to approximately 3.3 log CFU/mL—indicating a substantial reduction both the untreated control and the lower concentration group [22].
These findings highlight not only the dose-dependent antimicrobial activity of fucoidan but also its dual functional potential: as a growth inhibitor at moderate concentrations and as a bactericidal agent at higher doses. The progressive decrease in viable bacterial counts at 250 µg/mL suggests membrane disruption or interference with critical cellular processes, meriting further mechanistic studies. This research underscores the potential application of fucoidan as an effective natural antimicrobial, especially for targeting oral pathogens like S. mutans, and potentially extending its utility to foodborne pathogens and biofilm-related contamination in food systems [32].
In addition to its inhibitory effects on biofilm formation, fucoidan also downregulated genes (flaA, fbp, prfA, hlyA, and agrA) in L. monocytogenes biofilms. These genes play critical roles in bacterial communication, biofilm formation, and pathogenicity. The observed downregulation suggests that fucoidan interferes with these molecular pathways, preventing biofilm formation and destabilizing pre-existing biofilms [36]. These findings align with previous research suggesting that natural polysaccharides, like fucoidan, can act as potent antibiofilm agents by targeting QS, stress response, and pathogenecity mechanisms [37,38,39].
The seafood sector is facing substantial issues, which offer significant food safety and global health hazards. It reveals fucoidan’s potential as an environmental therapy for various difficulties. Fucoidan has various advantages over typical compound sanitizers, including the fact that it is naturally sourced, low in toxicity, and environmentally benign [30,40]. Furthermore, its efficacy at sub-MIC levels makes it more industrially applicable by lowering costs and minimizing the possibility of antibiotic resistance [41].
In comparison, fucoidan (Fucus vesiculosus) has recently garnered attention for its wide-ranging antimicrobial and antibiofilm actions. Fucoidan has shown efficacy hostile to both Gram-positive and Gram-negative bacteria, involving Salmonella spp., with MICs ranging between 125 and 150 µg/mL [8,22]. Like quercetin, fucoidan can inhibit biofilm formation on food-contact surfaces such as SS, HG, and SR, and it also modulates bacterial gene expression related to quorum sensing, stress tolerance, and virulence [8]. A sensory assessment was performed on fucoidan-treated groups, incorporating various concentrations of fucoidan over multiple storage intervals [42]. Findings indicated butter samples with 0.5% fucoidan showed a marked decrease in acid and peroxide values, thereby enhancing shelf life, as confirmed through sensory testing [43]. On the first day, no differences in taste were detected across all samples at fucoidan applications, there were no negative impacts on flavor. As fucoidan levels increased, the butter’s color became marginally lighter, and textural improvements such as greater softness and spreadability were observed. By the 40th day of storage, a slight development of a rancid taste was present in all samples, with the control exhibiting the most intense off-flavor. In contrast, samples treated with higher levels of fucoidan retained acceptable sensory attributes and did not develop undesirable organoleptic qualities [44]. Overall, the shelf life—the time frame throughout food product remains safe, nutritious, and sensorially acceptable—was significantly extended in the fucoidan-treated butter.
Given their different molecular structures and bioactivities—polyphenolic versus polysaccharide—comparative research into natural compounds like quercetin and fucoidan is critical to identify effective, safe, and sustainable biofilm control strategies in food systems. This study builds on this growing body of knowledge by further exploring fucoidan’s antibiofilm effects, with particular attention to its mechanism of action and practical implications in seafood processing environments.
5. Limitations and Future Directions
Several limitations need to be addressed. The efficacy of fucoidan should be validated in seafood handling environments, as the current study was conducted in a controlled laboratory setting. Additionally, further research is required to evaluate the cost-effectiveness and potential impacts on the sensory attributes of seafood products [45,46]. Furthermore, it is essential to comprehensively assess the sensory implications of applying fucoidan to seafood products. Despite its natural origin and low toxicity, fucoidan may alter key organoleptic attributes—such as flavor, texture, aroma, and visual appearance—which could influence consumer preference and acceptance. Future investigations should therefore include sensory evaluation to ensure that the quality of seafood products remains unaffected following fucoidan treatment.
