Abstract
Isabella, an ancient hybrid grape originating from Vitis labrusca and Vitis vinifera genotypes, is widely cultivated for various food products and is considered a superfood due to its nutritional profile and high polyphenol content. To overcome the unsustainability of intensive agriculture and establish a new route towards more sustainable and socially fair superfood production, this work validated the establishment of undifferentiated in vitro cultures of V. labrusca var. Isabella. Two callus cell lines have been obtained on two different solid media, exhibiting distinct morpho-chemical characteristics. The total phenolic content and antioxidant activity of the callus juices were statistically different in the two cell lines. The subsequent qualitative–quantitative LC-MS analysis revealed the presence of seven stilbenoid derivatives in one cell line and three in the other; likewise, the total stilbenoid content was statistically different between the two cell lines (5.76 and 23.24 µg/mL of juice in the two cell lines on the 28th day of growth). The Isabella cell cultures possess nutritionally valuable profiles. These results suggest that plant cell culture technology can be a sustainable and viable option for the production of complementary, added-value food materials.
1. Introduction
In recent years, there has been a growing interest among consumers in adopting healthier dietary habits, accompanied by an increased awareness of food quality, safety, and environmental sustainability [1]. Plant-based foods may support these preferences, as they offer a combination of nutrients, micronutrients, and phytochemicals that are believed to promote overall health [2]. The understanding of the relationship between diet and health among customers led to an increasing consumption, especially by Western countries, of the so-called superfoods [3]. While the term “superfood” does not have an official, scientific, or regulatory definition, it is frequently used to describe foods that are inherently rich in beneficial nutrients. This appellative is mainly associated with agri-products that contain high amounts of nutrients or bioactive molecules, exhibiting specific biological properties and positive effects on physical and emotional health [4].
Among these superfoods, grapes particularly stand out and are often referred to as a superfruit. The most cultivated grapes (Vitis spp. L.), which are the most consumed fruits throughout the world in their natural or processed form [5,6], belong to the Euvitis sub-genus, including two particularly economically important species, Vitis vinifera (Eurasian group) and Vitis labrusca (the American group). Although V. vinifera is the most widely used and is especially known for its use in industrial wine production, V. labrusca is widely cultivated in North and South America and is a significant source of polyphenols in the Western diet [1]. V. labrusca and its hybrids, or natural hybrids with V. vinifera grapes (e.g., ‘Isabel’, ‘Concord’, ‘Bordô’, ‘Niagara’), are the main cultivars used in the grape processing industry among the world 10 major producers and, in Brazil, these account for around 50% of the total grape production [1]. In Turkey, Isabella (V. labrusca var. Isabella, also known as Isabel or Isabella) is the preferred grape due to its low susceptibility to major fungal diseases that commonly affect the vine, as well as its high production capacity [7]. The unique characteristics of colour, taste, and flavour of the Isabella berry have prompted the extension of its cultivation beyond wine production, especially for grape juice production [8,9]. Grape juice is a non-fermented grape derivative that is highly appreciated worldwide for its distinctive taste and associated health benefits [1]. Currently, grape juice ranks third in the world’s most exported juices, after orange juice and apple juice, and is a product of high commercial value [10].
Grapes are a rich source of phenols, along with other essential vitamins, minerals, dietary fibre, healthy carbohydrates, antioxidants, and a moderate amount of protein, as well as a high water content that helps keep the body hydrated [11]. Even if the nature and composition of different berry grapes can vary across species, cultivars, cultivation practices, and different processing [9], grapes as a whole and their extracts can be used as a source of antioxidants by the industry and utilised as nutraceuticals [12].
However, the growing demand for many of these recently recognised as superfood products means that they are often no longer produced using traditional and local production practices but instead revert to intensive agricultural production practices, with important social and environmental impacts [13]. The increase in demand for natural products also placed considerable pressure on traditional methods, such as wild harvesting, which had long served as a primary source of plant-based bioactive compounds. The current necessity of exacerbating wild-harvesting to meet demand can lead to biodiversity loss, habitat degradation, and the depletion of natural resources [14].
At the same time, providing this plant-based food in adequate amounts and quality will be increasingly complex [15] as reported by the Food and Agriculture Organization (FAO-2009) [16] and the United Nations (UN-2017) [17], the global population will increase by over a third between 2009 and 2050, reaching around 10 billion by 2050. Between 60% and 110% more food may be needed by 2050; therefore, a substantial expansion of agriculture is expected, as it is unlikely that all production increases will come from current agricultural land [18]. Dealing with the issue that high-intensity agriculture is already a huge environmental burden, responsible for 20–25% of global emissions, together with relying on fertiliser and pesticides, one of the greatest challenges of the 21st century is to meet society’s growing food needs whilst simultaneously reducing agriculture’s environmental impact [15].
Moreover, while crop yields should be increased to meet the growing food demand, the yields of major crops have plateaued in recent years. A potential yield increase through conventional agricultural practices is limited due to climate change, land degradation, and water scarcity, as well as the consequences of agricultural commodities that contribute to desertification, negatively impacting existing farming systems [19].
Throughout the decades, several strategies have been proposed to improve plant production efficiencies, among which “plant cellular agriculture” is a more modern and sophisticated form of agriculture [20].
Plant cell culture (PCC) technology is a new approach to consider for plant-based food production. In analogy to the radical invention of “cultured meat”, but to an even greater extent, bioreactor-grown plant cells could be exploited as an entirely new food biomass for human consumption. Since its introduction, this biotechnology has influenced several sectors, from the harvesting of crops with improved agronomic and nutritional characteristics, to the commercial cultivation of plant cells in bioreactors, rather than in the field, for the production of phytochemicals used as pharmaceuticals, pigments, and ingredients for cosmetics and food. If the aforementioned usages are well established, the possibility of using the entire in vitro-obtained biomass as a food alternative is still emerging [21,22,23].
The need for a new generation of sustainable and healthy plant-based food, and given that plant cell culture as a food ingredient is a largely unexplored area with enormous scientific and daily life applications [24], in this work, V. labrusca var. Isabella leaves were proposed as the starting material for the establishment of undifferentiated in vitro cultures.
This study focused on the obtainment of two solid undifferentiated cell lines using two basal media, the evaluation of the morphological and secondary metabolite differences between the two cell lines, and, eventually, since PCC can be a reliable technology for the production of high-value food materials in a fully controlled environment, independently of environmental or seasonal factors [20], we highlighted the nutritional content of the obtained biomasses.
2. Materials and Methods
2.1. Chemicals
HPLC-grade methanol and acetonitrile, and analytical-grade ethanol and acetic acid were purchased from VWR-BDH Chemicals (Milan, Italy). Ultrapure water was obtained using a Sartorius Arium system (Sartorius Italy, Varedo, Italy). Trans-resveratrol reference standard was purchased from Merck (Milan, Italy). Basal medium components, vitamins, hormones, and agar for the preparation of Isabella cell culture media were purchased from Duchefa (Micropoli, Milan, Italy).
2.2. Callus Cell Line Establishment
V. labrusca var. Isabella plants were purchased by a local nursery in Padova. Plants were planted in soil and, once they reached an appropriate growth level, the leaves were harvested at different vegetative growth stages and used as starting material (Table 1). Plant material has been washed accurately with tap water and then treated with a detergent. The sterilisation process has been carried out by dipping the plant material in 80% ethanol and then in 15% sodium hypochlorite plus a wetting agent. The leaves were rinsed three times in sterile distilled water under a laminar flow cabinet. Four trials were carried out; harvesting time and sterilisation conditions are reported in Table 1.

Table 1.
Trials on V. labrusca. EtOH means ethanol; NaClO means sodium hypochlorite.
