Wastewater Treatment Using a Combination of Pumpkin seed Waste After Extraction of Essential Oils (Bio-Coagulant) and Ferric Chloride (Chemical Coagulant): Optimization and Modeling Using a Box–Behnken Design
Abstract
1. Introduction
2. Materials and Methods
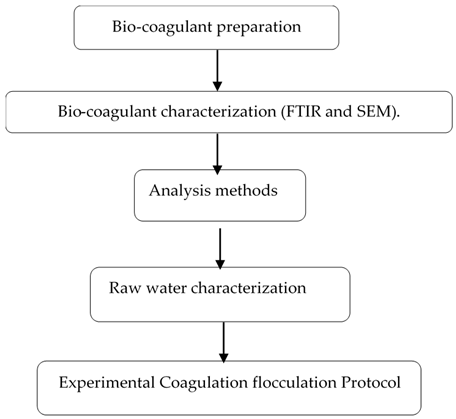
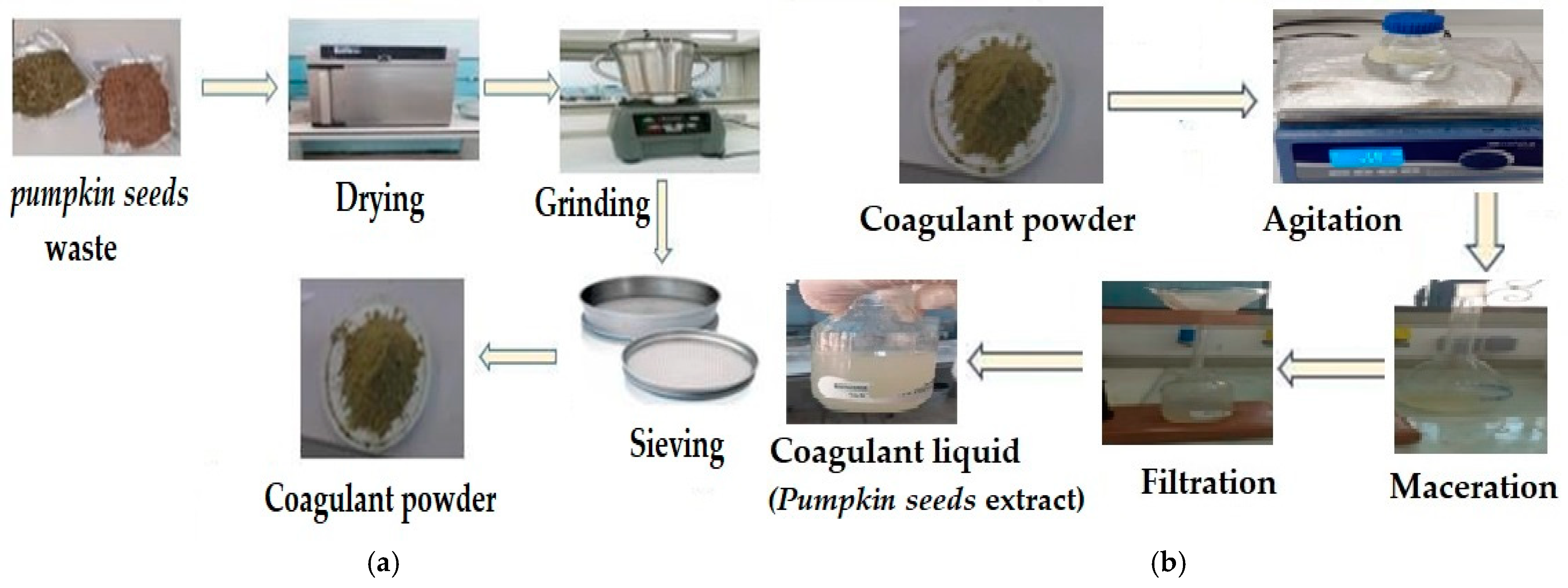
2.1. Preparation of the Bio-Coagulant
2.2. Analytical Methods
2.3. Raw Wastewater
2.4. Experimental Coagulation–Flocculation Protocol
3. Results and Discussion
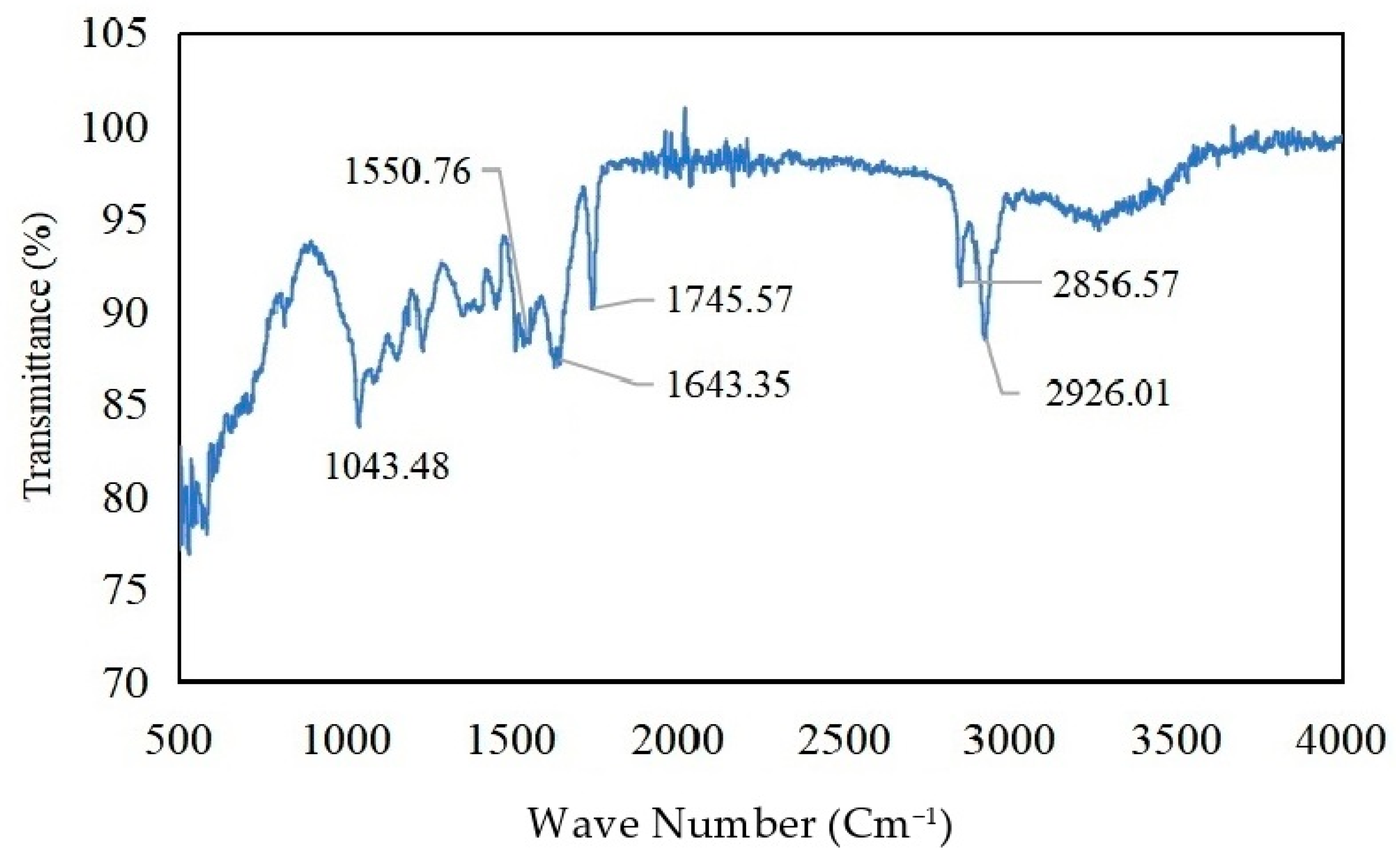
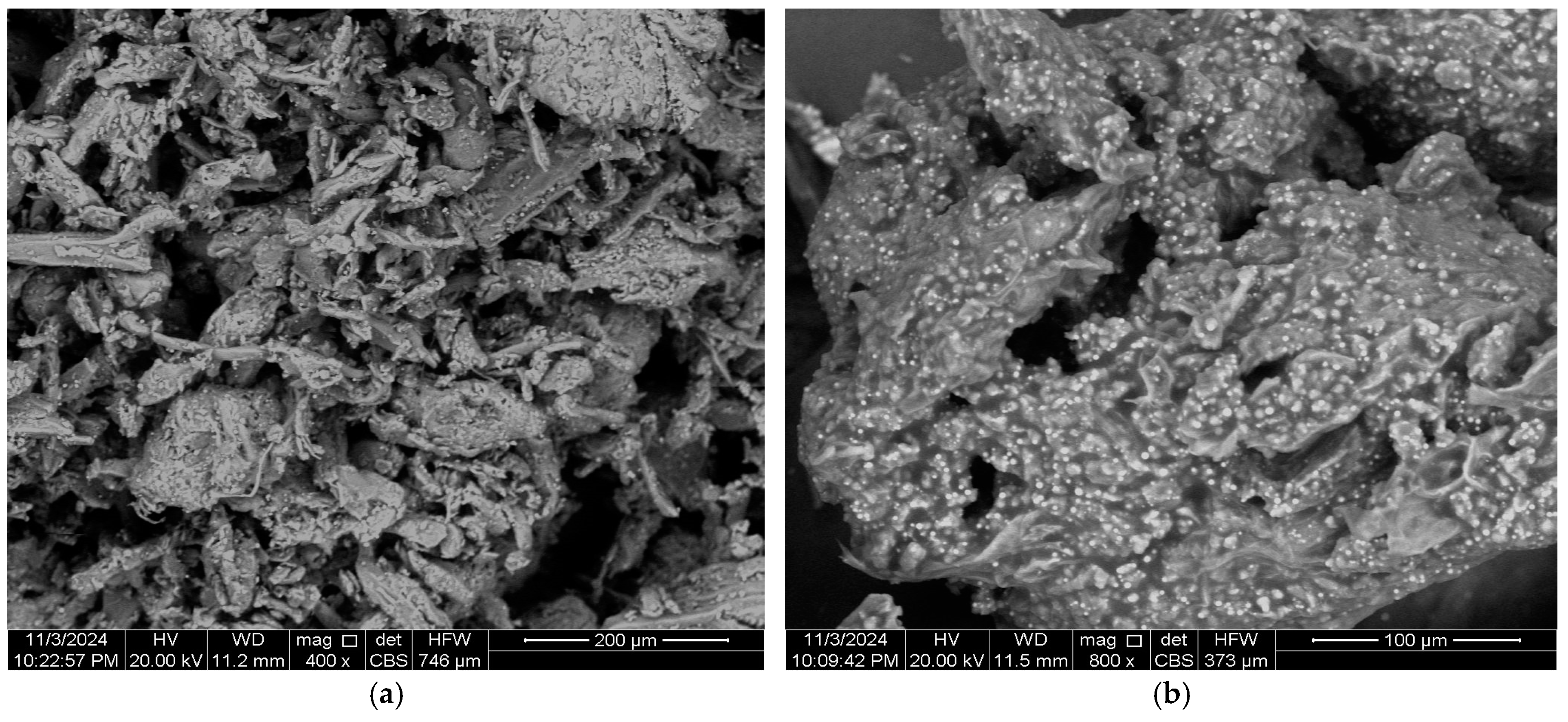
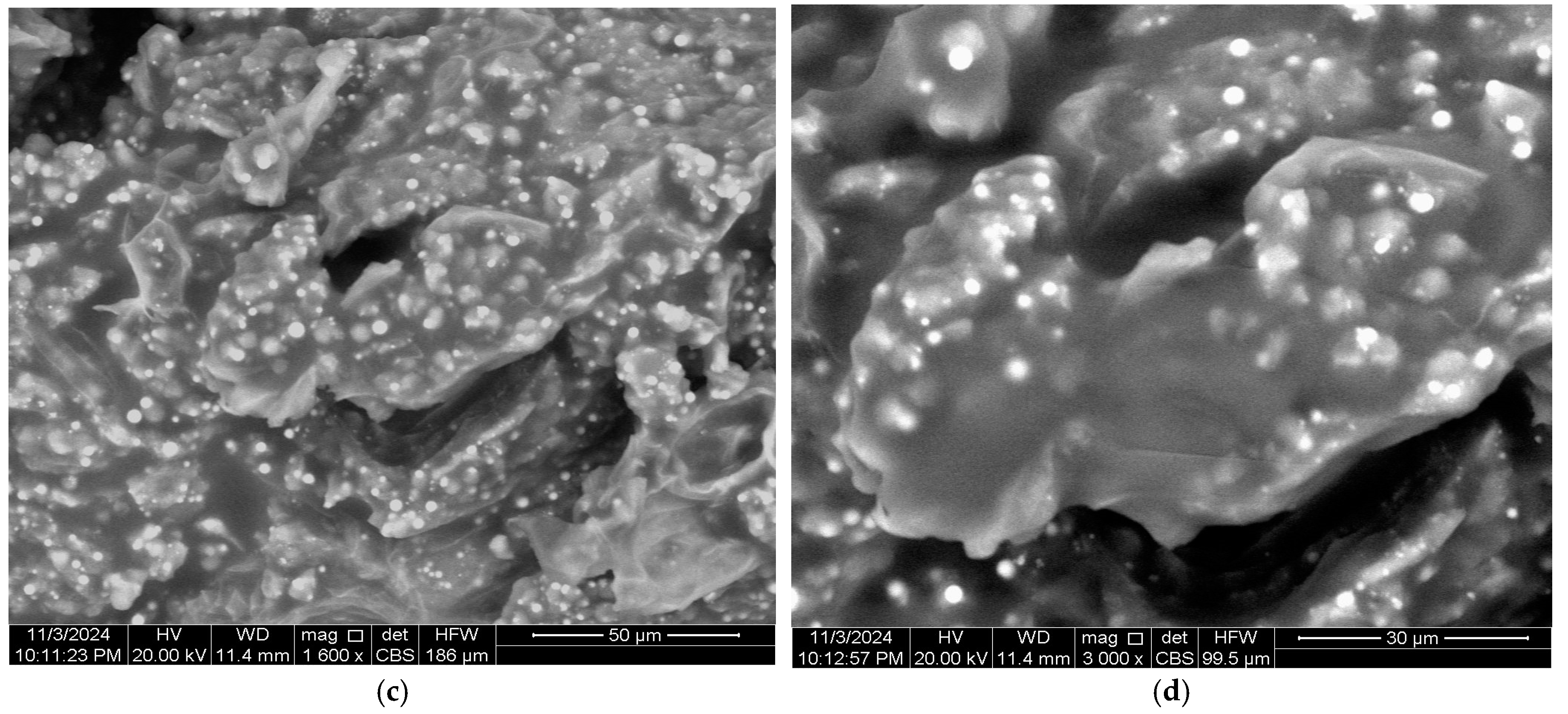
3.1. Characterization of Bio-Coagulant
3.2. Optimization and Modeling of Coagulation–Flocculation Process
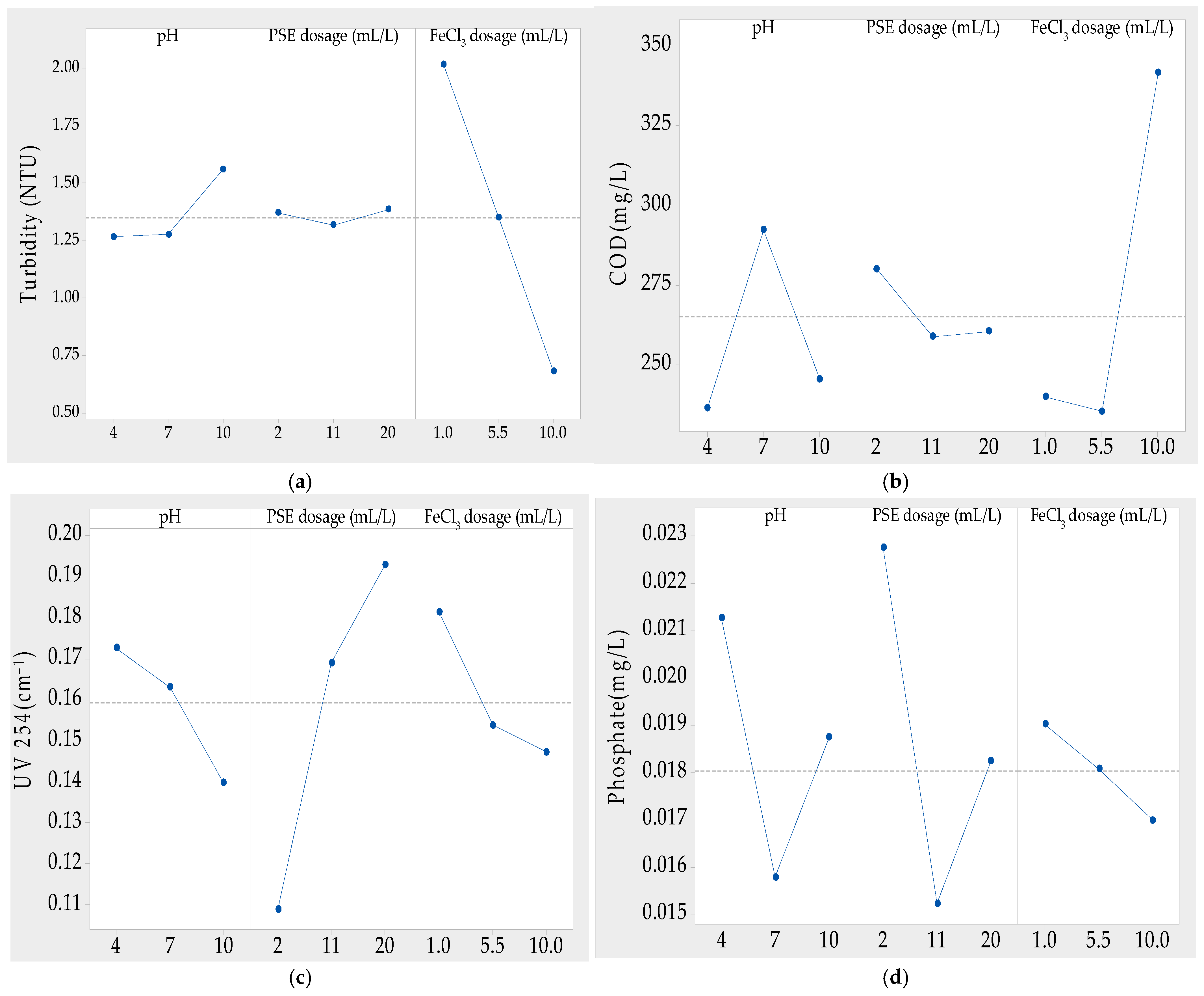
3.3. Effects Graphs
3.3.1. Effect of pH
3.3.2. Effect of Pumpkin seed Extract Dose
3.3.3. Effect of Chemical Coagulant FeCl3
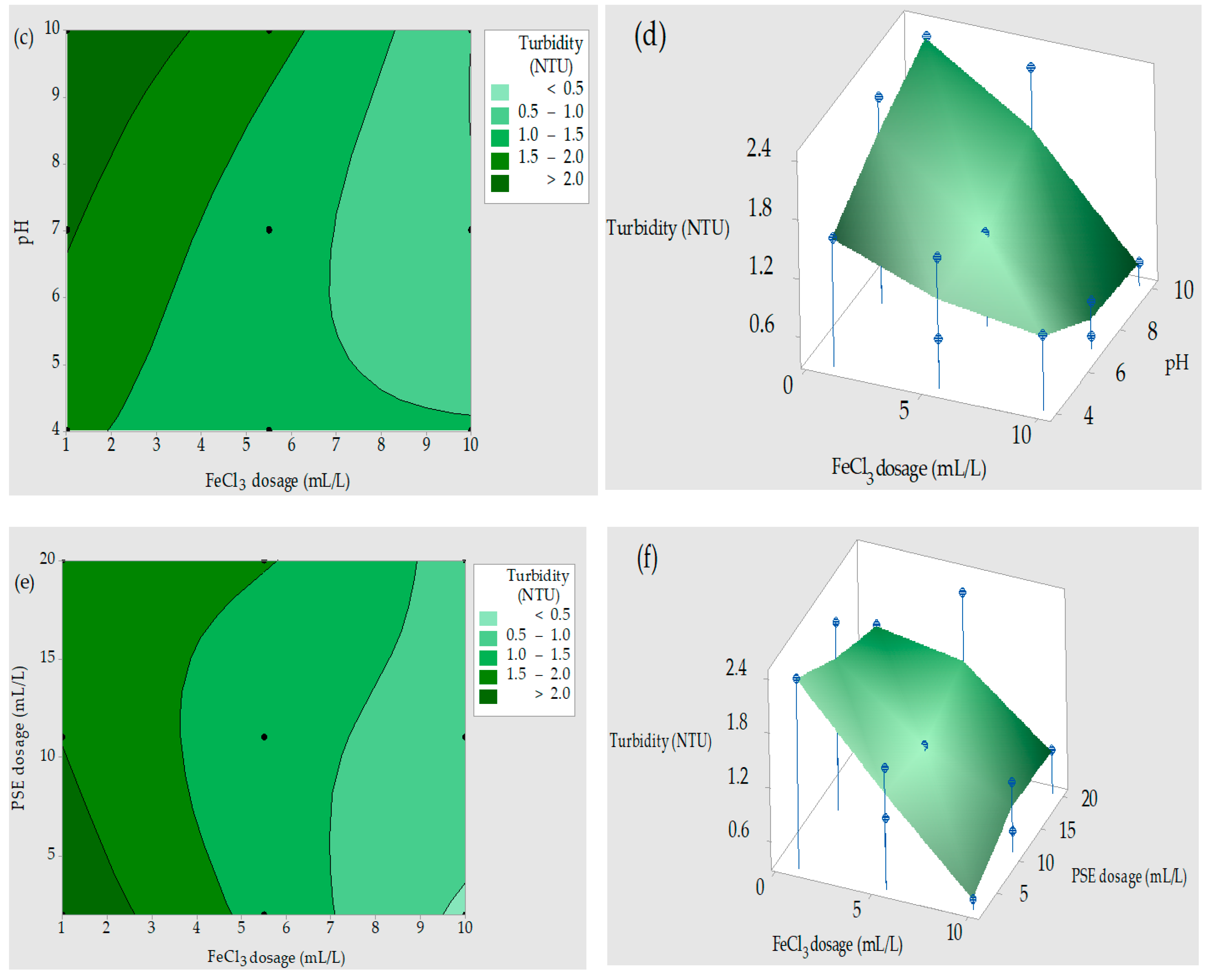
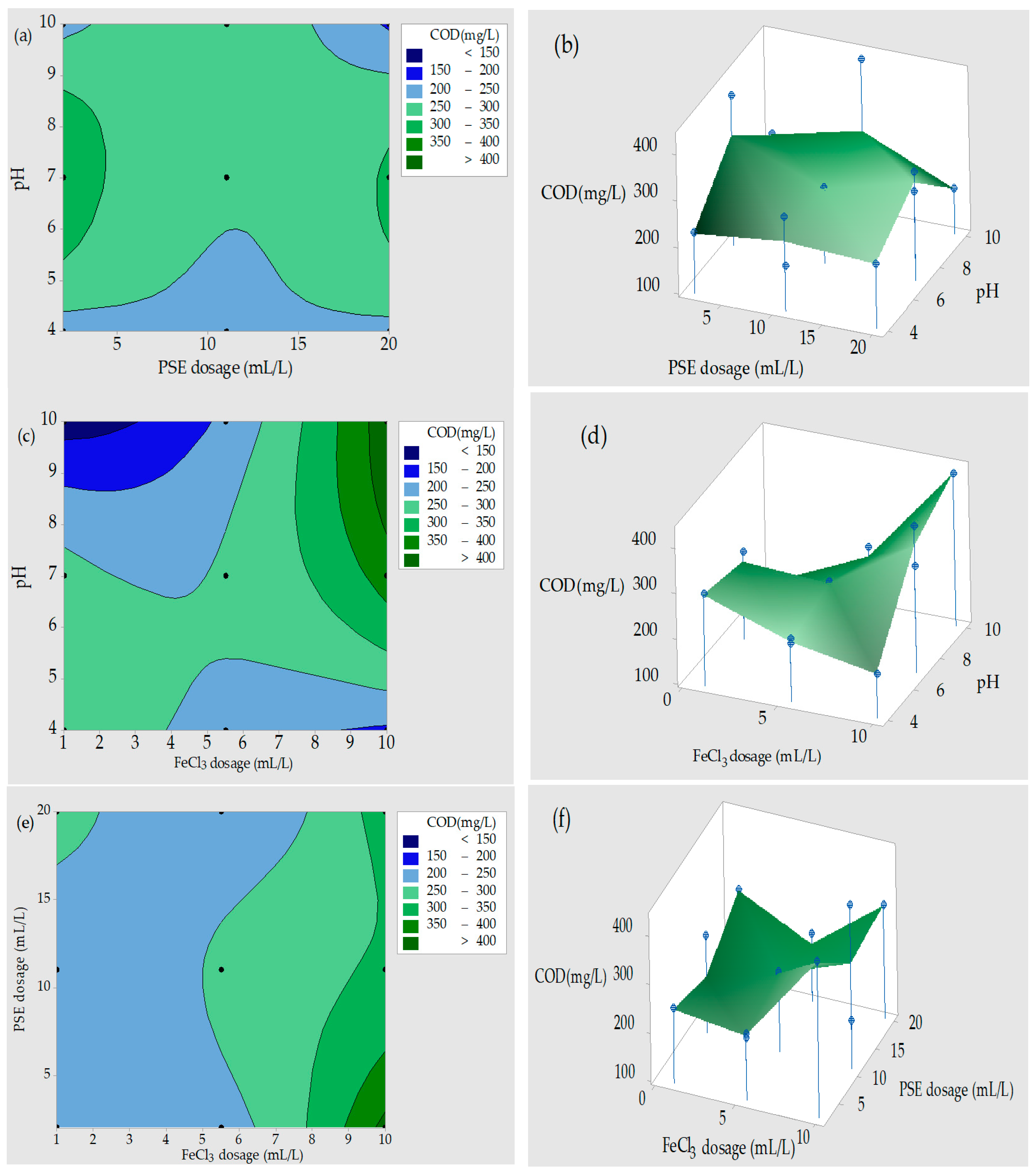
3.4. Contour Plots (2D) and Response Surface Plots (3D)
3.4.1. Turbidity Removal
3.4.2. COD Removal
3.4.3. UV 254 Removal
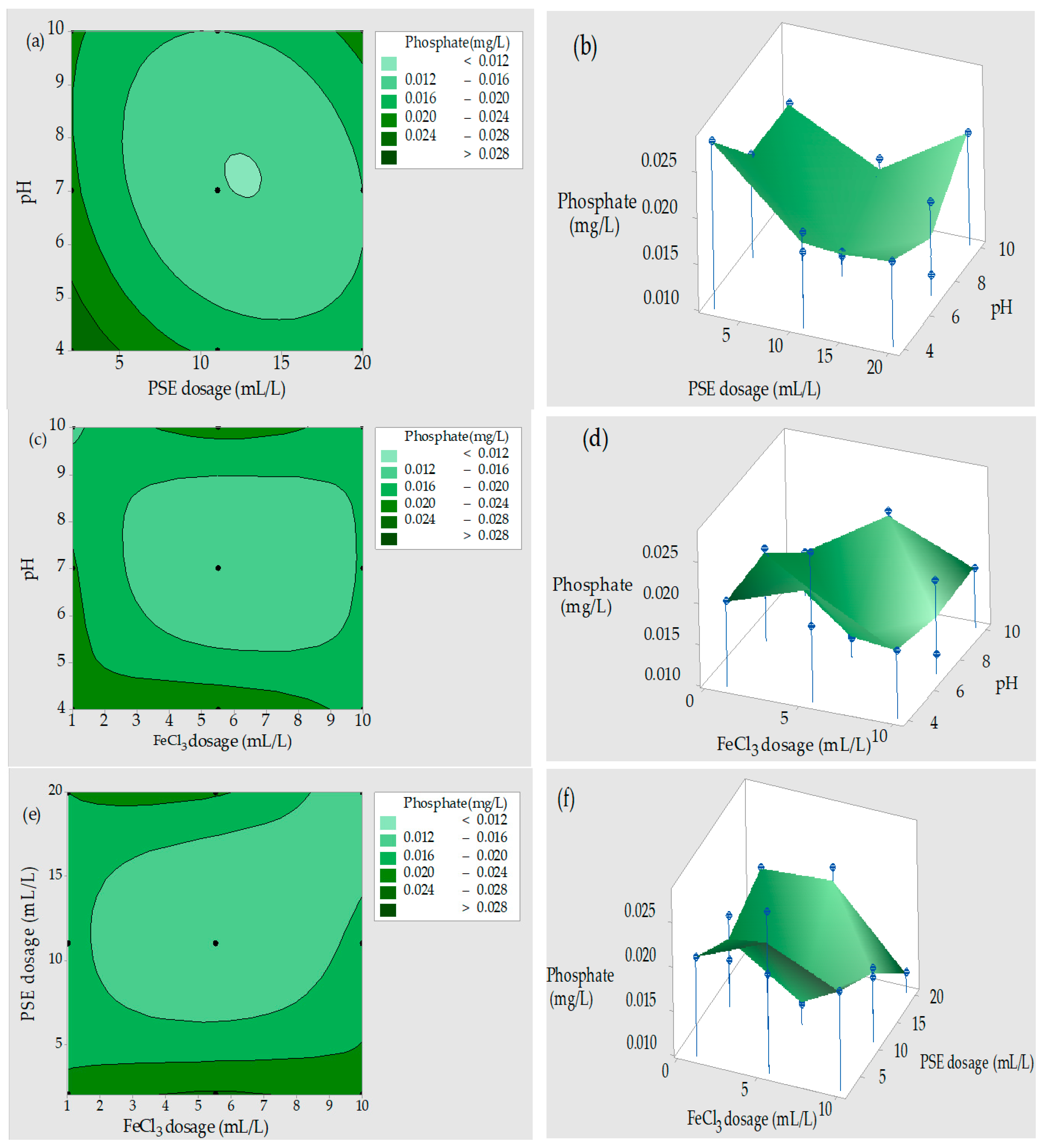
3.4.4. Phosphate Removal
3.5. Analysis of Variance (ANOVA)
3.6. Optimization
3.7. Optimization and Model Validation of Experimental Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PS | Pumpkin seeds |
| PSE | Pumpkin seeds extract |
| BBD | Box–Behnken design |
| COD | chemical oxygen demand |
| FeCl3 | Ferric chloride |
| FTIR | Fourier transform infrared spectrometer |
| HCl | dihydrogen chloride |
| NaCl | sodium chloride |
| NaOH | sodium hydroxide |
| RSM | response surface methodology |
| SEM | scanning electron microscope |
| UV 254 | aromatic organic matter |
References
- Lemessa, F.; Simane, B.; Seyoum, A.; Gebresenbet, G. Assessment of the Impact of Industrial Wastewater on the Water Quality of Rivers around the Bole Lemi Industrial Park (BLIP), Ethiopia. Sustainability 2023, 15, 4290. [Google Scholar] [CrossRef]