In addition, practical challenges related to fucoidan’s formulation and application need to be thoroughly explored. Its physicochemical stability under different processing conditions—including variations in temperature, pH, and interaction with other food-grade compounds—must be characterized. Suitable delivery systems, such as edible films, surface sprays, or wash treatments, should be developed and refined to guarantee consistent and efficient application for optimal biofilm suppression. Moreover, the industrial viability of fucoidan depends on the ability to produce it at scale with consistent purity and bioactivity. Establishing standardized manufacturing protocols is therefore critical for ensuring reproducibility and enabling commercial implementation.
The antimicrobial effectiveness of fucoidan in environments containing complex food residues, such as proteins or lipids, remains unexplored and represents a critical gap in its practical application. Proteins and fats may bind to or shield bacterial cells, potentially reducing the availability and efficacy of fucoidan at the site of microbial adhesion or biofilm formation.
Therefore, future studies should be designed to assess fucoidan’s antibacterial and antibiofilm performance under conditions that closely mimic actual food processing environments. Investigating its stability and activity in the presence of meat exudates, fish oils, or dairy residues will provide valuable insights into its functional reliability. This will help determine whether higher concentrations or alternative formulations are required to maintain its antimicrobial potency under such challenging conditions. Addressing this limitation is essential for validating fucoidan’s suitability for use in food industry applications where organic contaminants are routinely encountered.
Future studies should also explore the synergistic effects of fucoidan with other natural antimicrobials to enhance its efficacy against biofilm-associated infections.
Therefore, this study suggests that fucoidan could serve as a safe and effective natural antibiofilm agent to combat L. monocytogenes on food-contact surfaces. It offers a viable strategy for improving food safety and minimizing microbial contamination in the seafood industry. Further research is needed to expand its application to other foodborne pathogens and industrial contexts, maximizing its potential as a natural antimicrobial intervention.
6. Conclusions
Fucoidan exhibits significant antimicrobial and antibiofilm activity against L. monocytogenes on seafood surfaces, leading to a reduction in bacterial counts by 2.91 log CFU/cm2 on HG, 2.45 log CFU/cm2 on SR, and 2.11 log CFU/cm2 on SS at sub-MIC (31.25–125 µg/mL). The downregulation of the virulence gene indicated that it has antimicrobial effects against foodborne pathogens. Due to its natural origin, low toxicity, and effectiveness at sub-MIC levels, fucoidan presents a promising, sustainable alternative to conventional chemical disinfectants in the seafood industry. Further studies are required to evaluate its practical applications in industrial settings, explore potential synergistic effects with other antimicrobials, and assess its impact on seafood product quality. These findings highlight fucoidan’s potential as an effective tool for enhancing food safety and minimizing microbial contamination in the seafood sector.