Leaf explants (8 × 12 mm pieces), half with the abaxial face and half with the adaxial face adhering to the media, were cultured on MS [24] and B5 [25] agarized (9 g/L) basal media, supplemented with sucrose (3%), and with a pH of 5.7 before autoclaving. The basal media were added with 1.3 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D), 0.25 mg/L of kinetin (K), and 0.25 mg/L of naphtaleneacetic acid (NAA). MSA is the MS basal medium added of the plant growth regulators, and B5A is the B5 basal medium added of the plant growth regulators. After the sterilisation process in an autoclave (Fedegari, Pavia, Italy) for 30 min at 121 °C, 20 mL of each medium were transferred to 90 mm Petri dishes under a laminar flow cabinet, prior to utilisation.
All the experiments were performed using eight explants per dish. The explants were cultivated in a growth chamber at 25 ± 1 °C under a 16/8 h photoperiod, and the obtained biomass was subcultured every 6 weeks. The material responses were recorded weekly, and the cells were regularly observed under a S9i stereomicroscope (Leica, Wetzlar, Germany).
2.3. Preliminary Colourimetric Assays
For the juice obtainment, stabilised calli were harvested from Petri dishes and maintained at −18 °C. Once thawed, the material was squeezed and put in the ultrasound bath for 40 min. After the centrifugation, at 13,200 rpm, the supernatant of each sample (juice, hereafter) was taken and analysed. For the analysis of total phenol content and antioxidant activity during the growth cycle, the juices were obtained by squeezing the material harvested on the 14th, 28th, and 42nd days.
The total phenol content (TPC) was determined using the Folin–Ciocalteu colourimetric assay as described by Li et al. [26]. Gallic acid (Fluka, Buchs, Switzerland) was used for the standard calibration curve. Appropriately diluted juice or standard solution (200 µL) was added to 1 mL of 1:10 diluted Folin–Ciocalteu reagent (Fluka-Buchs, Switzerland). After 4 min, 800 µL of saturated sodium carbonate (75 g/L) was added. Following a 30 min incubation at room temperature, the absorbance at 765 nm was measured using a HeλIOS (Thermo Electron Corporation, Waltham, MA, USA) spectrophotometer. The calibration curve (y = 0.1089 × −0.0055, R2 = 0.9997) was constructed with gallic acid concentration from 1 µg/mL to 10 µg/mL. The samples were analysed in two independent experiments, each performed in duplicate, and the results are reported as means ± standard deviation (SD).
The antioxidant activity was tested using the DPPH method assay [27]. A total of 100 µL of appropriately diluted juice or ascorbic acid (Sigma-Aldrich, Milan, Italy), used as a positive control, was mixed with 400 µL of DPPH (Sigma-Aldrich, Milan, Italy) solution (0.1 mM). After 30 min of incubation, the absorbance at 517 nm was measured using a HeλIOS (Thermo Electron Corporation, Waltham, MA, USA) spectrophotometer. The antioxidant activity was calculated as a percentage of inhibition based on the discolouration of the DPPH solution in the presence of the antioxidant. The samples were analysed in two independent experiments, each performed in duplicate, and the results are reported as means ± SD. The results were compared to ascorbic acid calibration curve solutions (y = 11.768 × −0.9896, R2 = 0.9994) ranging within 0.8–6 µg/mL.
2.4. Quali-Quantitative Analyses
The quali-quantitative analyses by a liquid chromatography coupled with a mass spectrometer (LC-MS/MS) and a high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD) were performed on the juices obtained from calli in the same way as reported above, from the material harvested on the 14th and 28th days.
The LC-MS/MS analysis was performed using an Agilent 1290 Infinity II combined with the Agilent 6550 mass spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA). The chromatographic separation was performed using a Gemini 5 μm C6-Phenyl column (250 × 4.6 mm), with the same precolumn (Phenomenex, Bologna, Italy), and set at 40 °C. The optimised gradient programme was the following: eluent A, water with 0.1% (v/v) formic acid, eluent B, acetonitrile; 0–8 min, 97% A; 8–26.5 min, 75% A; 26.5–40 min, 20% A; 40–42 min 97% A. The flow rate was 0.75 mL/min, with an injection volume of 5 μL; chromatograms were acquired at 265 and 325 nm, and UV-Vis spectra were recorded in the 190–700 nm range. For MS and MS2 detection, the Dual AJS ESI source was operated in negative ion mode. The gas temperature was set at 300 °C with a flow rate of 5 L/min, while the sheath gas temperature was maintained at 250 °C with a flow rate of 11 L/min. The nebuliser pressure was set at 35 psi, and the capillary and fragmentor voltages were 3500 V and 260 V, respectively. In the case of MS2 analysis, the compound fragmentation patterns were recorded at different collision energies (0, 10, and 20 eV) with an isolation width of 4 m/z. The MassHunter Workstation Data Acquisition 10.0 (Agilent Technologies Inc., Santa Clara, CA, USA) programme was used for data acquisition, while the MassHunter Qualitative Analysis 10.0 (Agilent Technologies Inc., Santa Clara, CA, USA) software was used for data processing.
HPLC-DAD analyses were performed using an Agilent 1100 HPLC Series System (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a degasser, quaternary gradient pump, column thermostat, and UV-Vis detector. The chromatographic separation was performed using a Gemini 5 μm C6-Phenyl column (250 × 4.6 mm), with the same precolumn (Phenomenex, Bologna, Italy), and set at 40 °C. The mobile phase consisted of 0.15% acetic acid in water (A) and acetonitrile (B), with the following gradient elution programme: 97% A at 0–6 min, 75% A at 15 min, 75% A at 20 min, 20% A at 30 min, and 97% A at 40 min. The flow rate was 1 mL/min, with an injection volume of 10 µL; chromatograms were acquired at 325 nm, and UV-Vis spectra were recorded in the 190–700 nm range.
For the quantification of stilbenoid derivatives, the chromatograms acquired at the wavelength of 325 nm were used. The content was expressed as trans-resveratrol equivalents. Trans-resveratrol authentic commercial standard (Merck-Milan, Italy) solution (1 mg/mL) was prepared in methanol, and the calibration curve (y = 80.268 ×−21.156 R2 = 0.9993) was obtained with six concentration levels in a concentration range of 1.5–125 µg/mL. Peak areas were plotted against corresponding concentrations. The samples were analysed in two independent experiments, each performed in duplicate, and the results are expressed as mean ± SD.
2.5. Nutritional Analyses
2.5.1. Elemental Analysis and Protein Content During the Growth Cycle
Calli collected during the growth cycle (14, 28, and 42 days) were dried for 48 hrs in the freeze dryer (Lyovapor-L-200, Büchi-Milan, Italy). On the ground powder, carbon (C), hydrogen (H), nitrogen (N), and sulphur (S) were determined using a vario MicroCube elemental analyser (Elementar, Como, Italy). The samples (analysed in two independent experiments, each performed in duplicate), were exactly weighed into a tin container and placed in the loading assembly for the analysis. The elemental composition is expressed as the percentage in dry calli. The total protein content was determined by the nitrogen-to-protein conversion factor (N × 6.25) [28]. The protein content is expressed as the percentage in dry calli.
2.5.2. Nutritional Label
The nutritional value has been assessed on fresh callus samples by “EPTANORD food analysis and consulting” (Conselve, Padova, Italy). The moisture content, ash content, and fat content were measured according to ISTISAN 1996/34, and expressed in g/100 g [29]. The protein content was measured according to ISO 1871:2009 and expressed in g/100 g [30]. The fibre content was measured according to AOAC Official Method 985.29 1986 and expressed in g/100 g [31]. The total carbohydrates were calculated according to ISTISAN 1996/34 and expressed in g/100 g [29]. The energy value was determined according to Reg UE 1169/2011 (25 October 2011) and expressed in kilocalories (kcal/100 g).
