1. Introduction
Cookies and biscuits are one of the most popular quick snacks worldwide due to their ready-to-eat nature, affordability, wide availability, relatively long shelf-life, and assortment diversity [
1,
2]. Although widely appreciated and consumed by different age and socio-economic groups, cookies are rich in sugar, sodium, saturated fats, and energy value and are poor in micronutrients, fiber, and bioactive compounds [
3]. As a result, their excessive consumption can lead to chronic non-communicable diseases such as cardiovascular diseases, cancer, and diabetes [
4,
5]. As consumers become more health-aware and interested in the active role of food to prevent lifestyle diseases, there is a need to improve the nutritional and functional value of these food products while maintaining good sensory and structural qualities [
1,
6]. Cookies have proven to be a good vehicle for the delivery of dietary fiber and bioactive compounds from the fruit processing industry to consumers [
7,
8]. The fruit processing industry generates large amounts of by-products with valuable nutritional and functional properties, as justified by their richness in fiber, micronutrients, and bioactive compounds [
9]. Currently, there is a growing trend towards their valorization to produce dietary supplements and nutraceuticals or to be used as natural food ingredients [
6,
10]. A good strategy in cookie and biscuit production involves the inclusion of functional ingredients in their composition by partially replacing the wheat flour with powders from fruit processing by-products [
11]. Many studies have been dedicated to the innovation of cookies and biscuits by partially replacing wheat flour with fruit processing by-products such as pomace flour from orange [
12], avocado, kiwifruit, pineapple and pomegranate [
2,
3,
13], apple [
14,
15,
16,
17,
18,
19], tomato [
20,
21], blueberry [
1,
8,
22,
23], melon [
14], grape [
5,
24,
25,
26], grapefruit [
6], pear [
11], and raspberry, red currants, and strawberry [
8,
27].
Bilberry (
Vaccinium myrtillus L.), also known as huckleberry, whortleberry, or European blueberry, is a perennial dwarf shrub belonging to the
Ericaceae family, genus
Vaccinium, originating from northern Europe and North America [
28]. From ancient times, bilberries, the blue/black fruits of bilberry, are known for their benefits in treating various conditions such as hemorrhoids, vomiting, diarrhea, skin ulcers, and mucosal tissue inflammation [
29]. The health-promoting effects of bilberries have been attributed to their phenolic compound content, comprising anthocyanins, terpenoids, flavonols, tannins, coumarins, phenolic acids, and resveratrol. Delphinidin and cyanidin are predominant among anthocyanins, followed by petunidin, peonidin, and malvidin, which are mostly glycosylated by galactose, glucose, and arabinose [
30]. Due to the abundance of anthocyanins and of the other health-promoting compounds with antioxidant, antimicrobial, anti-inflammatory, anti-diabetic, cardioprotective, neuroprotective, and anticancer activities, bilberries have been awarded the title of “super food” or “functional food” [
31,
32,
33,
34]. Bilberries are available fresh, dried, or frozen, but most often, they are used to produce juices, jams, alcoholic beverages, violet pigments, or food supplements [
35]. The residue from bilberry juice processing is the pomace, consisting mainly of skins and seeds, which is a valuable source of vitamins, fibers, and minerals. But what distinguishes bilberry pomace is its huge content of anthocyanins and other phenolic compounds with strong antioxidant properties. Moreover, bilberry pomace was reported to contain significant amounts of polyunsaturated fatty acids (including n-3 PUFAs) from the seed oil [
36]. Muceniece et al. [
37] obtained the highest yields of polyphenols in extracts from wild bilberries among five
Vaccinium spp. berry pomaces and demonstrated the strong antioxidative, hypoglycemic, and hepatoprotective properties of bilberry pomace extract. Other authors reviewed the anti-inflammatory, anti-obesity, antidiabetic, cardioprotective, and antiproliferative effects of bilberry anthocyanins [
38,
39]. Regardless of their high functional value, pomaces are usually used as animal feed or fertilizer or are even discarded by the food industry, resulting in environmental pollution and increasing the costs of the fruit processing industry [
40]. However, after drying to increase shelf life and reduce disposal, pomace could be used in various food applications for enhancing the biological value and functional properties while reducing fruit waste [
13,
20]. Previously, Syrpas et al. [
30] studied the enzyme-assisted extraction of bilberry pomace for further valorization and Nemetz et al. [
41] proposed the application of crude bilberry pomace powder as a coloring foodstuff, while Blejan et al. [
35,
42] developed fruit leathers and corn-based extruded snacks enriched with bilberry pomace powder. Recently, Nour [
43] optimized the direct extraction of the bioactive compounds from bilberry pomace in apple juice, while Frum et al. [
44] investigated the encapsulation of bilberry pomace powder to produce a new and sustainable dietary supplement.