Author Contributions
Conceptualization, A.R.; methodology, A.R. and P.K.R.; software, A.R.; validation, A.R. and P.K.R.; formal analysis, A.R. and P.K.R.; investigation, A.R.; resources, A.R.; data curation, A.R. and P.K.R.; writing—original draft preparation, A.R. and P.K.R.; writing—review and editing, A.R. and P.K.R.; visualization, A.R.; supervision, S.Y.P.; project administration, S.Y.P.; funding acquisition, S.Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, grand number 2021R1I1A3A04037468. This research was also supported by the National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Korea (R2025055).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rohilla, A.; Kumar, V.; Ahire, J.J. Unveiling the persistent threat: Recent insights into Listeria monocytogenes adaptation, biofilm formation, and pathogenicity in foodborne infections. J. Food Sci. Technol. 2024, 61, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Dawan, J.; Zhang, S.; Ahn, J. Recent advances in biofilm control technologies for the food industry. Antibiotics 2025, 14, 254. [Google Scholar] [CrossRef]
- Roy, P.K.; Kim, S.H.; Jeon, E.B.; Park, E.H.; Park, S.Y. Inhibition of Listeria monocytogenes cocktail culture biofilms on crab and shrimp coupons and the expression of biofilm-related genes. Antibiotics 2023, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Huamán, B.R.H.; Vega-Sánchez, A.; Rolón-Verdún, P.; Gervilla-Cantero, G.; Rodríguez-Jerez, J.J.; Ripolles-Avila, C. Effect of Cinnamomum cassia essential oil combined with enzymes on the elimination and regrowth potential of Listeria monocytogenes and Salmonella enterica biofilms formed on stainless steel surfaces. Food Control 2025, 172, 111120. [Google Scholar] [CrossRef]
- Yang, P.; Huo, Y.; Yang, Q.; Zhao, F.; Li, C.; Ju, J. Synergistic anti-biofilm strategy based on essential oils and its application in the food industry. World J. Microbiol. Biotechnol. 2025, 41, 81. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Younce, F.; Rasco, B.; Ryu, D.; Tang, J.; Zhu, M.J. Enhanced steam-sanitizer strategies for eliminating Listeria biofilms on food-contact surfaces. Food Control 2025, 169, 111020. [Google Scholar] [CrossRef]
- CDC. Listeria Infection (Listeriosis). Available online: https://www.cdc.gov/listeria/ (accessed on 15 March 2025).
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Giaouris, E.; Habimana, O. The battle against biofilms: A focus on novel antimicrobial strategies and their mechanisms of action. Antibiotics 2025, 14, 111. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Ashrafudoulla, M.; Nahar, S.; Joo, H.J.; Jahid, I.K.; Park, S.H.; Kim, K.S.; Ha, S.D. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT 2020, 118, 108864. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Yamik, D.Y.; Vetboocha, N.; Vongkamjan, K. Effect of novel phage cocktail on Salmonella recovered from broiler sources and its anti-biofilm effect on food contact surface model. Food Control 2025, 169, 111000. [Google Scholar] [CrossRef]
- Alsuwat, M.A.; Shah, A.A.; Ullah, S.; Khan, R.U.; Alissa, M.; Khan, M.S. Microbial biofilm formation to mitigate foodborne pathogens strategies and control measures. Indian. J. Microbiol. 2025, 65, 1–16. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Kang, M.G.; Jo, D.M.; Cho, K.J.; Kim, Y.M. Inhibition of polymicrobial biofilms of Candida albicans-Staphylococcus aureus/Streptococcus mutans by fucoidan-gold nanoparticles. Mar. Drugs 2023, 21, 123. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Shang, N.; Wu, K.; Liao, W. Recent advances in the structure, extraction, and biological activity of sargassum fusiforme polysaccharides. Mar. Drugs 2025, 23, 98. [Google Scholar] [CrossRef]
- Vladkova, T.G.; Staneva, A.D.; Avramova, I.A.; Ivanova, I.A.; Gospodinova, D.N. Fucoidan-containing, low-adhesive siloxane coatings for medical applications: Inhibition of bacterial growth and biofilm development. Materials 2023, 16, 3651. [Google Scholar] [CrossRef]