2.6. Statistical Analysis
Results were reported as mean ± SD of two independent experiments, each performed in duplicate. Statistical analysis was performed using one-way analysis of variance (ANOVA) with GraphPad Prism v 7.05 (San Diego, CA, USA); p values < 0.05 were considered statistically significant. Different Latin letters (which denote significant differences at p < 0.05) are attributed in alphabetic order from the richest sample to the poorest sample.
3. Results and Discussion
3.1. Callus Cell Lines Establishment
To valorise V. labrusca var. Isabella as a source of polyphenols, Isabella leaves were used as starting material for establishing in vitro dedifferentiated cell cultures. The most responsive and least contaminated material, after the sterilisation process, was the one harvested in May 2022.
The choice of medium, as well as the plant growth regulators, is based on the literature data, the genus or species under investigation, considering, for example, whether the species is herbaceous or woody, as well as on the operator’s empirical experience. In fact, if no information is available in the literature, it is good practice to start with the widely used standard medium and then experiment by varying the plant growth regulators [32].
For the media choice, we took into consideration the published literature: there are several studies regarding V. vinifera, whereas information about V. labrusca is almost missing. The main media used for culturing grapevine cells are the Murashige and Skoog medium (MS) and the Gamborg medium (B5) [33,34,35,36,37], all with different combinations of auxin and cytokinin hormones [38]. Since, to the best of our knowledge, no studies on in vitro culture are available for the Isabella variety, we decided to use the two most commonly used media for grapevine, MS and B5 media, which are also those with higher macronutrient concentrations [32,39]. In addition, B5 medium has already been used for the cell culture establishment of two different V. labrusca varieties [37,40]. Both basal media were supplemented with a combination of two auxins (2,4-D and NAA) and kinetin as cytokinin (MS medium supplemented with plant growth regulators, MSA hereafter; B5 medium supplemented with plant growth regulators, B5A hereafter). The effects induced by the phytohormones depend on the concentration used, the target tissue, and their inherent activity; the most commonly used classes are auxins and cytokinins. These two classes are often added together in the media, and the ratio of their concentrations determines the type of biomass produced by the cultured cells or tissues [32,39,41].
The uncontaminated explants (80%), just a few days after, started to bend and become bullous and swollen; in fifteen days, the callogenesis started, mainly on the surface of the abaxial leaf blade of the explants cultured on MSA, and in the cutting area of the explants cultured on B5A. No differences in callogenesis initiation were observed in the explants’ abaxial or adaxial faces in B5A medium. In both media the callogenesis was 100%, and the calli kept growing in the two media, maintaining their respective features and ending up, after 10 subcultures of stabilisation, in two different cell lines in the two media: green and juicy in MSA (V MSA, hereafter) and mostly white and brownish in B5A medium (V B5A, hereafter) (Figure 1).
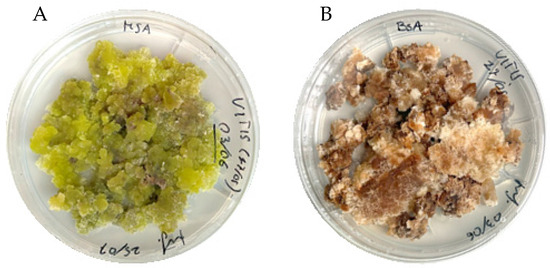
Figure 1.
V MSA—green and juicy callus cultured in MSA medium (A); V B5A—white and brownish callus cultured in B5A medium (B).
The different responses obtained in the two culture media can be fundamentally attributed to the two basal media, as the carbon source is qualitatively and quantitatively the same (sucrose 30 g/L), as well as the hormonal balance (2,4-D 1.3 mg/L; NAA 0.25 mg/L; K 0.25 mg/L), pH value, and explant type. The MS has a high concentration of nitrogen, particularly ammonium nitrate, compared to B5 medium, while B5 is richer in vitamins, especially thiamine [42]. The composition of the basal medium can have a different impact depending on the species being examined. However, it is known that nitrogen forms a major component of proteins and chlorophylls, and this could explain the formation of green calli in MSA. Instead, the brownish colour exhibited by the calli grown on B5A could be associated with the presence and partial oxidation of phenolic compounds [43].
Depending on the part of the plant that is cultured, you can refer to cell culture (cell suspension, gametic cells, and protoplast culture), tissue culture (callus and differentiated tissues), and organ culture (any organ, such as zygotic and somatic embryos, roots, shoots, and anthers, among others) [44]. Within these, callus cultures and cell suspensions offer a wide range of usages in the pharma and food sectors [45]. The callus culture, based on the dedifferentiated in vitro technology, has numerous applications in basic research and in environmental and commercial fields. Moreover, it allows the production of metabolites of pharmacological, cosmetic, and food interest [46], which is the reason why we decided to evaluate the possible potential of Isabella cultures.
3.2. Preliminary Colourimetric Essays
As a preliminary analysis, to highlight possible differences between the two cell lines obtained after a period of stabilisation (12 subcultures), we harvested the material and analysed it for total phenol content (TPC) and antioxidant activity. The TPC and antioxidant activities were measured in the juices obtained from the material harvested three times during the calli growth cycle. The growth cycle is the period between two successive subcultures, during which plant cells undergo three main phases (latency, exponential, and stationary), and each phase can be characterised by changes in plant metabolism [47,48,49]. Figure 2 shows the TPC of V MSA and V B5A juices, each harvested after 14, 28, and 42 days of cell growth. The TPC (Figure 2) is expressed as µg of gallic acid equivalent per mL of juice.

Figure 2.
TPC of V MSA and V B5A juices during the growth cycle. The results are reported as means ± SD. The significant differences at p < 0.05 are denoted by different Latin letters.
As reported in Figure 2, the TPC of V B5A juices increases in the mid-cycle and decreases at the end of the growth. The TPC of V B5A juice on the 28th day of growth resulted in the statistically highest content, and the total phenolic content of V B5A juices were quite always twice the content of V MSA; in fact, at 28 days of growth for V B5A the content reached 228 µg/mL of juice, but in V MSA only 131 µg/mL of juice. For V MSA, the content of the 28-day-old juice resulted statistically similar to that at 14 days, whereas the juice at 42 days resulted not only as the poorest within the V MSA line, but also among all the samples. The V B5A juice on the 42nd day, although statistically poorer than those at 14 and 28 days, resulted in a higher TPC compared to all the V MSA juices.
In the same way as for callus morphological characteristics, also for the production of phenolic compounds, the different biosynthetic capability of the two cell lines may only be attributed to the basal medium composition. It is well known that macro- and microelements can indirectly affect biosynthetic pathways and secondary metabolite production in cell cultures, as well as vitamins can act as catalysts in metabolic processes [42]. However, it is challenging to provide an unequivocal and definitive explanation for our results; certainly, the characteristics of the species and its propensity to adapt and respond to in vitro conditions represent key factors. Focusing on the nitrogen source, we would have expected higher metabolic activity in callus from MSA medium, given the high presence of ammonium nitrate, which can influence the production of secondary metabolites. At the same time, B5A contains an amount of thiamine 100 times higher than MSA, and thiamine also plays a vital role as a cofactor in the metabolic pathways of plant cells [42,50,51].
Plant cell and tissue cultures can be a feasible method for producing secondary metabolites shortly and efficiently (when the process is well-stabilised) compared to in vivo plants, which take several years to grow and reach the point of desired metabolite production [52,53,54]. Furthermore, cell culture technology offers several advantages compared to the in vivo materials, either from the point of view of cultivation, extraction, and production: the continuous year-round production independent of geographical and environmental constraints; the uniform quality material production free of pesticides; the possibility of high concentration of products of interest which can be produced by rapidly; the reduction in aggressive extraction solvents dangerous for the environment [22].
