Abstract
This research models a 20 MW PEM hydrogen plant. PEM units operate in the 60 to 80 °C range based on their location and size. This study aims to recover the waste heat from PEM modules to enhance the efficiency of the plant. In order to recover the heat, two systems are implemented: (a) recovering the waste heat from each PEM module; (b) recovering the heat from hot water to produce electricity utilizing an organic refrigerant cycle (ORC). The model is made by ASPEN® V14. After modeling the plant and utilizing the ORC, the module is optimized using Python to maximize the electricity produced by the turbine, therefore enhancing the efficiency. The system is a closed-loop cycle operating at 25 °C and ambient pressure. The 20 MW PEM electrolyzer plant produces 363 kg/hr of hydrogen and 2877 kg/hr of oxygen. Based on the higher heating value of hydrogen, the plant produces 14,302.2 kWh of hydrogen energy equivalents. The ORC is maximized by increasing the electricity output from the turbine and reducing the pump work while maintaining energy conservation and mass balance. The results show that the electricity power output reaches 555.88 kW, and the pump power reaches 23.47 kW.
1. Introduction
Hydrogen is a fundamental atom in many chemical compounds and processes. It is an intermediate molecule for many processes and a stand-alone energy carrier. In the last two decades, producing reasonably priced hydrogen without emitting tons of emissions has attracted attention among scientists and industry.
Hydrogen production pathways can be illustrated in many ways. This work illustrates two main pathways to reform feedstocks to hydrogen: (a) using thermal energy and heating processes; (b) using electricity to reform the feedstock. Basically, reforming the feedstock by burning and generating heat for refining purposes or using electricity to reform the feedstock. Electrified processes can be categorized as thermal and non-thermal. Steam methane reforming is the most convenient technology for producing hydrogen, utilizing 800 °C to crack natural gas, a fossil-based thermal-joule heating process. Results from research have shown that if electricity is used to reform the feedstock into hydrogen, there is a good opportunity to reduce emissions and be cost-competitive at scale production [1,2,3]. Figure 1 illustrates various hydrogen production pathways.
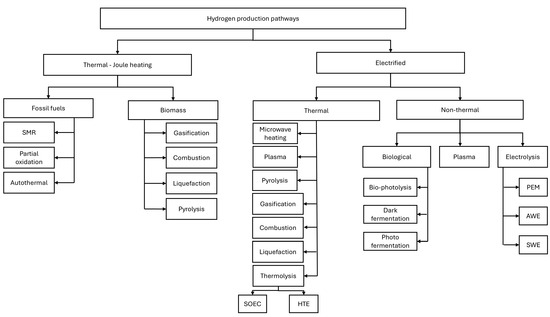
Figure 1.
Hydrogen production pathways.
Finding the fittest to-purpose-cost beneficial with minimum environmental impact for producing hydrogen depends on the geographical location and resources. The proper system can be deployed based on the availability of resources such as water or natural gas. Technologies such as plasma [3,4], electric heating [5], and electrolyzers [6] utilize electricity to reform the feedstock to hydrogen. This research uses proton exchange membrane (PEM) electrolyzers [7] to reform water into hydrogen. A heat recovery system coupled with an organic refrigerant cycle (ORC) [8] is integrated into the system to enhance overall efficiency by converting wasted heat from PEM modules into electricity.
In current systems, the heat generated by modules is released into the air. In fact, if a heat recovery system is utilized, the generated heat can be returned to the system to produce electricity. There is a scarcity of literature and studies on heat recovery from PEM for large-scale hydrogen production. Norbet and Assma have conducted thermo-economic studies of waste heat recovery in PEMs using the Rankine cycle utilizing ethane and R1233zd(E) [9]. Els van and his collaborators looked at utilizing heat at a 2.5 MW PEM plant. They recommended expanding the model to a larger scale using industrial data, which this study tried to use [10].
