Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies
Abstract
1. Introduction
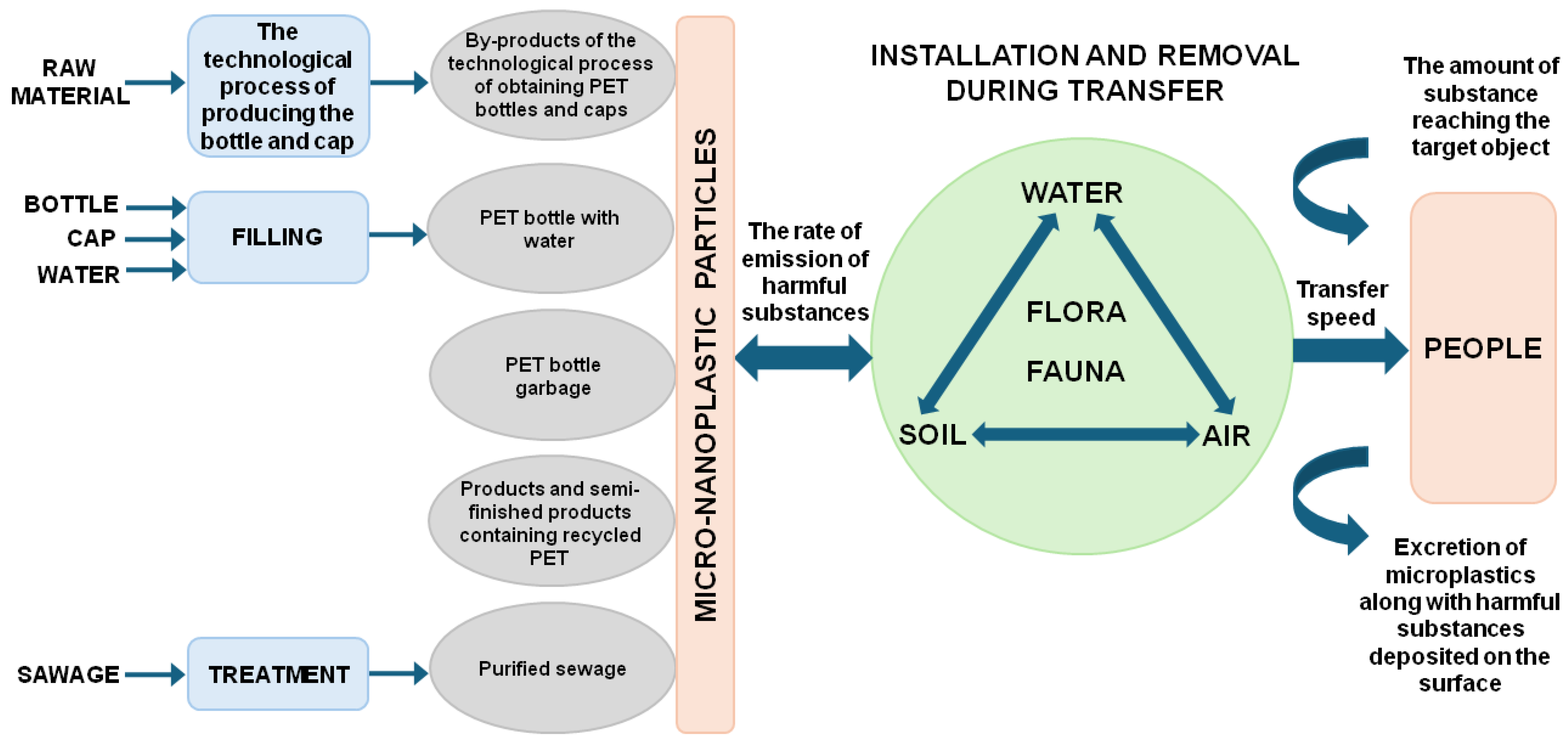
2. Significance of Environmental and Food Pollution by Microplastics
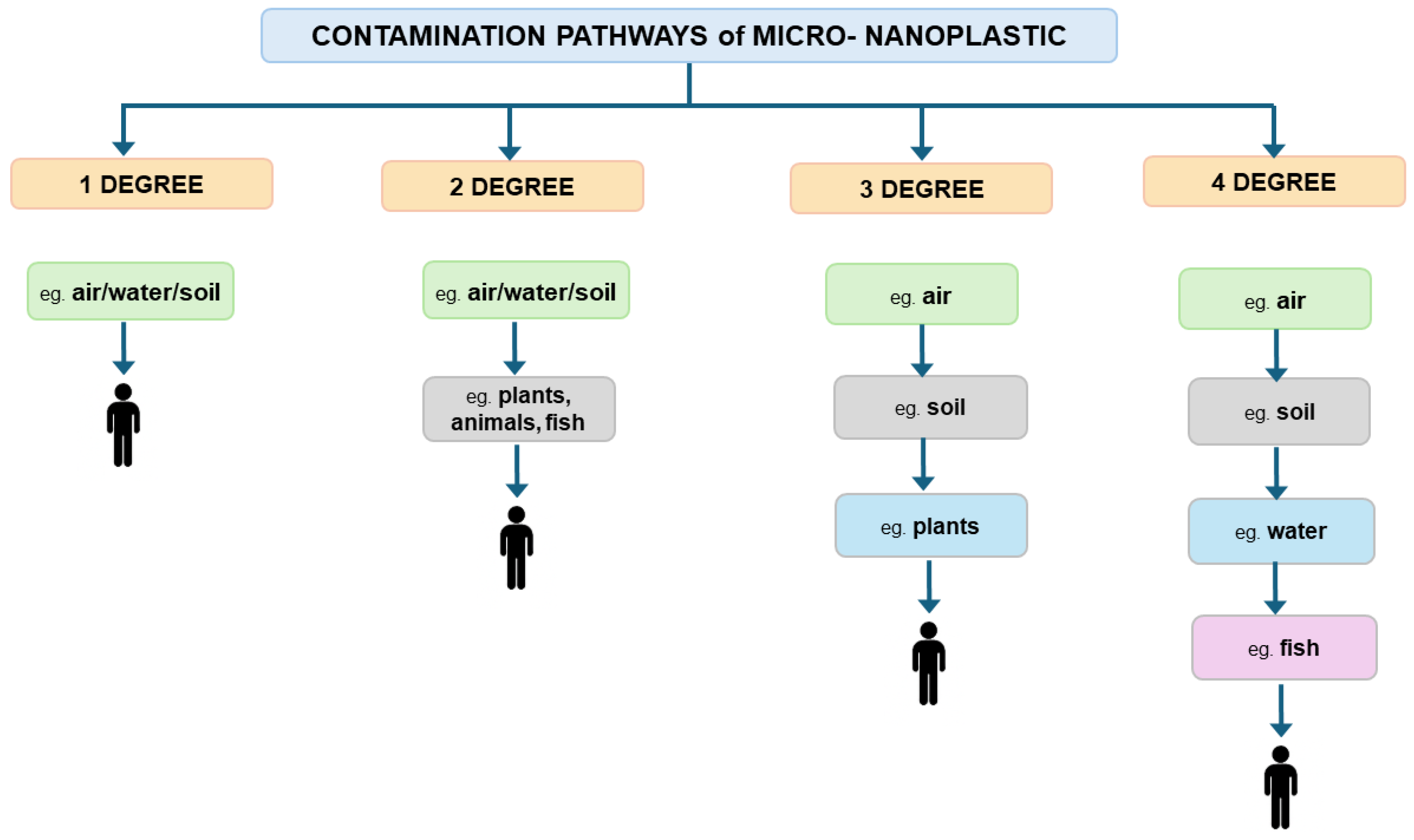
- Contamination path I degree—contamination reaches the target object very quickly because the contamination distribution chain consists of object I (e.g., air, water) and the target object.