Among the secondary metabolite classes, polyphenols play a vital role in plants, including the attraction of pollinators, structural functions, UV radiation protection, and defence against biotic and abiotic stress. Plants can increase their production as a means of defence to cope with their environment [55,56]. Phenolics are a very heterogeneous class of aromatic compounds with at least one aromatic ring to which one or more OH groups are connected. They are naturally occurring in many plant-based foods, where they display a great variety of structures, from simple phenol derivatives to complex polymeric structures [56,57].
Besides the importance of the plant environment interactions and responses, the phenolic content is one of the most important factors that evaluate the health effects of plants [58]; the potential health benefits of dietary plant polyphenols in healthy promotion, as well as against several diseases, are well known and studied [56,57,59].
Grapes are among the most studied fruits due to their secondary metabolite content, especially for the phenolic fractions, which strongly contribute to the properties and health benefits of grapes [5,6].
In a Brazilian study [60], where Isabella is the most commonly used grape variety for juice production, the authors found a higher TPC in grapes harvested in September (more than 900 µg/mL) compared to those harvested in May (around 800 µg/mL). In another study, where the authors still analysed a Brazilian Isabella juice, obtained with the same extraction method as the previous one, they found almost the same TPC as in the material harvested in May of the previous work [61]. The TPC of Isabella was also monitored during ripening, highlighting its increase in overripe berries versus unripe ones, with a content three times lower in the latter (1.15 mg/g of fresh weight) [8]. In a comparative study with a V. vinifera variety [5], Isabella pomace was found to have a greater total phenolic content (470 µg/g of fresh weight). Considering our results, we found a total phenol content that is half to five times lower than that found in Isabella juice or berries, as far as V B5A juices. The lesser TPC found in our material could be explained by the different extraction method, which, in our protocol, consisted of a simple squeezing of the material, compared to the steam extraction method used for 60 min at a temperature maintained at 75–85 °C for the two Isabella juices mentioned earlier [60,61]. Moreover, the highest phenol content in those works can be attributed to the presence of the peel during extraction. In fact, the peel has a significantly higher content (approximately 22 times higher) than that in the pulp, which is similar to that of V MSA juices. In any case, these results confirmed the potential of in vitro material, in which different metabolic pathways can be activated.
Together with the TPC, the antioxidant capacity of this class was also measured due to its wide range of antioxidant activity. The DPPH method was employed based on phenols’ capacity to neutralise free radicals [55], along with scavenging reactive oxygen species (ROS), including the superoxide anion, hydroxyl radical, and hydrogen peroxide, which can help prevent or alleviate oxidative stress-related diseases [58,62].
Figure 3 shows the antioxidant activity of V MSA and V B5A juices, each harvested after 14, 28, and 42 days of cell growth. The antioxidant activity is expressed as the percentage of DPPH inhibition (discolouration) of 25 µL of juice.
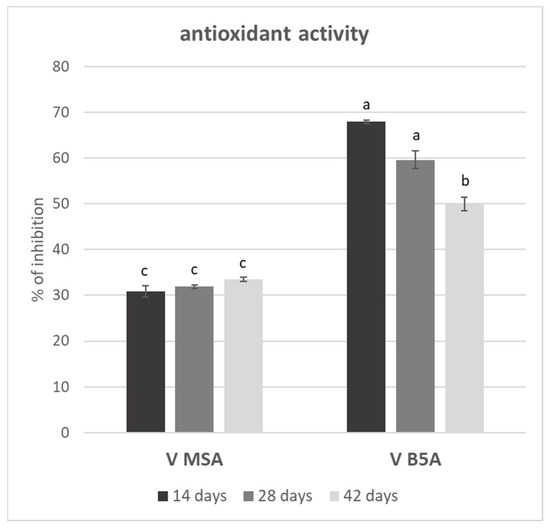
Figure 3.
Percentage of DPPH inhibition of 25 µL of V MSA and V B5A juices during the growth cycle. The results are reported as means ± SD. The significant differences at p < 0.05 are denoted by different Latin letters.
V B5A juices showed more activity than juices from V MSA; in fact, for V MSA juices, the inhibition is around 30%, whereas for B5A, it is about 60%. These data are in accordance with the TPC results reporting a phenol content in V B5A juices that was almost twice as compared with the juices from V MSA. V B5A juice on the 28th day of growth resulted in the most active, together with the juice on the 14th, and the one on the 42nd was the less active, whereas all the V MSA juices showed statistically the same activity. Looking at the activity, it is difficult to compare our results with other papers, due to different protocols [63], but in any case the antioxidant activity of V MSA juices, for all the growth cycle points, is comparable to less than 1.5 μg of ascorbic acid, tested in the same conditions; instead, for V B5A it is comparable to 2.93 and 2.17 μg of ascorbic acid, related, respectively, to juices harvested at 14 and 42 days of the growth cycle.
3.3. Quali-Quantitative Analyses
Based on the previous UV spectra obtained by the HPLC-DAD analysis, which suggested the major presence of stilbenoid class in the juice, the identification focused on these compounds. Seven compounds were identified in V B5A callus juice and three compounds in V MSA juice, based on compound molecular weights, fragmentation patterns, and UV spectra characterisation, further supported by the literature [64,65,66]. Therefore, the MS data were compared to chromatograms acquired at 325 nm at HPLC-DAD for the appropriate identification. The retention times, both of LC-MS analyses and HPLC-DAD, the maxima of UV spectra, the parental ions expressed as [M-H]-m/z, the fragmentations, and the tentative compound characterisation are reported in Table 2. The presence or absence of a compound in the two cell lines is marked with “×” or “-“, respectively.

Table 2.
Compounds tentatively identified by LC-MS/MS and HPLC-DAD analyses.
Figure 4 shows the V B5A chromatogram, which is the qualitatively richest, acquired at 325 nm, with the peaks numbered as reported in Table 2.

Figure 4.
A HPLC-DAD chromatogram of V B5A juice, acquired at 325 nm, with the identified peaks numbered.
In V MSA juice two isomers of resveratrol (compounds 7 and 8) and a resveratrol dimer diglucoside (compound 2) were found, whereas in V B5A juice two other resveratrol diglucoside dimers (compounds 4 and 5), an oxidised resveratrol dimer (compound 3), and a stilbenoid derivative (compound 1, based on the UV spectrum), were found. For the glycosylated forms, since the fragmentation is nearly identical between pallidol and ε-viniferin, their identity should be confirmed by comparison with pure standards [67].
Stilbenes, phenolic acids, and flavonoids are the most important polyphenols; stilbenes, which are phytoalexins, are synthesised in plants as a defence mechanism against external stresses. The limited presence of stilbenes in the human diet can be attributed to their restricted distribution in the plant kingdom. This is because the stilbene synthase, the enzyme involved in the last step, is not expressed in all plant species. As a result, stilbenes are found in only a few food sources such as grapes, red wine, peanuts, and some types of berries. In the past decades, the interest in this compound class has been continuously growing, especially in the scientific community, because of their potential health benefits on human health, especially resveratrol, which has been extensively studied during the past 20 years due to its wide range of biological activity [68,69,70]. One of the main sources of stilbenoids is the Vitaceae family, with V. vinifera at the head, followed by eight other genera that are known to produce stilbenoids, and V. labrusca is one of these. Considering the health and economic importance of stilbenes, and the extremely high variability of their content in plants, determined by several factors [68], the discovery that Isabella calli had mainly activated the stilbenes biosynthetic pathway could highlight this material as a source for stilbene production. Moreover, the different qualitative content in the two cell lines, with V B5A richer compared to V MSA, confirms that growth media could have, in some species, a very strong influence on secondary metabolite production.