- Owhonka, A.; Fubara, E.F.; Justice, O.B. Wastewater Quality—It’s Impact on the Environment and Human Physiology: A Review. Int. J. Adv. Res. Innov. 2021, 9, 43–58. [Google Scholar] [CrossRef]
- Li, X.; Bao, L.; Wei, Y.; Zhao, W.; Wang, F.; Liu, X.; Su, H.; Zhang, R. Occurrence, Bioaccumulation, and Risk Assessment of Microplastics in the Aquatic Environment: A Review. Water 2023, 15, 1768. [Google Scholar] [CrossRef]
- Hammami, S. Étude de Dégradation Des Colorants de Textile Par Les Procédés d’Oxydation Avancée. Application à La Dépollution Des Rejets Industriels. Ph.D. Thesis, Université de Marne la Vallée, Champs-sur-Marne, France, 2008. [Google Scholar]
- Mansour, H.B.; Boughzala, O.; Barillier, D.; Mosrati, R. Les Colorants Textiles Sources de Contamination de l’eau: CRIBLAGE de La Toxicité et Des Méthodes de Traitement. Rev. Sci. L’Eau 2011, 24, 209–238. [Google Scholar] [CrossRef]
- Kasperchik, V.P.; Yaskevich, A.L.; Bil’Dyukevich, A.V. Wastewater Treatment for Removal of Dyes by Coagulation and Membrane Processes. Pet. Chem. 2012, 52, 545–556. [Google Scholar] [CrossRef]
- Yao, S.; Fabbricino, M.; Race, M.; Ferraro, A.; Pontoni, L.; Aimone, O.; Chen, Y. Study of the Digestate as an Innovative and Low-Cost Adsorbent for the Removal of Dyes in Wastewater. Processes 2020, 8, 852. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. A Hybrid Adsorption Membrane Process for Removal of Dye from Synthetic and Actual Wastewater. Chem. Eng. Process.—Process Intensif. 2020, 157, 108113. [Google Scholar] [CrossRef]