Blackcurrant (
Ribes nigrum L.) is a branched woody shrub from the
Grossulariaceae family bearing dark purple bittersweet berries, which are particularly rich in phenolic compounds, including anthocyanins, phenolic acids, flavonoids, and condensed and hydrolyzable tannins [
45]. Anthocyanins represent the dominant phenolic group in blackcurrants, accounting for around 80% of the total phenolic content [
46]. Several in vivo and in vitro experiments have demonstrated the anti-inflammatory, antimicrobial, anticarcinogenic, vasomodulatory, and immunomodulatory activities provided by the blackcurrant intake [
47]. The strong astringency and acid taste and high perishability make blackcurrants seldom eaten in their fresh state, with the fruits mostly being processed in juices, jams, jellies, and alcoholic beverages [
48,
49]. In blackcurrant juice processing, around 20% of the fruits are discarded as pomace [
48,
50,
51]. Blackcurrant pomace has been recognized as a valuable source of fibers, fruit acids, and phenolic compounds, especially anthocyanins [
50,
52,
53]. Giving the blackcurrant pomace a further use makes it possible to recover its high fiber, vitamin, and bioactive content and to increase the sustainability of the fruit processing industry. Studies regarding potential applications of blackcurrant pomace have mainly been focused on anthocyanins [
54] and pectin [
55] extraction, as well as on the addition of blackcurrant extracts and powders in crackers [
56], fruit leather [
35], meat products [
57], pasta [
58], and yogurt [
59].
The purpose of this study was to enhance the fiber and bioactive compound contents of cookies by partially replacing wheat flour with bilberry and blackcurrant pomace powders obtained from by-products of juice production. The influence of the pomace powder addition on dough rheology and processing properties was evaluated, while control and supplemented cookies were analyzed for their physicochemical, bioactive, and sensory characteristics.
2. Materials and Methods
2.1. Raw Materials
Wild bilberries (Vaccinium myrtillus L.) and blackcurrants (Ribes nigrum L.) harvested from the hills of Vâlcea county, Oltenia Region, South-West Romania (44°49′ N 24°15′ E), were processed into juice without enzymatic treatment (juice yields of 57.6% and 49.3% for bilberry and blackcurrant, respectively) in a small-scale fruit juice factory located in Vaideeni (Vâlcea county, Romania). The pomaces (two batches of 3 kg for each berry) obtained as juice by-products were collected, packed in polyethylene bags, transported, and stored at −18 °C until use. The bilberry and blackcurrant pomaces were thawed in the air at room temperature (20 °C) and convectively dried in thin layers at 57 °C in a dehydrator (Deca +SS Design, Profimatic, Cluj-Napoca, Romania). The dried pomaces were thoroughly ground into powders using an electric coffee grinder (Bosch TSM6A011W, Bosch, Munich, Germany), passed through a 0.3 mm sieve, and kept in polyethylene bags at ambient temperature (20 °C) until cookies manufacturing.