After identifying the compounds of interest, quantitative analysis was performed during the growth cycle. The peak areas of the compounds were plotted against the trans-resveratrol standard curve at 325 nm. Considering that in vitro cell metabolism is plastic during the growth cycle, as a result of cell activity, quantitative analysis was also performed on the material harvested three times during the growth cycle, as reported above, on the 14th, 28th, and 42nd days.
Since the total phenol content decreases during the growth cycle by 42 days, quantitative analyses are referred to only for 14- and 28-day-old materials. The metabolite content, expressed as the sum of individual compounds, is expressed as µg/mL of juice, µg/g of fresh callus, and µg/g or mg/g of dry callus. This comparison has been possible because when the juice was extracted, we recorded both the volume of the juice and the weight of the harvested calli. Moreover, a portion of calli at each time point was lyophilised, which allowed for comparison between the juice, fresh, and dry material content. The total stilbenoid contents in the juice, fresh callus and dry callus, expressed as the sum of the single compounds, are reported in Table 3, and, to better appreciate the ratio among the compounds, Figure 5 shows the relative content, expressed as average, of each compound in the respective juice.

Table 3.
Total stilbenoid content, at 14 and 28 days of growth cycle, expressed as µg/mL of juice, µg/mL of fresh calli, and µg/g of dry calli. The results are reported as means ± SD, and the significant differences at p < 0.05, within the same column, are denoted by different Latin letters.
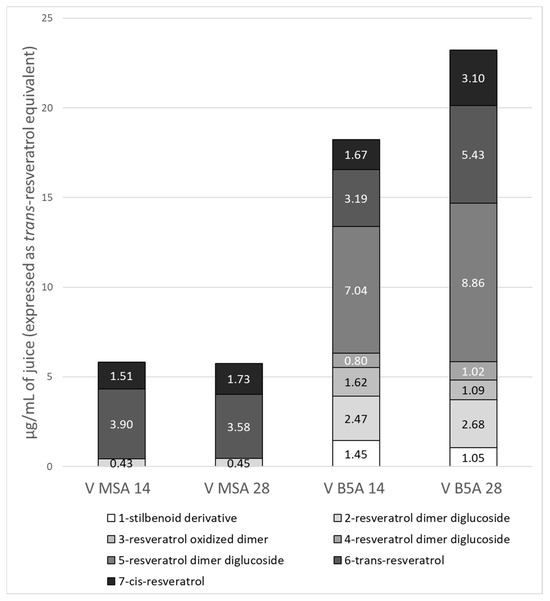
Figure 5.
Relative content of each compound in V MSA and V B5A juices at 14 and 28 days of the growth cycle. The results are reported as means.
As reported in Table 3, V B5A contents on the 14th day were always the richest, either in juice or fresh and dry callus, followed by the same cell line content at 14 days of growth. All the V MSA contents, either at 14 or 28 days, resulted in significant comparability among them, but were poorer compared to the V B5A contents (Table 3). These data confirmed the preliminary results of the TPC.
Stilbenoids are synthesised through the phenylpropanoid pathway, where stilbene synthase (STS) directly catalyses the reaction of trans-resveratrol formation [71]. Fewer articles focused on altering the stilbene biosynthetic properties of Vitis plant cells cultivated in vitro, showing an increase in resveratrol content in transgenic STS-overexpressing callus compared to the control [72]. Besides the genetic modification, different biotic and abiotic agents on the induced synthesis of resveratrol in plant tissues have also been studied widely, among which the use of chemicals and elicitors is particularly promising [37,73,74,75,76,77]. We found the highest total stilbenoid content in calli from B5A medium, whereas the authors of a work on the stilbene production in callus cultures of a grapevine variety found an increase in the total phenol content, including stilbenes, in MS medium added with 6-benzylaminopurine as cytokinin [77]. Moreover, MS medium presents ammonium nitrate (NH4NO3) as the main source of nitrogen, where it plays an important role in the growth of tissue cell lines and the production of phenolic compounds, often increasing their production, but sometimes also reducing it [78]. Even if Sae-Lee et al. [78] have demonstrated that treatment with ammonium nitrate in V. vinifera cv. Pok Dum cell suspensions could induce phenolics and resveratrol production, in our experiment, the material in the medium with ammonium nitrate as the nitrogen source (MSA) resulted in a lower total stilbenoid content compared to the material from the B5A medium, which is free of ammonium nitrate. The results confirm the different effects of culture media from one species to another and from one compound to another, showing that the optimal conditions for a specific culture are the result of systematic trials and experimentations [32,39,79].
From Figure 5, it is clear what was already reported: the qualitative richness of V B5A juice compared to V MSA. V B5A showed a greater amount of a resveratrol dimer diglucosylated (compounds 2), both at 14 and 28 days, and a greater content of the trans-resveratrol (compound 6), at 28 days of growth, compared to the other juices. Additionally, the content of cis-resveratrol (compound 7) was almost twofold at 28 days in V B5A, compared to the other juices. Moreover, V B5A juice exhibits an interesting amount of compound 5, not present in V MSA, which is a diglucoside derivative of resveratrol. The glycosylated bioactive compounds can exhibit increased water solubility, physicochemical stability, intestinal absorption, and biological half-life, thereby improving their bio- and pharmacological properties. The glycosylation of stilbenoids can overcome the water insolubility of these compounds, thereby increasing their pharmacological potential [80].
For the Vitis genus, the literature on stilbenoid presence is abundant but difficult to compare, mainly because many different clones, cultivars, and hybrids have been used, particularly with V. vinifera. As reported by [81], the comparison between quantification studies has some drawbacks because of variability, particularly when comparing solid materials such as grape skins or stems. Some problems may involve extraction rates of different solvents, extraction conditions, the occurrence of composition or isomer conversion, and variations in the preparation of plant material. Moreover, the literature on stilbenoid composition is mainly and almost exclusively focused on the quantification of trans-resveratrol. Dani et al. [82] reported the content of trans-resveratrol in 70% ethanol extracts of leaves from organic (0.714 mg/g extract) and conventional farming (0.062 mg/g extract). In V. labrusca var. Isabella trans-resveratrol was detected in peel (12.96 µg/g fresh weight) and seeds (2.35 µg/g fresh weight), but not in pulp [12]. On the other hand, Rockenbach et al. [83] did not find trans-resveratrol in the skin extract of Isabel. Cazarin et al. [60] observed an increase in the presence of trans-resveratrol in Isabella juice harvested in Brazil in September, compared to that in March, 0.73 µg/mL and 0.57 µg/mL, respectively. Also, less content was found by other authors in Isabella fresh weight. A higher content was found by other authors [6] that quantified resveratrol in pulp and seeds of plants V. labrusca var. Isabella from three different geographical regions and reported a content ranging from 12.30 to 28.85 mg/100 g pulp FW and from 18.77 to 37.50 mg/100 g seeds DW. Considering the data about the trans-resveratrol content of our callus juices, especially in V B5A at 28 days (5.43 µg/mL of juice), together with the other stilbene derivatives, that, as far as we know, are not yet been considered in Isabella material, in our opinion, in vitro Isabella calli can represent a new strategy for the production of stilbenes for biological and commercial evaluation.
3.4. Nutritional Analysis
3.4.1. Elemental Analysis and Protein Content During the Growth Cycle
The elemental composition (C, N, H, and S) and protein content of V MSA and V B5A, during the growth cycle, expressed as a percentage of dry calli, is reported in Table 4.

Table 4.
Elemental analysis on V MSA and V B5A during the growth cycle. The results are reported as means ± SD. The significant differences at p < 0.05 are denoted by different Latin letters.
