A Statistical Procedure for Exploring a Skeletal Age-Explicative Tool for Growing Patients
Abstract
1. Introduction
2. Materials and Methods
- Age between 6 and 16 years old;
- OPG;
- X-ray of the second phalanx of the third finger of the right hand;
- Collection of biometric data such as height, weight, and wrist circumference;
- All data must have been collected within a time window of a maximum of three months.
- Systemic pathologies affecting growth;
- Bone pathologies and/or previous fractures of the upper limbs;
- Multiple dental agenesia or oligodontia.
- Data collection
- Statistical analysis
3. Results
- SM1: 27.3% female; 62.7% male;
- SM2: 52.4% female; 47.6% male;
- SM3: 66.7% female 33.3% male;
- SM4: 64.0% female; 36.0% male.
4. Discussion
5. Conclusions
- Sex has a high explicative role for the assessment of skeletal age;
- Height and weight are explicative of skeletal age, while their combination in BMI is not significant for skeletal maturation explication;
- The degree of skeletal maturation and ossification of the median palatine suture is also indicative, suggesting a higher score for skeletal age.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perinetti, G.; Contardo, L. Reliability of Growth Indicators and Efficiency of Functional Treatment for Skeletal Class II Malocclusion: Current Evidence and Controversies. Biomed Res. Int. 2017, 2017, 1367691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perinetti, G.; Perillo, L.; Franchi, L.; Di Lenarda, R.; Contardo, L. Maturation of the middle phalanx of the third finger and cervical vertebrae: A comparative and diagnostic agreement study. Orthod. Craniofacial Res. 2014, 17, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Franchi, L.; McNamara, A.J. The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin. Orthod. 2005, 11, 119–129. [Google Scholar] [CrossRef]
- Malmgren, O.; Ömblus, J.; Hägg, U.; Pancherz, H. Treatment with an appliance system in relation to treatment intensity and growth periods. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 143–151. [Google Scholar] [CrossRef]
- El Refaei, A.K.; Fayed, M.M.; Heider, A.M.; Mostafa, Y.A. Treatment of a complex malocclusion in a growing skeletal Class II patient. J. Clin. Orthod. 2014, 48, 181–189. [Google Scholar] [PubMed]
- Tanner, J.M. Normal growth and techniques of growth assessment. Clin. Endocrinol. Metab. 1986, 15, 411–451. [Google Scholar] [CrossRef] [PubMed]
- Frysz, M.; Gregory, J.S.; Aspden, R.M.; Paternoster, L.; Tobias, J.H. The effect of pubertal timing, as reflected by height tempo, on proximal femur shape: Findings from a population-based study in adolescents. Bone 2020, 131, 115179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malina, R.M. Skeletal age and age verification in youth sport. Sports Med. 2011, 41, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Alkhal, H.A.; Wong, R.W.; Rabie, A.B. Correlation between chronological age, cervical vertebral maturation and Fishman’s skeletal maturity indicators in southern Chinese. Angle Orthod. 2008, 78, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist; Stanford University Press: Redwood City, CA, USA, 1959. [Google Scholar]
- Franchi, L.; Baccetti, T.; McNamara, J.A., Jr. Mandibular growth as related to cervical vertebral maturation and body height. Am. J. Orthod. Dentofac. Orthop. 2000, 118, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Franchi, L.; McNamara, J.A., Jr. An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Hezenci, Y. Is hand-wrist radiography still necessary in orthodontic treatment planning? BMC Oral Health 2024, 24, 616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szeląg, E.; Paradowska-Stolarz, A.; Noga, L.; Pietruszka, K.; Szumko, M.; Ogiński, T. Does the Baccetti’s Method of Establishing of Skeletal Age Have Clinical importance? Dent. Med. Probl. 2013, 50, 449–453. [Google Scholar]
- Hashim, H.A.; Mansoor, H.; Mohamed, M.H.H. Assessment of Skeletal Age Using Hand-Wrist Radiographs following Bjork System. J. Int. Soc. Prev. Community Dent. 2018, 8, 482–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hägg, U.; Taranger, J. Maturation indicators and the pubertal growth spurt. Am. J. Orthod. 1982, 82, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Fishman, L.S. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod. 1979, 49, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Kansal, S. A comparison of modified MP3 stages and the cervical vertebrae as growth indicators. J. Clin. Orthod. 2002, 36, 398–406. [Google Scholar] [PubMed]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A new system of dental age assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar] [PubMed]
