Analysis of Volatile Flavor Compounds in Four Commercial Beverages Using Static Headspace Gas Chromatography/Mass Spectrometry: A Qualitative Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Instrumental Analysis of Volatile Compounds and Peak Identification
3. Results and Discussion
3.1. Qualitative Identification of Flavor Compounds in Commercial Beverages
3.2. VOC Flavor Profile of Concentrated Apple Juice
3.3. VOC Flavor Profile of Commercial Tea
3.4. VOC Flavor Profile of Carbonated Lemon Drink
3.5. VOC Flavor Profile of Flavored Sparkling Water
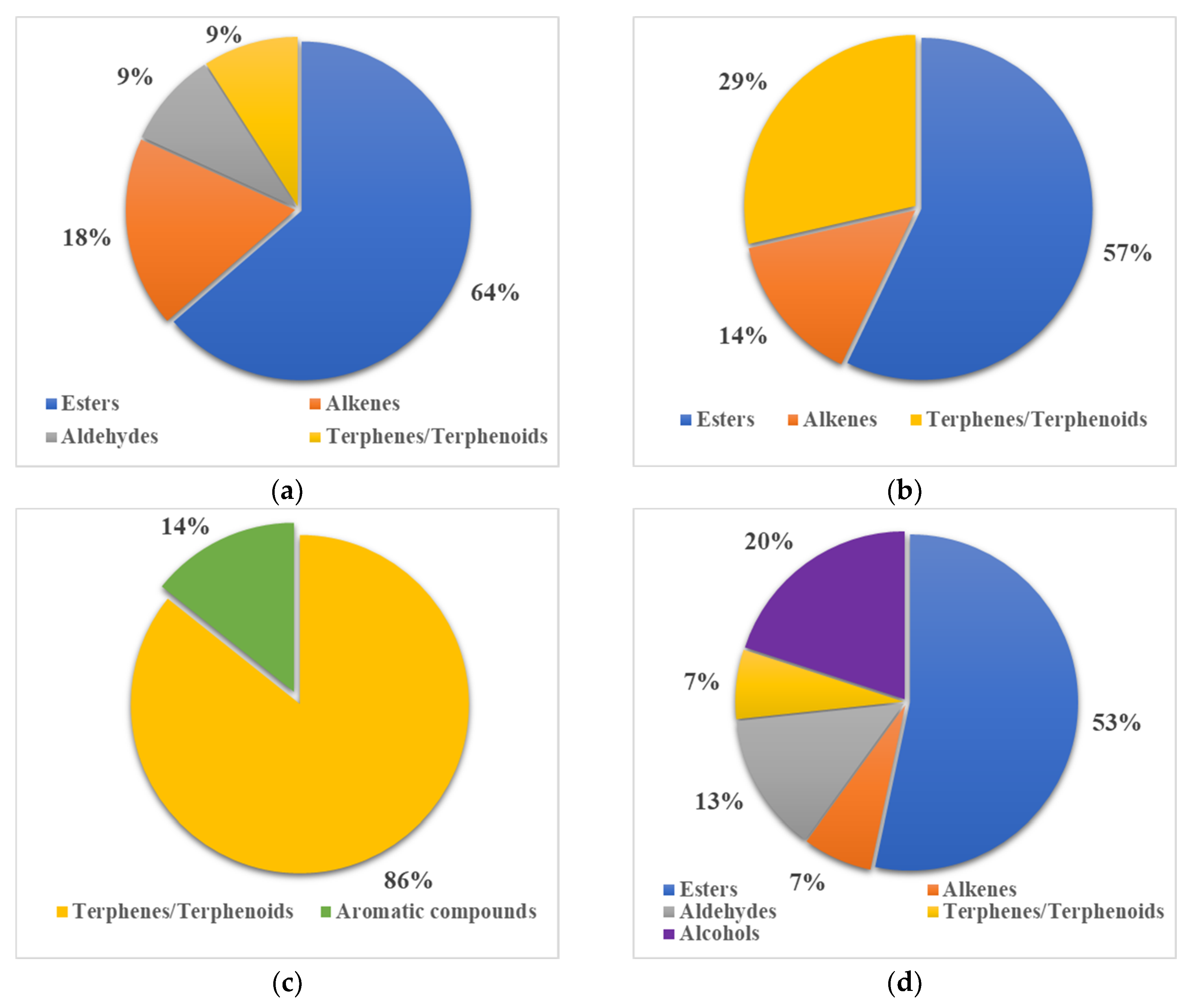
3.6. Flavor Compound Distribution in Commercial Beverages
3.7. Consumption of Commercial Drinks and Current Regulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its preservatives, additives and applications. Int. J. Chem. Biochem. Sci. 2012, 1, 36–47. [Google Scholar]
- Silva, M.M.; Lidon, F. Food preservatives–An overview on applications and side effects. Emir. J. Food. Agric. 2016, 26, 366–373. [Google Scholar] [CrossRef]
- Mortensen, A. Sweeteners permitted in the European Union: Safety aspects. Scand. J. Food. Nutr. 2006, 50, 104–116. [Google Scholar] [CrossRef]
- Brinsden, H.; Lobstein, T. Comparison of nutrient profiling schemes for restricting the marketing of food and drink to children. Pediatr. Obes. 2013, 8, 325–337. [Google Scholar] [CrossRef]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef] [PubMed]
- West, S. Production of flavours, flavour enhancers and other protein-based speciality products. In Novel Enzyme Technology for Food Applications; Woodhead Publishing: Cambridge, UK, 2007; pp. 183–204. [Google Scholar]
- Li, H.; Wang, C.; Zhu, L.I.; Huang, W.; Yi, B.; Zhang, L.; Xu, D. Variations of flavor substances in distillation process of Chinese Luzhou-flavor liquor. J. Food. Process. Eng. 2012, 35, 314–334. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef]
- Jadhav, D.; Rekha, B.N.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- Carmines, E.L. Evaluation of the potential effects of ingredients added to cigarettes. Part 1: Cigarette design, testing approach, and review of results. Food Chem. Toxicol. 2002, 40, 77–91. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene-a review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Comm. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Gianotti, A. Volatilome changes during probiotic fermentation of combined soy and rice drinks. Food Funct. 2021, 12, 3159–3169. [Google Scholar] [CrossRef]
- Aydar, E.F.; Mertdinç, Z.; Demircan, E.; Çetinkaya, S.K.; Özçelik, B. Kidney bean (Phaseolus vulgaris L.) milk substitute as a novel plant-based drink: Fatty acid profile, antioxidant activity, in-vitro phenolic bio-accessibility and sensory characteristics. Innov. Food Sci. Emerg. Technol. 2023, 83, 103254. [Google Scholar] [CrossRef]
- Wildenradt, H.L.; Christensen, E.N.; Stackler, B.; Caputi, A.; Slinkard, K.; Scutt, K. Volatile constituents of grape leaves. I. Vitis vinifera variety ‘Chenin blanc’. Am. J. Enol. Vitic. 1975, 26, 148–153. [Google Scholar] [CrossRef]