- Contamination path II degree—contamination reaches the target object at a moderate pace because contamination is transferred to object I, from which it goes to object II (e.g., plants, vegetables, fruit, animals) and reaches the target object.
- Contamination path III degree—contamination reaches the target object slowly because contamination is transferred to object I (e.g., air), then to object II (e.g., soil), from which it gets to object III (e.g., plants, vegetables, fruit, animals) and reaches the target object.
- Contamination path IV degree—contamination reaches the target object very slowly, as the contamination is transferred to object I (e.g., air), then to object II (e.g., soil), from which it passes to object III (e.g., water) and to object IV (e.g., fish, seafood, plants) and reaches the target object.
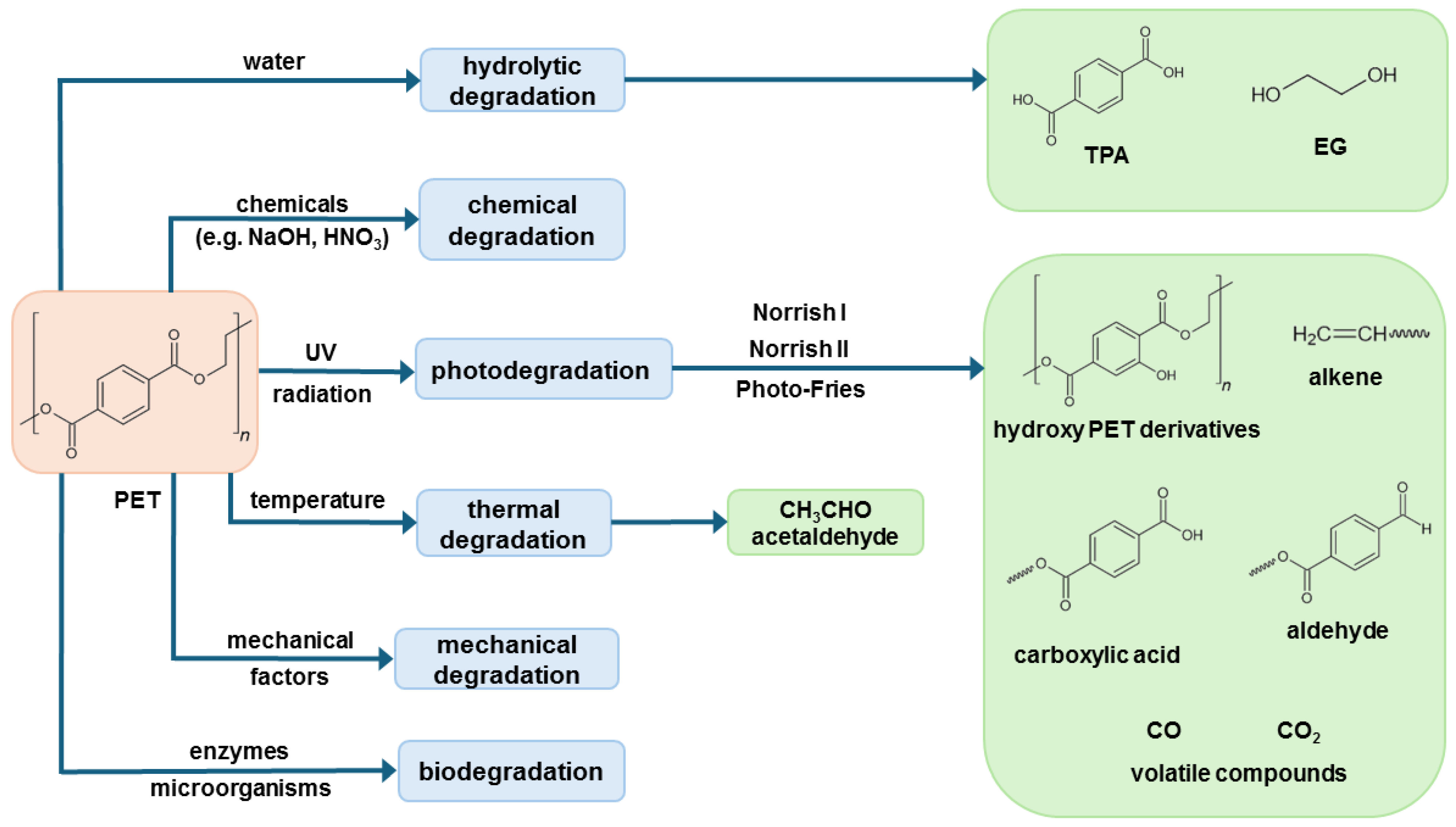
3. Mechanism and Factors Affecting PET Degradation
- Thermal degradation (factor: temperature),
- Photo-degradation (UV radiation),
- Hydrolytic degradation (water),
- Chemical degradation (chemical substances),
- Mechanical degradation (mechanical factors and stress),
- Biochemical degradation (enzymes or microorganisms),
- Combination of factors above.