The wheat flour type 650 (moisture—13.90%; protein—11.93%; fat—1.42%; and ash—0.65%) used for dough and cookie preparation was produced by the Maripan Cereal S.R.L mills (Tuglui, Dolj County, Romania). Vegetable fat for pastry (99.7% fat, containing refined palm and sunflower oils, emulsifier—E471, and antioxidant—E320, E321) was provided by Shchedro LLC (Zaporizhzhia, Ucraina), while the baking powder (Alpina) used in the formulation was purchased from Zeelandia S.R.L. (Iasi, Romania). Salt (sodium chloride) and granulated sugar were procured from the local market.
2.2. Chemicals
Gallic acid, Folin–Ciocalteu’s reagent, 2,2-diphenyl−1-picrylhydrazyl (DPPH), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (Steinheim, Germany), while anhydrous sodium carbonate, sodium hydroxide of analytical grade, and methanol were purchased from Merck (Darmstadt, Germany). Distilled water was used to prepare all the aqueous solutions.
2.3. Preparation of Cookies
The recipe of the control cookies (labeled CC) contained 500 g white wheat flour, 187 g sugar, 218 g vegetable fat, 4 g baking powder, 1 g sodium chloride, and 156 mL water. In the supplemented cookies, 2.5%, 5%, and 10% of the wheat flour was replaced with bilberry (labeled as CBI2.5, CBI5.0, and CBI10.0) and blackcurrant (labeled as CBC2.5, CBC5.0, and CBC10.0) pomace powder, respectively, while keeping all the other ingredients constant. The replacement levels were chosen based on previous works [
19,
27] and preliminary tests. Cookie dough was manufactured in a planetary mixer (Rohnson R586, 700 W, Praha, Czech Republic). Fat and sugar were thoroughly creamed with the flat beater for 12 min at medium speed; then, water containing the sodium chloride and baking powder was added and mixed for 6 min at high speed to obtain a homogenous cream. Finally, the wheat flour was gradually added to the creaming mass under continuous mixing and further kneaded for 20 min. The dough was sheeted to a thickness of 6.13 mm by using an automatic dough sheeter (Flamic, Waico Group, Isola Vicentina, Italy). Round-shaped cookies were cut from the dough sheet using a cookie cutter of 5 cm in diameter. The cookies were transferred to a baking tray and baked at 200 °C for 10 min in an electrically heated deck oven (MIWE Condo, Arnstein, Germany). After baking, the cookies were left to cool on racks to room temperature (20 °C) and packed in airtight Ziploc bags for further analysis. Cookies from each formulation were manufactured in two independent batches. The determinations of the color parameters, proximate composition, and physical, textural, and sensory properties of the cookies were carried out 24 h after baking.
2.4. Proximate Composition and Physicochemical Properties of Bilberry (BIPP) and Blackcurrant (BCPP) Pomace Powders
The proximate composition of BIPP and BCPP (dry matter, protein, fat, fiber, and ash content) was determined following standard methods [
60].
The water solubility index (WSI) of the pomace powders was determined according to the method provided by AACC [
61] and was calculated as follows:
The water absorption capacity (WAC) was determined following the method described by Jose et al. [
2]. Briefly, the mixture of 1 g of the sample and 10 mL of distilled water was kept for 30 min at ambient temperature (20 °C) and were then centrifuged for 30 min at 3000 rpm. The water absorption capacity was calculated as follows:
2.5. Proximate Composition of Cookies
Cookies were analyzed for dry matter, crude protein, crude fat, crude fiber, and ash content according to the methods presented in AACC [
61]. The analyses were carried out in duplicate, and the average values are reported.
2.6. Physical Characteristics of Cookies
The thickness and diameter were measured 1 h after baking on ten randomly selected cookies for each formulation using a digital Vernier caliper, as previously presented by De Toledo et al. [
14]. Two perpendicular diameters were measured for each cookie sample. The average values were reported in millimeters [
61]. The spread ratio was determined based on the ratio of the average diameter and average thickness of the cookies [
61]. The weight of the cookies was recorded by using a Kern balance (model ABJ 220 4M, Kern and Sohn Gmbh, Balingen, Germany).