- Zou, H.; Ma, W.; Wang, Y. A Novel Process of Dye Wastewater Treatment by Linking Advanced Chemical Oxidation with Biological Oxidation. Arch. Environ. Prot. 2015, 41, 33–39. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A Comparative Study on Oxidation of Disperse Dyes by Electrochemical Process, Ozone, Hypochlorite and Fenton Reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D.; Muresan, A.; Muresan, R.; Popescu, A. Textile Wastewater Treatment by Homogeneous Oxidation with Hydrogen Peroxide. Environ. Eng. Manag. J. 2009, 8, 1359–1369. [Google Scholar] [CrossRef]
- Yildiz, B.S. Water and Wastewater Treatment: Biological Processes. In Metropolitan Sustainability: Understanding and Improving the Urban Environment; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2012; pp. 406–428. [Google Scholar] [CrossRef]
- Chen, G.; Ekama, G.A.; van Loosdrecht, M.C.M.; Brdjanovic, D. Biological Wastewater Treatment; Taylor & Francis: London, UK, 2020; pp. 1–840. [Google Scholar] [CrossRef]
- Exley, C. La Toxicité de l’aluminium Chez l’homme. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Rondeau, V.; Commenges, D.; Jacqmin-gadda, H.; Dartigues, J. Relation between Aluminum Concentrations in Drinking Water and Alzheimer’s Disease: An 8-Year Follow-up Study. Am. J. Epidemiol. 2000, 152, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zengjin, W.; Xiaomin, W.; Junlin, Y.; Jinning, S.; Jingyi, C.; Xianchen, L.; Zhao, X. Chronic Exposure to Aluminum and Risk of Alzheimer’s Disease: A Meta-Analysis. Neurosci. Lett. 2016, 610, 200–206. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Quality Of Water Treated By Coagulation Using Moringa Oleifera Seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Megersa, M.; Beyene, A.; Ambelu, A.; Triest, L. Comparison of Purified and Crude Extracted Coagulants from Plant Species for Turbidity Removal. Int. J. Environ. Sci. Technol. 2019, 16, 2333–2342. [Google Scholar] [CrossRef]
- Benalia, A. Extraction et Valorisation Des Produits Actifs Des Plantes Naturelles En Tant Que Bio Coagulants Utiles Dans L’amelioration de Qualite Des Eaux. Doctorat Thesis, University of Constantine 3, El Khroub, Algeria, 2023. [Google Scholar]