- Nan, G.; Zhang, L.; Liu, Z.; Liu, Y.; Du, Y.; Zhao, H.; Zheng, S. Quantitative Determination of p-Cymene, Thymol, Neryl Acetate, and β-Caryophyllene in Different Growth Periods and Parts of Eupatorium fortunei Turcz by GC-MS/MS. J. Anal. Methods Chem. 2021, 2021, 2174667. [Google Scholar] [CrossRef]
- Nisperos-Carriedo, M.O.; Shaw, P.E. Comparison of volatile flavor components in fresh and processed orange juices. J. Agric. Food Chem. 1990, 38, 1048–1052. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Vicente, A.M. Composition of the essential oils from flowers and leaves of vervain [Aloysia triphylla (L’Herit.) Britton] grown in Portugal. J. Essent Oil Res. 2005, 17, 73–78. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Dembitsky, V.M.; Moussaieff, A. Comparative study of volatile compounds in the fresh fruits of Mandragora autumnalis. Acta Chromatogr. 2006, 17, 151–160. [Google Scholar]
- Sanz, C.; Ansorena, D.; Bello, J.; Cid, C. Optimizing headspace temperature and time sampling for identification of volatile compounds in ground roasted Arabica coffee. J. Agric. Food Chem. 2001, 49, 1364–1369. [Google Scholar] [CrossRef]
- Zanin, R.C.; Smrke, S.; Kurozawa, L.E.; Yamashita, F.; Yeretzian, C. Novel experimental approach to study aroma release upon reconstitution of instant coffee products. Food Chem. 2020, 317, 126455. [Google Scholar] [CrossRef]
- Sinharoy, P.; McAllister, S.L.; Vasu, M.; Gross, E.R. Environmental Aldehyde Sources and the Health Implications of Exposure. Adv. Exp. Med. Biol. 2019, 1193, 35–52. [Google Scholar] [PubMed]
- Xiao, Z.; Zhang, S.; Zhu, J.; Niu, Y.; Xiong, W.; Chen, F. Identification of key aromas of grapefruit juice and study of their contributions to the enhancement of sweetness perception. Eur. Food Res. Technol. 2023, 249, 537–551. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, L.; Tu, K. Aroma in freshly squeezed strawberry juice during cold storage detected by E-nose, HS–SPME–GC–MS and GC-IMS. Food Meas. 2023, 17, 3309–3322. [Google Scholar] [CrossRef]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. Trends Analyt Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Steingass, C.B.; Carle, R.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef]
- Martins, A.B.; Friedrich, J.L.; Rodrigues, R.C.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A. Optimized butyl butyrate synthesis catalyzed by Thermomyces lanuginosus lipase. Biotechnol. Prog. 2013, 29, 1416–1421. [Google Scholar] [CrossRef]
- Touaibia, M. Composition and anti-inflammatory effect of the common myrtle (Myrtus communis L.) essential oil growing wild in Algeria. Phytotherapie 2018, 16, S143–S148. [Google Scholar] [CrossRef]
- Wang, L.; Deng, W.; Wang, P.; Huang, W.; Wu, J.; Zheng, T.; Chen, J. Degradations of aroma characteristics and changes of aroma related compounds, PPO activity, and antioxidant capacity in sugarcane juice during thermal process. J. Food Sci. 2020, 85, 1140–1150. [Google Scholar] [CrossRef]