- Saraç, F.; Baydemir Kılınç, B.; Çelikel, P.; Büyüksefil, M.; Yazıcı, M.B.; Şimşek Derelioğlu, S. Correlations between Dental Age, Skeletal Age, and Mandibular Morphologic Index Changes in Turkish Children in Eastern Anatolia and Their Chronological Age during the Pubertal Growth Spurt Period: A Cross-Sectional Study. Diagnostics 2024, 14, 887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghergie, M.; Ciobotaru, C.D.; Pop, R.; Colceriu-Șimon, I.; Bunta, O.; Pastrav, M.; Feștilă, D. Correlation Between Dental Age, Chronological Age, and Cervical Vertebral Maturation in Patients with Class II Malocclusion: A Retrospective Study in a Romanian Population Group. Children 2025, 12, 398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahmasbi, S.; Seifi, M.; Soleymani, A.A.; Mohamadian, F.; Alam, M. Comparative study of changes in the airway dimensions following the treatment of Class II malocclusion patients with the twin-block and Seifi appliances. Dent. Med. Probl. 2023, 60, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Pathak, A.; Jain, R.L. Assessment of skeletal age using MP3 and hand-wrist radiographs and its correlation with dental and chronological ages in children. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tiegs-Heiden, C.A.; Howe, B.M. Imaging of the Hand and Wrist. Clin. Sports Med. 2020, 39, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, H.M. The assessment and interpretation of Demirjian, Goldstein and Tanner’s dental maturity. Ann. Hum. Biol. 2012, 39, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Jourieh, A.; Khan, H.; Mheissen, S.; Assali, M.; Alam, M.K. The dorrelation between dental stages and skeletal maturity stages. Biomed. Res. Int. 2021, 2021, 9986498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angelieri, F.; Cevidanes, L.H.; Franchi, L.; Gonçalves, J.R.; Benavides, E.; McNamara, J.A., Jr. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanner, J.M.; Whitehouse, R.H.; Takaishi, M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch. Dis. Child. 1966, 41, 454–471. [Google Scholar] [CrossRef]
- Bedogni, G.; Borghi, A.; Battistini, N.C. Manuale di valutazione antropometrica dello stato nutrizionale. Edra 2001, 41, 454–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eknoyan, G. Adolphe Quetelet (1796–1874)--the average man and indices of obesity. Nephrol. Dial. Transplant. 2008, 23, 47–51. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D. Conditional logit analysis of qualitative choice behavior. Front. Econom. 1974, 1, 105–142. [Google Scholar]
- Franchi, L.; Pavoni, C.; Faltin, K., Jr.; McNamara, J.A., Jr.; Cozza, P. Long-term skeletal and dental effects and treatment timing for functional appliances in Class II malocclusion. Angle Orthod. 2013, 83, 334–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hedesiu, M.; Marcu, M.; Salmon, B.; Pauwels, R.; Oenning, A.C.; Almasan, O.; Roman, R.; Baciut, M.; Jacobs, R.; DIMITRA Research Group. Irradiation provided by dental radiological procedures in a pediatric population. Eur. J. Radiol. 2018, 103, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.T. ALARA concept-now a requirement. Radiol. Technol. 1980, 51, 525. [Google Scholar] [PubMed]
- Lee, H.; Badal, A. A Review of Doses for Dental Imaging in 2010–2020 and Development of a Web Dose Calculator. Radiol. Res. Pract. 2021, 2021, 6924314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.G.; Lin, J.; Jiang, J.H.; Wang, Q.; Ng, S.H. Validity and reliability of a method for assessment of cervical vertebral maturation. Angle Orthod. 2012, 82, 229–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grippaudo, M.M.; Quinzi, V.; Manai, A.; Paolantonio, E.G.; Valente, F.; La Torre, G.; Marzo, G. Orthodontic treatment need and timing: Assessment of evolutive malocclusion conditions and associated risk factors. Eur. J. Paediatr. Dent. 2020, 21, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.S.; Scott, P.; DiBiase, A.T. Compliance: Getting the most from your orthodontic patients. Dent. Update 2007, 34, 565–566, 569–570, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, S.; Zhou, C.; Jin, Z.; He, H.; Bai, Y.; Li, W.; Wang, J.; Hu, M.; Cao, Y.; et al. Expert consensus on early orthodontic treatment of class III malocclusion. Int. J. Oral Sci. 2025, 17, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Proffit, W.R.; Fields, H.W. Modern Orthodontics, 2nd ed.; Masson: Milano, Italy, 2001. [Google Scholar]
- Sizonenko, P.C. Physiology of puberty. J. Endocrinol. Investig. 1989, 12 (Suppl. S3), 59–63. [Google Scholar] [PubMed]
- Satoh, M.; Hasegawa, Y. Factors affecting prepubertal and pubertal bone age progression. Front. Endocrinol. 2022, 13, 967711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karlberg, J.; Kwan, C.W.; Gelander, L.; Albertsson-Wikland, K. Pubertal growth assessment. Horm. Res. 2003, 60 (Suppl. S1), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.B.; Roche, A.F.; Wagner, B. Pubertal spurts in cranial base and mandible. Comparisons within individuals. Angle Orthod. 1985, 55, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D. Impact of obesity on female puberty and pubertal disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 91, 102400. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Radiation Protection 180, Medical Radiation Exposure for the European Population; Office for Official Publications of the European Communities: Luxembourg, 2014. Available online: https://ec.europa.eu/energy/en/radiation-protection-publications (accessed on 1 December 2014).
