Exploring the Interplay of Uric Acid and Advanced Oxidation Protein Products Following Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
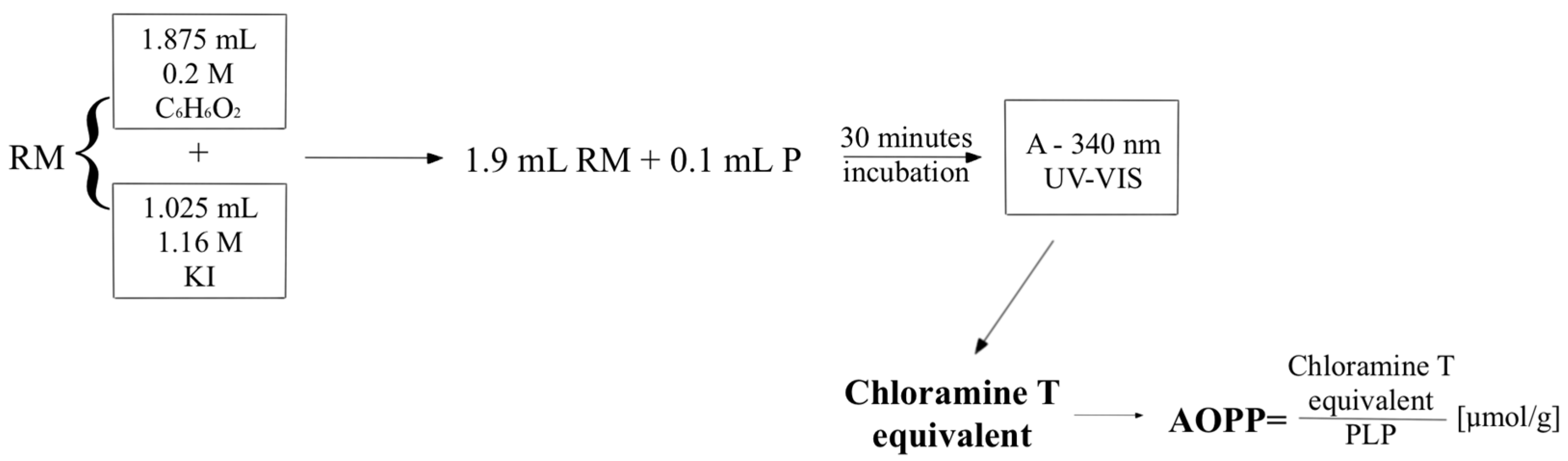
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
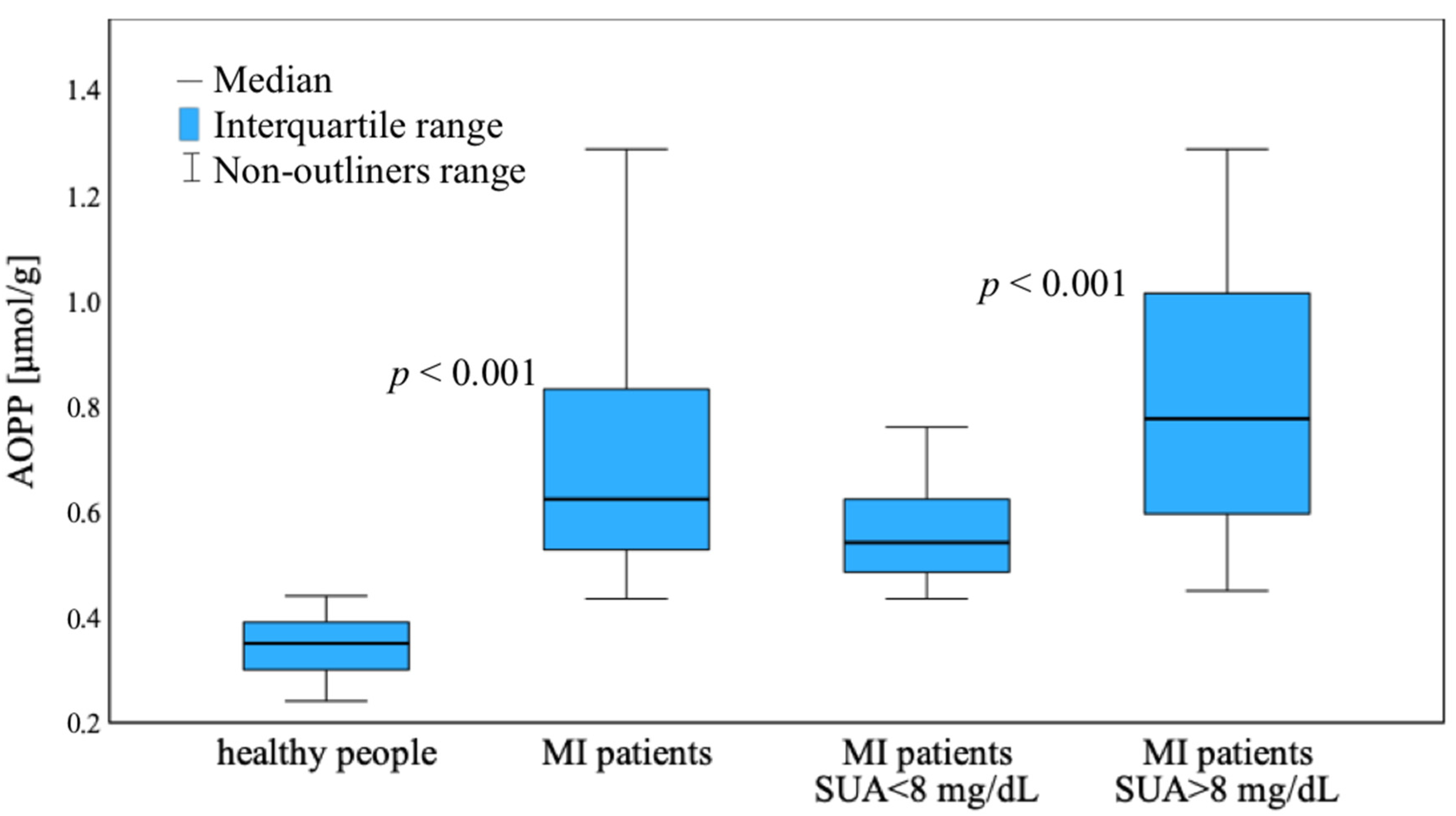
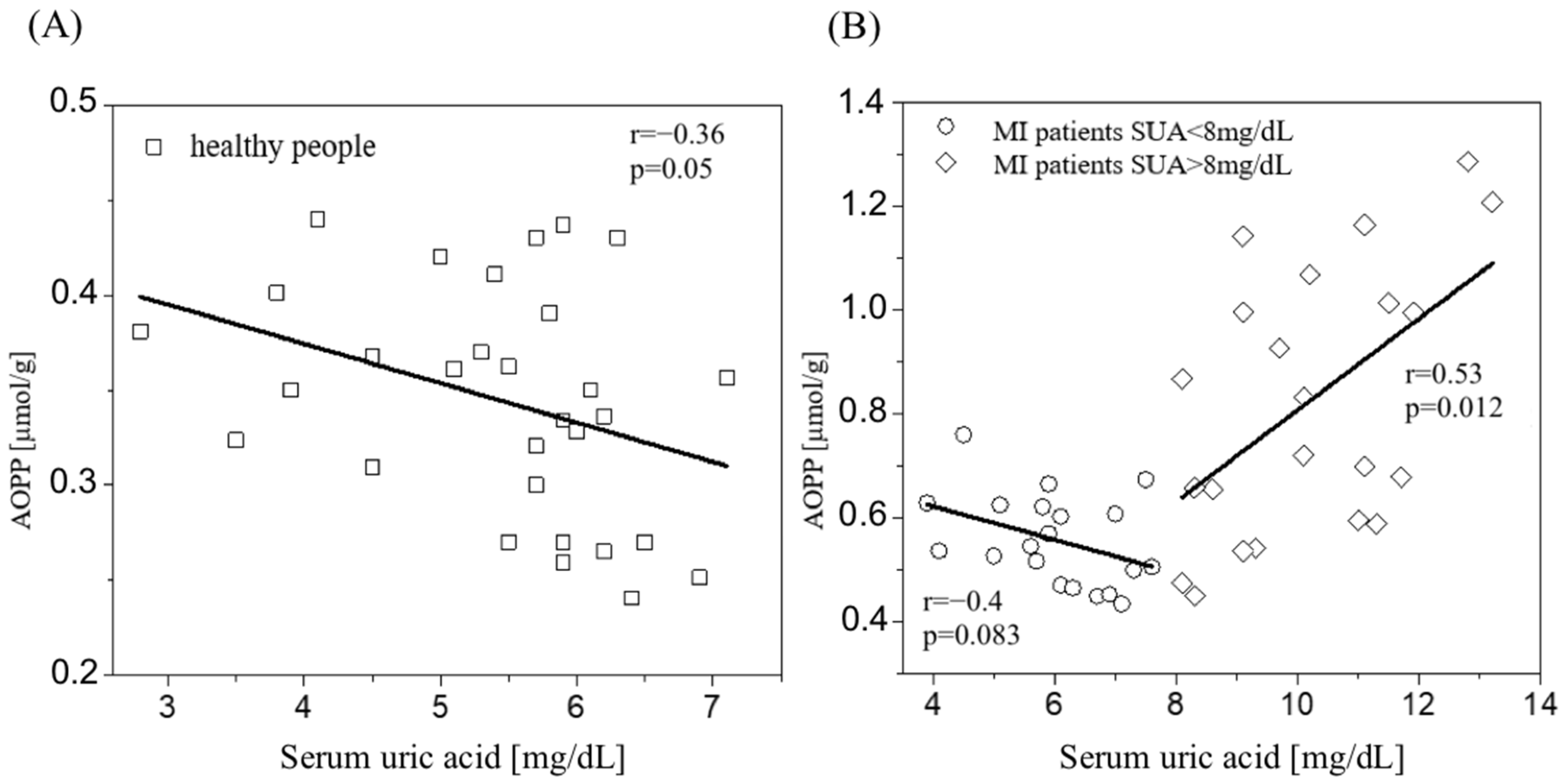
3.2. AOPP Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | absorbance |
| ACS | acute coronary syndrome |
| AOPP | advanced oxidation protein products |
| BMI | Body mass index |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| CK-MB | creatine phosphokinase-MB |
| CRP | C-reactive protein |
| CVD | cardiovascular diseases |
| DAMPs | damage-associated molecular patterns |
| DM | diabetes mellitus |
| DNA | deoxyribonucleic acid |
| EDTA | ethylenediaminetetraacetic acid |
| HDL | high-density lipoprotein |
| HF | heart failure |
| HIF | hypoxia-inducible factors |
| HIF-1 alfa | hypoxia-inducible factors-1 alfa |
| HOCl | hypochlorous acid |
| LDL | low-density lipoprotein cholesterol |
| MI | acute myocardial infarction |
| MPO | myeloperoxidase |
| mtDNA | mitochondrial deoxyribonucleic acid |
| NETs | neutrophil extracellular traps |
| NO | nitric oxide |
| NSTEMI | Non-ST elevation MI |
| NYHA | New York Heart Association Classification |
| OS | oxidative stress |
| P | plasma |
| PCI | percutaneous coronary intervention; |
| PPC | plasma protein concentration |
| PLP | protein level in plasma |
| RM | reactant mixture |
| ROS | reactive oxygen species |
| SD | standard deviation |
| STEMI | ST-segment elevation MI |
| SUA | serum uric acid |
| UA | uric acid |
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Turell, L.; Carballal, S.; Botti, H.; Radi, R.; Alvarez, B. Oxidation of the Albumin Thiol to Sulfenic Acid and Its Implications in the Intravascular Compartment. Braz. J. Med. Biol. Res. 2009, 42, 305–311. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Partyka, Ł.; Hartwich, J.; Drożdż, W.; Gruca, A.; Jopek, R.; Karcz, D.; Dembińska-Kieć, A. Changes in Oxidative Stress Parameters in Patients with Peripheral Vascular Disease in Response to Conservative and Surgical Treatment. Acta Angiol. 2001, 7, 29–41. [Google Scholar]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent. Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Majewski, W.; Strzyzewski, K.W.; Kasprzak, M.P.; Iskra, M. Advanced Oxidation Protein Products and Carbonylated Proteins as Biomarkers of Oxidative Stress in Selected Atherosclerosis-Mediated Diseases. Biomed. Res. Int. 2017, 2017, 4975264. [Google Scholar] [CrossRef] [PubMed]
