Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Thermal Treatment
2.3. K232 and K268 Calculated
2.4. Preparation of Methanolic Extracts of the Oil
2.5. FTIR-ATR Measurements
2.6. UV and Fluorescence Spectra
2.7. Quantification of Thymoquinone by HPLC
2.8. DPPH Assay
2.9. Data Evaluation and Statistical Analysis
3. Results and Discussion
3.1. Oxidative Stability of BCSO
3.1.1. Specific Absorbance Coefficients
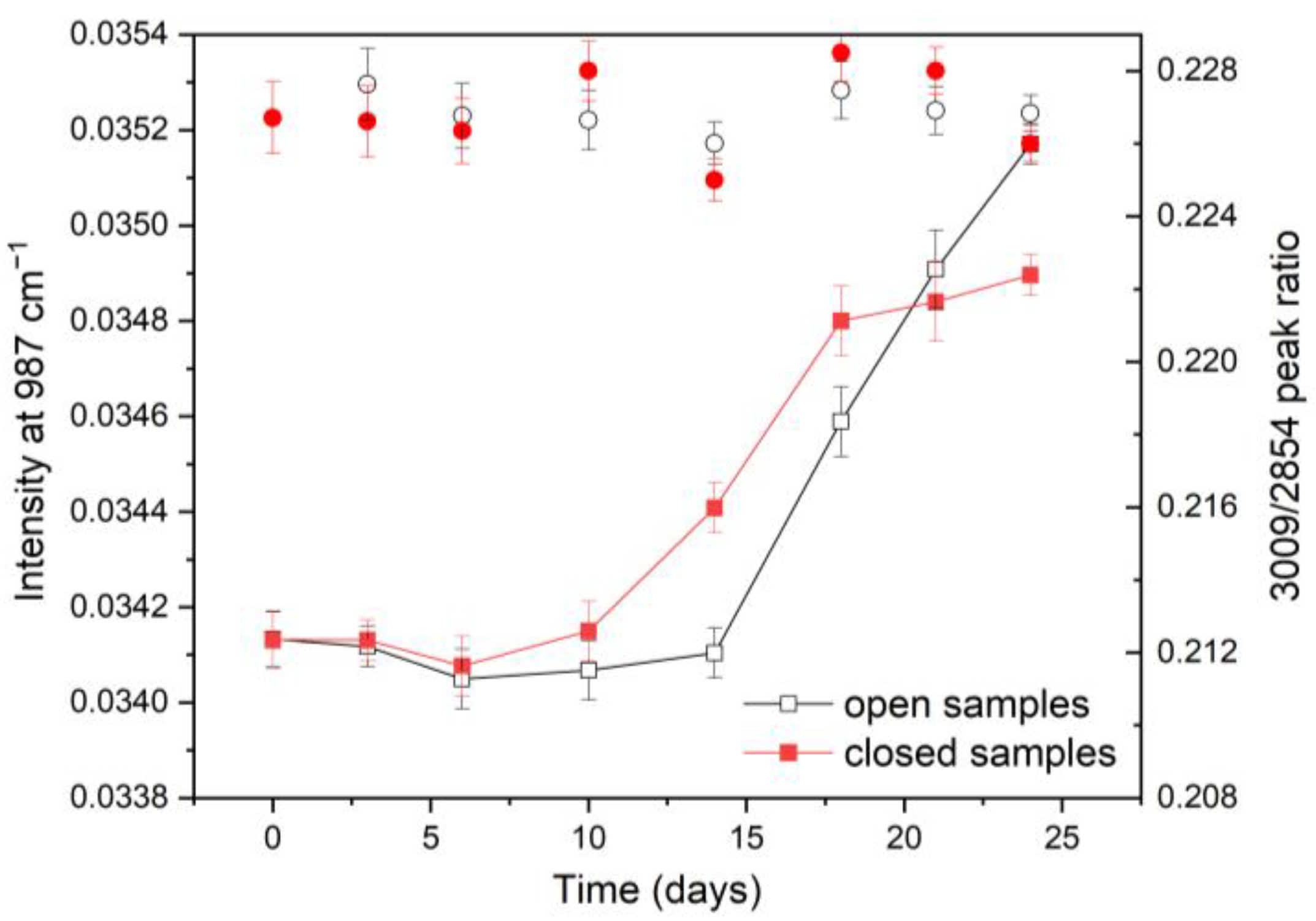
3.1.2. FTIR Spectra
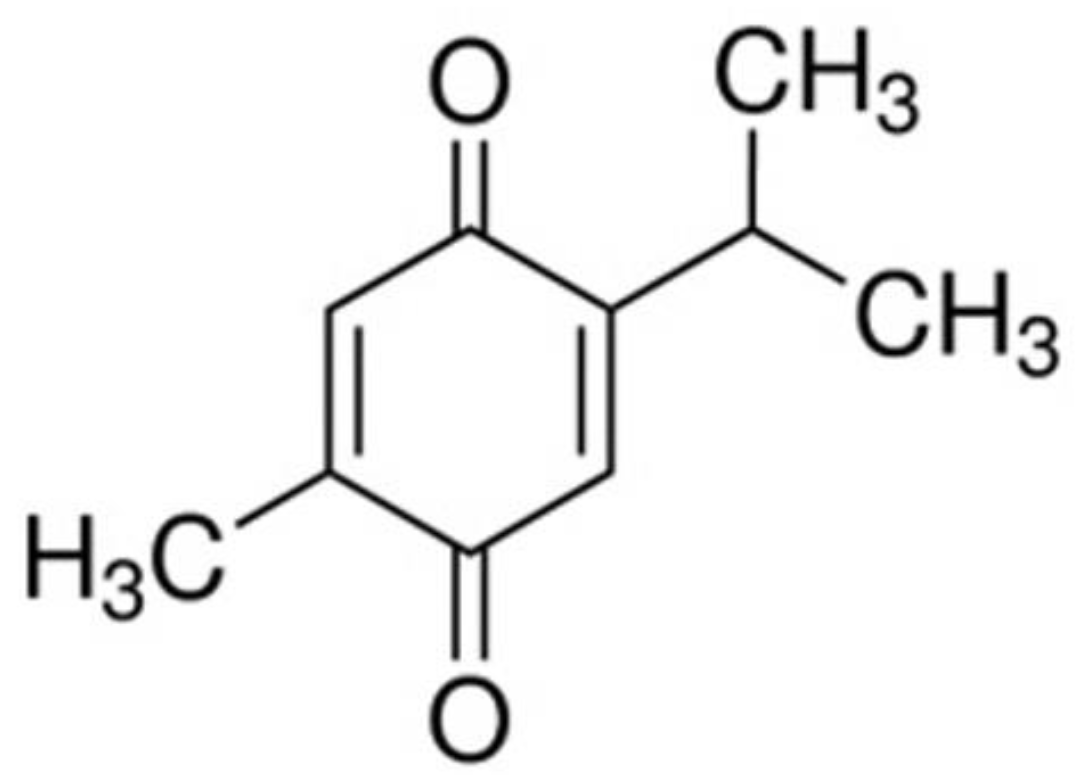
3.2. TQ Content Estimation
3.2.1. Specification of Used Methods
3.2.2. Changes in TQ Content During Thermal Treatment of BCSO
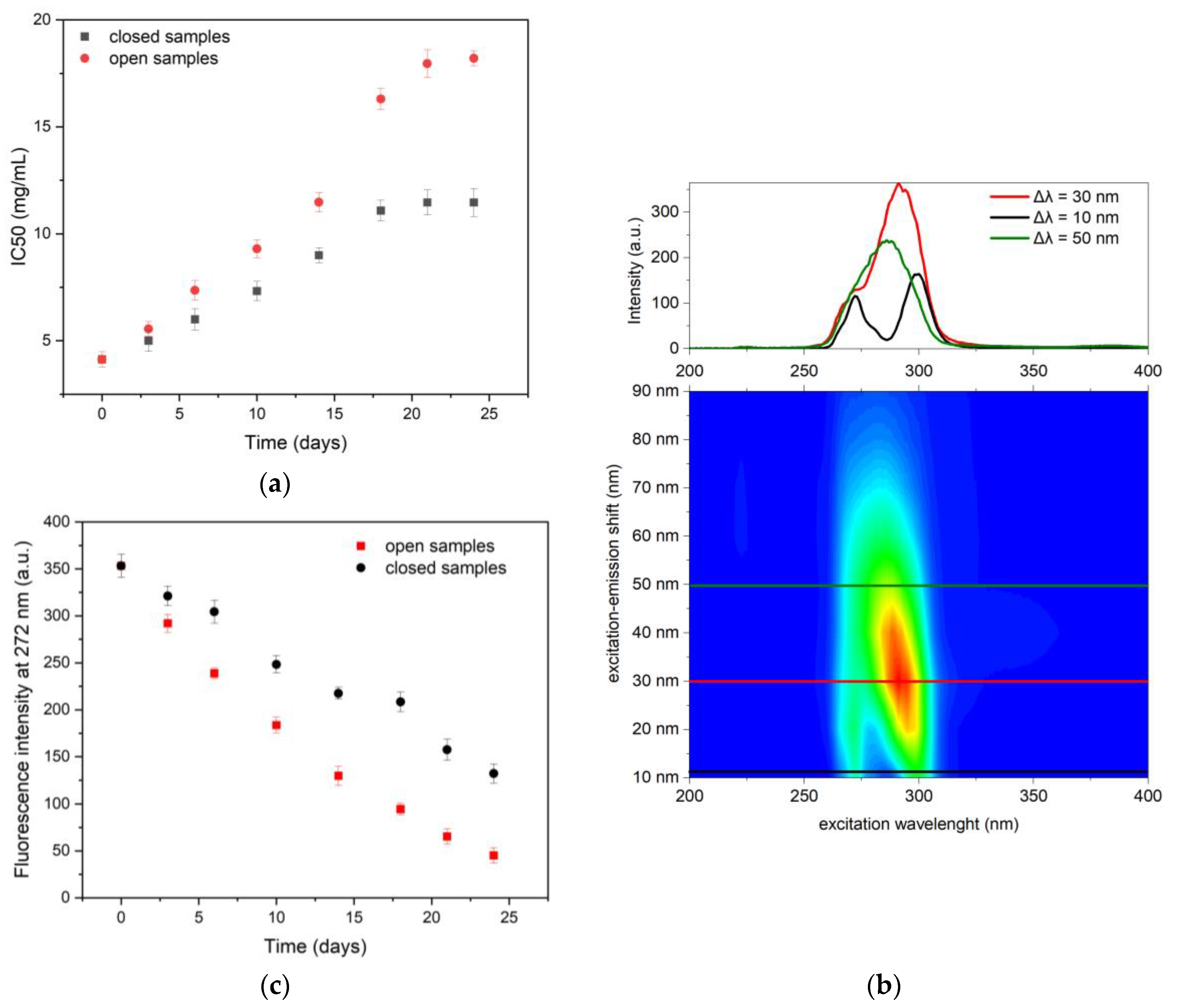
3.3. DPPH Radical Scavenging Activity
3.3.1. No Treatment BCSO and TQ Probe
3.3.2. The Role of TQ in BCSO Radical Scavenging Activity
3.3.3. Association Between the Phenolic Compound Content and BCSO Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef]
- Zielińska, M.; Dereń, K.; Polak-Szczybyło, E.; Stępień, A.E. The role of bioactive compounds of nigella sativa in rheuma-toid arthritis therapy—Current reports. Nutrients 2021, 13, 3369. [Google Scholar] [CrossRef]
- Kiralan, M.; Ulaş, M.; Özaydin, A.; Özdemır, N.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Blends of Cold Pressed Black Cumin Oil and Sunflower Oil with Improved Stability: A Study Based on Changes in the Levels of Volatiles, Tocopherols and Thymoquinone during Accelerated Oxidation Conditions. J. Food Biochem. 2017, 41, e12272. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Ahmady, S.H. Prediction of thymoquinone content in black seed oil using multivariate analysis: An efficient model for its quality assessment. Ind. Crop. Prod. 2018, 124, 626–632. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Hussin, A.S.M. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid. Based Complement. Altern. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Kartal, M.; Erdem, S.A. Quantitative analysis of thymoquinone in Nigella sativa L. (Black cumin) seeds and commercial seed oils and seed oil capsules from Turkey. J. Fac. Pharm. Ankara/Ankara Ecz. Fak. Derg. 2017, 41, 34–41. [Google Scholar] [CrossRef]
- Kurowska, N.; Madej, M.; Strzalka-Mrozik, B. Thymoquinone: A Promising Therapeutic Agent for the Treatment of Colorectal Cancer. Curr. Issues Mol. Biol. 2024, 46, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef]
- Elyasi, R.; Majdi, M.; Krause, S.T.; Kücükay, N.; Azizi, A.; Degenhardt, J. Identification and functional characterization of a γ-terpinene synthase in Nigella sativa L (black cumin). Phytochemistry 2022, 202, 113290. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sakulkittiyut, B.; Ngamprasertsith, S.; Sawangkeaw, R. Volatile Compound Fingerprints of Black Cumin (Nigella sativa L.) Seed Oil Extracted by Supercritical Carbon Dioxide. Biol. Life Sci. Forum 2021, 6, 31. [Google Scholar] [CrossRef]
- Edris, A.E. Evaluation of the volatile oils from different local cultivars of Nigella sativa L. Grown in Egypt with emphasis on the effect of extraction method on thymoquinone. J. Essent. Oil-Bearing Plants 2010, 13, 154–164. [Google Scholar] [CrossRef]