- Dixon, J.; Kuldell, N. BioBuilding: Using banana-scented bacteria to teach synthetic biology. Meth. Enzymol. 2011, 497, 255–271. [Google Scholar]
- Filipiak, W.; Sponring, A.; Filipiak, A.; Baur, M.; Ager, C.; Wiesenhofer, H.; Amann, A. Volatile Organic Compounds (VOCs) Released by Pathogenic Microorganisms In Vitro: Potential Breath Biomarkers. In Volatile Biomarkers; Elsevier: Amsterdam, The Netherlands, 2013; Volume 463. [Google Scholar]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food. Bioproc. Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochem 2021, 190, 112857. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Obeidat, S.M.; Afifi, F.U.; Abu Zarga, M.H. Aroma Profile of Two Populations of Salvia verbenaca Collected from Two Bio-Geographical Zones from Jordan. Chem. Biodivers 2020, 17, e1900553. [Google Scholar] [CrossRef]
- Novak, J.; Bitsch, C.; Langbehn, J.; Pank, F.; Skoula, M.; Gotsiou, Y.; Franz, C.M. Ratios of cis-and trans-sabinene hydrate in Origanum majorana L. and Origanum microphyllum (Bentham) Vogel. Biochem. Syst. Ecol. 2000, 28, 697–704. [Google Scholar] [CrossRef]
- Evans, W.C.; Evans, D. Volatile oils and resins. In Trease and Evans’ Pharmacognosy, 16th ed.; WB Saunders: Orlando, FL, USA, 2009; Chapter 22; pp. 263–303. [Google Scholar]
- Derwich, E.; Benziane, Z.; Manar, A.; Boukir, A.; Taouil, R. Phytochemical analysis and in vitro antibacterial activity of the essential oil of origanum vulgare from Morocco. Am. Eurasian J. Sci. Res. 2010, 5, 120–129. [Google Scholar]
- Schulz, H.; Baranska, M. Fruits and vegetables. In Infrared Spectroscopy for Food Quality Analysis and Control; Academic Press: Cambridge, MA, USA, 2009; pp. 321–353. [Google Scholar]
- Juana, F.L.; Angel, P.A.J.; Manuel, V.M. Beneficial health effects of bioactive compounds present in spices and aromatic herbs. Stud. Nat. Prod. Chem. 2012, 37, 115–134. [Google Scholar]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of odor-active volatiles and odor contribution based on binary interaction effects in mango and vodka cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef]
- Romanenko, E.P.; Tkachev, A.V. Identification by GC/MS of cymene isomers and 3, 7,7-trimethylcyclohepta-1, 3, 5-triene in essential oils. Chem. Nat. Compd. 2006, 42, 699–701. [Google Scholar] [CrossRef]
- Úbeda, C.; Callejón, R.M.; Troncoso, A.M.; Rojas, J.M.; Peña, F.; Morales, M.L. Impact Odorants in Strawberry Vinegars. In Flavour Science; Academic Press: Cambridge, MA, USA, 2014; pp. 177–181. [Google Scholar]
- Zheng, L.Y.; Sun, G.M.; Liu, Y.G.; Lv, L.L.; Yang, W.X.; Zhao, W.F.; Wei, C.B. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef]
- European Comission. 2013. Available online: https://joint-research-centre.ec.europa.eu/system/files/2013-02/FinRep-FAD-2010-0015.pdf (accessed on 12 December 2023).