- Azouaou Toualbi, L.; Mounir, A.; Wafa, B.; Medina, A.; Abderrezak, K.; Chahine, T.; Henni, C.; Abdelghani, B.; Atmane, S. Implications of Advanced Oxidation Protein Products and Vitamin E in Atherosclerosis Progression. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, 135–144. [Google Scholar] [CrossRef]
- Shang, X.L.; Fan, F.H.; Zhi, J.G.; Nagai, R.; Wei, R.Z.; Zhi, Q.L.; Zhan, M.Z.; Zhou, M.; Xie, D.; Guo, B.W.; et al. Advanced Oxidation Protein Products Accelerate Atherosclerosis Through Promoting Oxidative Stress and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1156–1162. [Google Scholar] [CrossRef]
- Servettaz, A.; Guilpain, P.; Goulvestre, C.; Chéreau, C.; Hercend, C.; Nicco, C.; Guillevin, L.; Weill, B.; Mouthon, L.; Batteux, F. Radical Oxygen Species Production Induced by Advanced Oxidation Protein Products Predicts Clinical Evolution and Response to Treatment in Systemic Sclerosis. Ann. Rheum. Dis. 2007, 66, 1202–1209. [Google Scholar] [CrossRef]
- Kaneda, H.; Taguchi, J.; Ogasawara, K.; Aizawa, T.; Ohno, M. Increased Level of Advanced Oxidation Protein Products in Patients with Coronary Artery Disease. Atherosclerosis 2002, 162, 221–225. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Witko-Sarsat, V.; Nguyen-Khoa, T.; Nguyen, A.T.; Gausson, V.; Mothu, N.; London, G.M.; Jungers, P. Advanced Oxidation Protein Products as Risk Factors for Atherosclerotic Cardiovascular Events in Nondiabetic Predialysis Patients. Am. J. Kidney Dis. 2005, 45, 39–47. [Google Scholar] [CrossRef]
- Żurawska-Płaksej, E.; Grzebyk, E.; Marciniak, D.; Szymańska-Chabowska, A.; Piwowar, A. Oxidatively Modified Forms of Albumin in Patients with Risk Factors of Metabolic Syndrome. J. Endocrinol. Invest. 2014, 37, 819–827. [Google Scholar] [CrossRef]
- Klafke, J.Z.; Porto, F.G.; Batista, R.; Bochi, G.V.; Moresco, R.N.; da Luz, P.L.; Viecili, P.R.N. Association between Hypertriglyceridemia and Protein Oxidation and Proinflammatory Markers in Normocholesterolemic and Hypercholesterolemic Individuals. Clin. Chim. Acta 2015, 448, 50–57. [Google Scholar] [CrossRef]
- Holme, I.; Aastveit, A.H.; Hammar, N.; Jungner, I.; Walldius, G. Uric Acid and Risk of Myocardial Infarction, Stroke and Congestive Heart Failure in 417,734 Men and Women in the Apolipoprotein MOrtality RISk Study (AMORIS). J. Intern. Med. 2009, 266, 558–570. [Google Scholar] [CrossRef]
- Chen, J.H.; Chuang, S.Y.; Chen, H.J.; Wen-Ting, Y.E.H.; Wen-Harn, P.A.N. Serum Uric Acid Level as an Independent Risk Factor for All-Cause, Cardiovascular, and Ischemic Stroke Mortality: A Chinese Cohort Study. Arthritis Rheum. 2009, 61, 225–232. [Google Scholar] [CrossRef]
- Trkulja, V.; Car, S. On-Admission Serum Uric Acid Predicts Outcomes after Acute Myocardial Infarction: Systematic Review and Meta-Analysis of Prognostic Studies. Croat. Med. J. 2012, 53, 162–172. [Google Scholar] [CrossRef]