4. Methods for Detecting of Microplastic Particles
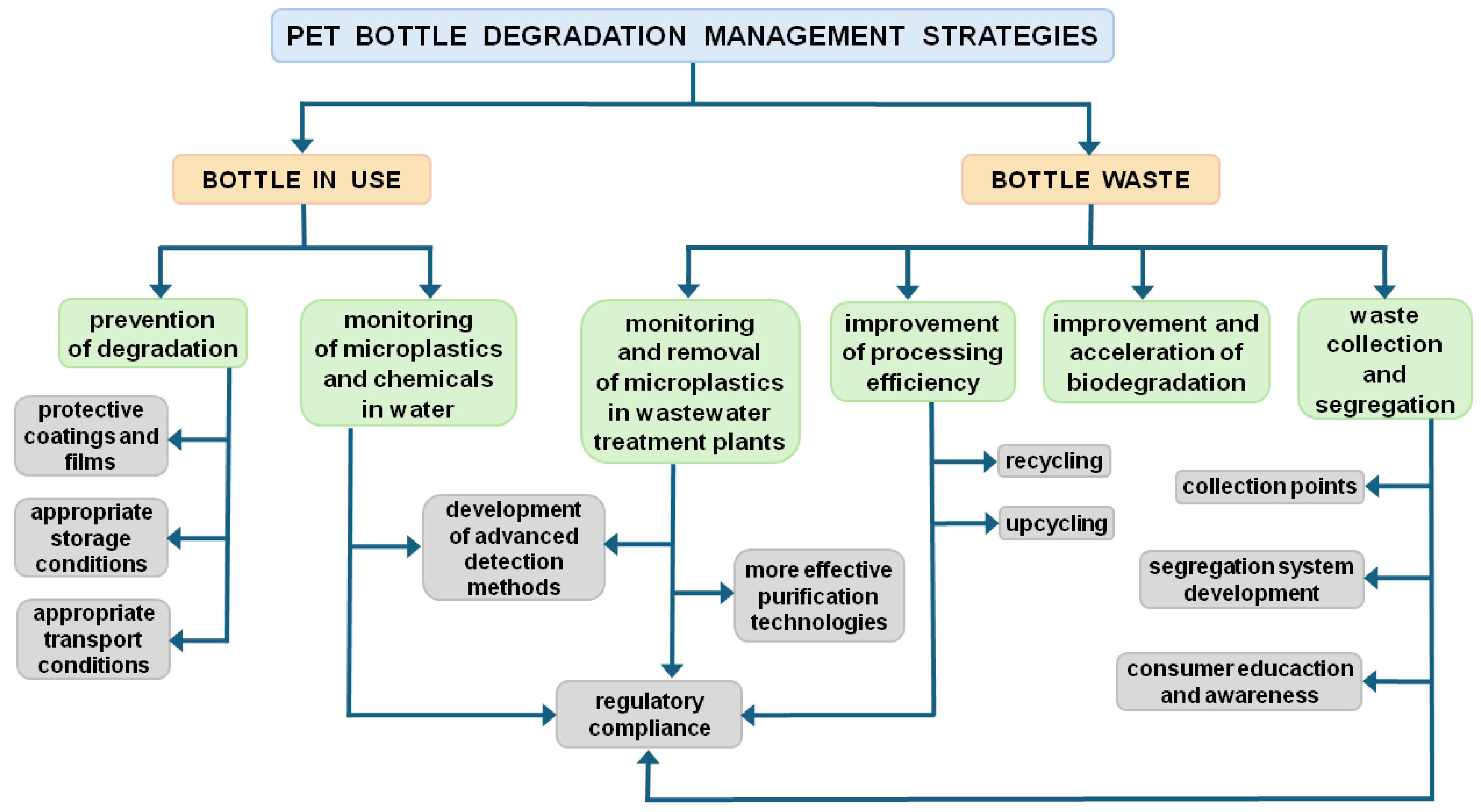
5. PET Bottle Degradation Management Strategies
5.1. Preventing PET Bottle Degradation
5.2. Improving the Efficiency of Waste Management
5.2.1. Biodegradation and Biological Recycling
5.2.2. Mechanical and Chemical Recycling
5.2.3. Upcycling
5.2.4. Monitoring and Removing Microplastics in Wastewater Treatment Plants
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| BHET | bis(hydroxyethyl)terephthalate |
| CAGR | compound annual growth rate |
| CVD | chemical vapor deposition |
| DLC | diamond-like carbon |
| DMT | dimethyl terephthalate |
| DRS | deposit return schemes |
| EG | ethylene glycol |
| EPR | extended producer responsibility |
| EU | European Union |
| LCC | leaf-branch compost cutinase |
| MBR | membrane bioreactor |
| MHET | mono(2-hydroxyethyl) terephthalate |
| MMA-MAc-DAM-MA | methacrylate-methyl acrylate-diallyl maleate-maleic |
| MP | microplastic |
| MPF | microplastic fiber |
| µ-FTIR | FTIR microscopy |
| µ-Raman | Raman microscopy |
| O-PTIR | optical photothermal infrared spectroscopy |
| OVPOSS | octavinyl-polyhedral oligomeric silsesquioxane |
| PET | polyethylene terephthalate |
| PICVD | plasma impulse chemical vapor deposition |
| PPWR Regulation | EU Packaging and Packaging Waste Regulation |
| PU | polyurethane |
| PVOH | Poly(vinyl alcohol) |
| py-GC-MS | pyrolysis gas chromatography-mass spectrometry |
| QCL-IR | tunable mid-infrared quantum cascade laser technology |
| rPET | recycled PET |
| RSF | rapid sand filtration |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SMA | stone mastic asphalt |
| SUP Directive | EU Directive on single-use plastics |
| TPA | terephthalic acid |
| UV | ultraviolet |
| VOC | volatile organic compound |
| WCCG | quadruple variant of LC-cutinase |
References
- Panou, A.; Karabagias, I. Migration and Safety Aspects of Plastic Food Packaging Materials: Need for Reconsideration? Coatings 2024, 14, 168. [Google Scholar] [CrossRef]
- Muhib, M.I.; Uddin, M.K.; Rahman, M.M.; Malafaia, G. Occurrence of microplastics in tap and bottled water, and food packaging: A narrative review on current knowledge. Sci. Total Environ. 2023, 865–876, 161274. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, M.; Zhao, T.; Cui, J.; Ye, H.; Zhou, C.; Ye, L.; Zhou, L. Polystyrene microplastics trigger colonic inflammation in rats via the TLR4/NF-κB/COX-2 pathway and modulation of intestinal microbiota. Toxicology 2025, 513, 154090. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Li, B.; Zhang, J.; Guan, D.; Hu, Y.; Fan, H.; Sun, Y.; Wang, H.; Guo, L. New insights into microplastics inhibiting kitchen waste dry digestion: Digestion efficiency, microbial communities and microplastic aging mechanisms. Chem. Eng. J. 2025, 508, 160907. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Fu, Z.; Guan, D.; Zhang, D.; Zhang, H.; Zhang, Q.; Xie, J.; Sun, Y.; Wang, D. Innovative overview of the occurrence, aging characteristics, and ecological toxicity of microplastics in environmental media. Environ. Pollut. 2024, 346, 123623. [Google Scholar] [CrossRef]
- PET Bottle Market Report by Capacity (High, Medium, Low), Distribution Channel (Business to Business, Retail), Color (Transparent, Coloured), Technology (Stretch Blow Molding, Injection Molding, Extrusion Blow Molding, Thermoforming, and Others), End-Use (Packaged Water, Carbonated Soft Drinks, Food Bottles & Jars, Non-Food Bottles & Jars, Fruit Juice, Beer, and Others), and Region 2025–2033. Available online: https://www.imarcgroup.com/pet-bottle-manufacturing-plant (accessed on 21 April 2025).