- Benalia, A.; Derbal, K.; Amrouci, Z.; Baatache, O.; Khalfaoui, A.; Pizzi, A. Application of Plant-Based Coagulants and Their Mechanisms in Water Treatment: A Review. J. Renew. Mater. 2024, 12, 667–698. [Google Scholar] [CrossRef]
- Prathna, T.C.; Srivastava, A. Ferric Chloride for Odour Control: Studies from Wastewater Treatment Plants in India. Water Pract. Technol. 2021, 16, 35–41. [Google Scholar] [CrossRef]
- Poon, C.S.; Chu, C.W. The Use of Ferric Chloride and Anionic Polymer in the Chemically Assisted Primary Sedimentation Process. Chemosphere 1999, 39, 1573–1582. [Google Scholar] [CrossRef]
- Dabhi, Y.M. Physicochemical Treatment of Dairy Plant Wastewater. Int. J. Basic Appl. Chem. Sci. 2013, 3, 9–14. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2012; ISBN 978-087553-0130. [Google Scholar]
- Bouteflika, A. Valeurs Limites Des Paramètres de Rejets d’effluents Liquides Industriels. J. Off. République Algérienne 2006, 26, 1–27. [Google Scholar]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 37–41. [Google Scholar] [CrossRef]
- Almeida, M.; Erthal, R.; Padua, E.; Silveira, L.; Am, L. Talanta Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Ouiem, B.; Kerroum, D.; Benalia, A.; Khalfaoui, A.; Pizzi, A. Optimization and Modeling of Bio-coagulation Using Pine Cone as a Natural Coagulant: Jar Test and Pilot-Scale Applications. Water Air Soil Pollut. 2024, 235, 770. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Pine Cone as Bio-Coagulant for Heavy Metal Removal from Industrial Wastewater: Use of Box—Behnken Design. Ind. Crops Prod. 2024, 210, 118185. [Google Scholar] [CrossRef]