- Nazari, M.; Taheri, M.; Nouri, F.; Bahmanzadeh, M.; Alikhani, M.Y. The antimicrobial and antibiofilm effects of gentamicin, imipenem, and fucoidan combinations against dual-species biofilms of Staphylococcus aureus and Acinetobacter baumannii isolated from diabetic foot ulcers. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Improving potential strategies for biological activities of phlorotannins derived from seaweeds. Crit. Rev. Food Sci. Nutr. 2025, 65, 833–855. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of bacterial biofilm: Insights into antibiotic resistance mechanisms and therapeutic intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef]
- Jeong, G.J.; Khan, F.; Tabassum, N.; Cho, K.J.; Kim, Y.M. Strategies for controlling polymicrobial biofilms: A focus on antibiofilm agents. Int. J. Antimicrob. Agents 2024, 64, 107243. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.; Munawar, A.; Nawaz, Z.; Hussain, N.; Hafeez, A.B.; Szweda, P. Antibiotic resistance and preventive strategies in foodborne pathogenic bacteria: A comprehensive review. Food Sci. Biotechnol. 2025, 34, 2101–2129. [Google Scholar] [CrossRef]
- Elkhalifa, A.M.E.; Nazar, M.; Ali, S.I.; Khursheed, I.; Taifa, S.; Ahmad Mir, M.; Shah, I.H.; Malik, M.; Ramzan, Z.; Ahad, S.; et al. Novel therapeutic agents for management of diabetes mellitus: A hope for drug designing against Diabetes mellitus. Life 2024, 14, 99. [Google Scholar] [CrossRef]
- Roy, A.; Roy, P.K.; Cho, S.R.; Park, S.Y. Effects of fucoidan on the inhibition of biofilm formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on seafoods and its molecular antibiofilm mechanisms. Microorganisms 2025, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Anestopoulos, I.; Kiousi, D.E.; Klavaris, A.; Maijo, M.; Serpico, A.; Suarez, A.; Sanchez, G.; Salek, K.; Chasapi, S.A.; Zompra, A.A.; et al. Marine-derived surface active agents: Health-promoting properties and blue biotechnology-based applications. Biomolecules 2020, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Song, M.G.; Jeon, E.B.; Kim, S.H.; Park, S.Y. Antibiofilm efficacy of quercetin against Vibrio parahaemolyticus biofilm on food-contact surfaces in the food industry. Microorganisms 2022, 10, 1902. [Google Scholar] [CrossRef] [PubMed]
- McGurrin, A.; Suchintita Das, R.; Soro, A.B.; Maguire, J.; Flórez Fernández, N.; Dominguez, H.; Torres, M.D.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobial activities of polysaccharide-rich extracts from the Irish seaweed Alaria esculenta, generated using green and conventional extraction technologies, against foodborne pathogens. Mar. Drugs 2025, 23, 46. [Google Scholar] [CrossRef]
- Kim, S.H.; Roy, P.K.; Park, S.Y. Synergistic effects of combined flavourzyme and floating electrode-dielectric barrier discharge plasma on reduction of Escherichia coli biofilms in squid (Todarodes pacificus). Microorganisms 2024, 12, 1188. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Park, J.; Toushik, S.H.; Shaila, S.; Ha, A.J.W.; Rahman, M.A.; Park, S.H.; Ha, S.D. Synergistic mechanism of UV-C and postbiotic of Leuconostoc mesenteroides (J. 27) combination to eradicate Salmonella thompson biofilm in the poultry industry. Food Control 2024, 164, 110607. [Google Scholar] [CrossRef]
- Roy, P.K.; Kim, G.; Fang, X.; Hassan, B.; Soysa, M.D.; Shin, S.T.; Cho, J.K. Optimization of post-activation systems to improve the embryonic development in porcine parthenogenesis and somatic cell nuclear transfer. J. Embryo Transf. 2017, 32, 95–104. [Google Scholar] [CrossRef]
- Roy, P.K.; Fang, X.; Hassan, B.; Shin, S.T.; Cho, J.K. Effects of roscovitine on in vitro development of porcine oocyte using brilliant cresyl blue. J. Embryo Transf. 2017, 32, 111–122. [Google Scholar] [CrossRef]