1.1. Water Electrolyzer
There are various electrolyzer technologies for producing hydrogen, including alkaline electrolyzers (AWE) [11], polymer electrolyte membranes (PEMs) [11], solid oxide cells (SCOECs) [12], anion exchange membranes (AEMs) [11], supercritical water electrolysis (SWE), high-pressure electrolysis (HPE), and high-temperature electrolysis (HTE) [11]. Table 1 briefly compares these technologies.

Table 1.
Electrolyzer types.
1.2. Proton Exchange Membrane Electrolyzer
Proton exchange membrane electrolyzers are a type of equipment that utilizes a solid polymer electrolyte to produce hydrogen through the electrolysis of water. The main advantages of using PEM electrolysis are its compact design, high efficiency, high rate of hydrogen production, rapid response to power fractionation, and high hydrogen purity. The main challenges associated with PEM are material costs, water purity sensitivity, and the durability of the membrane and catalysts. PEM electrolysis occurs in three key steps:
- (a)
- Water splits at the anode:
2H2O → 4H+ + 4e− + O2,
- (b)
- Protons migrate through the PEM toward the cathode.
- (c)
- The protons (H+) recombine with electrons supplied from the external circuit to form molecular hydrogen gas:
4H+ + 4e → 2H2,
The overall reaction:
2H2O → 2H2 + O2,
The schematic of PEM electrolysis process is shown in Figure 2.
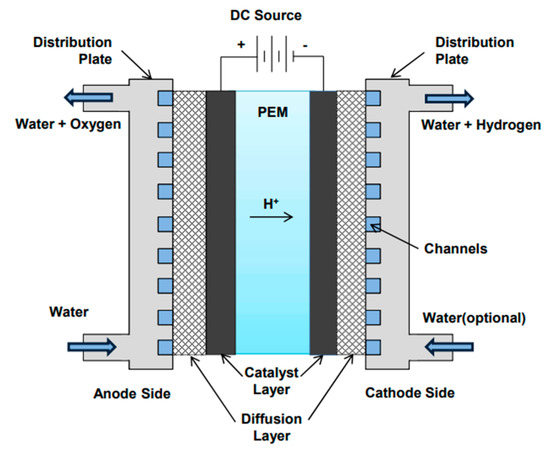
Figure 2.
PEM electrolysis process [13].
1.3. Organic Refrigerant Cycle
Organic refrigerant cycles (ORCs) are thermodynamic processes that use organic working fluids to transfer heat. These cycles are particularly advantageous when dealing with low-to-moderate-temperature heat sources [14]. ORCs operate similarly to the traditional Rankine cycle but uses organic fluids with lower boiling points, such as hydrocarbons or refrigerants, instead of water. ORCs can be used in various applications such as waste heat recovery, geothermal power generation, and solar thermal power.
Choosing the appropriate working fluid depends on the application, thermodynamic properties, environmental impacts, safety, and economic concerns. Fluids like R123, R245fa, and R245ca are commonly selected based on their favorable characteristics.
Mojtaba Aghajani and his colleagues have surveyed R245fa and R134fa for the heat recovery of PEM units. For both working fluids (R−134a and R−245fa), they noticed that increasing the thermal load decreases the net power output while the efficiency of the hybrid system decreases [15]. Mohammad Ali Abdelkareem and his colleagues have reviewed the recovery of waste heat from PEM and fuel cells and discussed various applications for the system [16]. Huy and his colleagues discussed PEM fuel cell thermal management solutions [17]. Jie and his colleagues looked at PEM system waste heat recovery using an ORC utilizing R123, R245fa, R134a, water, and ethanol. They found that the maximum efficiency of the recovery system is 4.73% [18].
The efficiency of a typical with no heat recovery modules, PEM hydrogen production is in the range of 60 to 70% when considering the lower heating value of hydrogen [19]. In this research, a 20 MW plant is modeled to evaluate its efficiency after adopting ORC and waste-to-heat modules.