- Benalia, A.; Atime, L.; Baatache, O.; Khalfaoui, A.; Fadia, A. Removal of Lead in Water by Coagulation Flocculation Process Using Cactus—Based Natural Coagulant: Optimization and Modeling by Response Surface Methodology (RSM). Environ. Monit. Assess. 2024, 196, 244. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Mhuka, V.; Nindi, M.M. Bioremediation of Cd (II), Pb (II) and Cu (II) from Industrial Effluents by Moringa Stenopetala Seed Husk. J. Environ. Sci. Health Part A 2019, 54, 337–351. [Google Scholar] [CrossRef]
- Dao, M.; Nguyen, V.; Tran, T.; Nguyen, X.; Vo, D.; Nguyen, V.; Hoang, L. Pilot-Scale Study of Real Domestic Textile Wastewater Treatment Using Cassia fistula Seed-Derived Coagulant. J. Chem. 2021, 2021, 7608856. [Google Scholar] [CrossRef]
- Saritha, V.; Karnena, M.K.; Dwarapureddi, B.K. Competence of Blended Coagulants for Surface Water Treatment. Appl. Water Sci. 2020, 10, 20. [Google Scholar] [CrossRef]
- Getahun, M.; Asaithambi, P.; Befekadu, A.; Alemayehu, E. Chemical Oxygen Demand Removal from Wet Coffee Processing Wastewater Using Indigenous Natural Coagulants: Optimization through Response Surface Methodology. Desalination Water Treat. 2024, 317, 100217. [Google Scholar] [CrossRef]
- Desta, W.M.; Bote, M.E. Wastewater Treatment Using a Natural Coagulant (Moringa Oleifera Seeds): Optimization through Response Surface Methodology. Heliyon 2021, 7, e08451. [Google Scholar] [CrossRef]
- Getahun, M.; Befekadu, A.; Alemayehu, E. Coagulation Process for the Removal of Color and Turbidity from Wet Coffee Processing Industry Wastewater Using Bio-Coagulant: Optimization through Central Composite Design. Heliyon 2024, 10, e27584. [Google Scholar] [CrossRef] [PubMed]
- Benalia, A.; Derbal, K. Etude Expérimentale et Modélisation Du Processus de La Coagulation Floculation: Application Aux Eaux Destinée a La Consommation. Master’s Thesis, University of Constantine 3, El Khroub, Algeria, 2015. [Google Scholar]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Luc, T.Q.; Hoang, H.V.; Cao, H.T.; Tran, L.P.T. Treatment Surface Water Using a Novel Pumpkin Seed-Based Natural Bio-Coagulant: Optimization by CCD and Toxicity Evaluation. Glob. Nest J. 2023, 25, 142–148. [Google Scholar] [CrossRef]
- Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein Fractionation of Seeds of Moringa Oleifera Lam and Its Application in Superficial Water Treatment. Sep. Purif. Technol. 2017, 180, 114–124. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Amin, M.M.; Hajizadeh, Y.; Pourzamani, H.; Memarzadeh, M.; Mahvi, A.H.; Mahdavi, M. Filter Backwash Water Treatment by Coagulation: A Comparison Study by Polyaluminium Ferric Chloride and Ferric Chloride. Desalination Water Treat. 2017, 66, 320–329. [Google Scholar] [CrossRef]
- Muniz, G.L.; da Silva, T.C.F.; Borges, A.C. Assessment and Optimization of the Use of a Novel Natural Coagulant (Guazuma Ulmifolia) for Dairy Wastewater Treatment. Sci. Total Environ. 2020, 744, 140864. [Google Scholar] [CrossRef]
- Gohari Ardabili, A.; Farhoosh, R.; Haddad Khodaparast, M.H. Chemical Composition and Physicochemical Properties of Pumpkin Seeds (Cucurbita Pepo Subsp. Pepo Var. Styriaka) Grown in Iran. J. Agric. Sci. Technol. 2011, 13, 1053–1063. [Google Scholar]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A Preliminary Study on Jatropha Curcas as Coagulant in Wastewater Treatment. Environ. Technol. 2011, 32, 971–977. [Google Scholar] [CrossRef]
- Al-gheethi, A.; Mohamed, R.; Wurochekke, A.; Nurulainee, N.; Mas Rahayu, J.; Amir Hashim, M. Efficiency of Moringa Oleifera Seeds for Treatment of Laundry Wastewater. MATEC Web Conf. 2017, 103, 1–8. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Gómez Castaño, J.A.; Cifuentes, G.R. Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia Ficus-Indica Fruit Peel Mucilage and FeCl3. Polymers 2023, 15, 217. [Google Scholar] [CrossRef]