- What We Learned About Recycling by Detecting 40 Billion Waste Objects in 2024. Available online: https://www.greyparrot.ai/resource-hub/blog/2024-recycling-data (accessed on 21 April 2025).
- Single-Use Plastics. Available online: https://environment.ec.europa.eu/topics/plastics/single-use-plastics_en (accessed on 5 March 2025).
- Packaging Waste—EU Rules on Packaging and Packaging Waste. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/packaging-waste_en (accessed on 23 April 2025).
- Economic Study of Returnable Refillable PET in the EU Soft Drinks Industry. Available online: https://unesda.eu/wp-content/uploads/2024/04/PwC-Economic-study-of-returnable-refillables-PET_2022.pdf (accessed on 5 March 2025).
- AENOR Certifies the 100% rPET Recycled Plastic Content in the Aguas Danone Bottles. Available online: https://www.en.aenor.com/conocenos/sala-de-informacion-aenor/notas-de-prensa/aenor-certifica-el-contenido-plastico-reciclado-rpet-en-las-botellas-de-aguas-danone (accessed on 21 April 2025).
- Coca-Cola in Western Europe Transitions to 100% Recycled Plastic (rPET) Bottles in Two More Markets. Available online: https://www.cocacolaep.com/news-and-stories/coca-cola-in-western-europe-transitions-to-100-recycled-plastic-rpet-bottles-in-two-more-markets/ (accessed on 21 April 2025).
- TOMRA Is Ready for the Expansion of the Deposit Return System in Germany from January 2022. Available online: https://www.tomra.com/news-and-media/news/2022/expansion-of-deposit-return-systems-in-germany (accessed on 21 April 2025).
- Deposit-Refund System in Poland. Available online: https://www.gov.pl/web/climate/deposit-refund-system-in-poland (accessed on 21 April 2025).
- Recycling Revelations: Study Calls for “Super Cleaning” Investment Amid rPET Bottle Contamination Concerns. Available online: https://www.packaginginsights.com/news/recycling-revelations-study-calls-for-super-cleaning-investment-amid-rpet-bottle-contamination-concerns.html (accessed on 21 April 2025).
- Bach, C.; Dauchy, X.; Chagnon, M.C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef]
- MacDonald, W.A. New advances in poly(ethylene terephthalate) polymerization and degradation. Polym. Int. 2002, 51, 923–930. [Google Scholar] [CrossRef]
- Whelan, T. Polymer Technology Dictionary; Chapman & Hall: London, UK, 1994; p. 327. [Google Scholar]
- European Commission. Commission Delegated Decision (EU) 2024/1441 of 11 March 2024 Supplementing Directive (EU) 2020/2184 of the European Parliament and of the Council by Laying Down a Methodology to Measure Microplastics in Water Intended for Human Consumption (Notified Under Document C(2024) 1459). Available online: https://eur-lex.europa.eu/eli/dec_del/2024/1441/oj (accessed on 27 March 2025).
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Pinlova, B.; Nowack, B. From cracks to secondary microplastics—surface characterization of polyethylene terephthalate (PET) during weathering. Chemosphere 2024, 352, 141305. [Google Scholar] [CrossRef] [PubMed]
- Horne, F.; Liggat, J.; MacDonald, W.; Sankey, S. Photo-oxidation of poly(ethylene terephthalate) films intended for photovoltaic backsheet. J. Appl. Polym. Sci. 2020, 137, 48623. [Google Scholar] [CrossRef]
- Fechine, G.; Rabello, M.; Souto Maior, R.M.; Catalani, L.H. Surface characterization of photodegraded poly(ethylene terephthalate). The effect of ultraviolet absorbers. Polymer 2004, 45, 2303–2308. [Google Scholar] [CrossRef]
- Rostampour, S.; Cook, R.; Jhang, S.; Li, Y.; Fan, C.; Sung, L. Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions. Polymers 2024, 16, 2249. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Amin, A.A.; Karimi, H.; Pirsaheb, M.; Kim, H.; Hossini, H. Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J. Water Process Eng. 2021, 39, 101708. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Hagelskjær, O.; Hagelskjær, F.; Margenat, H.; Yakovenko, N.; Sonke, J.E.; Le Roux, G. Majority of potable water microplastics are smaller than the 20 μm EU methodology limit for consumable water quality. PLoS Water 2025, 4, e0000250. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Taheri, S.; Shoshtari-Yeganeh, B.; Pourzamani, H.; Ebrahimpour, K. Investigating the pollution of bottled water by the microplastics (MPs): The effects of mechanical stress, sunlight exposure, and freezing on MPs release. Environ. Monit. Assess. 2023, 195, 62. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Boutroy, N.; Pernel, Y.; Rius, J.; Auger, F.; Bardeleben, H.; Cantin, J.; Abel, F.; Zeinert, A.; Casiraghi, C.; Ferrari, A.; et al. Hydrogenated amorphous carbon film coating of PET bottles for gas diffusion barriers. Diam. Relat. Mater. 2006, 15, 921–927. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. SiOx layer as functional barrier in polyethylene terephthalate (PET) bottles against potential contaminants from post-consumer recycled PET. Food Addit. Contam. 2008, 25, 788–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef]
- Giraldo-Narcizo, S.; Guenani, N.; Sánchez-Pérez, A.M.; Guerrero, A. Accelerated Polyethylene Terephthalate (PET) Enzymatic Degradation by Room Temperature Alkali Pre-treatment for Reduced Polymer Crystallinity. ChemBioChem 2023, 24, 1–6. [Google Scholar] [CrossRef]
- Gerassimidou, S.; Lanska, P.; Hahladakis, J.N.; Lovat, E.; Vanzetto, S.; Geueke, B.; Groh, K.J.; Muncke, J.; Maffini, M.; Martin, O.V.; et al. Unpacking the complexity of the PET drink bottles value chain: A chemicals perspective. J. Hazard. Mater. 2022, 430, 128410. [Google Scholar] [CrossRef]
- Europe Bottled Water Market Report by Product Type (Still, Carbonated, Flavored, Mineral), Distribution Channel (Supermarkets and Hypermarkets, Convenience Stores, Direct Sales, On-Trade, and Others), Packaging Type (PET Bottles, Metal Cans, and Others), and Country 2025–2033. Available online: https://www.imarcgroup.com/europe-bottled-water-market (accessed on 11 March 2025).