As the efficiency of the PEM module has increased, less attention has been focused on the desired heat from the modules. In typical PEM systems, the heat dissipates to the surroundings. In large-scale applications, the amount of wasted heat becomes more viable and cost-effective for recovery and use. In this design, the extra wasted energy is recovered through water flow and then passed through the ORC to enhance the efficiency of the system by generating electricity via a turbine. Moreover, the water returns to PEM units at ambient temperature, so the water for cooling the system is within a closed loop.
Figure 3 illustrates the organic refrigerant cycle module. The module includes a pump, an evaporator, a turbine, and a condenser. An organic working fluid is heated in a boiler until it is converted to vapor. Then, the vapor enters the turbine, where it is expanded and produces work. The working fluid from the turbine passes through a pump to be condensed. At this stage, the fluid’s pressure rises, making it ready to enter the evaporator again.
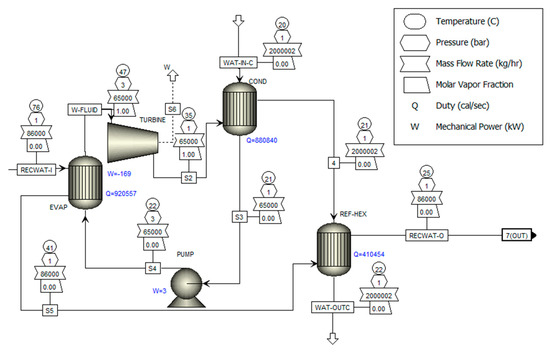
Figure 3.
Organic refrigerant cycle.
1.4. Aim and Novelty
As the PEM system is utilized to produce hydrogen, an excessive amount of heat will be released through the modules. This study aims to capture that heat via the ORC and use it to enhance the system’s efficiency. The novelty of this study lies in modeling a 20 MW electrolysis system with ORC. The ORC module is capable of producing electricity from waste heat. Additionally, optimization enhances system efficiency and the total efficiency of the ORC system by reducing pump power while increasing turbine power in the refrigerant unit.
2. Methods
The software Aspen Plus® V14 is used to model and simulate 20 MW electrolysis processes with a cooling system for heat recovery modules, and the efficiency is compared with conventional systems. PENG−ROB library is hired as the property method.
The total system efficiency () is calculated by Equation (4), defined by the total heat transfer from the system (), the total required electricity for hydrogen production (), the heat loss from the electrolyzer stack for cooling the hydrogen and oxygen (), the electricity requirements for the electrical pump ( and ), and the electricity input to the total system (). The total efficiency of the ORC system can be calculated by the difference between the turbine work and pump work, divided by the transferred heat into the system, as shown in Equation (5).
2.1. Technical Analysis
The electrolyzer model is built to analyze a 20 MW process utilizing ORC heat recovery modules with conventional systems. Technical analyses aim to investigate the quality and quantity of recoverable waste heat available from the electrolysis process and compare it with previously built systems. In each scenario, the amount of heat waste and, therefore, the efficiency of the system are calculated.
2.2. Model Development
As shown in Figure 4, the 20 MW plant includes waste heat capture, and an ORC module is modeled. The plant includes ten 2 MW PEM units, water waste heat recovery, and the ORC module. Each of the 2 MW modules are illustrated in Figure 5. As shown in each 2 MW module, each module includes a PEM unit, three heat exchangers, and two separators. The PEM stack is fed with two streams: (a) 2 MW of electricity; (b) water at ambient temperature and pressure conditions (20 °C and 1 bar). The water flows through the PEM units and is divided into oxygen and hydrogen. Hydrogen is separated from oxygen at a separation column (H2-SEP). The generated heat is absorbed by the water, which is heat water recovery (Waste-HE). The hydrogen passes through a heat exchanger (B1) to recover the heat from the produced hydrogen. The separated oxygen is mixed with some water. The water and oxygen separate at the O2−SEP, and the water returns to the PEM unit. The oxygen passes through a heat exchanger (O2HEX) and recovers heat (HOTW2). All recovered heat water streams, HOTW, HOTW2, and HOTW3, are mixed and enter the ORC module.