Concerning the elemental composition, in both the cell lines the major elements were carbon (C), followed by hydrogen (H), nitrogen (N), and sulphur (S). V MSA resulted in the cell line with the highest content of N, especially at 42 days, and followed by the other two time points. The content of S, similar in all the juices and time points, showed an interesting tendency to increase on the 42nd day in V B5A, where it doubled the values of the other two time points on the 42nd day. It is known that sulphur plays important roles in the plant’s growth and development; it is also a component linked to plant tolerance to biotic and abiotic stress. However, it is difficult to draw conclusions about the increase observed at the end of the cell growth cycle because, so far, its role in plant cell management, compared to other elements, remains obscure [84].
V MSA, thanks to its highest content of N, also resulted in samples richer in protein, ranging from 27.19% at 28 days to 32.19% (the highest value among the samples) on the 42nd day. The V B5A protein contents at 14 and 28 days were almost half that of V MSA, resulting also in the poorest juices among the samples. Both the cell lines showed an increase in protein content on the 42nd day. As for the previous experiment, the data on the protein content confirmed the differences between the two Isabella cell lines, even if, contrary to the results on the secondary metabolites, the protein content of V MSA doubled V B5A, and even if the trend during the growth cycle for both cell lines resulted similar pattern. The nitrogen-to-protein conversion factor of 6.25 [28] is commonly used to determine the crude protein content in various matrices. The use of conversion factors is sensitive to errors due to non-proteinaceous nitrogen variability in different biomasses; however, their use avoids difficult extraction protocols, which can also affect the veracity and reproducibility of the results. Besides the intrinsic error, it is still widely applied in the majority of studies, and it is also recognised as a method for nutritional labelling in Europe (ISO 1871:2009) [30]. Eventually, this method permits a true comparison among the samples analysed. In one of the few available data on plant cell cultures [15], the protein contents of different berries’ culture resulted from 13.7 and 18.9% (DW) through the amino acid composition analyses, whereas if applying the nitrogen-to-protein conversion factor (as was performed for our samples) on the nitrogen from the elemental analyses, the protein content would be from 23.75 to 45.00%, similar to our cell lines, especially for V MSA. The total protein content of two cell lines was also studied by [85] through amino acid composition, and their data align with the previously reported data for dry weights. The protein content also resulted in comparable or lower values, applying the nitrogen-to-protein conversion factors, to the percentage of nitrogen found in a study investigating the potential of in vitro-grown industrial hemp calli, as a new generation of energy crop [86].
3.4.2. Nutritional Label
Based on the optimistic data on the elemental composition and protein content, we generated the nutritional label to evaluate plant cell cultures for the production of agricultural commodities for humankind.
The total content of moisture, ashes, carbohydrates, fibre, proteins, and fats, expressed as a percentage of fresh weight (FW) for each cell line, is reported in Table 5. Based on the percentage of moisture, the values have been calculated as a percentage of dry weight (DW), for a better comparison.

Table 5.
Moisture, ashes, carbohydrates, fibre, proteins, fats, and kcal expressed as percentage of fresh weight (FW) or dry weight (DW) of calli from each cell line.
As mentioned above, the applicability of in vitro plant cell culture material as a whole for food purposes is a concept that has only recently been introduced; therefore, comparisons with other papers are limited.
One of the major works on the nutritional characterisation of the nutritional value of PCC was pursued by Nordlund et al. [15], where three different in vitro biomasses derived from berries were evaluated in several contents, beyond the already cited protein content, including dry matter, elemental composition, carbohydrates, and fatty acids. The dry matter content of the samples ranged between 3% and 4%, data comparable with our samples, which are within the range of 3.9% to 4.1%. In our samples, the mass left behind after heating to 800 °C, associated with inorganic materials (hashes), was lower by about an order of magnitude compared to in vitro-grown industrial hemp calli, which ranged from 12.23% to 15.66% in dry weight. Meanwhile, the fibre contents of our samples (30.7 and 34.1% of dry weight for V MSA and V B5A, respectively) were comparable to those reported by Nordlund et al. [15].
Comparing the nutritional analyses of plant cell culture lines with those of their parental plants, we found that both the V MSA and V B5A resulted in higher water content compared to fruit, peel, and pulp (around 96% instead of 70–89%), whereas the ashes resulted in lower values. The protein content of fresh calli was comparable to that of fresh fruit pulp and peel, and in V MSA, it was also higher. The protein values on the dry material instead resulted in higher values than the fruit, especially for V MSA (26.4% in the nutritional label, and up to 32.19% on the 42nd day of the growth cycle) (Table 2), instead of values between 12 and 14% in dry V. labrusca fruits. The protein content was also higher than that of dry seeds, against which the total lipid content of plant cell culture (DW) resulted in more, 15.4 and 19.5%, respectively, for V MSA and V B5A instead of 10.84% in seeds [12,87].
Eventually, Isabella plant cell cultures were found to possess nutritionally valuable compounds, sometimes also exceeding the amounts of the reference material (fruits in this case).
4. Conclusions
This paper focused on the establishment of dedifferentiated plant cell cultures of V. labrusca var. Isabella, since grapes are among the most studied fruits due to their secondary metabolite content [5]. Unlike for V. vinifera, which is the other major grape, the literature is abundant, only recently V. labrusca has gained attention from the scientific community, thanks to the appellative of “superfruit”. We followed the in vitro culture technology route because high-tech production systems, such as PCC, may have advantages in the impact categories of water consumption and land use compared to products made by conventional farming practices [19]. We successfully established two cell lines, characterised by different morpho-chemical characteristics, with the V B5A line exhibiting the highest total phenol content, antioxidant activity, and total stilbenoid content.
Besides the use of PCC for secondary metabolite production in the pharmaceutical and cosmetic sectors, plant cell cultures have shown great potential for food uses, with relatively high protein, energy, and fibre contents. Even if it has been demonstrated the undifferentiated cells’ potential for food purposes, this technology, as far as now, is unlikely to cope with food-derived agriculture. Nevertheless, this technology can serve as an additional tool for global food production and opens up possibilities in places where traditional agriculture is constrained, such as due to climate or space limitations [85].
The V MSA and V B5A lines, obtained in this research, showed nutritional values comparable to the literature data on other cell cultures, and in some items, they were also better than those obtained from the parental plant. The results of our work can be considered as the groundwork for a further development of a commercial application of these materials; in fact, for the obtainment of PCC materials, often the starting protocols are species and even cultivar-specific, and most of the time based on the empirical experience of the researcher [79,88]. The literature on PCC-based food is still in its infancy, and few works have been published on this topic [15,89,90], and, until now, only two have performed a comprehensive environmental impact assessment on PCC systems [14,19]; therefore, the economic feasibility evaluation versus traditional farming products is relatively difficult. Further studies are needed to confirm these hypotheses after technical process optimisation of the Isabella in vitro-derived materials. Besides the novel food/food ingredient definitions vary broadly, showing the necessity of food regulation compliance to be met, and the necessity of studies on the ADME (absorption, distribution, metabolism, and excretion), another obstacle to overcome is consumer acceptance. The sensory evaluation for consumer acceptance, combined with safety assessment and knowledge sharing, could significantly enhance the application of plant cell culture technology as a sustainable tool and viable option for producing various added-value food materials [14,15,19,20,85,88,89,90].
