Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
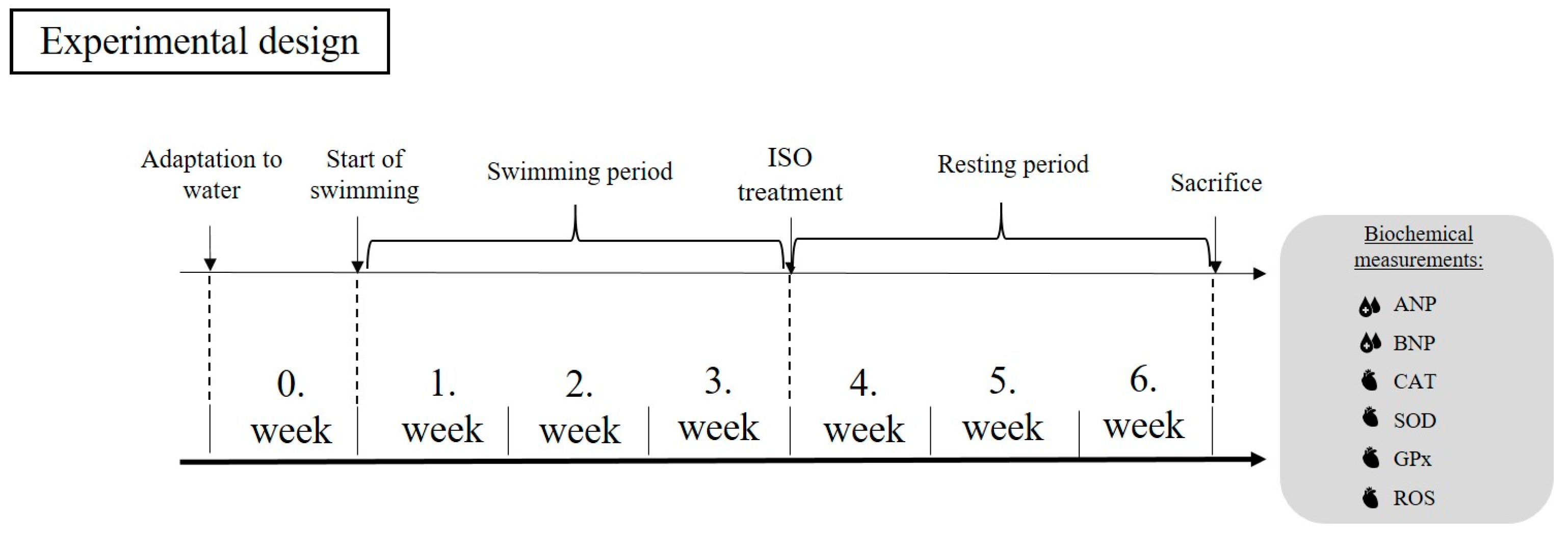
2.2. Exercise Protocol
2.3. ELISA Measurements
2.4. BCA Protein Measurements
2.5. Statistical Analysis
3. Results
3.1. Serum ANP and BNP Concentrations
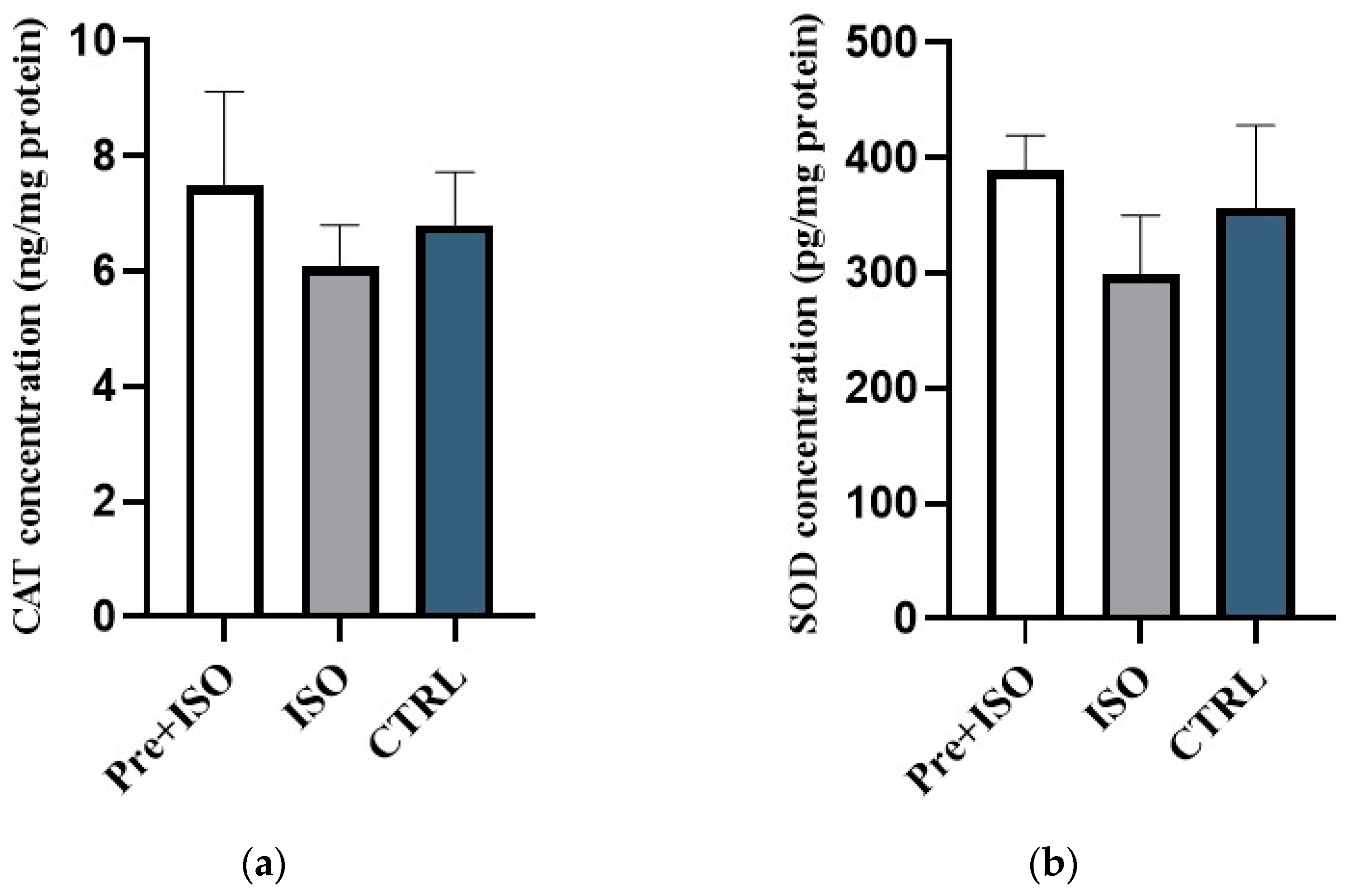
3.2. Cardiac CAT and SOD Concentrations
3.3. Cardiac GPx Concentrations
3.4. Cardiac ROS Concentrations
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edgardo Olvera Lopez, B.D.B.; Jan, A. Cardiovascular Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative Stress in Ischemic Heart Disease. Oxid. Med. Cell. Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef] [PubMed]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.X.; Raza, S.; Shah, A.M. Redox signaling in the cardiomyocyte: From physiology to failure. Int. J. Biochem. Cell Biol. 2016, 74, 145–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, S.; Yoshimura, M.; Nakayama, M.; Mizuno, Y.; Harada, E.; Ito, T.; Nakamura, S.; Abe, K.; Yamamuro, M.; Sakamoto, T.; et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: A long-term follow-up analysis. Circulation 2004, 110, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, F.; Zhang, C.; Zheng, L.R.; Yang, J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. BioMed Res. Int. 2020, 2020, 2018035. [Google Scholar] [CrossRef] [PubMed]
- Borzsei, D.; Szabo, R.; Hoffmann, A.; Harmath, A.; Sebestyen, J.; Osman, J.; Juhasz, B.; Priksz, D.; Varga, C.; Posa, A. Multiple Applications of Different Exercise Modalities with Rodents. Oxid. Med. Cell. Longev. 2021, 2021, 3898710. [Google Scholar] [CrossRef]
- Elokda, A.S.; Nielsen, D.H. Effects of exercise training on the glutathione antioxidant system. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 630–637. [Google Scholar] [CrossRef]
- Yan, Z.; Spaulding, H.R. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020, 32, 101508. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Demircan, B.; Taysi, S.; Oztasan, N.; Gumustekin, K.; Siktar, E.; Polat, M.F.; Akar, S.; Akcay, F.; Dane, S. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 143, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Borzsei, D.; Karacsonyi, Z.; Gesztelyi, R.; Nemes, K.; Berko, A.M.; Veszelka, M.; Torok, S.; Kupai, K.; Varga, C.; et al. Postconditioning-like effect of exercis: New paradigm in experimental menopause. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H400–H407. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.K.; Farrar, R.P.; Starnes, J.W. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am. J. Physiol. 1992, 263, H804–H809. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Nikolic Turnic, T.; Zivkovic, V.; Pindovic, B.; Chichkova, N.V.; Fisenko, V.P.; Nikolic, M.; Stijak, L.; Yurievna, L.E.; Veselinovic, M.; et al. The Enhanced Effects of Swimming and Running Preconditioning in an Experimental Model of Myocardial Ischemia/Reperfusion Injury. Medicina 2023, 59, 1995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, Y.; Tan, Y.; Zhang, Z.; Hou, Z.; Gao, C.; Feng, P.; Zhang, X.; Yi, W.; Gao, F. Short-Duration Swimming Exercise after Myocardial Infarction Attenuates Cardiac Dysfunction and Regulates Mitochondrial Quality Control in Aged Mice. Oxid. Med. Cell. Longev. 2018, 2018, 4079041. [Google Scholar] [CrossRef]
- Song, X.J.; Yang, C.Y.; Liu, B.; Wei, Q.; Korkor, M.T.; Liu, J.Y.; Yang, P. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int. J. Med. Sci. 2011, 8, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Lyngbakken, M.N.; Myhre, P.L.; Rosjo, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef]
- He, J.; Lu, Y.; Song, X.; Gong, X.; Li, Y. Inhibition of microRNA-146a attenuated heart failure in myocardial infarction rats. Biosci. Rep. 2019, 39, BSR20191732. [Google Scholar] [CrossRef]
- Horakova, D.; Azeem, K.; Benesova, R.; Pastucha, D.; Horak, V.; Dumbrovska, L.; Martinek, A.; Novotny, D.; Svagera, Z.; Hobzova, M.; et al. Total and High Molecular Weight Adiponectin Levels and Prediction of Cardiovascular Risk in Diabetic Patients. Int. J. Endocrinol. 2015, 2015, 545068. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef]
- Rao, P.R.; Viswanath, R.K. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2007, 12, 179–187. [Google Scholar] [PubMed]