- Gagliardi, A.C.M.; Miname, M.H.; Santos, R.D. Uric Acid: A Marker of Increased Cardiovascular Risk. Atherosclerosis 2009, 202, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Puig, J.G. Uric Acid and Oxidative Stress: Relative Impact on Cardiovascular Risk? Nutr. Metab. Cardiovasc. Dis. 2007, 17, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.J. Cardiovascular Drugs and Serum Uric Acid. Cardiovasc. Drugs Ther. 2003, 17, 397–414. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Franchini, M.; Favaloro, E.J.; Targher, G. The Paradoxical Relationship between Serum Uric Acid and Cardiovascular Disease. Clin. Chim. Acta 2008, 392, 1–7. [Google Scholar] [CrossRef]
- Sakai, H.; Tsutamoto, T.; Tsutsui, T.; Tanaka, T.; Ishikawa, C.; Horie, M. Serum Level of Uric Acid, Partly Secreted from the Failing Heart, Is a Prognostic Marker in Patients with Congestive Heart Failure. Circ. J. 2006, 70, 1006–1011. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart Failure after Myocardial Infarction: Incidence and Predictors. ESC Heart Fail. 2021, 8, 222. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Shao, J.; Lin, L.; Jiang, M.; Wang, L.; Lu, X.; Zhang, H.; Chen, Y.; Zhang, R. Immune and Inflammation in Acute Coronary Syndrome: Molecular Mechanisms and Therapeutic Implications. J. Immunol. Res. 2020, 2020, 4904217. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling Pathways and Targeted Therapy for Myocardial Infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef]
- Tomandlova, M.; Parenica, J.; Lokaj, P.; Ondrus, T.; Kala, P.; Miklikova, M.; Helanova, K.; Helan, M.; Malaska, J.; Benesova, K.; et al. Prognostic Value of Oxidative Stress in Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Prospective Cohort Study. Free Radic. Biol. Med. 2021, 174, 66–72. [Google Scholar] [CrossRef]
- Hanasand, M.; Omdal, R.; Norheim, K.B.; Gøransson, L.G.; Brede, C.; Jonsson, G. Improved Detection of Advanced Oxidation Protein Products in Plasma. Clin. Chim. Acta 2012, 413, 901–906. [Google Scholar] [CrossRef]
- Cvetkovic, T.; Saric, S.; Stefanovic, N.; Stojiljkovic, V.; Djordjevic, B.; Stojanovic, D.; Cvetkovic, M.; Deljanin Ilic, M. Plasma Advanced Oxidation Products as an Additional Tool in Assessment of Post-Infarction Heart Failure. J. Int. Med. Res. 2022, 50, 030006052211397. [Google Scholar] [CrossRef]
- Skvarilová, M.; Bulava, A.; Stejskal, D.; Adamovská, S.; Bartek, J. Increased Level of Advanced Oxidation Products (AOPP) as a Marker of Oxidative Stress in Patients with Acute Coronary Syndrome. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2005, 149, 83–87. [Google Scholar] [CrossRef]