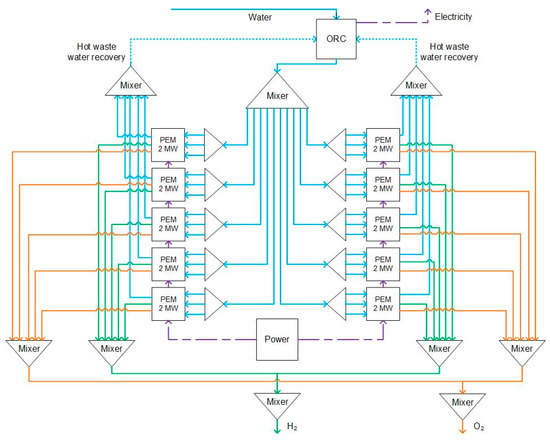
Figure 4.
20 MW PEM hydrogen production with heat recovery and ORC module. Purple, blue, green, and orange indicate electricity, water, hydrogen, and oxygen flows, respectively.
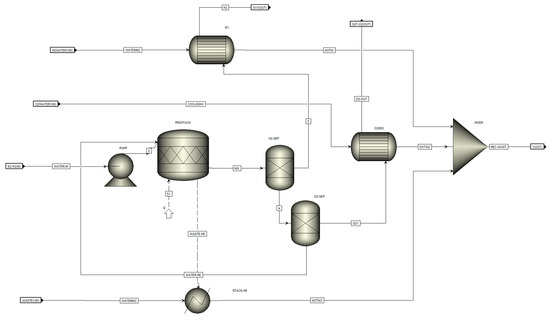
Figure 5.
2 MW PEM module.
The Peng Robinson method is used to model the plant via ASPEN V.14®. Hydrogen, water, oxygen, and R245fa are the components of the system. The ambient temperature and pressure are assumed to be 25 °C and 1.01325 bar, respectively. Model’s statistics are represented in Appendix A Table A1.
Based on experimental results, the operation temperature of the PEM is 70 °C at a pressure of 30 bar [20,21]. Inside the PEM stack, the model simulates the electrolysis reactions shown in Equations (1)–(3), where the water splits into hydrogen and oxygen. The fraction reaction conversion in ASPEN is assumed to be 0.75. The maximum number of iterations to convert equations is 30, with an error tolerance of 0.0001. Currently, manufacturers such as FEST GmbH in Germany, ITM Power in United Kingdom, IMI VIVO in Italy, Nel Hydrogen in Norway, Siemens in Germany, Hele Titanium in China, Plug Power in USA and many more offer 2 MW PEM electrolyzers.
Table 2 summarizes the operating parameters of the 2 MW PEM electrolyzer and the heating capture system. In this operation, all water runs at ambient pressure and temperature conditions to optimize the energy consumption of cooling systems.

Table 2.
2 MW PEM electrolyzer operation parameters.
The hydrogen production mass flow rate and output pressure are compared to evaluate the performance of the modeled 2 MW PEM electrolyzer and the available PEM electrolyzer benchmarked technologies.
In order to further increase the operation efficiency, water captures heat from each stack and flows out of each module. Then, the total water enters the ORC. The R245fa working fluid is utilized to recover heat, which is appropriate for recovering low-temperature heat [22,23]. Another advantage of R245fa is the coolant’s low viscosity, which reduces the pumping power required in the ORC system. The input parameters for ORC are summarized in Table 3.

Table 3.
ORC operation condition.
The results show that the work performed by the turbine is sufficient to provide the energy needed for cooling hydrogen or oxygen water. After the heat exchange, the water leaves the evaporator at 41.73 °C and passes through a turbine. At this stage, electricity will be produced from the turbine coupled with a generator. In the next step, the water will pass through a condenser. The cold water will be pumped back into the evaporator for the next cycle.
The total efficiency of the ORC system can be calculated by the difference between the turbine and pump works, divided by the transferred heat into the system, as shown in Equation (5). The operation condition for the 20 MW plant is summarized in Table 4.