- STATISTICS2022. Available online: https://naturalmineralwaterseurope.org/statistics/ (accessed on 11 March 2025).
- Europe Bottled Water Market Size & Outlook, 2024–2030. Available online: https://www.grandviewresearch.com/horizon/outlook/bottled-water-market/europe (accessed on 2 February 2025).
- UNU-INWEH. Global Bottled Water Industry: A Review of Impacts and Trends. 2023. Available online: https://collections.unu.edu/eserv/UNU:9106/BottledWater_Report_Final_-compressed.pdf (accessed on 29 April 2025).
- Top Bottled Water Consuming Countries. Available online: https://www.worldatlas.com/articles/top-bottled-water-consuming-countries.html (accessed on 5 February 2025).
- Orset, C.; Barret, N.; Lemaire, A. How consumers of plastic water bottles are responding to environmental policies? Waste Manag. 2017, 61, 13–27. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- SaveMoneyCutCarbon. Available online: https://www.savemoneycutcarbon.com/learn-save/how-long-does-it-take-for-plastic-to-biodegrade/ (accessed on 30 April 2025).
- Dhaka, V.; Singh, S.; Anil, A.G.; Sunil Kumar Naik, T.S.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.; Singh, J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef]
- Elvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554–116568. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris; NOAA Technical Memorandum NOS—OR R—30; NOAA: Silver Spring, MD, USA, 2009; pp. 1–49. [Google Scholar]
- Aini, S.A.; Syafiuddin, A.; Benet, G.A. The presence of microplastics in air environment and their potential impacts on health. Environ. Toxicol. Manag. 2022, 2, 31–39. [Google Scholar] [CrossRef]
- Faure, F.; Saini, C.; Potter, G.; Galgani, F.; de Alencastro, L.F.; Hagmann, P. An evaluation of surface micro- and mesoplastic pollution in pelagic ecosystems of the Western Mediterranean Sea. Environ. Sci. Pollut. Res. 2015, 22, 12190–12197. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 13–24. [Google Scholar] [CrossRef]
- Alfaro-Nunez, A.; Astorga, D.; Caceres-Farias, L.; Bastidas, L.; Villegas, C.S.; Macay, K.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galapagos. Sci. Rep. 2021, 11, 6424–6432. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Sun, Y.; Asia, L.; Wong-Wah-Chung, P.; Doumenq, P.; Moulin, P. Microplastics in different water samples (seawater, freshwater, and wastewater): Removal efficiency of membrane treatment processes. Water Res. 2023, 232, 119673–119696. [Google Scholar] [CrossRef]
- Roslan, N.; Lee, Y.; Ibrahim, Y.; Tuan Anuar, S.; Yusof, K.; Lai, L.; Brentnall, T. Detection of microplastics in human tissues and organs: A scoping review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Sun, J.; Sui, M.; Wang, T.; Teng, X.; Sun, J.; Chen, X. Detection and quantification of various microplastics in human endometrium based on laser direct infrared spectroscopy. Sci. Total Environ. 2024, 906, 167760–167770. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total Environ. 2023, 854, 158699–158709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; AN, L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci. Total Environ. 2023, 856, 159060–159070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, C.; Liu, Y.; Li, W.; Li, S.; Peng, L.; Kang, L.; Ullah, S.; Gong, Z.; Li, Z.; et al. Microplastics are detected in human gallstones and have the ability to form large cholesterol-microplastic heteroaggregates. J. Hazard. Mater. 2024, 467, 133631–133642. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wang, X.; Li, D.; Wang, H.; Xu, H.; Liu, Y.; Kang, R.; Chen, Q.; Zheng, L.; et al. Discovery and analysis of microplastics in human bone marrow. J. Hazard. Mater. 2024, 477, 135266–135277. [Google Scholar] [CrossRef]
- Xu, H.; Dong, C.; Yu, Z.; Ozaki, Y.; Hu, Z.; Zhang, B.; Yao, W.; Yu, J.; Xie, Y. Detection and analysis of microplastics in tissues and blood of human cervical cancer patients. Environ. Res. 2024, 259, 119498–119509. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Feng, Z.; Wang, Z.; Lv, M.; Chang, J.; Chen, L.; Wang, C. Human Microplastics Exposure and Potential Health Risks to Target Organs by Different Routes: A Review. Curr. Pollut. Rep. 2023, 9, 468–485. [Google Scholar] [CrossRef]
- Brown, J.; Zeman, K.; Bennett, W. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am. J. Respir. Crit. Care Med. 2002, 166, 1240–1247. [Google Scholar] [CrossRef]
- Henneberger, A.; Zareba, W.; Ibald-Mulli, A.; Rückerl, R.; Cyrys, J.; Couderc, J.; Mykins, B.; Woelke, G.; Wichmann, H.; Peters, A. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ. Health Perspect. 2005, 113, 440–446. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.; Fabris, C.; Yurkow, E.J.; Stapleton, P. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55–66. [Google Scholar] [CrossRef]
- Medley, E.; Spratlen, M.; Yan, B.; Herbstman, J.; Deyssenroth, M. A Systematic Review of the Placental Translocation of Micro- and Nanoplastics. Curr. Environ. Health Rep. 2023, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Piyumali, H.; Wijesekara, H.; Sonne, C.; Lam, S.; Mahatantila, K.; Vithanage, M. Nanoplastic occurrence, transformation and toxicity: A review. Environ. Chem. Lett. 2023, 21, 363–381. [Google Scholar] [CrossRef]
- Li, X.; Li, Z. Perspectives on the Toxic Effects of Micro- and Nanoplastics on the Environment: A Bibliometric Analysis of the 2014 to 2023 Period. Toxics 2024, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Chojnacka-Puchta, L.; Zapór, L.; Miranowicz-Dzierżawska, K.; Skowroń, J. The exposure routes of micro-and nanoplastics and their potential toxic effects on human health. Med. Pr. Work. Health Saf. 2024, 75, 81–96. [Google Scholar] [CrossRef]
- Jones, R.; Wilks, E.; Metanomski, W.; Kahovec, J.; Hess, M.; Stepto, R.; Kitayama, T. Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations 2008; Royal Society of Chemistry: Cambridge, UK, 2009; p. 253. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Nayak, S.G.; Labde, J.V.; Gharal, P.R.; Rao, K.; Kelkar, A.K. Degradation and Recyclability of Poly (Ethylene Terephthalate); IntechOpen: Rijeka, Croatia, 2012; Chapter 4; pp. 75–98. [Google Scholar] [CrossRef]