Author Contributions
Conceptualisation, V.D.C., A.P., P.B. and R.F.; methodology, V.D.C. and R.F.; software, V.D.C. and P.B.; validation, V.D.C. and R.F.; formal analysis, V.D.C.; investigation, V.D.C. and R.F.; resources, A.P., P.B. and R.F.; data curation, V.D.C. and P.B.; writing—original draft preparation, V.D.C. and R.F.; writing—review and editing, V.D.C., A.P., P.B. and R.F.; visualisation, V.D.C. and R.F.; supervision, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Toaldo, I.M.; Cruz, F.A.; Alves, T.D.L.; De Gois, J.S.; Borges, D.L.G.; Cunha, H.P.; Da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive Potential of Vitis labrusca L. Grape Juices from the Southern Region of Brazil: Phenolic and Elemental Composition and Effect on Lipid Peroxidation in Healthy Subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Scognamiglio, M.; Gallicchio, M.; Potenza, N.; Piccolella, S.; Russo, A.; Monaco, P.; Fiorentino, A. Metabolic Profiling of Strawberry Grape (Vitis × Labruscana Cv. ‘Isabella’) Components by Nuclear Magnetic Resonance (NMR) and Evaluation of Their Antioxidant and Antiproliferative Properties. J. Agric. Food Chem. 2011, 59, 7679–7687. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, Y.D.; Mahale, S.V.; Zohra, B.; Nayik, G.A.; Dar, A.H.; Khan, K.A.; Abdi, G.; Karabagias, I.K. Nutritional Profile and Potential Health Benefits of Super Foods: A Review. Sustainability 2021, 13, 9240. [Google Scholar] [CrossRef]
- Fernández-Ríos, A.; Laso, J.; Aldaco, R.; Margallo, M. Superfoods: A Super Impact on Health and the Environment? Curr. Opin. Environ. Sci. Health 2023, 31, 100410. [Google Scholar] [CrossRef]
- Keskin, N.; Bilir Ekbic, H.; Kaya, O.; Keskin, S. Antioxidant Activity and Biochemical Compounds of Vitis vinifera L. (Cv. ‘Katıkara’) and Vitis labrusca L. (Cv. ‘Isabella’) Grown in Black Sea Coast of Turkey. Erwerbs-Obstbau 2021, 63, 115–122. [Google Scholar] [CrossRef]
- Kavgacı, M.; Yukunc, G.O.; Keskin, M.; Can, Z.; Kolaylı, S. Comparison of Phenolic Profile and Antioxidant Properties of Pulp and Seeds of Two Different Grapes Types (Vitis vinifera L. and Vitis labrusca L.) Grown in Anatolia: The Amount of Resveratrol of Grape Samples. Chem. Afr. 2023, 6, 2463–2469. [Google Scholar] [CrossRef]
- Yamamoto, L.Y.; De Assis, A.M.; Roberto, S.R.; Bovolenta, Y.R.; Nixdorf, S.L.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Application of Abscisic Acid (S-ABA) to Cv. Isabel Grapes (Vitis vinifera × Vitis labrusca) for Color Improvement: Effects on Color, Phenolic Composition and Antioxidant Capacity of Their Grape Juice. Food Res. Int. 2015, 77, 572–583. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Hayirlioglu-Ayaz, S.; Kostadinović Veličkovska, S.; Ilieva, F.; Esatbeyoglu, T.; Ayaz, F.A. Accumulation of Phenolic Compounds and Antioxidant Capacity during Berry Development in Black ‘Isabel’ Grape (Vitis vinifera L. × Vitis labrusca L.). Molecules 2020, 25, 3845. [Google Scholar] [CrossRef]
- Ozkan, K.; Karadag, A.; Sagdic, O.; Ozcan, F.S.; Ozer, H. The Effects of Different Drying Methods on the Sugar, Organic Acid, Volatile Composition, and Textural Properties of Black ‘Isabel’ Grape. Food Meas. 2023, 17, 1852–1861. [Google Scholar] [CrossRef]
- Spinelli, F.R.; André, J.A.; Celso, P.G.; Vicenzi, M.S.; Kinast, E.J.; Lamb, C.R.C.; Bertoldo, G. Soluble Solids Profile of Brazilian Vitis labrusca and Hybrid Grape Musts, from the 2012–2022 Harvest. J. Food Compos. Anal. 2024, 125, 105797. [Google Scholar] [CrossRef]
- Colak, N.; Bengu, A.S. Ripening-Related Changes in the Nutritional Profile of a Little-Known Red ‘Isabel’ Grape (Vitis vinifera L. × Vitis labrusca L.). Appl. Fruit Sci. 2024, 66, 1877–1889. [Google Scholar] [CrossRef]
- Santos, L.P.; Morais, D.R.; Souza, N.E.; Cottica, S.M.; Boroski, M.; Visentainer, J.V. Phenolic Compounds and Fatty Acids in Different Parts of Vitis labrusca and V. vinifera Grapes. Food Res. Int. 2011, 44, 1414–1418. [Google Scholar] [CrossRef]
- Magrach, A.; Sanz, M.J. Environmental and Social Consequences of the Increase in the Demand for ‘Superfoods’ World-wide. People Nat. 2020, 2, 267–278. [Google Scholar] [CrossRef]
- Oluyemi, G.F.; Afolabi, R.O.; Zamora, S.C.; Li, Y.; McElroy, D. Environmental Impact Assessment of a Plant Cell-Based Bio-Manufacturing Process for Producing Plant Natural Product Ingredients. Sustainability 2024, 16, 8515. [Google Scholar] [CrossRef]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.-M.; Heiniö, R.-L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant Cells as Food—A Concept Taking Shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. World Summit on Food Security. Rome 16–18 November 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 2 July 2025).
- Department of Economic and Social Affairs—Population Division. World Population Prospects. The 2017 Revision Volume I: Comprehensive Tables. United Nations, New York, 2017. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2017_world_population_prospects-2017_revision_volume-i_comprehensive-tables.pdf (accessed on 2 July 2025).
- Molotoks, A.; Stehfest, E.; Doelman, J.; Albanito, F.; Fitton, N.; Dawson, T.P.; Smith, P. Global Projections of Future Cropland Expansion to 2050 and Direct Impacts on Biodiversity and Carbon Storage. Glob. Change Biol. 2018, 24, 5895–5908. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kärkkäinen, E.; Häkkinen, S.T.; Nohynek, L.; Ritala, A.; Rischer, H.; Tuomisto, H.L. Life Cycle Assessment of Plant Cell Cultures. Sci. Total Environ. 2022, 808, 151990. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Mastrobuono, V.; Pagliarello, R.; Bennici, E.; Tavazza, R.; Verardi, A.; Presenti, O.; Panozzo, M.; Sangiorgio, P.; Massa, S. Consumer Acceptance of Edible Hydrogels Obtained by Plant Cell Culture Technology and By-Products Valorization: An Italian Case Study for Future Innovation of the Plate. Innov. Food Sci. Emerg. Technol. 2025, 100, 103893. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent Applications of Plant Cell Culture Technology in Cosmetics and Foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Davies, K.M.; Deroles, S.C. Prospects for the Use of Plant Cell Cultures in Food Biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef]
- Eibl, R.; Senn, Y.; Gubser, G.; Jossen, V.; Van Den Bos, C.; Eibl, D. Cellular Agriculture: Opportunities and Challenges. Annu. Rev. Food Sci. Technol. 2021, 12, 51–73. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Experimental Cell Research. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.X.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Kaewkod, T.; Promputtha, I.; Desvaux, M.; Tragoolpua, Y. Evaluation of Antioxidant and Antibacterial Activities of White Mulberry (Morus alba L.) Fruit Extracts. Plants 2021, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins. Circular 1931, 183, 1–16. [Google Scholar]
- Baldini, M.; Fabietti, F.; Giammaroli, S.; Onori, R.; Orefice, L.; Stacchini, A. Methods of analysis for the chemical control of foods. In ISTISAN 96/34; Istituto Superiore di Sanità: Rome, Italy, 1996. [Google Scholar]
- ISO 1871:2009; Food and Feed Products. ISO: Geneva, Switzerland, 2009.
- AOAC 985.29-1986; Total Dietary Fiber in Foods. Enzymatic-Gravimetric Method. Association of Official Agricultural Chemists: Gaithersburg, MA, USA, 2003.