Table 4.
20 MW operation parameters summary.
2.3. Maximizing ORC Efficiency
In order to increase the efficiency of the process, the ORC module is optimized to maximize the produced electricity and enhance the cycle’s efficiency. Therefore, the objective function is defined as
where the quality penalty is defined as
And
represents the cycle’s work, calculates the turbine’s work, and measures the pump’s work.
For limiting feasible solutions and meeting actual physical properties, the constraints below have been used to avoid any system failures during the process:
Superheat constraint:
Subcooling constraint:
Pressure constraints:
Net positive suction head (NPSH) constraint:
NPSH is calculated using the equations below:
To make sure these calculations are correct, the overall energy balance for ORC is formulated as below:
The evaporator heat transfer, condenser heat transfer, and recuperator heat transfer using the effectiveness-NTU method are calculated as follows:
The effectiveness-NTU is expressed as below:
LMTD represents the lean mean temperature difference (LMTD) method for heat exchangers:
In the end, the thermal efficiency of the cycle is calculated using Equation (26):
3. Results
3.1. Hydrogen Production and Heat Recovery at a 20 MW PEM Electrolyzer Plant
ASPEN V14 is used to model a 20 MW hydrogen production plant that utilizes waste heat recovery and an ORC to produce electricity. The system is a closed-loop cycle operating at 25 °C and ambient pressure. The 20 MW PEM electrolyzer model produces 363 kg/hr of hydrogen and 2877 kg/hr of oxygen. Based on the higher heating value (HHV) of hydrogen, the plant produces 14,302.2 kWh hydrogen energy equivalents. Table 5 represents the results for producing hydrogen and streams for each 2 MW stack.

Table 5.
Results for each 2 MW PEM electrolyzer.
As a result, each PEM stack rejects 546.36 kW of thermal energy. Therefore, the heat recovery captured from ten 2 MW stacks equals 5463.65 kW. This means that from the electricity provided to separate hydrogen and oxygen, 5463.65 kW of heat is lost, which is 27.32% of the power input and is available for recovery. The cooling water runs through the plant so as to (a) capture rejected heat from PEM units, (b) cool down the produced hydrogen, and (c) cool down the produced oxygen. Table 6 represents the results for cooling water for each 2 MW PEM electrolyzer.

Table 6.
Results for the cooling system of the 2 MW electrolyzer.
The cumulative amount of required electricity for producing hydrogen, recovered waste heat from stacks, and heat loss while producing oxygen and hydrogen are calculated. Table 7 provides the summary results.

Table 7.
Energy portfolio of the units.
Figure 6 presents the energy flow within the PEM stacks and demonstrates the energy flow for producing hydrogen. As it is clear, the majority of energy within the system is present in the form of hydrogen. The total electrical system conversion efficiency is 71.41%. By capturing waste heat, the thermal efficiency of the system will increase, and therefore, the system efficiency will be further enhanced. An ORC module is added to the plant to maintain the 25 °C cooling water recirculating through the plant and producing some electricity from the recovered heat, which is 27.32% of total input energy. In the following sections, the electricity produced and optimizing ORC is explained.
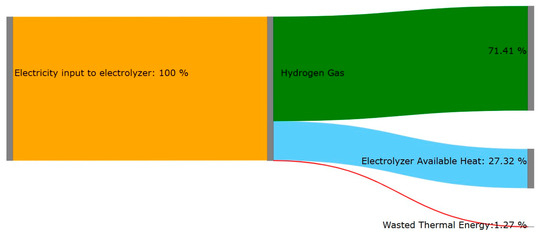
Figure 6.
Sankey diagram of energy flow within the PEM process.
3.2. Organic Refrigerant Cycle
After collecting the waste heat via water from PEM stacks, the ambient temperature of the water reaches 76.48 °C, which is the operation temperature of the typical PEM fuel cells. The water enters the ORC module to return to ambient temperature, 25 °C. The water passes through an evaporator to exchange heat with the R245fa refrigerant. The refrigerant working fluid passes through the turbine and produces electricity, as Figure 3 shows. The water exits the evaporator and cools down to 25 °C to recirculate through the plant. Table 8 presents the results for the ORC module.

