The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
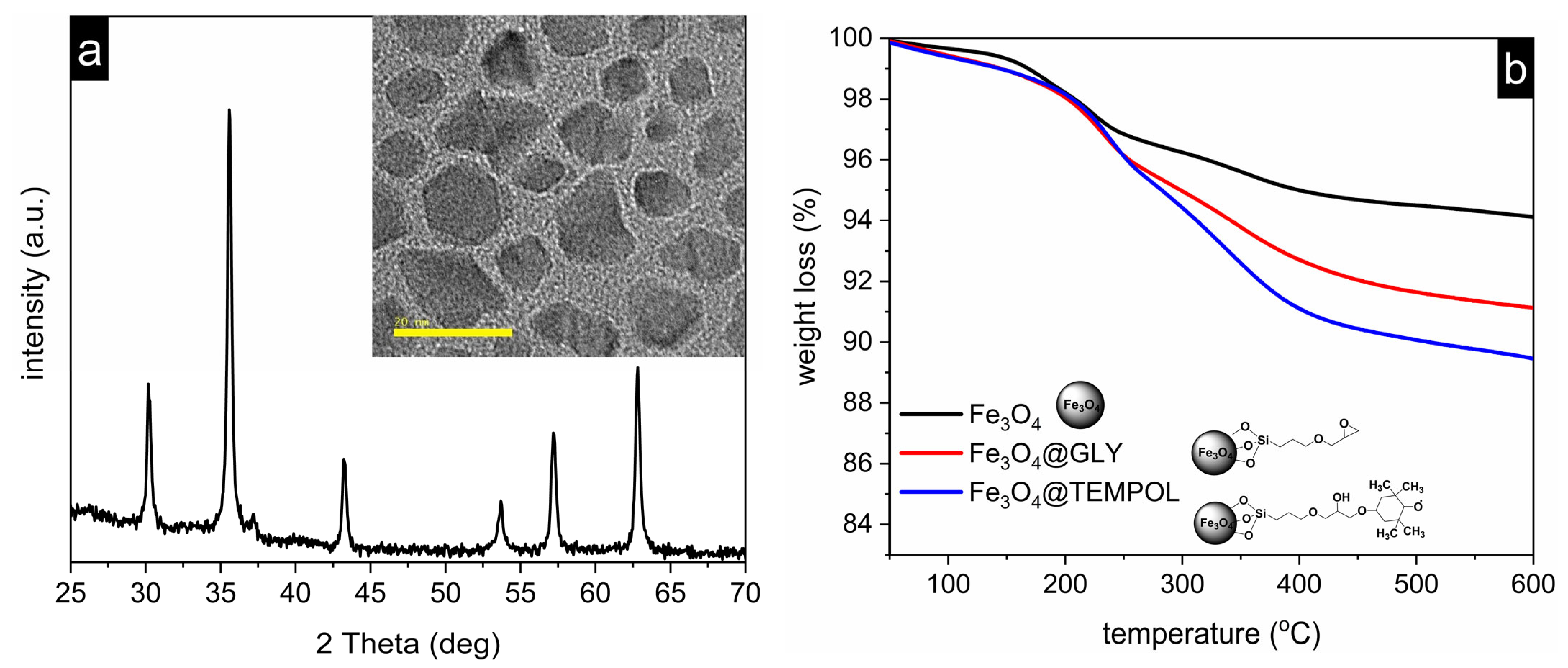
2.1.1. Synthesis Procedure
2.1.2. Yeast Cells Preparation
2.2. Methods
2.2.1. Nanoparticles Characterization