- Nadkar, M.Y.; Jain, V.I. Serum Uric Acid in Acute Myocardial Infarction. J. Assoc. Physicians India 2008, 56, 759–762. [Google Scholar]
- Gordon, T.; Kannel, W.B. Drinking and Its Relation to Smoking, BP, Blood Lipids, and Uric Acid. The Framingham Study. Arch. Intern. Med. 1983, 143, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wietlisbach, V.; Bovet, P.; Shamlaye, C.; Riesen, W.; Paccaud, F.; Burnier, M. Prevalence of Hyperuricemia and Relation of Serum Uric Acid with Cardiovascular Risk Factors in a Developing Country. BMC Public Health 2004, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric Acid Shown to Contribute to Increased Oxidative Stress Level Independent of Xanthine Oxidoreductase Activity in MedCity21 Health Examination Registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Giordano, M. Hyperuricemia and Endothelial Function: Is It a Simple Association or Do Gender Differences Play a Role in This Binomial? Biomedicines 2022, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Duan, X.M.; Liu, Y.; Yu, J.; Tang, Y.L.; Liu, Z.L.; Jiang, S.; Zhang, C.P.; Liu, J.Y.; Xu, J.X. Uric Acid Induces Endothelial Dysfunction by Activating the HMGB1/RAGE Signaling Pathway. Biomed. Res. Int. 2017, 2017, 391920. [Google Scholar] [CrossRef]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Marsche, G.; Frank, S.; Hrzenjak, A.; Holzer, M.; Dirnberger, S.; Wadsack, C.; Scharnagl, H.; Stojakovic, T.; Heinemann, A.; Oettl, K. Plasma-Advanced Oxidation Protein Products Are Potent High-Density Lipoprotein Receptor Antagonists in Vivo. Circ. Res. 2009, 104, 750–757. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Z.; Mo, Z.; Xiao, J. The Characteristics and Roles of Advanced Oxidation Protein Products in Atherosclerosis. Cardiovasc. Toxicol. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Bagyura, Z.; Takács, A.; Kiss, L.; Dósa, E.; Vadas, R.; Nguyen, T.D.; Dinya, E.; Soós, P.; Szelid, Z.; Láng, O.; et al. Level of Advanced Oxidation Protein Products Is Associated with Subclinical Atherosclerosis. BMC Cardiovasc. Disord. 2022, 22, 5. [Google Scholar] [CrossRef]
- Villalpando Sánchez, D.C.; Alvarez Aguilar, C.; Gómez García, A. Advanced Oxidation Protein Products and Their Relationship with Cardiovascular Risk Factors in Young Apparently Healthy People. Clin. Investig. Arterioscler. 2017, 29, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-Induced Activation of Human Neutrophil and Monocyte Oxidative Metabolism: A Potential Target for N-Acetylcysteine Treatment in Dialysis Patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gorudko, I.V.; Grigorieva, D.V.; Shamova, E.V.; Kostevich, V.A.; Sokolov, A.V.; Mikhalchik, E.V.; Cherenkevich, S.N.; Arnhold, J.; Panasenko, O.M. Hypohalous Acid-Modified Human Serum Albumin