Table 8.
ORC module stream results.
As the ORC cools down the water to ambient temperature, the wasted heat is used to produce electricity from phase changes in the working fluid. A summary of results for the ORC is presented in Table 9.

Table 9.
ORC results for 86,000 kg/hr water at 76 °C.
3.3. Maximizing ORC Efficiency
The ORC is being maximized by increasing the electricity output from the turbine and reducing pump work while maintaining energy conservation and mass balance. Solving Equation (6) resulted in the electricity power output reaching 555.88 kW and the pump power reaching 23.47 kW. Therefore, the electricity power output increased by 555.88 kW from 169.97 kW. In order to achieve maximum efficiency, the mass flow rate of working fluid, cooling water, and hot water are also optimized. Based on the optimized value, the working fluid and cooling water mass flow rates are 61,200 kg/hr and 1,033,092 kg/hr, respectively. Additionally, this optimization improves the total system efficiency (η), calculated using Equation (4), from 72.95 to 74.80, and increases the ORC system’s total efficiency from 3.06 to 10.13 based on Equation (5). Table 10 compares the results before and after optimizing the ORC.

Table 10.
ORC variables before and after optimization.
Figure 7 illustrates the pressure enthalpy diagram for the ORC. In this optimization, the efficiency of the turbine and pump is assumed to be 70%, which is a conservative assumption.
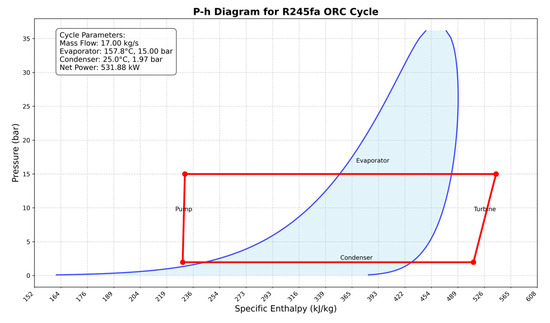
Figure 7.
Pressure–enthalpy diagram for the optimized organic Rankine cycle (ORC).
4. Discussion
This study aimed to enhance the efficiency of the PEM hydrogen plant by incorporating heat recovery modules into the system. Heat is utilized throughout the system: (a) the heat recovered from each PEM module; (b) the heat recovered from the refrigerant cycle to generate electricity and enhance the system’s efficiency.
The 20 MW PEM electrolyzer plant produces 363 kg/hr of hydrogen and 2877 kg/hr of oxygen. Based on the higher heating value of hydrogen, the plant produces 14,302.2 kWh of hydrogen energy equivalent. The ORC is being maximized by increasing the electricity output from the turbine and reducing pump work while maintaining energy conservation and the mass balance.
The results prove that 5463.65 kW of energy is available to recover from ten 2 MW PEM modules. This heat is recovered through a flow of water. The heated water passes through the ORC module and a turbine unit to heat the working fluid, R245fa, producing electricity. The heat recovery system and ORC units make the water cooling system for the plant a closed loop since the exit water from the ORC unit returns to the initial point of the plant at the same temperature, which is 25 °C.
The results show that the work performed by the turbine is sufficient to provide the energy needed for cooling hydrogen or oxygen water. As the B1 unit requires 7.2 kW, the O2 Hex unit requires 3.7 kW, and there are 10 PEM modules, the total duty work required by them is equal to 109 kW, which is less than the total electricity produced from the turbine. Comparing these results with previous works utilizing ORCs [24] shows this system operates at ambient temperature, using cooling fluid water. Moreover, a lower water mass flow rate is required to cool down the system.