- Cao, J.; Qin, L.; Liu, M.; Yao, M.; Wang, K.; Lin, H.; Qu, C.; He, Y.; Xue, C.; Miao, J. Fucoidan from sea cucumber cooking liquid: Structural analysis, physicochemical properties, and anti-Helicobacter pylori potential. Int. J. Biol. Macromol. 2025, 306, 141593. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Gemba, M.; Rosiak, E.; Kołożyn-Krajewska, D. Development of predictive models of biofilm formation by C. sakazakii, E. cloacae on surfaces used in the food industry and medicine. Int. J. Food Microbiol. 2025, 434, 111131. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, J.; Viswanathan, P. Anti-bacterial and antibiofilm properties of seaweed polysaccharide-based nanoparticles. Aquac. Int. 2023, 31, 2799–2823. [Google Scholar] [CrossRef]
- Khan, F.; Manivasagan, P.; Lee, J.-W.; Pham, D.T.N.; Oh, J.; Kim, Y.-M. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar. Drugs 2019, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Rizzo, M.G.; Spano, A.; Spagnuolo, D.; Di Martino, A.; Morabito, M.; Manghisi, A.; Genovese, G.; Guglielmino, S.; Calabrese, G.; et al. Effects of crude polysaccharides from marine macroalgae on the adhesion and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Algal Res. 2022, 63, 102646. [Google Scholar] [CrossRef]
- Han, S.; Choi, M.W.; Byun, K.H.; Kim, B.H.; Song, M.S.; Kang, I.; Ha, S.D. Characterization of Salmonella ser. Enteritidis-specific bacteriophages and biocontrol strategy to reduce S. Enteritidis on egg products using bacteriophages and essential oil compounds. Food Control 2024, 160, 110304. [Google Scholar] [CrossRef]
- Shivaprasad, D.P.; Kaushik, A.; Taneja, N.K.; Lakra, A.; Bharadwaj, D.K.; Juneja, V.K.; Taneja, P.; Chauhan, K.; Oberoi, H.S. Breaking the biofilm barrier: Vitamin C as a novel strategy against multidrug-resistant Salmonella typhimurium. Int. J. Food Sci. Technol. 2025, 60, vvae082. [Google Scholar] [CrossRef]
- Lawal, H.; Saeed, S.I.; Gaddafi, M.S.; Kamaruzzaman, N.F. Green nanotechnology: Naturally sourced nanoparticles as antibiofilm and antivirulence agents against infectious diseases. Int. J. Microbiol. 2025, 8746754. [Google Scholar] [CrossRef]
- Galinskaitė, A.; Gruškienė, R.; Kavleiskaja, T.; Stanevičienė, R.; Servienė, E.; Sereikaitė, J. Bioactive fucoidan-based three-component colloidal particles for food safety. Food Bioprocess. Technol. 2025, 18, 5621–5633. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Thirugnanasambantham, M.K.; Khan, F.; Mani, A.K. Bacteria-inspired synthesis of silver-doped zinc oxide nanocomposites: A novel synergistic approach in controlling biofilm and quorum-sensing-regulated virulence factors in Pseudomonas aeruginosa. Antibiotics 2025, 14, 59. [Google Scholar] [CrossRef]
- Krishnan, L.; Ravi, N.; Mondal, A.K.; Akter, F.; Kumar, M.; Ralph, P.; Kuzhiumparambil, U. Seaweed-based polysaccharides–review of extraction, characterization, and bioplastic application. Green. Chem. 2024, 26, 5790–5823. [Google Scholar] [CrossRef]
- Alipour, A.; Marhamatizadeh, M.H.; Mohammadi, M. Studying the shelf life of butter containing fucoidan, by evaluating sensory and chemical properties. Food Sci. Nutr. 2023, 11, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z.; Kačániová, M. The effect of the addition of hemp seeds, Amaranth, and Golden flaxseed on the nutritional value, physical, sensory characteristics, and safety of poultry Pâté. Appl. Sci. 2022, 12, 5289. [Google Scholar] [CrossRef]
- Pittia, P.; Heer, M. Space food for the future: Nutritional challenges and technological strategies for healthy and high-quality products. In In-Space Manufacturing and Resources: Earth and Planetary Exploration Applications; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 58, pp. 251–268. [Google Scholar]
- Palafox Félix, S.; Sandoval Larios, G.; Cabrera, R.; García-Galaz, A.; Huerta-Ocampo, J.Á.; Guzmán-Partida, A.M.; Armenta Corral, R.I.; Sarabia-Sainz, J.A.; Ramos Clamont Montfort, G. Effects of fucoidan and fucoidan oligosaccharides in growth and quorum sensing mediated virulence factor of Campylobacter Jejuni. Polysaccharides 2025, 6, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).