2.7. Color Analysis
Color analysis was conducted 24 h after baking using a PCE-CSM1 reflectance colorimeter (PCE Instruments, Meschede, Germany). The following CIEL*a*b* color parameters were recorded on the upper surface of cookies: L* indicating lightness, a* indicating redness (+) to greenness (−), and b* indicating yellowness (+) to blueness (−). Hue angle (h*) and chroma (C*) were calculated as arctan(b*/a*) and (a*2 + b*2)1/2, respectively. Three randomly selected cookies from each formulation were analyzed, with five readings at different points of the central area of each sample.
2.8. Titratable Acidity and pH
Titratable acidity was measured in the extract made from 10 g of finely ground cookie, homogenized, and diluted to 100 mL with distilled water. The extract was titrated with 0.1 N NaOH until the pH reached 8.1 [
60], and the results were expressed as a % of citric acid. Triplicate extracts were prepared for each formulation. The pH measurements were taken in triplicate at 20 °C according to the official method of AOAC [
60] by using a Hanna pH-meter HI255 (Hanna Instruments, Padova, Italy).
2.9. Rheological Analysis
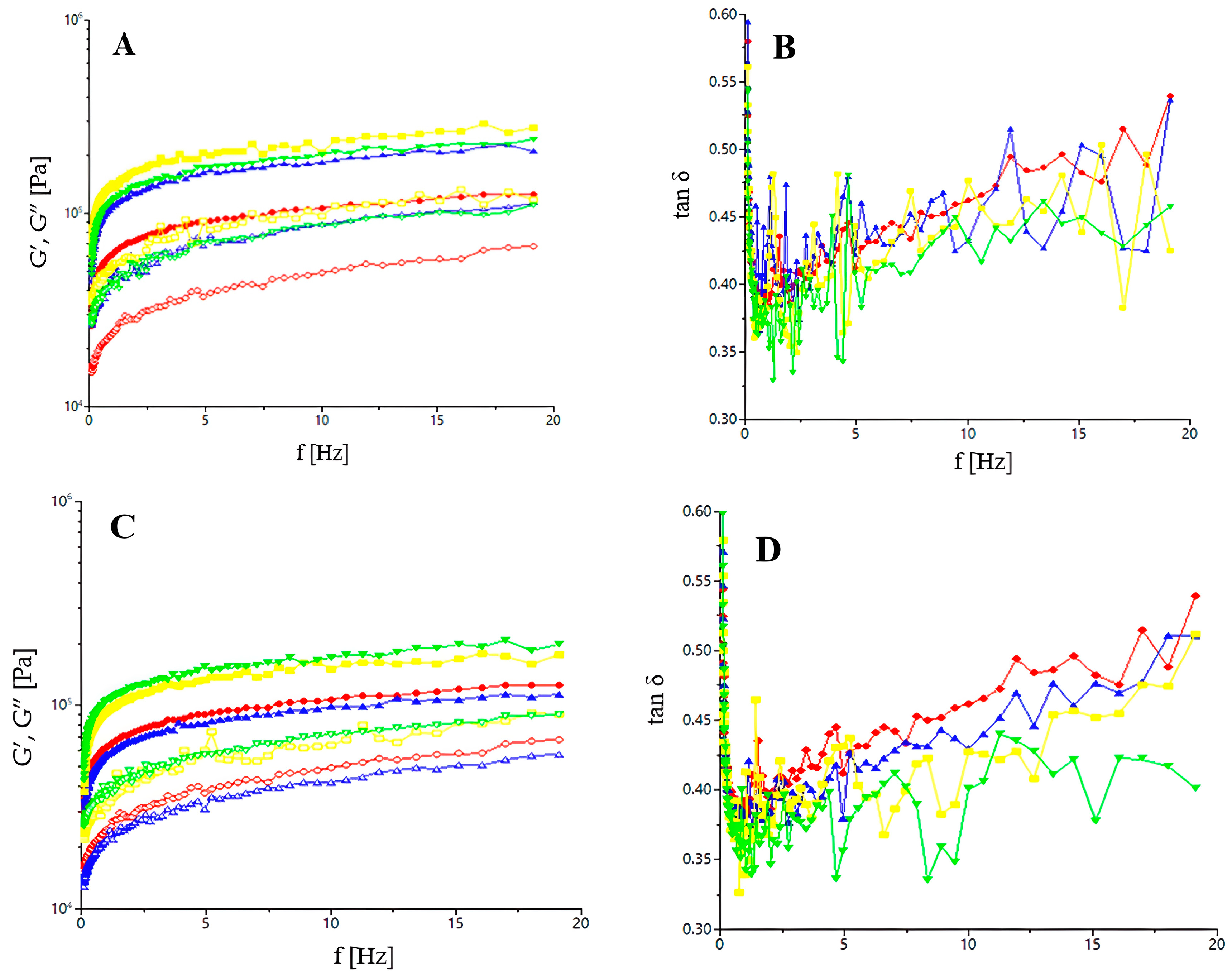
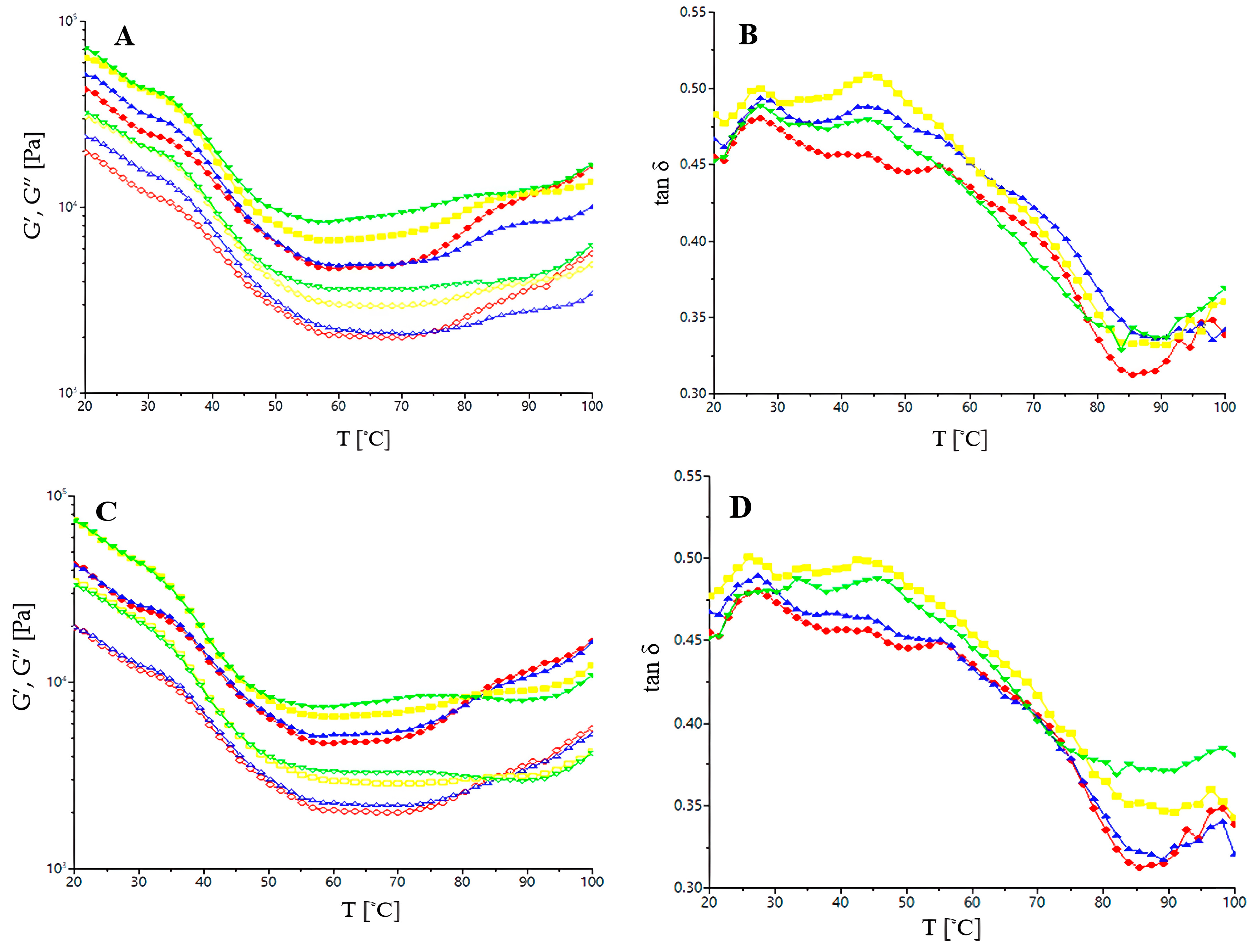
The dynamic moduli, storage (G′), loss (G″), and loss tangent (tan δ) were evaluated using a HAAKE MARS 40 rheometer (Termo-HAAKE, Karlsruhe, Germany). For this test, a gap of 2 mm was set and a plate of 40 mm in diameter was used. The dough samples were placed between the rheometer plates after a previous rest of 5 min