The ORC module effectively utilizes waste heat from the PEM stacks, reducing the water temperature from 76.47 °C to 25 °C, generating 169.97 kW of electricity in its initial configuration. Optimization efforts further enhanced this performance, increasing the ORC power output to 555.88 kW and improving the total system efficiency to 74.80%. By comparing the achieved efficiency and the previous work summarized in Table 1, it can be concluded that efficiency has improved by 4.8%. These improvements stem from optimizing the mass flow rates of both the working fluid and cooling water while maintaining energy conservation principles.
The waste heat recovery system enhances efficiency and supports sustainable energy utilization by reducing the cooling load and repurposing otherwise lost thermal energy. This integration is particularly beneficial for large-scale hydrogen production facilities, where efficiency gains translate. Optimizing the ORC not only reduced the volume of required water for cooling but also increased the net efficiency of the refrigeration cycle. Performing techno-economic analyses would be a great next step in evaluating the use of ORC waste heat recovery for producing hydrogen.
Author Contributions
S.S.N.: Conceptualization (lead); methodology (equal); software (lead); validation (lead); formal analysis (lead); investigation (lead).; resources (lead); data curation (lead); writing—original draft preparation (lead); writing—review and editing (equal); visualization (equal); supervision (lead); project administration (lead). F.M.: Conceptualization (supporting); methodology (equal); software (supporting); validation (supporting); formal analysis (supporting); (writing—original draft preparation (supporting); writing—review and editing (equal); visualization (equal). E.P.: Review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Texas A&M Energy Institute for supporting this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations and Symbols
The following abbreviations are used in this manuscript:
| AEM | Anion exchange membrane |
| HTE | High-temperature electrolysis |
| ORC | Organic refrigerant cycle |
| PEMI | Proton exchange membrane |
| SCOECs | Solid Oxide Electrolyzer Cells |
| SWE | Supercritical water electrolysis |
| HHV | Higher heating value |
| MW | Megawatt |
| kW | Kilowatt |
| kg/hr | Kilograms per hour |
| °C | Degrees Celsius |
| bar | Pressure unit |
| η | Efficiency |
| H2 | Hydrogen |
| O2 | Oxygen |
| e− | Electron |
| H+ | Proton |
| R245fa | Organic refrigerant used in ORC |
| h | Enthalpy |
| Q | Heat transfer |
| W | Work output |
| T | Temperature |
| P | Pressure |
Appendix A

Table A1.
Model statistics.
Table A1.
Model statistics.
| Statistic | |
| Number of variables | 6270 |
| Number of incident variables | 6263 |
| Number of fixed variables | 563 |
| Number of free variables | 5707 |
| Number of equations | 5707 |
| Number of excluded equations | 0 |
| Number of non-zeros | 20,563 |
| Number of incidents non-zeros | 19,457 |
| Number of incomplete connections | 0 |
References
- Nyangon, J.; Darekar, A. Advancements in hydrogen energy systems: A review of levelized costs, financial incentives and technological innovations. Innov. Green Dev. 2024, 3, 100149. [Google Scholar] [CrossRef]
- Wolfram, P.; Kyle, P.; Fuhrman, J.; O’Rourke, P.; McJeon, H. The hydrogen economy can reduce costs of climate change mitigation by up to 22%. One Earth 2024, 7, 885–895. [Google Scholar] [CrossRef]
- Niknezhad, S.S.; Staack, D.; Pistikopoulos, E.N. Hydrogen Production Plant Via an Intensified Plasma-Based Technology. Available online: https://ssrn.com/abstract=4889037 (accessed on 1 May 2025).