- Dockal, E.R.; Cass, Q.B.; Brocksom, T.J.; Brocksom, U.; Corrêa, A.G. A simple and efficient synthesis of thymoquinone and methyl p-benzoquinone. Synth. Commun. 1985, 15, 1033–1036. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Fotooh-Abadi, E.; Ghasemi, Y. Biotransformation of monoterpenes by immobilized microalgae. J. Appl. Phycol. 2011, 23, 975–981. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Soliman, R.M.; Abdel Salam, R.A.; Eid, B.G.; Khayyat, A.; Neamatallah, T.; Mesbah, M.K.; Hadad, G.M. Stability study of thymoquinone, carvacrol and thymol using HPLC-UV and LC-ESI-MS. Acta Pharm. 2020, 70, 325–342. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, M. Changes in volatile compounds of black cumin (Nigella sativa L.) seed oil during thermal oxidation. Int. J. Food Prop. 2014, 17, 1482–1489. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Mohamed Ahmed, I.A.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, F.; Wu, G.; Wang, X. Nutritional Composition and Volatile Compounds of Black Cumin (Nigella sativa L.) Seed, Fatty Acid Composition and Tocopherols, Polyphenols, and Antioxidant Activity of Its Essential Oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Amin, H.H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Abdul Aziz, J.M.; Rahman, H.S. Structural Characterization, Antimicrobial Activity, and In Vitro Cytotoxicity Effect of Black Seed Oil. Evid.