for relaxation. Frequency sweep tests were performed at 25 °C from 1 to 20 Hz in a range of linear viscoelasticity. For the frequency sweep tests, the dynamic moduli and tan δ were carried out at a constant stress of 15 Pa during heating from 20 to 100 °C at a heating rate of 4 °C per min, a frequency of 1 Hz, and a fixed strain of 0.001.
2.10. Texture Analysis
The textural properties of the doughs were evaluated with a compression test by using the TVT 6700 texturometer (Perten Instruments, Hägersten, Sweden) equipped with a 35 mm diameter cylindrical probe. The sample height was 35 mm, and the starting distance from the sample was 5 mm. The compression was made up to 40% of the initial height of the sample. The trigger force was 20 g, the test speed was 5 mm/s, and the recovery time between compressions was 12 s. Hardness (N), adhesiveness (J), resilience (dimensionless), stringiness (mm), stickiness (g), cohesiveness (dimensionless), chewiness (N), and gumminess (N) were measured.
The textural properties of cookies were determined using a Perten TVT 6700 texturometer (Perten Instruments, Hägersten, Sweden). A double compression test was performed to determine hardness (N), springiness (dimensionless), cohesiveness (dimensionless), gumminess (N), and chewiness (N) parameters. For this test, the texturometer was equipped with a 10 kg load cell and the sample height was 7 mm. The compression was made up to 10% of the initial height of the sample by using a 20 mm diameter cylinder. The starting distance from the sample was 3 mm, the trigger force was 10 g, and the test speed was 2 mm/s. The recovery time between compressions was 5 s.
The fracturability (mm) of the cookies was assessed by a 3-point bend single-cycle breaking test. The geometry used for this test was made up of an aluminum break probe of 70 mm and a 3-point bend rig. The gap was carefully chosen between the support plates so that they could support the sample. The sample height was 7 mm, while the starting distance of the knife was 2 mm above the sample. The test was performed at a speed of 3 mm/s. A trigger force of 50 g was applied. A single-cycle compression test was performed to determine the force required to penetrate the sample (puncture force, N). For these determinations, the texturometer was equipped with a stainless steel cylinder probe, 45 mm in height and 2 mm in diameter. The average sample height was 7 mm, and the starting distance from the sample was 10 mm. The measurement parameters were set as follows: the compression, 2 mm; the initial speed, 1 mm/s; the test speed, 0.5 mm/s; the retract speed, 10 mm/s; and the trigger force, 5 g. The distance above the trigger was 40 mm. The shear force (N) (the force required to cut through the sample to a certain distance) was assessed by a single-cycle cutting test. The texturometer was equipped with a break probe 55 mm high in aluminum. The measurements were made in the center of each sample. The sample height was 7 mm, the starting distance from the sample was 5 mm, the trigger force was 25 g, and the test speed was 2 mm/s.