- Horner, K. Review article: Radiation protection in dental radiology. Br. J. Radiol. 1994, 67, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Masthoff, M.; Gerwing, M.; Masthoff, M.; Timme, M.; Kleinheinz, J.; Berninger, M.; Heindel, W.; Wildgruber, M.; Schülke, C. Dental imaging—A basic guide for the radiologist. Rofo 2019, 191, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Posadzy, M.; Desimpel, J.; Vanhoenacker, F. Cone beam CT of the musculoskeletal system: Clinical applications. Insights Imaging 2018, 9, 35–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hegde, R.J.; Shigli, A.L.; Gawali, P.N.; Mune, B.B. Assessment of the relationship between body mass index, dental age, chronological age, and skeletal maturity among 6-12-year children in Pune, India. J. Indian Soc. Pedod. Prev. Dent. 2025, 43, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ballanti, F.; Lione, R.; Fiaschetti, V.; Fanucci, E.; Cozza, P. Low-dose CT protocol for orthodontic diagnosis. Eur. J. Paediatr. Dent. 2008, 9, 65–70. [Google Scholar] [PubMed]
- Hezenci, Y.; Bulut, M. Correlation of skeletal development and midpalatal suture maturation. Eur. J. Med. Res. 2024, 29, 461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, S.M.N.; Castilho, J.C.M.; Ortolani, C.L.F.; David, A.F.; Manhães Junior, L.R.C.; Matsui, R.H. Evaluation and measurement of midpalatal suture through the digitalized occlusal radiography in patients submitted to rapid maxillary expansion. Rev. Dent. Press Ortod. Ortopedia Facial 2009, 14, 62–68. [Google Scholar] [CrossRef]
- Gilli, G. The assessment of skeletal maturation. Horm Res. 1996, 45 (Suppl. S2), 49–52. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, G.; Irurita Olivares, J.; Alemán Aguilera, I. Age estimation in infant skeletal remains by measurements of the pars lateralis. Int. J. Leg. Med. 2022, 136, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