- Khataee, A.R.; Zarei, M.; Fathinia, M.; Jafari, M.K. Photocatalytic Degradation of an Anthraquinone Dye on Immobilized TiO2 Nanoparticles in a Rectangular Reactor: Destruction Pathway and Response Surface Approach. Desalination 2011, 268, 126–133. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S. Model Prediction of Coagulation by Magnetised Rice Starch for Wastewater Treatment Using Response Surface Methodology (RSM) with Artificial Neural Network (ANN). Sci. Afr. 2022, 17, e01282. [Google Scholar] [CrossRef]




| Parameter | Value | Algerian Standards [25] |
|---|---|---|
| Turbidity (NTU) | 250 | / |
| COD (mg/L) | 640 | 120 |
| UV 254 | 0.893 | / |
| Temperature (°C) | 24 | 30 |
| Phosphate (mg/L) | 0.115 | / |
| Salinity (mg/L) | 5.5 | / |
| Conductivity (mS/cm) | 9.15 | |
| pH | 8.25 | 6.5–8.5 |
| Sulfate (mg/L) | 2.35 | / |
| TAC (mg/L CaCO3) | 100 | / |
| Chloride (mg/L) | 4.2 | / |
| Standard Run | pH (X1) | PSE Dosage (mL/L) (X2) | FeCl3 Dosage (mL/L) (X3) |
|---|---|---|---|
| 4 | 10 | 20 | 5.5 |
| 1 | 4 | 2 | 5.5 |
| 6 | 10 | 11 | 1 |
| 14 | 7 | 11 | 5.5 |
| 5 | 4 | 11 | 1 |
| 15 | 7 | 11 | 5.5 |
| 10 | 7 | 20 | 1 |
| 12 | 7 | 20 | 10 |
| 2 | 10 | 2 | 5.5 |
| 13 | 7 | 11 | 5.5 |
| 8 | 10 | 11 | 10 |
| 11 | 7 | 2 | 10 |
| 7 | 4 | 11 | 10 |
| 9 | 7 | 2 | 1 |
| 3 | 4 | 20 | 5.5 |
| R2 | R2 Adjusted | p | |
|---|---|---|---|
| Phosphate (mg/L) | 97.02% | 91.65% | 0.003 |
| COD (mg/L) | 98.41% | 95.56% | 0.001 |
| Turbidity (NTU) | 97.21% | 92.18% | 0.002 |
| UV 254 (Cm−1) | 98.62% | 96.15% | 0 |
| Factor | Responses | |||||
|---|---|---|---|---|---|---|
| pH | PSE dosage (mL/L) | FeCl3 dosage (mL/L) | Turbidity (NTU) | COD (mg/L) | UV 254 (cm−1) | Phosphate (mg/L) |
| 4 | 17.8182 | 10 | 0.7546 | 190.882 | 0.00283 | 0.01487 |
| Parameter | Unit | Raw Water | Y Predicted | Y Observed | Error |
|---|---|---|---|---|---|