Induces Neutrophil NADPH Oxidase Activation, Degranulation, and Shape Change. Free Radic. Biol. Med. 2014, 68, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Yariswamy, M.; Yoshida, T.; Valente, A.J.; Kandikattu, H.K.; Sakamuri, S.S.V.P.; Siddesha, J.M.; Sukhanov, S.; Saifudeen, Z.; Ma, L.; Siebenlist, U.; et al. Cardiac-Restricted Overexpression of TRAF3 Interacting Protein 2 (TRAF3IP2) Results in Spontaneous Development of Myocardial Hypertrophy, Fibrosis, and Dysfunction. J. Biol. Chem. 2016, 291, 19425–19436. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric Acid Provides an Antioxidant Defense in Humans against Oxidant- and Radical-Caused Aging and Cancer: A Hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric Acid Stimulates Vascular Smooth Muscle Cell Proliferation and Oxidative Stress via the Vascular Renin-Angiotensin System. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; He, F.; Cheng, C.; Xu, B.L.; Sheng, J.L. Uric Acid Aggravates Myocardial Ischemia–Reperfusion Injury via ROS/NLRP3 Pyroptosis Pathway. Biomed. Pharmacother. 2021, 133, 110990. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Papežíková, I.; Pekarová, M.; Kolářová, H.; Klinke, A.; Lau, D.; Baldus, S.; Lojek, A.; Kubala, L. Uric Acid Modulates Vascular Endothelial Function through the down Regulation of Nitric Oxide Production. Free Radic. Res. 2013, 47, 82–88. [Google Scholar] [CrossRef]
- Țăpoi, L.; Șalaru, D.L.; Sascău, R.; Stătescu, C. Uric Acid—An Emergent Risk Marker for Thrombosis? J. Clin. Med. 2021, 10, 2062. [Google Scholar] [CrossRef]
- Omidvar, B.; Ayatollahi, F.; Alasti, M. The Prognostic Role of Serum Uric Acid Level in Patients with Acute ST Elevation Myocardial Infarction. J. Saudi Heart Assoc. 2012, 24, 73–78. [Google Scholar] [CrossRef][Green Version]
- Ndrepepa, G.; Braun, S.; Haase, H.U.; Schulz, S.; Ranftl, S.; Hadamitzky, M.; Mehilli, J.; Schömig, A.; Kastrati, A. Prognostic Value of Uric Acid in Patients with Acute Coronary Syndromes. Am. J. Cardiol. 2012, 109, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, A.G.; Brennan, D.M.; Hoar, B.M.; Hazen, S.L.; Hoogwerf, B.J. Serum Uric Acid Is an Independent Predictor of All-Cause Mortality in Patients at High Risk of Cardiovascular Disease: A Preventive Cardiology Information System (PreCIS) Database Cohort Study. Arthritis Rheum. 2008, 58, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Ambrose, J.A. Understanding Myocardial Infarction. F1000Res 2018, 7, F1000 Faculty Rev-1378. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury—From Basic Science to Clinical Bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Gu, J.; Liu, R.; Xu, F.; Qian, H.; He, Q.; Meng, W. Release of Mitochondrial DNA Correlates with Peak Inflammatory Cytokines in Patients with Acute Myocardial Infarction. Anatol. J. Cardiol. 