- Rostamzadeh, F.; Najafipour, H.; Aminizadeh, S.; Jafari, E. Therapeutic effects of the combination of moderate-intensity endurance training and MitoQ supplementation in rats with isoproterenol-induced myocardial injury: The role of mitochondrial fusion, fission, and mitophagy. Biomed. Pharmacother. 2024, 170, 116020. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P.; Demirel, H.A.; Smuder, A.J. Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radic. Res. 2014, 48, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, R.; Ranjbar, K.; Salehi, I.; Komaki, A.; Zarrinkalam, E.; Amiri, P. Comparison of the preconditioning effect of different exercise training modalities on myocardial ischemia-reperfusion injury. PLoS ONE 2023, 18, e0295169. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch. Biochem. Biophys. 1996, 331, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ascensao, A.; Magalhaes, J.; Soares, J.; Ferreira, R.; Neuparth, M.; Marques, F.; Oliveira, J.; Duarte, J. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int. J. Cardiol. 2005, 100, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börzsei, D.; Kiss, V.; Nagy, A.; Hoffmann, A.; Török, S.; Almási, N.; Veszelka, M.; Varga, C.; Szabó, R. Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart. Appl. Sci. 2024, 14, 2073. https://doi.org/10.3390/app14052073
Börzsei D, Kiss V, Nagy A, Hoffmann A, Török S, Almási N, Veszelka M, Varga C, Szabó R. Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart. Applied Sciences. 2024; 14(5):2073. https://doi.org/10.3390/app14052073
Chicago/Turabian StyleBörzsei, Denise, Viktória Kiss, András Nagy, Alexandra Hoffmann, Szilvia Török, Nikoletta Almási, Médea Veszelka, Csaba Varga, and Renáta Szabó. 2024. "Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart" Applied Sciences 14, no. 5: 2073. https://doi.org/10.3390/app14052073
APA StyleBörzsei, D., Kiss, V., Nagy, A., Hoffmann, A., Török, S., Almási, N., Veszelka, M., Varga, C., & Szabó, R. (2024). Moderate-Intensity Swimming Alleviates Oxidative Injury in Ischemic Heart. Applied Sciences, 14(5), 2073. https://doi.org/10.3390/app14052073