-Based Complement. Altern. Med. 2019, 2019, 6515671. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, S.; Li, Q.; Zhang, L. An indirect analytical approach based on ATR-FTIR spectroscopy for determining the FFA content in vegetable oils. RSC Adv. 2020, 10, 24073–24078. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Analysis of Olive Oils by Fluorescence Spectroscopy: Methods and Applications. In Olive Oil: Constituents, Quality, Health Properties and Bioconversions; IntechOpen: London, UK, 2007; pp. 63–88. ISBN 0000957720. [Google Scholar]
- Siejak, P.; Neunert, G.; Kamińska, W.; Dembska, A.; Polewski, K.; Siger, A.; Grygier, A.; Tomaszewska-Gras, J. A crude, cold-pressed oil from elderberry (Sambucus nigra L.) seeds: Comprehensive approach to properties and characterization using HPLC, DSC, and multispectroscopic methods. Food Chem. 2025, 464, 141758. [Google Scholar] [CrossRef] [PubMed]
- Papoti, V.T.; Tsimidou, M.Z. Looking through the qualities of a fluorimetric assay for the total phenol content estimation in virgin olive oil, olive fruit or leaf polar extract. Food Chem. 2009, 112, 246–252. [Google Scholar] [CrossRef]
- Tura, M.; Ansorena, D.; Astiasarán, I.; Mandrioli, M.; Toschi, T.G. Evaluation of Hemp Seed Oils Stability under Accelerated Storage Test. Antioxidants 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Method Ch 5-91 Specific extinction of oils and fats, ultraviolet absorption (Ch 5-91). In Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 2009; pp. 5–91. ISBN 978-1-893997-74-5.