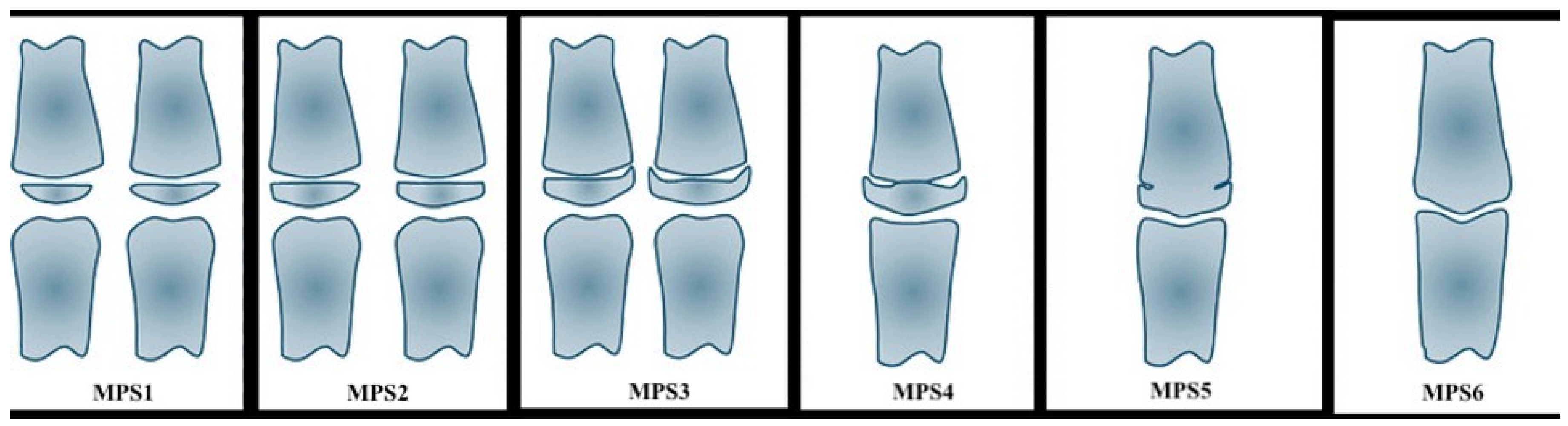

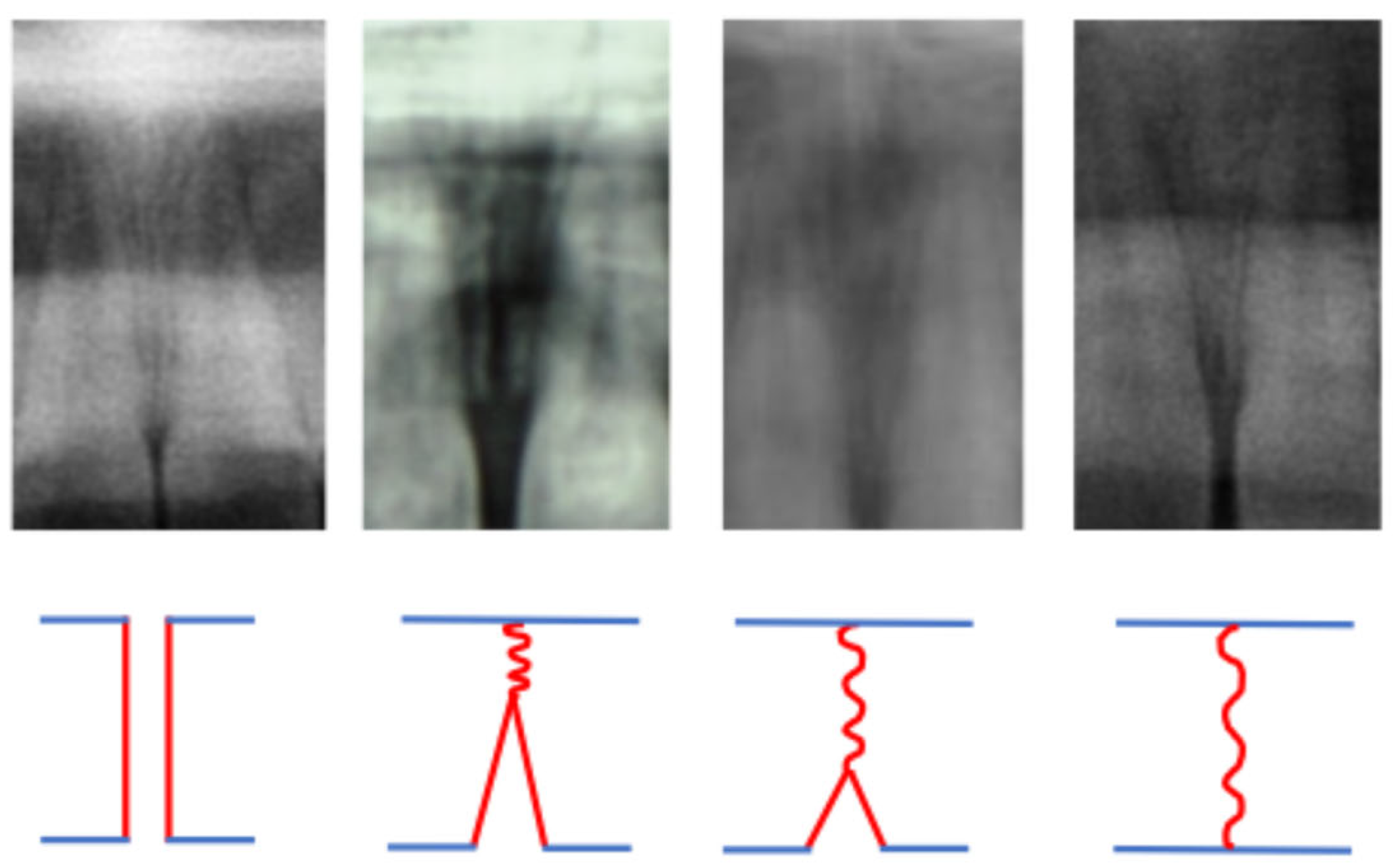
| Stage | Bones’ Maturation | Phase of the Growth |
|---|---|---|
| MPS1 | the epiphysis is narrower than the metaphysis | the patient is more than one year before the pubertal growth spurt |
| MPS2 | the epiphysis is at least as wide as the metaphysis | the patient is one year before the onset of the pubertal growth spurt |
| MPS3 | the epiphysis is either as wide as or wider than the metaphysis | the patient is at coincidence of the pubertal growth spurt |
| MPS4 | the epiphysis begins to fuse with the metaphysis | the patient is during the deceleration of the curve of growth |
| MPS5 | the epiphysis is mostly, but not completely fused with the metaphysis | the patient toward the end of the pubertal growth spurt |
| MPS6 | the epiphysis totally fused with the metaphysis | the patient is at the end of the pubertal growth spurt |
| MPS Method Categories | Categories Used for the Present Analysis |
|---|---|
| MPS-1 | SM1 |
| MPS-2 | |
| MPS-3 | SM2 |
| MPS-4 | SM3 |
| MPS-5 | SM4 |
| MPS-6 |
| Variables | Mean | SD |
|---|---|---|
| Age | 137.4 | 25.6 |
| Height (cm) | 149.1 | 13.5 |
| Weight (kg) | 45.9 | 13.7 |
| BMI | 20.3 | 4.2 |
| Wrist circumference | 1.86 | 1.113 |
| Skeletal Age | |
|---|---|
| Weight | ρ: 0.5903 ** |
| p: <0.001 | |
| Height | ρ: 0.6188 ** |
| p: <0.001 | |
| BMI | ρ: 0.3165 ** |
| p: <0.001 | |
| Wrist circumference | ρ: 0.3881 ** |
| p: <0.001 |
| Skeletal Age | Odds Ratio | SD | z | p > |z| | Confidence Interval 95% | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Sex | 0.03 | 0.02 | −4.57 | <0.001 | −5.2 | −2.08 |