2017, 17, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Wurm, R.; Ruhittel, S.; Lenz, M.; Huber, K.; Wojta, J.; Heinz, G.; Hülsmann, M.; Speidl, W.S. Release of Mitochondrial DNA Is Associated with Mortality in Severe Acute Heart Failure. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gaál Kovalčíková, A.; Janovičová, L.; Hodosy, J.; Bábíčková, J.; Vavrincová-Yaghi, D.; Vavrinec, P.; Boor, P.; Podracká, L.; Šebeková, K.; Celec, P.; et al. Extracellular DNA Concentrations in Various Aetiologies of Acute Kidney Injury. Sci. Rep. 2022, 12, 16812. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, T.; Scarlatescu, A.I.; Sanda, G.M.; Toma, L.; Stancu, C.S.; Dorobantu, M.; Micheu, M.M.; Sima, A.V.; Niculescu, L.S. Mitochondrial DNA Together with MiR-142-3p in Plasma Can Predict Unfavorable Outcomes in Patients after Acute Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 9947. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Yuan, K.; Yuan, J. MtDNA in the Pathogenesis of Cardiovascular Diseases. Dis. Markers 2021, 2021, 7157109. [Google Scholar] [CrossRef]
- Korabecna, M.; Zinkova, A.; Brynychova, I.; Chylikova, B.; Prikryl, P.; Sedova, L.; Neuzil, P.; Seda, O. Cell-Free DNA in Plasma as an Essential Immune System Regulator. Sci. Rep. 2020, 10, 17478. [Google Scholar] [CrossRef] [PubMed]
- Singel, K.L.; Grzankowski, K.S.; Khan, A.N.M.N.H.; Grimm, M.J.; D’Auria, A.C.; Morrell, K.; Eng, K.H.; Hylander, B.; Mayor, P.C.; Emmons, T.R.; et al. Mitochondrial DNA in the Tumour Microenvironment Activates Neutrophils and Is Associated with Worse Outcomes in Patients with Advanced Epithelial Ovarian Cancer. Br. J. Cancer 2018, 120, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants 2022, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Witko-Sarsat, V. Importance of Oxidatively Modified Proteins in Chronic Renal Failure. Kidney Int. Suppl. 2001, 78, S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Capeillère-Blandin, C.; Gausson, V.; Descamps-Latscha, B.; Witko-Sarsat, V. Biochemical and Spectrophotometric Significance of Advanced Oxidized Protein Products. Biochim. Biophys. Acta Mol. Basis Dis. 2004, 1689, 91–102. [Google Scholar] [CrossRef]
- Wybranowski, T.; Napiórkowska, M.; Bosek, M.; Pyskir, J.; Ziomkowska, B.; Cyrankiewicz, M.; Pyskir, M.; Pilaczyńska-Cemel, M.; Rogańska, M.; Kruszewski, S.; et al. Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences. Int. J. Mol. Sci. 2022, 23, 10103. [Google Scholar] [CrossRef]