2.11. Extraction of Phenolic Compounds
After grinding the cookies with a pestle and mortar, 1 g of ground sample was homogenized with 10 mL methanol. After 60 min of sonication in a Bandelin Sonorex Digital 10P ultrasonic bath (Bandelin Electronic GmbH, Berlin, Germany), the homogenates were centrifuged at 2500× g for 5 min. The recovered supernatants were filtered through Whatman No. 1 filter paper and utilized for the subsequent analyses of the total phenolic content and DPPH radical scavenging activity.
2.12. Total Phenolic Content
The total phenolic content was assessed in the extracts by the spectrophotometric Folin–Ciocalteu method given by Singleton et al. [
62] using a calibration curve prepared with gallic acid. Aliquots (0.1 mL) of the extract or standard solution of gallic acid were pipetted in a test tube; then, 6 mL of distilled water and 0.5 mL of the Folin–Ciocalteu reagent (freshly diluted 1:1 with distilled water) were added. After 3 to 8 min, 1.5 mL of 20% sodium carbonate solution were added, and a 10 mL volume was made up with water. The content was vortexed for 1 min and stored in the dark for 30 min at 40 °C. The absorbance of the reaction mixture was then measured at 765 nm on a Varian Cary 50 UV spectrophotometer (Varian Co., Cary, NC, USA). The determinations were performed in three repetitions, and the results were reported as milligrams of gallic acid equivalents (GAE) per 100 g.
2.13. DPPH Radical Scavenging Activity
The antioxidant activity of the pomace powders and cookies was assessed as the ability to scavenge the DPPH free radical following the spectrophotometric method proposed by Brand-Williams et al. [
63] with slight modifications. This procedure involves reacting aliquots of 50 μL extract with 3 mL of 0.004% DPPH methanolic solution. After shaking them and keeping them in the dark for 30 min, the absorbance was recorded at 517 nm against methanol using a Varian Cary 50 UV spectrophotometer (Varian Co., NC, USA). The DPPH radical scavenging activity was calculated as the percent of inhibition by using the following formula: DPPH scavenging activity (%) = [1 − A
sample/A
control] × 100, where A
sample is the absorbance of the test sample and A
control is the absorbance of the control negative reaction without extract. Trolox was selected as a standard, and the results were expressed as milimoles of Trolox per 100 g of sample.
2.14. Sensory Analysis
A panel of 24 members (15 females and 9 males, aged 24 to 59 years), comprising master’s students and teaching staff from the Department of Horticulture and Food Science, University of Craiova, participated in the sensory evaluation. The cookies were evaluated 24 h after baking for appearance, color, taste, flavor, texture, and overall acceptability. The panelists were invited to complete a nine-point hedonic scale (1 being “dislike extremely” and 9 being “like extremely”). The test was conducted in two sessions, one for each berry pomace added to the cookies. In each session, the panelists received four units of cookies, referring to the pomace powder percentage replacing the wheat flour in the formulation (CC, CBI2.5, CBI5.0, and CBI10.0 in the first session, and CC, CBC2.5, CBC5.0, and CBC10.0 in the second session). The samples identified by code numbers were randomly served to panelists on plastic plates. The analysis was conducted under daylight conditions at 20 °C, water was provided, and the panelists were asked to rinse their mouth between the evaluations. This study was approved by the Committee on Ethics of University of Craiova (no. 482 on 21 February 2025) based on the informed consent from the participants.