- Roslaniec, Z.; Pietkiewicz, D. Synthesis and Characteristics of Polyester-Based Thermoplastic Elastomers: Chemical Aspects: Sections 1–5; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; Chapter 13; pp. 579–629. [Google Scholar] [CrossRef]
- Pirzadeh, E.; Zadhoush, A.; Haghighat, M. Hydrolytic and Thermal Degradation of PET Fibers and PET Granule: The Effects of Crystallization, Temperature, and Humidity. J. Appl. Polym. Sci. 2007, 90, 1544–1549. [Google Scholar] [CrossRef]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Hurley, C.; Leggett, G. Quantitative investigation of the photodegradation of polyethylene terephthalate film by friction force microscopy, contact-angle goniometry, and X-ray photoelectron spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 1688–1697. [Google Scholar] [CrossRef]
- Hurley, R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, L.; Huang, H.; Tian, Y.; Gong, Z.; Liu, P.; Wu, X.; Li, W.; Gao, S. Formation of Nano- and Microplastics and Dissolved Chemicals During Photodegradation of Polyester Base Fabrics with Polyurethane Coating. Environ. Sci. Technol. 2023, 57, 1894–1906. [Google Scholar] [CrossRef]
- Colnik, M.; Pecar, D.; Knez, Z.; Gorsek, A.; Skerget, M. Kinetics Study of Hydrothermal Degradation of PET Waste into Useful Products. Processes 2021, 10, 24. [Google Scholar] [CrossRef]
- Grossetête, T.; Rivaton, A.; Gardette, J.; Hoyle, C.; Ziemer, M.; Fagerburg, D.; Clauberg, H. Photochemical degradation of poly(ethylene terephthalate)-modified copolymer. Polymer 2000, 41, 3541–3554. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- McMahon, W.; Birdsall, H.A.; Johnson, G.R.; Camilli, C.T. Degradation Studies of Polyethylene Terephthalate. J. Chem. Eng. Data 1959, 4, 57–79. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Taheri, S.; Zadhoush, A.; Mehrabani-Zeinabad, A. Hydrolytic degradation of poly(ethylene terephthalate). J. Appl. Polym. Sci. 2007, 103, 2304–2309. [Google Scholar] [CrossRef]
- Lionetto, F.; Lionetto, M.G.; Mele, C.; Corcione, C.E.; Bagheri, S.; Udayan, G.; Maffezzoli, A. Autofluorescence of Model Polyethylene Terephthalate Nanoplastics for Cell Interaction Studies. Nanomaterials 2022, 12, 1560. [Google Scholar] [CrossRef]
- Helmberger, S.M.; Frame, K.M.; Grieshop, M. Counterstaining to Separate Nile Red-Stained Microplastic Particles from Terrestrial Invertebrate Biomass. Environ. Sci. Technol. 2020, 54, 5580–5588. [Google Scholar] [CrossRef]
- Majcen, A.; Tassoti, S.; Spitzer, P. Lighting Up for Learning—Fluorescence Analysis of Microplastic Particles by Secondary School Students Using Nile Red. J. Chem. Educ. 2023, 100, 4007–4012. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Sharma, A.; Sharma, S.; Nagraik, R. An insight into different microplastic detection methods. Int. J. Environ. Sci. Technol. 2022, 19, 5721–5730. [Google Scholar] [CrossRef]
- Lin, X.; Gowen, A.A.; Chen, S.; Xu, J.L. Baking releases microplastics from polyethylene terephthalate bakeware as detected by optical photothermal infrared and quantum cascade laser infrared. Sci. Total Environ. 2024, 924, 171408. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pavlidis, G.; Dillon, E.; Beltran, V.; Schwartz, J.J.; Thoury, M.; Borondics, F.; Sandt, C.; Kjoller, K.; Berrie, B.H.; et al. Micro to Nano: Multiscale IR Analyses Reveal Zinc Soap Heterogeneity in a 19th-Century Painting by Corot. Anal. Chem. 2022, 94, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Beltran, V.; Nuyts, G.; Borondics, F.; De Meyer, S.; Van Bos, M.; Jaroszewicz, J.; Otten, E.; Debulpaep, M.; De Wael, K. Novel optical photothermal infrared (O-PTIR) spectroscopy for the noninvasive characterization of heritage glass-metal objects. Sci. Adv. 2022, 8, eabl6769. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Godejohann, M.; Gerdts, G. Rapid Identification and Quantification of Microplastics in the Environment by Quantum Cascade Laser-Based Hyperspectral Infrared Chemical Imaging. Environ. Sci. Technol. 2020, 54, 15893–15903. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 μm in the Environment. Environ. Sci. Technol. 2020, 54, 15594–15603. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC—Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Rivera-Rivera, D.M.; Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Sánchez-Álvarez, A.; Rodríguez-Delgado, J.M.; Cedillo-González, E.I.; Kaushik, G.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques. Biosensors 2025, 15, 44. [Google Scholar] [CrossRef]
- Behera, A.; Mahapatra, S.R.; Majhi, S.; Misra, N.; Sharma, R.; Singh, J.; Singh, R.P.; Pandey, S.S.; Singh, K.R.; Kerry, R.G. Gold nanoparticle assisted colorimetric biosensors for rapid polyethylene terephthalate (PET) sensing for sustainable environment to monitor microplastics. Environ. Res. 2023, 234, 116556. [Google Scholar] [CrossRef] [PubMed]
- Marcela Melo Cardozo, I.; Pereira dos Anjos, J.; Oliveira Campos da Rocha, F.; de Andrade, J.B. Exploratory analysis of the presence of 14 carbonyl compounds in bottled mineral water in polyethylene terephthalate (PET) containers. Food Chem. 2021, 365, 130475. [Google Scholar] [CrossRef] [PubMed]
- Soufizadeh, M.; Morello, R.; Ferraro, A.; Spasiano, D.; Fratino, U. Technical and Economic Feasibility Investigation for the Treatment of Microplastic-Contaminated Marine Sediments Through an Environmentally Sustainable Separation Process. Sustainability 2025, 17, 1258. [Google Scholar] [CrossRef]
- Bellasi, A.; Binda, G.; Pozzi, A.; Boldrocchi, G.; Bettinetti, R. The extraction of microplastics from sediments: An overview of existing methods and the proposal of a new and green alternative. Chemosphere 2021, 278, 130357. [Google Scholar] [CrossRef]
- European Council. Directive (EU) 2018/852 of the European Parliament and of the Council of 30 May 2018 Amending Directive 94/62/EC on Packaging and Packaging Waste. Available online: https://eur-lex.europa.eu/eli/dir/2018/852/oj (accessed on 2 March 2025).
- European Council. Directive (Eu) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 2 March 2025).
- Council Decision (EU, Euratom) 2020/2053 of 14 December 2020 on the System of Own Resources of the European Union and Repealing Decision 2014/335/EU, Euratom. Available online: https://eur-lex.europa.eu/eli/dec/2020/2053/oj (accessed on 2 March 2025).