- Qi, Y.; Uchida, T.; Yamamoto, M.; Yamamoto, Y.; Kido, K.; Ito, H.; Ohno, N.; Asahara, M.; Yamada, Y.; Yamaguchi, O.; et al. Perioperative Elevation in Cell-Free DNA Levels in Patients Undergoing Cardiac Surgery: Possible Contribution of Neutrophil Extracellular Traps to Perioperative Renal Dysfunction. Anesthesiol. Res. Pract. 2016, 2016, 2794364. [Google Scholar] [CrossRef]
- Wu, H.; Dai, R.; Wang, M.; Chen, C. Uric Acid Promotes Myocardial Infarction Injury via Activating Pyrin Domain-Containing 3 Inflammasome and Reactive Oxygen Species/Transient Receptor Potential Melastatin 2/Ca2+pathway. BMC Cardiovasc. Disord. 2023, 23, 10. [Google Scholar] [CrossRef]
- Shohet, R.V.; Garcia, J.A. Keeping the Engine Primed: HIF Factors as Key Regulators of Cardiac Metabolism and Angiogenesis during Ischemia. J. Mol. Med. 2007, 85, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Heck-Swain, K.L.; Koeppen, M. The Intriguing Role of Hypoxia-Inducible Factor in Myocardial Ischemia and Reperfusion: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2023, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; Chen, C. HIF-1α in Myocardial Ischemia-reperfusion Injury (Review). Mol. Med. Rep. 2021, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.R.; Print, C.; Farahi, N.; Peyssonnaux, C.; Johnson, R.S.; Cramer, T.; Sobolewski, A.; Condliffe, A.M.; Cowburn, A.S.; Johnson, N.; et al. Hypoxia-Induced Neutrophil Survival Is Mediated by HIF-1α–Dependent NF-ΚB Activity. J. Exp. Med. 2005, 201, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.L.; Fan, F.H.; Zhang, X.; Ping, Y.C.; Shang, X.L.; Jian, X.F.; Zhi, Q.L.; Yue, X.S.; Guo, B.W.; Zhan, M.Z.; et al. Advanced Oxidation Protein Products Accelerate Renal Fibrosis in a Remnant Kidney Model. J. Am. Soc. Nephrol. 2007, 18, 528–538. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, X.; Shu, S.; Zhang, C.; Liang, Y.; Jiang, T.; Zhang, W.; Guo, T.; Liang, X.; Tang, X. Advanced Oxidation Protein Products Inhibit the Autophagy of Renal Tubular Epithelial Cells. Exp. Ther. Med. 2018, 15, 3908–3916. [Google Scholar] [CrossRef]
- Wu, Y.W.; Ho, S.K.; Tseng, W.K.; Yeh, H.I.; Leu, H.B.; Yin, W.H.; Lin, T.H.; Chang, K.C.; Wang, J.H.; Wu, C.C.; et al. Potential Impacts of High-Sensitivity Creatine Kinase-MB on Long-Term Clinical Outcomes in Patients with Stable Coronary Heart Disease. Sci. Rep. 2020, 10, 5638–5639. [Google Scholar] [CrossRef]
- Babuin, L.; Jaffe, A.S. Troponin: The Biomarker of Choice for the Detection of Cardiac Injury. Can. Med. Assoc. J. 2005, 173, 1191–1202. [Google Scholar] [CrossRef]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in Acute Myocardial Infarction: Current Perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Men or women | Diabetes mellitus |
| Age 18–80 years | Obesity (BMI > 30) |
| Provision of informed consent before any study-specific procedures | Pregnancy |