2.15. Statistical Analysis
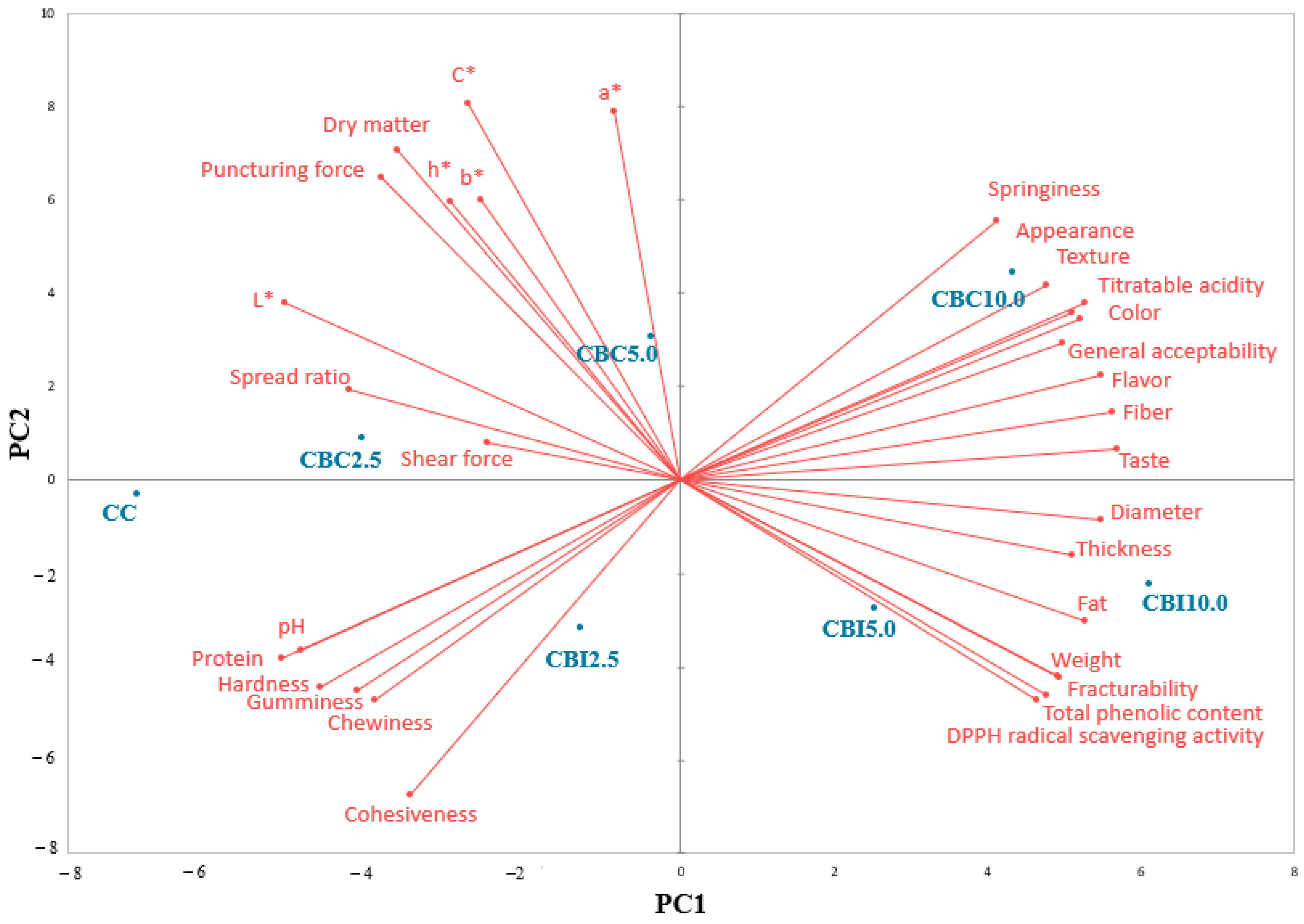
All measurements were carried out at least in triplicate. Mean values and standard deviation were calculated by using Statgraphics Centurion software (version XVI.I) from StatPoint Technologies, Inc. (The Plains, VA, USA). Analysis of variance (ANOVA) was conducted, and the means were compared using Fisher’s Least Significant Difference test (LSD) and the multiple range test with a confidence level set at 5% (p < 0.05). In order to perform principal component analysis (PCA), XLSTAT 2021.2.1 (New York, NY, USA) software was used.