| Age | 3.42 | 1.21 | 3.47 | 0.001 | 0.53 | 1.93 |
| Height (cm) | 1.1 | 0.047 | 2.33 | 0.02 | 0.02 | 0.18 |
| Weight (kg) | 1.13 | 0.06 | 2.11 | 0.035 | 0.01 | 0.23 |
| Wrist circumference | 0.79 | 0.33 | −0.57 | 0.569 | −1.06 | 0.58 |
| Midpalate suture | ||||||
| 2 | 3.52 | 2.56 | 1.73 | 0.084 | −0.17 | 2.68 |
| 3 | 15.25 | 15.58 | 2.67 | 0.008 | 0.72 | 4.73 |
| 4 | 0.81 | 0.86 | −0.2 | 0.845 | −2.29 | 1.87 |
| Lower first premolar −34 | 3.23 | 2.68 | 1.41 | 0.16 | −0.46 | 2.80 |
| Skeletal age < cut 1 | 20.69 | 6.62 | - | - | 7.71 | 33.68 |
| cut1 < Skeletal age < cut2 | 23.64 | 6.89 | - | - | 10.13 | 37.16 |
| Cut2 < Skeletal age < cut3 | 24.19 | 6.94 | - | - | 10.59 | 37.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepedino, M.; Esposito, R.; Delvecchio, M.; Ciavarella, D.; Rofrano, G.; Masedu, F. A Statistical Procedure for Exploring a Skeletal Age-Explicative Tool for Growing Patients. Appl. Sci. 2025, 15, 5593. https://doi.org/10.3390/app15105593
Tepedino M, Esposito R, Delvecchio M, Ciavarella D, Rofrano G, Masedu F. A Statistical Procedure for Exploring a Skeletal Age-Explicative Tool for Growing Patients. Applied Sciences. 2025; 15(10):5593. https://doi.org/10.3390/app15105593
Chicago/Turabian StyleTepedino, Michele, Rosa Esposito, Maurizio Delvecchio, Domenico Ciavarella, Giuseppe Rofrano, and Francesco Masedu. 2025. "A Statistical Procedure for Exploring a Skeletal Age-Explicative Tool for Growing Patients" Applied Sciences 15, no. 10: 5593. https://doi.org/10.3390/app15105593
APA StyleTepedino, M., Esposito, R., Delvecchio, M., Ciavarella, D., Rofrano, G., & Masedu, F. (2025). A Statistical Procedure for Exploring a Skeletal Age-Explicative Tool for Growing Patients. Applied Sciences, 15(10), 5593. https://doi.org/10.3390/app15105593