- Ramadan, M.F.; Mörsel, J.T. Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils upon stripping. Eur. J. Lipid Sci. Technol. 2004, 106, 35–43. [Google Scholar] [CrossRef]
- Pooja, C.; Sukhneet, S. Chauhan Polar Compounds in Frying Oils: A Review. Appl. Ecol. Environ. Sci. 2021, 9, 21–29. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, H.J. Methods for Measuring Lipid Oxidation. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2020; pp. 1–27. [Google Scholar]
- Prabowo, S.; Muflihah; Rohman, A. Monitoring oxidative levels of frying oils using ftir spectroscopy and multivariate calibration. Int. J. Appl. Pharm. 2018, 10, 82–87. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Rexhepi, F.; Behrami, A.; Samaniego-SÃ, C.; Rebezov, M.; Shariati, M.A.; da Silva, A.B.; de Souza, C.K. Chemical changes of pumpkin seed oils and the impact on lipid stability during thermal treatment: Study by ftir-spectroscopy. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e5839. [Google Scholar] [CrossRef]
- Gaca, A.; Kludská, E.; Hradecký, J.; Hajšlová, J.; Jeleń, H.H. Changes in volatile compound profiles in cold-pressed oils obtained from various seeds during accelerated storage. Molecules 2021, 26, 285. [Google Scholar] [CrossRef] [PubMed]
- Raschi, A.B.; Romano, E.; Benavente, A.M.; Altabef, A.B.; Tuttolomondo, M.E. Structural and vibrational analysis of thymoquinone. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2010, 77, 497–505. [Google Scholar] [CrossRef]
- Pagola, S.; Benavente, A.; Raschi, A.; Romano, E.; Molina, M.A.A.; Stephens, P.W. Crystal structure determination of thymoquinone by high-resolution x-ray powder diffraction. AAPS PharmSciTech 2004, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.L.Y.; Xin, C.K.; Akowuah, G.A. Effect of different extraction solvents on the content of Thymoquinone content of Nigella sativa L. seeds using UV-Visible spectroscopy and evaluation of the free radical scavenging activity. Indian. J. Nat. Prod. Resour. 2023, 14, 22–27. [Google Scholar] [CrossRef]
- Rohman, A.; Wibowo, D.; Sudjadi; Lukitaningsih, E.; Rosman, A.S. Use of fourier transform infrared spectroscopy in combination with partial least square for authentication of black seed oil. Int. J. Food Prop. 2015, 18, 775–784. [Google Scholar] [CrossRef]
- Farhan, N.; Salih, N.; Salimon, J. Physiochemical properties of Saudi Nigella sativa L. (‘Black cumin’) seed oil. OCL Oilseeds Fats Crop. Lipids 2021, 28, 11. [Google Scholar] [CrossRef]
- Lutterodt, H.; Luther, M.; Slavin, M.; Yin, J.J.; Parry, J.; Gao, J.M.; Yu, L.L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT 2010, 43, 1409–1413. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders. Oxid. Med. Cell. Longev. 2018, 2018, 1209801. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Tesarova, H.; Svobodova, B.; Kokoska, L.; Marsik, P.; Pribylova, M.; Landa, P.; Vadlejch, J. Determination of oxygen radical absorbance capacity of black cumin (Nigella sativa) seed quinone compounds. Nat. Prod. Commun. 2011, 6, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Khither, H.; Sobhi, W.; Khenchouche, A.; Mosbah, A.; Benboubetra, M. In-Vitro Antioxidant Effect of Thymoquinone. Annu. Res. Rev. Biol. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phyther. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Sandhya, A.; Kannayiram, G. Pharmacological, bioactive screening of medicinal plant nigella sativa and the derived compound thymoquinone: An invitro study. Int. J. Res. Pharm. Sci. 2020, 11, 2458–2465. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur. Food Res. Technol. 2013, 236, 1099–1105. [Google Scholar] [CrossRef]