- European Chemicals Agency (ECHA) 361. Available online: https://echa.europa.eu/ro/substance-information/-/substanceinfo/100.002.361 (accessed on 12 December 2023).
- Tiitinen, K.; Hakala, M.; Kallio, H. HS volatiles from frozen berries of sea buckthorn (Hippophaë rhamnoides L.) varieties. Eur. Food. Res. Technol. 2006, 223, 455–460. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA) 072. Available online: https://echa.europa.eu/ro/substance-information/-/substanceinfo/100.027.072 (accessed on 12 December 2023).
- TGSC Information System. Available online: http://www.thegoodscentscompany.com/data/rw1524131.html (accessed on 12 December 2023).
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Tsang, S. RIFM fragrance ingredient safety assessment, trans-2-Hexenol, CAS Registry Number 928-95-0. Food. Chem. Toxicol. 2018, 118, S49–S58. [Google Scholar] [CrossRef]
- Schmidt, L.; Göen, T. Human metabolism of α-pinene and metabolite kinetics after oral administration. Arch. Toxicol. 2017, 91, 677–687. [Google Scholar] [CrossRef]
- National Institute of Statistics. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/consumul_de_bauturi_in_anul_2021.pdf (accessed on 12 December 2023).
| Peak No. | RT (min) | Compound | CAS No. | Probability (%) | Flavor/Fragrance |
|---|---|---|---|---|---|
| 1 | 4.428 | Pentyl acetate | 628-63-7 | 66.8 | Banana/apple |
| 2 | 7.562 | trans-2-Pentene | 646-04-8 | 65.2 | - |
| 3 | 7.898 | Ethyl butyrate | 105-54-4 | 73.0 | Pineapple |
| 4 | 8.250 | Tetramethylene diacetate | 628-67-1 | 70.6 | Pineapple |
| 5 | 8.775 | Ethyl 2-methylbutyrate | 7452-79-1 | 65.1 | Strawberries |
| 6 | 9.490 | 2-Methylbutyl acetate | 624-41-9 | 68.7 | Sweet banana |
| 7 | 10.044 | 3,3-Dimethyl-1-pentene | 3404-73-7 | 66.8 | - |
| 8 | 10.200 | 3-Furaldehyde | 498-60-2 | 67.6 | Woody |
| 9 | 11.568 | Butyl butyrate | 109-21-7 | 69.0 | Sweet pineapple |
| 10 | 11.930 | Hexyl acetate | 142-92-7 | 75.4 | Sweet pear/banana |
| Peak No. | RT (min) | Compound | CAS No. | Probability (%) | Flavor/Fragrance |
|---|---|---|---|---|---|
| 1 | 4.429 | Pentyl acetate | 628-63-7 | 70.3 | Banana/apple |
| 2 | 7.898 | Ethyl butyrate | 105-54-4 | 78.0 | Pineapple |
| 3 | 9.449 | Isopentyl acetate | 123-92-2 | 80.0 | Banana/pear |
| 4 | 11.928 | 1-Methylcyclopentene | 693-89-0 | 70.1 | - |
| 5 | 12.607 | Pentyl butyrate | 540-18-1 | 67.7 | Banana/apricot |
| 6 | 12.756 | α-Terpineol | 586-62-9 | 72.6 | Pine/woody |
| 7 | 13.839 | (+)-α-Pinene | 80-56-8 | 74.7 | Citrus/spicy |
| Peak No. | RT (min) | Compound | CAS No. | Probability (%) | Flavor/Fragrance |
|---|---|---|---|---|---|
| 1 | 10.916 | (+)-Sabinene | 3387-41-5 | 77.9 | Herb |
| 2 | 11.770 | (+)-Limonene | 5989-27-5 | 73.1 | Fruity |