| Diagnosis of Myocardial Infarction | Active bleeding |
| History of moderate or severe hepatic impairment | |
| History of major surgery or severe trauma (within three months) | |
| Kidney disease requiring dialysis | |
| Respiratory failure | |
| History of severe chronic heart failure (NYHA class III or IV) | |
| Taking drugs increasing uric acid production: diuretics, antitubercular drugs, immunosuppressant agents, testosterone, xylitol, nicotinic acid, and lactate infusion. |
| Characteristic | Mean ± SD | Reference Values |
|---|---|---|
| Age | 69.36 ± 12.175 | not applicable |
| Gender (male) | 60% | not applicable |
| Troponin I | 5.04 ± 7.505 | <0.04 ng/mL |
| CK-MB | 28.23 ± 20.917 | <25 U/L |
| Total protein | 6.49 ± 0.702 | 6–8 g/dL |
| CRP | 30.85 ± 44.303 | <5.00 mg/L |
| Cholesterol | 204.09 ± 62.687 | <190 mg/dL |
| LDL cholesterol | 125.58 ± 48.677 | <115 mg/dL |
| HDL cholesterol | 45.12 ± 12.538 | >40 mg/dL |
| Triglycerides | 127.30 ± 108.171 | <150 mg/dL |
| Variable | Ndrepepa et al. [52] | Ioachimescu et al. [53] | ||||||
|---|---|---|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | Q 1 | Q 2 | Q 3 | Q 4 | |
| Number of patients | 1271 | 1261 | 1300 | 1292 | 755 | 802 | 751 | 790 |
| UA level, [mg/dL] | 1.3–5.3 | 5.3–6.3 | 6.3–7.5 | 7.5–18.4 | 0.4–4.9 | 5.0–5.9 | 6.0–7.0 | 7.1–13.9 |
| Primary endpoint, [1 year mortality] | 80 deaths | 77 deaths | 72 deaths | 221 deaths | 20 deaths | 28 deaths | 36 deaths | 71 deaths |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, J.; Wybranowski, T.; Karczmarska-Wódzka, A.; Sobczak, P.; Kruszewski, S.; Włodarczyk, Z. Exploring the Interplay of Uric Acid and Advanced Oxidation Protein Products Following Myocardial Infarction. Appl. Sci. 2024, 14, 1983. https://doi.org/10.3390/app14051983
Sikora J, Wybranowski T, Karczmarska-Wódzka A, Sobczak P, Kruszewski S, Włodarczyk Z. Exploring the Interplay of Uric Acid and Advanced Oxidation Protein Products Following Myocardial Infarction. Applied Sciences. 2024; 14(5):1983. https://doi.org/10.3390/app14051983
Chicago/Turabian StyleSikora, Joanna, Tomasz Wybranowski, Aleksandra Karczmarska-Wódzka, Przemysław Sobczak, Stefan Kruszewski, and Zbigniew Włodarczyk. 2024. "Exploring the Interplay of Uric Acid and Advanced Oxidation Protein Products Following Myocardial Infarction" Applied Sciences 14, no. 5: 1983. https://doi.org/10.3390/app14051983
APA StyleSikora, J., Wybranowski, T., Karczmarska-Wódzka, A., Sobczak, P., Kruszewski, S., & Włodarczyk, Z. (2024). Exploring the Interplay of Uric Acid and Advanced Oxidation Protein Products Following Myocardial Infarction. Applied Sciences, 14(5), 1983. https://doi.org/10.3390/app14051983