- Sielicka, M.; Malecka, M.; Purlan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Hossen, J.; Ali, M.A.; Reza, S. Theoretical investigations on the antioxidant potential of a non-phenolic compound thymoquinone: A DFT approach. J. Mol. Model. 2021, 27, 173. [Google Scholar] [CrossRef] [PubMed]
- Armutcu, F.; Akyol, S.; Akyol, O. The interaction of glutathione and thymoquinone and their antioxidant properties. Electron. J. Gen. Med. 2018, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Laukagalis, V.; Paulauskienė, A.; Baltušnikienė, A.; Meškinytė, E. Quality Changes of Cold-Pressed Black Cumin (Nigella sativa L.), Safflower (Carthamus tinctorius L.), and Milk Thistle (Silybum marianum L.) Seed Oils During Storage. Plants 2023, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crop. Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Rapid synchronous fluorescence method for virgin olive oil adulteration assessment. Food Chem. 2007, 105, 369–375. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Sikorska, E.; Wójcicki, K.; Kozak, W.; Gliszczynska-Swigło, A.; Khmelinskii, I.; Górecki, T.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A. Front-face fluorescence spectroscopy and chemometrics for quality control of cold-pressed rapeseed oil during storage. Foods 2019, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Aydeniz, B.; Yilmaz, E. Performance of different natural antioxidant compounds in frying oil. Food Technol. Biotechnol. 2016, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. Res. 2009, 48, 31–41. [Google Scholar]


| Day of Storage | Open Samples | Closed Samples | ||
|---|---|---|---|---|
| K232 | K268 | K232 | K268 | |
| 0 | 6.02 ± 0.58 a | 1.34 ± 0.18 ab | 6.02 ± 0.58 a | 1.34 ± 0.18 a |
| 3 | 5.99 ± 0.33 a | 1.43 ± 0.11 a | 6.56 ± 0.51 a | 1.38 ± 0.53 a |
| 6 | 5.76 ± 0.11 a | 1.31 ± 0.05 abc | 6.02 ± 0.43 a | 1.28 ± 0.47 a |
| 10 | 5.83 ± 0.59 a | 1.11 ± 0.15 bcd | 6.33 ± 0.42 a | 1.61 ± 0.17 a |
| 14 | 5.91 ± 0.09 a | 1.12 ± 0.03 bcd | 6.78 ± 0.32 ab | 1.59 ± 0.17 a |
| 18 | 7.17 ± 1.05 ab | 1.02 ± 0.19 d | 6.78 ± 0.39 ab | 1.66 ± 0.15 a |
| 21 | 8.54 ± 0.72 b | 1.04 ± 0.08 cd | 7.01 ± 1.01 ab | 1.39 ± 0.25 a |
| 24 | 8.57 ± 0.62 b | 1.09 ± 0.04 d | 7.46 ± 0.52 b | 1.25 ± 0.30 a |
| Sample | IC50 [µg/mL] | Reference | |
|---|---|---|---|
| This Study | Literature | ||
| Trolox | 5.60 ± 0.22 | 3.70 ± 0.08 | [41] |
| Tocopherol | 2.21 ± 0.12 | ||
| TQ | 72.31 ± 0.26 | 125.65 ± 0.76 | [42] |
| 121.56 ± 0.66 | [44] | ||
| 211.0 ± 0.0 | [43] | ||
| IC50 [mg/mL] | |||
| BCSO | 4.13 ± 0.36 | 2.30 ± 0.02 | [5] |
| 0.46 ± 0.00 | [43] | ||
| 4.02 ± 0.04 | [18] | ||
| Methanolic extract of BCSO | 5.07 ± 0.38 | 11.26 ± 0.93 (seeds) | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neunert, G.; Kamińska, W.; Nowak-Karnowska, J. Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments. Appl. Sci. 2025, 15, 377. https://doi.org/10.3390/app15010377
Neunert G, Kamińska W, Nowak-Karnowska J. Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments. Applied Sciences. 2025; 15(1):377. https://doi.org/10.3390/app15010377
Chicago/Turabian StyleNeunert, Grażyna, Wiktoria Kamińska, and Joanna Nowak-Karnowska. 2025. "Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments" Applied Sciences 15, no. 1: 377. https://doi.org/10.3390/app15010377
APA StyleNeunert, G., Kamińska, W., & Nowak-Karnowska, J. (2025). Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments. Applied Sciences, 15(1), 377. https://doi.org/10.3390/app15010377