| 3 | 12.058 | p-Cymene | 535-77-3 | 83.4 | Fruity |
| 4 | 12.175 | cis-Sabinene hydrate | 15537-55-0 | 77.5 | Herb |
| 5 | 12.274 | γ-Terpinene | 99-85-4 | 71.2 | Turpentine odor |
| 6 | 15.723 | 3-Carene | 13466-78-9 | 72.2 | Piney |
| 7 | 12.756 | α- Terpineol | 586-62-9 | 81.3 | Pine/Woody |
| Peak No. | RT (min) | Compound | CAS No. | Probability (%) | Flavor/Fragrance |
|---|---|---|---|---|---|
| 1 | 6.423 | Methyl butyrate | 623-42-7 | 71.7 | Sweet apple/pineapple |
| 2 | 6.978 | Ethyl isobutyrate | 97-62-1 | 77.0 | Fruity |
| 3 | 7.411 | Methyl 2-methylbutyrate | 868-57-5 | 83.5 | Ethereal, fruity |
| 4 | 7.893 | Ethyl butyrate | 105-54-4 | 71.4 | Pineapple |
| 5 | 8.473 | cis-2-Hexenol | 928-94-9 | 72.1 | Fruity/green |
| 6 | 8.773 | Ethyl 2-methylbutyrate | 7452-79-1 | 71.8 | Strawberries |
| 7 | 10.032 | 2-Hexenal | 6728-26-3 | 78.1 | Green apple |
| 8 | 11.606 | Ethyl hexanoate | 123-66-0 | 75.7 | Sweet apple/pineapple |
| 9 | 12.417 | Propyl 3-methylbutyrate | 557-00-6 | 70.8 | Sweet apple/fruity |
| 10 | 13.397 | trans-Verbenol | 1820-09-3 | 89.4 | Herbal |
| 11 | 13.713 | cis-Verbenol | 1845-30-3 | 82.8 | Herbal |
| 12 | 15.407 | 3-Menthene | 500-00-5 | 72.3 | - |
| 13 | 15.720 | 3-Carene | 13466-78-9 | 76.1 | Piny |
| 14 | 16.569 | 2-Menthene | 5256-65-5 | 79.5 | - |
| 15 | 18.700 | trans-Methyl cinnamate | 1754-62-7 | 83.9 | Intense/aromatic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petre, V.A.; Cristea, N.I.; Cojocaru, V.C.; Pascu, L.F.; Chiriac, F.L. Analysis of Volatile Flavor Compounds in Four Commercial Beverages Using Static Headspace Gas Chromatography/Mass Spectrometry: A Qualitative Approach. Appl. Sci. 2024, 14, 1910. https://doi.org/10.3390/app14051910
Petre VA, Cristea NI, Cojocaru VC, Pascu LF, Chiriac FL. Analysis of Volatile Flavor Compounds in Four Commercial Beverages Using Static Headspace Gas Chromatography/Mass Spectrometry: A Qualitative Approach. Applied Sciences. 2024; 14(5):1910. https://doi.org/10.3390/app14051910
Chicago/Turabian StylePetre, Valentina Andreea, Nicolae Ionuț Cristea, Victor Constantin Cojocaru, Luoana Florentina Pascu, and Florentina Laura Chiriac. 2024. "Analysis of Volatile Flavor Compounds in Four Commercial Beverages Using Static Headspace Gas Chromatography/Mass Spectrometry: A Qualitative Approach" Applied Sciences 14, no. 5: 1910. https://doi.org/10.3390/app14051910
APA StylePetre, V. A., Cristea, N. I., Cojocaru, V. C., Pascu, L. F., & Chiriac, F. L. (2024). Analysis of Volatile Flavor Compounds in Four Commercial Beverages Using Static Headspace Gas Chromatography/Mass Spectrometry: A Qualitative Approach. Applied Sciences, 14(5), 1910. https://doi.org/10.3390/app14051910