- Mizeraczyk, J.; Jasiński, M. Plasma processing methods for hydrogen production. Eur. Phys. J. Appl. Phys. 2016, 75, 24702. [Google Scholar] [CrossRef]
- Yang, H.; Nuran Zaini, I.; Pan, R.; Jin, Y.; Wang, Y.; Li, L.; Caballero, J.J.B.; Shi, Z.; Subasi, Y.; Nurdiawati, A.; et al. Distributed electrified heating for efficient hydrogen production. Nat. Commun. 2024, 15, 3868. [Google Scholar] [CrossRef]
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the green hydrogen market–Current state and outlook on green hydrogen demand and electrolyzer manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Iglesias Garcia, S.; Ferreiro Garcia, R.; Carbia Carril, J.; Iglesias Garcia, D. A review of thermodynamic cycles used in low temperature recovery systems over the last two years. Renew. Sustain. Energy Rev. 2018, 81, 760–767. [Google Scholar] [CrossRef]
- Lümmen, N.; Karouach, A.; Tveitan, S. Thermo-economic study of waste heat recovery from condensing steam for hydrogen production by PEM electrolysis. Energy Convers. Manag. 2019, 185, 21–34. [Google Scholar] [CrossRef]
- Van der Roest, E.; Bol, R.; Fens, T.; van Wijk, A. Utilisation of waste heat from PEM electrolysers—Unlocking local optimisation. Int. J. Hydrogen Energy 2023, 48, 27872–27891. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, R.; Shao, Z.; Zhang, C. The economic analysis for hydrogen production cost towards electrolyzer technologies: Current and future competitiveness. Int. J. Hydrogen Energy 2023, 48, 13767–13779. [Google Scholar] [CrossRef]
- Jensen, S.H. Solid Oxide Electrolyser Cell. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2007. [Google Scholar]
- Sood, S.; Prakash, O.; Boukerdja, M.; Dieulot, J.Y.; Ould-Bouamama, B.; Bressel, M.; Gehin, A.L. Generic Dynamical Model of PEM Electrolyser under Intermittent Sources. Energies 2020, 13, 6556. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Lambrinos, G.; Frangoudakis, A.; Papadakis, G. Low-grade heat conversion into power using organic Rankine cycles–A review of various applications. Renew. Sustain. Energy Rev. 2011, 15, 3963–3979. [Google Scholar] [CrossRef]
- Sheshpoli, M.A.; Ajarostaghi, S.S.M.; Delavar, M.A. Thermodynamic analysis of waste heat recovery from hybrid system of proton exchange membrane fuel cell and vapor compression refrigeration cycle by recuperative organic Rankine cycle. J. Therm. Anal. Calorim. 2019, 135, 1699–1712. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Muhammad, I.; Alaswad, A.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Recovery of waste heat from proton exchange membrane fuel cells—A review. Int. J. Hydrogen Energy 2024, 52, 933–972. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Manag. 2020, 204, 112328. [Google Scholar] [CrossRef]
- He, T.; Shi, R.; Peng, J.; Zhuge, W.; Zhang, Y. Waste heat recovery of a PEMFC system by using organic rankine cycle. Energies 2016, 9, 267. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Wang, L.; Husar, A.; Zhou, T.; Liu, H. A parametric study of PEM fuel cell performances. Int. J. Hydrogen Energy 2003, 28, 1263–1272. [Google Scholar] [CrossRef]
- Nur Ozdemir, S.; Taymaz, I.; Okumuş, E.; Gül Boyacı San, F.; Akgün, F. Experimental investigation on performance evaluation of PEM electrolysis cell by using a Taguchi method. Fuel 2023, 344, 128021. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z.; Yu, B.; Ouyang, H.; Chen, J. Simultaneous experimental comparison of low-GWP refrigerants as drop-in replacements to R245fa for Organic Rankine cycle application: R1234ze(Z), R1233zd(E), and R1336mzz(E). Energy 2019, 173, 721–731. [Google Scholar] [CrossRef]
- Kajurek, J.; Rusowicz, A.; Grzebielec, A.; Bujalski, W.; Futyma, K.; Rudowicz, Z. Selection of refrigerants for a modified organic Rankine cycle. Energy 2019, 168, 1–8. [Google Scholar] [CrossRef]
- María Villarreal Vives, A.; Wang, R.; Roy, S.; Smallbone, A. Techno-economic analysis of large-scale green hydrogen production and storage. Appl. Energy 2023, 346, 121333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).