- Dixon, R.A. (Ed.) Isolation and Maintenance of Callus and Cell Suspension Cultures. In Plant Cell Culture—A Pratical Approach; IRL Press: Washington, DC, USA, 1985. [Google Scholar]
- Moriguchi, T.; Kozaki, I.; Matsuta, N.; Yamaki, S. Plant Regeneration from Grape Callus Stored under a Combination of Low Temperature and Silicone Treatment. Plant Cell Tiss. Organ Cult. 1988, 15, 67–71. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wen, P.-F.; Kong, W.-F.; Pan, Q.-H.; Zhan, J.-C.; Li, J.-M.; Wan, S.-B.; Huang, W.-D. Effect of Salicylic Acid on Phenylpropanoids and Phenylalanine Ammonia-Lyase in Harvested Grape Berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of Elicitors on the Production of Resveratrol and Viniferins in Cell Cultures of Vitis vinifera L. Cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef]
- Taurino, M.; Ingrosso, I.; D’amico, L.; De Domenico, S.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates Elicit Different Sets of Stilbenes in Vitis vinifera Cv. Negramaro Cell Cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef]
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor. Plants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, É. Use of Grapevine Cell Cultures for the Production of Phytostilbenes of Cosmetic Interest. Comptes Rendus Chim. 2016, 19, 1062–1070. [Google Scholar] [CrossRef]
- Gray, D.J.; Trigiano, R.N. Introducing to Plant Tissue Culture. In Plant Tissue Culture Concepts and Laboratory Exercises; Trigiano, R.N., Gray, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Chen, J.; Hall, D.E.; Murata, J.; De Luca, V. L-Alanine Induces Programmed Cell Death in V. labrusca Cell Suspension Cultures. Plant Sci. 2006, 171, 734–744. [Google Scholar] [CrossRef]
- Plant Tissue Culture for Biotechnology. In Plant Biotechnology and Agriculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 131–138. ISBN 978-0-12-381466-1.
- Hazrati, R.; Zare, N.; Asghari-Zakaria, R.; Sheikhzadeh, P.; Johari-Ahar, M. Factors Affecting the Growth, Antioxidant Potential, and Secondary Metabolites Production in Hazel Callus Cultures. AMB Expr. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Orcan, P.; Orcan, M.Y. Insights into Total Phenolic, Flavonoid, and Antioxidant Activity of Callus Subculture Frequency in Rare Endemic Ajuga xylorrhiza Kit Tan. Sci. Rep. 2024, 14, 31720. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. (Eds.) Plant Cell Culture Protocols; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2018; Volume 1815, ISBN 978-1-4939-8593-7. [Google Scholar]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Prashant, S.P.; Bhawana, M. An Update on Biotechnological Intervention Mediated by Plant Tissue Culture to Boost Secondary Metabolite Production in Medicinal and Aromatic Plants. Physiol. Plant. 2024, 176, e14400. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of Plant Secondary Metabolites: A Historical Perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.K.; Bisaria, V.S. Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; ShangGuan, Y.; Liu, H.; Xu, D. Callus Growth Kinetics and Accumulation of Secondary Metabolites of Bletilla striata Rchb.f. Using a Callus Suspension Culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef]
- Gomulski, J.; Krzemińska, M.; Jochymek, M.; Kiss, A.K.; Grzegorczyk-Karolak, I. The Influence of Basal Medium on Polyphenol Accumulation in Shoot Cultures of Clerodendrum trichotomum and Clerodendrum colebrookianum. Molecules 2024, 29, 5983. [Google Scholar] [CrossRef]
- Krasteva, G. Effect of Basal Medium on Growth and Polyphenols Accumulation by Gardenia jasminoides Ellis Cell Suspension. BIO Web Conf. 2022, 45, 02006. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent Advances towards Development and Commercialization of Plant Cell Culture Processes for the Synthesis of Biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in Vitro Technologies—Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic Compounds and Biological Rhythms: Who Takes the Lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of Phenolic Acid, Tannins, Stilbenes, Lignans and Flavonoids in Human Health—A Review. Int. J. Food Sci. Tech. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Correa, L.C.; Furlan, P.B.; Biasoto, A.C.T.; Pereira, G.E.; Rybka, A.C.P.; Junior, M.R.M. Tropical Isabella Grape Juices: Bioactive Compounds and Antioxidant Power Depends on Harvest Season. J. Food Sci. Eng. 2013, 3, 64–70. [Google Scholar]
- Da Silva, J.K.; Cazarin, C.B.B.; Correa, L.C.; Batista, Â.G.; Furlan, C.P.B.; Biasoto, A.C.T.; Pereira, G.E.; De Camargo, A.C.; Maróstica Junior, M.R. Bioactive Compounds of Juices from Two Brazilian Grape Cultivars. J. Sci. Food Agric. 2016, 96, 1990–1996. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Moss, R.; Mao, Q.; Taylor, D.; Saucier, C. Investigation of Monomeric and Oligomeric Wine Stilbenoids in Red Wines by Ultra-high-performance Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-flight Mass Spectrometry. Rapid Comm. Mass Spectrom. 2013, 27, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; Von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sharma, A.R.; Aniemena, C.; Roedel, K.; Henry, F.; Moussou, P.; Samuga, A.; Medina-Bolivar, F. Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba). Plants 2022, 12, 175. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A Rapid Quantification of Stilbene Content in Wine by Ultra-High Pressure Liquid Chromatography–Mass Spectrometry. Food Control 2020, 108, 106821. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A Review of Dietary Stilbenes: Sources and Bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application-A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S. Overexpression of Stilbene Synthase Genes to Modulate the Properties of Plants and Plant Cell Cultures. Biotech. App. Biochem. 2021, 68, 13–19. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Viniferins by an Elicited Grapevine Cell Culture in a 2 L Stirred Bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment Strategies for High Resveratrol Induction in Vitis vinifera L. Cell Suspension Culture. Biotechnol. Rep. 2014, 1–2, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol Production at Large Scale Using Plant Cell Suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Bonello, M.; Gašić, U.; Tešić, Ž.; Attard, E. Production of Stilbenes in Callus Cultures of the Maltese Indigenous Grapevine Variety, Ġellewża. Molecules 2019, 24, 2112. [Google Scholar] [CrossRef]
- Sae-Lee, N.; Kerdchoechuen, O.; Laohakunjit, N. Enhancement of Phenolics, Resveratrol and Antioxidant Activity by Nitrogen Enrichment in Cell Suspension Culture of Vitis vinifera. Molecules 2014, 19, 7901–7912. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.-M. Stilbenoid Chemistry from Wine and the Genus Vitis, a Review. OENO One 2012, 46, 57. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic Content of Grapevine Leaves (Vitis labrusca Var. Bordo) and Its Neuroprotective Effect against Per-oxide Damage. Toxicol. Vitr. 2010, 24, 148–153. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur Nutrition and Its Role in Plant Growth and Development. Plant Signal. Behav. 2023, 18, 2030082. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Nygren, H.; Nohynek, L.; Puupponen-Pimiä, R.; Heiniö, R.-L.; Maiorova, N.; Rischer, H.; Ritala, A. Plant Cell Cultures as Food—Aspects of Sustainability and Safety. Plant Cell Rep. 2020, 39, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, O.; Hesami, M.; Pepe, M.; Dutta, A.; Jones, A.M.P. In Vitro Plant Tissue Culture as the Fifth Generation of Bioenergy. Sci. Rep. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis vinifera and Vitis labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef]
- Ritala, A.; Heiniö, R.-L.; Häkkinen, S.T.; Lille, M.; Hyytiäinen-Pabst, T.; Rischer, H. Tailoring Sensory Properties of Plant Cell Cultures for Food Use. Food Res. Int. 2022, 157, 111440. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant Cell Culture Technology in the Cosmetics and Food Industries: Current State and Future Trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food Ingredients and Food Made with Plant Cell and Tissue Cultures: State-of-the Art and Future Trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).