- Bach, C.; Dauchy, X.; Severin, I.; Munoz, J.F.; Etienne, S.; Chagnon, M.C. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: Chemical analysis and potential toxicity. Food Chem. 2013, 139, 672–680. [Google Scholar] [CrossRef]
- Liu, R.; Liao, Z.; Zheng, J.; Wu, X.; Zongyi Tan and, H.O. Characterizing the photodegradation-induced release of volatile organic compounds from bottled water containers. Eco-Environ. Health 2024, 3, 145–153. [Google Scholar] [CrossRef]
- Idris, A.; Muntean, A.; Mesic, B.; Lestelius, M.; Javed, A. Oxygen Barrier Performance of Poly(vinyl alcohol) Coating Films with Different Induced Crystallinity and Model Predictions. Coatings 2021, 11, 1253. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, X.; Sun, J.; Gao, H.; Bai, Y.; Shao, L. Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property. Coatings 2021, 11, 1451. [Google Scholar] [CrossRef]
- Kang, T.; Tang, L.; Qu, J. Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane. Coatings 2018, 8, 411. [Google Scholar] [CrossRef]
- KHS on the Importance of Innovative PET Bottle Coatings. Available online: https://packagingeurope.com/khs-on-the-importance-of-innovative-pet-bottle-coatings/11048.article (accessed on 26 February 2025).
- Nakaya, M.; Kodama, K.; Yasuhara, S.; Hotta, A. Novel G as Barrier SiOC Coating to PET Bottles through a Hot Wire CVD Method. J. Polym. 2016, 2016, 4657193. [Google Scholar] [CrossRef]
- Shirakura, A.; Nakaya, M.; Koga, Y.; Kodama, H.; Hasebe, T.; Suzuki, T. Diamond-like carbon films for PET bottles and medical applications. Thin Solid Film. 2006, 494, 84–91. [Google Scholar] [CrossRef]
- Cheng, D.; Cai, G.; Wu, J.; Ran, J.; Wang, X. UV protective PET nanocomposites by a layer-by-layer deposition of TiO2 nanoparticles. Colloid Polym. Sci. 2017, 295, 2163–2172. [Google Scholar] [CrossRef]
- Kocayavuz, O.; Demirel, B.; Yaraş, A.; Akkurt, F.; Daver, F. A way to enhance the mechanical performance and uv visible-light barrier of polyethylene terephthalate packaging material: Synthesis and application of takedaite (ca3b2o6). Polym. Adv. Technol. 2022, 33, 3359–3367. [Google Scholar] [CrossRef]
- Inaner, N.B.; Demirel, B.; Yaras, A.; Akkurt, F.; Daver, F. Improvement of environmental stress cracking performance, load-carrying capacity, and UV light barrier property of polyethylene terephthalate packaging material. Polym. Adv. Technol. 2022, 33, 2352–2361. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- Pinter, E.; Welle, F.; Mayrhofer, E.; Pechhacker, A.; Motloch, L.; Lahme, V.; Grant, A.; Tacker, M. Circularity Study on PET Bottle-To-Bottle Recycling. Sustainability 2021, 13, 7370. [Google Scholar] [CrossRef]
- Brouwer, M.T.; Chacon, F.A.; van Velzen, E.U.T. Effect of recycled content and rPET quality on the properties of PET bottles, part III: Modelling of repetitive recycling. Packag. Technol. Sci. 2020, 33, 373–383. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Takehana, T.; Yoshida, S.; Hiraga, K.; Oda, K. Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly(ethylene terephthalate). Int. J. Syst. Evol. Microbiol. 2016, 66, 2813–2818. [Google Scholar] [CrossRef]
- Wallace, N.E.; Adams, M.C.; Chafin, A.C.; Jones, D.D.; Tsui, C.L.; Gruber, T.D. The highly crystalline PET found in plastic water bottles does not support the growth of the PETase-producing bacterium Ideonella sakaiensis. Environ. Microbiol. Rep. 2020, 12, 578–582. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Almdal, K.; Meyer, A.S. Signifiance of poly(ethylene terephthalate) (PET) substrate crystallinity on enzymatic degradation. New Biotechnol. 2023, 78, 162–172. [Google Scholar] [CrossRef]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Rational Protein Engineering of Thermo-Stable PETase from Ideonella sakaiensis for Highly Efficient PET Degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Agrawal, S. Machine learning-based enzyme engineering of PETase for improved efficiency in plastic degradation. J. Emerg. Investig. 2023, 6, 1–10. [Google Scholar] [CrossRef]
- Mrigwani, A.; Thakur, B.; Guptasarma, P. Conversion of polyethylene terephthalate into pure terephthalic acid through synergy between a solid-degrading cutinase and a reaction intermediate-hydrolysing carboxylesterase. Green Chem. 2022, 24, 6707–6719. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, J.; Chen, F.; Khalid, M.; Ye, J.; Romantschuk, M.; Hui, N. Hydrolase and plastic-degrading microbiota explain degradation of polyethylene terephthalate microplastics during high-temperature composting. Bioresour. Technol. 2024, 393, 130108. [Google Scholar] [CrossRef]
- Guo, B.; Lopez-Lorenzo, X.; Fang, Y.; Bäckström, E.; Capezza, A.J.; Vanga, S.R.; Furó, I.; Hakkarainen, M.; Syrénl, P. Fast Depolymerization of PET Bottle Mediated by Microwave Pre-Treatment and An Engineered PETase**. ChemSusChem 2023, 16, e202300742. [Google Scholar] [CrossRef]
- Joseph, T.M.; Azat, S.; Ahmadi, Z.; Jazani, O.M.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene terephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chena, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, J.; Wang, J. Chemical degradation and recycling of polyethylene terephthalate (PET): A review. RSC Sustain. 2025, 3, 2111–2133. [Google Scholar] [CrossRef]
- Mahler, A.S.H.; Lemming, M.; Jaime-Azuara, A.; Pedersen, T.H.; Hinge, M. Chemical recycling of polymer contaminated poly(ethylene terephthalate) by neutral hydrolysis. Waste Manag. 2025, 192, 12–19. [Google Scholar] [CrossRef]
- Cho, M.H.; Song, Y.J.; Rhu, C.J.; Go, B.R. Pyrolysis Process of Mixed Microplastics Using TG-FTIR and TED-GC-MS. Polymers 2023, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Chang, J.Y.; Yuan, X.; Khan, E.; Ok, Y.S.; Hou, C.H. Upcycling waste polyethylene terephthalate (PET) bottles into high-performance activated carbon for electrochemical desalination. Chemosphere 2024, 364, 143029. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Zinchenko, A. Conversion of waste bottles’ PET to a hydrogel adsorbent via PET aminolysis. Chemosphere 2021, 9, 106129. [Google Scholar] [CrossRef]
- Ahmadinia, E.; Zargar, M.; Karim, M.R.; Abdelaziz, M.; Ahmadinia, E. Performance evaluation of utilization of waste Polyethylene Terephthalate (PET) in stone mastic asphalt. Constr. Build. Mater. 2012, 36, 984–989. [Google Scholar] [CrossRef]