| Turbidity | NTU | 250 | 0.7546 | 0.77835 | 0.02375 |
| COD | mg/L | 640 | 190.882 | 194.382 | 3.5000 |
| UV 254 | cm⁻¹ | 0.893 | 0.00283 | 0.01385 | 0.01102 |
| Phosphate | mg/L | 0.115 | 0.01487 | 0.01538 | 0.00051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benalia, A.; Baatache, O.; Zerguine, K.E.; Khediri, A.; Derbal, K.; Ferroudj, N.; Khalfaoui, A.; Pizzi, A. Wastewater Treatment Using a Combination of Pumpkin seed Waste After Extraction of Essential Oils (Bio-Coagulant) and Ferric Chloride (Chemical Coagulant): Optimization and Modeling Using a Box–Behnken Design. Appl. Sci. 2025, 15, 5439. https://doi.org/10.3390/app15105439
Benalia A, Baatache O, Zerguine KE, Khediri A, Derbal K, Ferroudj N, Khalfaoui A, Pizzi A. Wastewater Treatment Using a Combination of Pumpkin seed Waste After Extraction of Essential Oils (Bio-Coagulant) and Ferric Chloride (Chemical Coagulant): Optimization and Modeling Using a Box–Behnken Design. Applied Sciences. 2025; 15(10):5439. https://doi.org/10.3390/app15105439
Chicago/Turabian StyleBenalia, Abderrezzaq, Ouiem Baatache, Katr Enada Zerguine, Amel Khediri, Kerroum Derbal, Nawal Ferroudj, Amel Khalfaoui, and Antonio Pizzi. 2025. "Wastewater Treatment Using a Combination of Pumpkin seed Waste After Extraction of Essential Oils (Bio-Coagulant) and Ferric Chloride (Chemical Coagulant): Optimization and Modeling Using a Box–Behnken Design" Applied Sciences 15, no. 10: 5439. https://doi.org/10.3390/app15105439
APA StyleBenalia, A., Baatache, O., Zerguine, K. E., Khediri, A., Derbal, K., Ferroudj, N., Khalfaoui, A., & Pizzi, A. (2025). Wastewater Treatment Using a Combination of Pumpkin seed Waste After Extraction of Essential Oils (Bio-Coagulant) and Ferric Chloride (Chemical Coagulant): Optimization and Modeling Using a Box–Behnken Design. Applied Sciences, 15(10), 5439. https://doi.org/10.3390/app15105439