- Veropalumbo, R.; Oreto, C.; Viscione, N.; Pirozzi, F.; Pontoni, L.; Trancone, G.; Race, M.; Russo, F. Exploring the effect on the environment of encapsulated micro- and nano-plastics into asphalt mastics for road pavement. Environ. Res. 2023, 216, 114466. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, X.; Ruan, J.; Wang, Y.; Zhang, J.; LeBlanc, G.A.; An, L. Ignored microplastic sources from plastic bottle recycling. Sci. Total Environ. 2022, 838, 156038. [Google Scholar] [CrossRef]
- Habib, R.Z.; Thiemann, T.; Kendi, R.A. Microplastics and Wastewater Treatment Plants—A Review. J. Water Resour. Prot. 2020, 12, 1–35. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setala, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Cho, S.J.; Choi, J.H.; Yoon, Y.S.; Um, N.I. Microplastic Pollution in Sewage Sludge from Wastewater Treatment Plants and Estimation of Microplastic Release. Water 2025, 17, 387. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, W.; Wu, S.; Ma, J.; Zhou, X.; Qian, X.; Xu, A.; Dong, W.; Jiang, M. Bioengineering Comamonas testosteroni CNB-1: A robust whole-cell biocatalyst for efcient PET microplastic degradation. Bioresour. Bioprocess. 2023, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.A.; Zhou, N.; Carroll, A.L.; Aryal, O.; Teitel, K.P.; Wilson, R.S.; Zhang, L.; Kapoor, A.; Castaneda, E.; Guss, A.M.; et al. Mechanisms of Polyethylene Terephthalate Pellet Fragmentation into Nanoplastics and Assimilable Carbons by Wastewater Comamonas. Environ. Sci. Technol. 2024, 58, 19338–19352. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpaa, M.; Sillanpaa, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- European Council. Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 Concerning Urban Wastewater Treatment. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj (accessed on 11 March 2025).

| Method | Key Parameters and Notes | Ref. |
|---|---|---|
| FTIR microscopy (µ-FTIR) | Microscopic imaging, particle identification based on FTIR spectra. Determination of number, size, shape and material of particles. Problematic detection of particles smaller than 20 μm. | [22,95] |
| Raman microscopy (µ-Raman) | Microscopic imaging, particle identification based on Raman spectra. Determination of number, size, shape and material of particles. | [22,30,31,32] |
| Autofluorescence measurements | Recording of fluorescence spectra of the bulk solution (does not directly provide the number of particles) or fluorescence microscopy. Limited to well defined samples, relatively cheap method but low selectivity. | [89] |
| Fluorescence measurements with dye Nile Red | Dye binding to microplastic particles followed by microscopic observation. Increased specificity compared to autofluorescence measurements. | [90,91,92,93,94] |
| Optical photothermal infrared spectroscopy (O-PTIR) | IR-irradiated sample thermally expands, refractive index change detected by visible light. High spatial resolution below 1 μm. | [96,97,98] |
| Tunable mid-infrared quantum cascade laser spectroscopy (QCL-IR) | Tunable lasers (1800–950 cm−1). Method fast, scans larger area, lower resolution vs O-PTIR. | [96,99] |
| Surface-enhanced Raman spectroscopy (SERS) | Sample placed on Klarite substrate and analyzed by Raman microscopy. High spatial resolution below 1 μm. | [100] |
| Pyrolysis gas chromatography-mass spectrometry (py-GC-MS) | Sample undergoes pyrolysis followed by gas chromatography separation and mass spectrometry identification. Determination of polymer type and total mass only; not particle number, size, or shape. | [101,102] |
| Biosensor with functionalized gold nanoparticles | Gold nanoparticles linked with a synthetic peptide exhibiting a high affinity for PET. Visual or instrumental, colorimetric detection. Theoretical concept has not yet been tested experimentally. | [104] |
| Preventive Measure | Key Characteristics and Potential Drawbacks | Ref. |
|---|---|---|
| Proper storage and transport conditions | Avoiding high temperatures and sunlight due to their impact on PET stability. | [28,112] |
| Surface modifications with polyvinyl alcohol (PVOH) | Improves durability, limits oxygen permeability. Limits PET degradation by oxygen. | [113] |
| Surface modifications with MMA-MAc-DAM-MA | Limits oxygen permeability, too thick layer has adverse effect. | [114] |
| Hybrid coating with octavinyl-polyhedral oligomeric silsesquioxane | Surface more resistant to mechanical and chemical damage. Coating has good thermal stability. | [115] |
| Silicon-based coatings | Protective layer of SiOx or hydrocarbon silicate applied to the inner side of the PET bottle. Reduced gas permeability, including oxygen. | [36,116,117] |
| Carbon coatings | PET surface modified by hydrogenated amorphous carbon coatings or diamond-like carbon coatings. Enhancing gas barrier properties, surface more resistant to mechanical and chemical damage. Bottles may be recycled. | [35,118] |
| TiO2 nanoparticles coatings | UV protection, increased thermal stability. | [119] |
| Addition of UV stabilizers (Tinuvin 1577w) | Improves durability, reduces formation of carboxyl groups. | [27] |
| PET composites with Ca3B2O6 or CaB2O4 | Improves mechanical properties, reduces UV permeability. | [120,121] |
| PET composites with polyurethane (PET-U) | Delays degradation but composite still generates microplastic. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawer, J.; Panuszko, A.; Kozłowski, D.; Juniewicz, J.; Szymikowski, J.; Brodnicka, E. Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies. Appl. Sci. 2025, 15, 5322. https://doi.org/10.3390/app15105322
Wawer J, Panuszko A, Kozłowski D, Juniewicz J, Szymikowski J, Brodnicka E. Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies. Applied Sciences. 2025; 15(10):5322. https://doi.org/10.3390/app15105322
Chicago/Turabian StyleWawer, Jarosław, Aneta Panuszko, Dawid Kozłowski, Jan Juniewicz, Jakub Szymikowski, and Elwira Brodnicka. 2025. "Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies" Applied Sciences 15, no. 10: 5322. https://doi.org/10.3390/app15105322
APA StyleWawer, J., Panuszko, A., Kozłowski, D., Juniewicz, J., Szymikowski, J., & Brodnicka, E. (2025). Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies. Applied Sciences, 15(10), 5322. https://doi.org/10.3390/app15105322