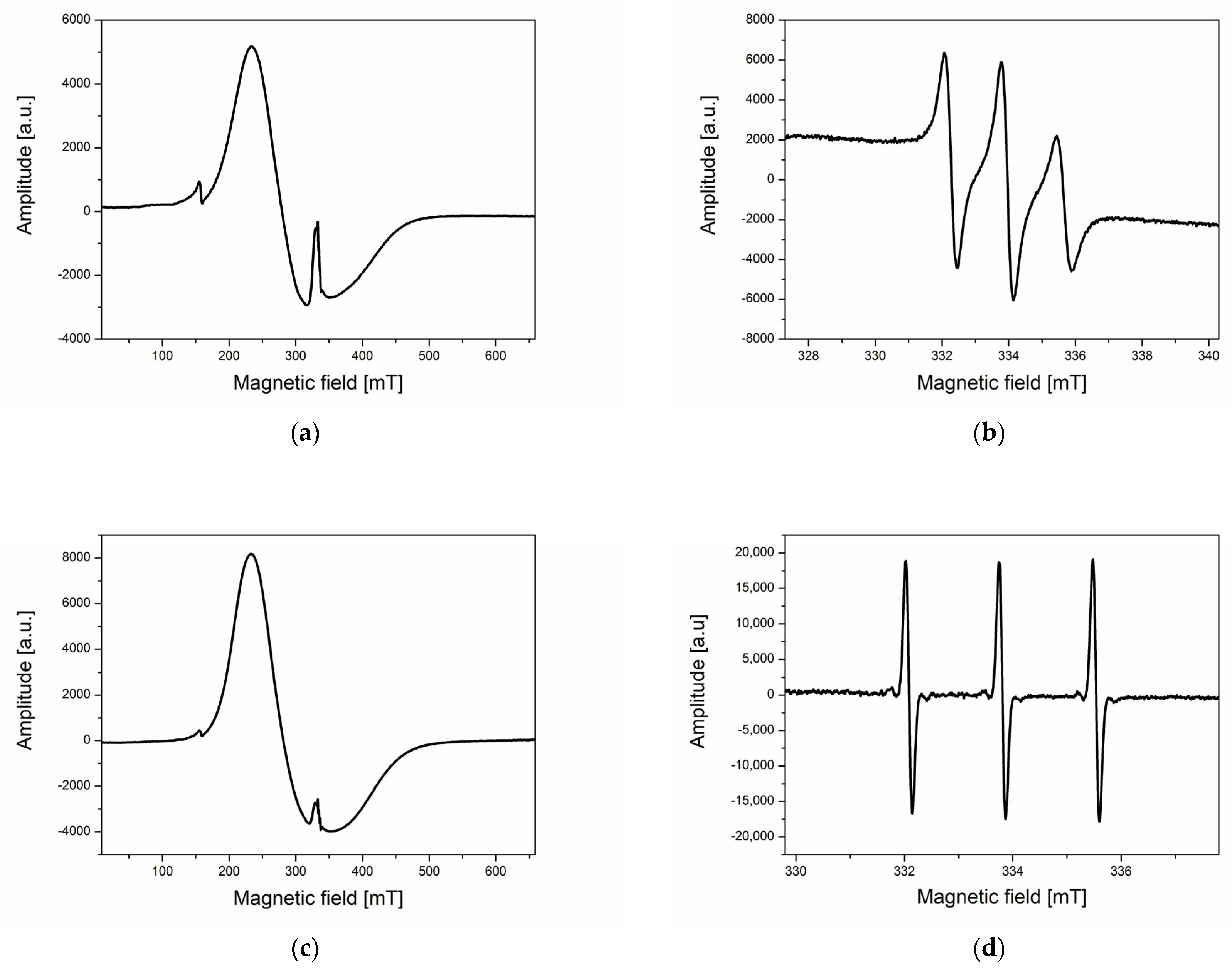
2.2.2. ESR Measurements
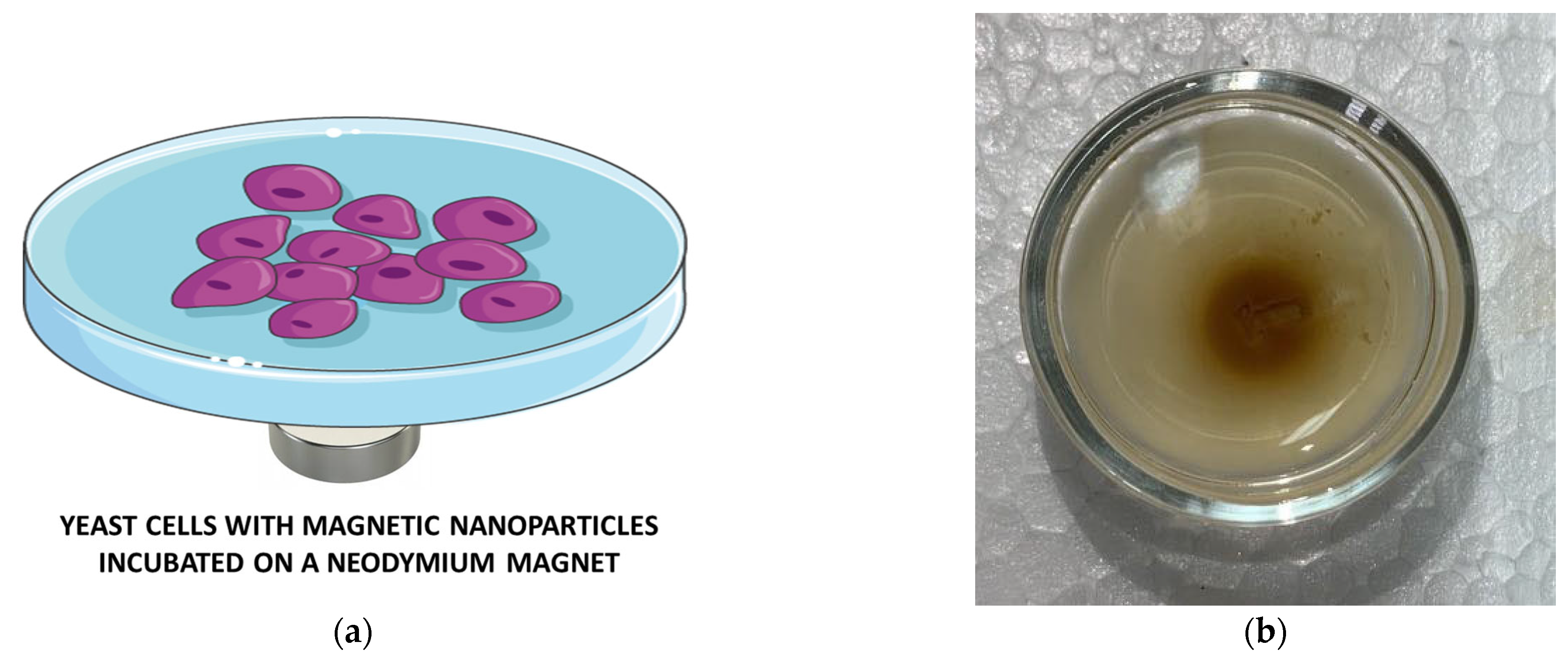
2.2.3. The Impact of a Stable Magnetic Field on Nanoparticle Uptake by Yeast Cells
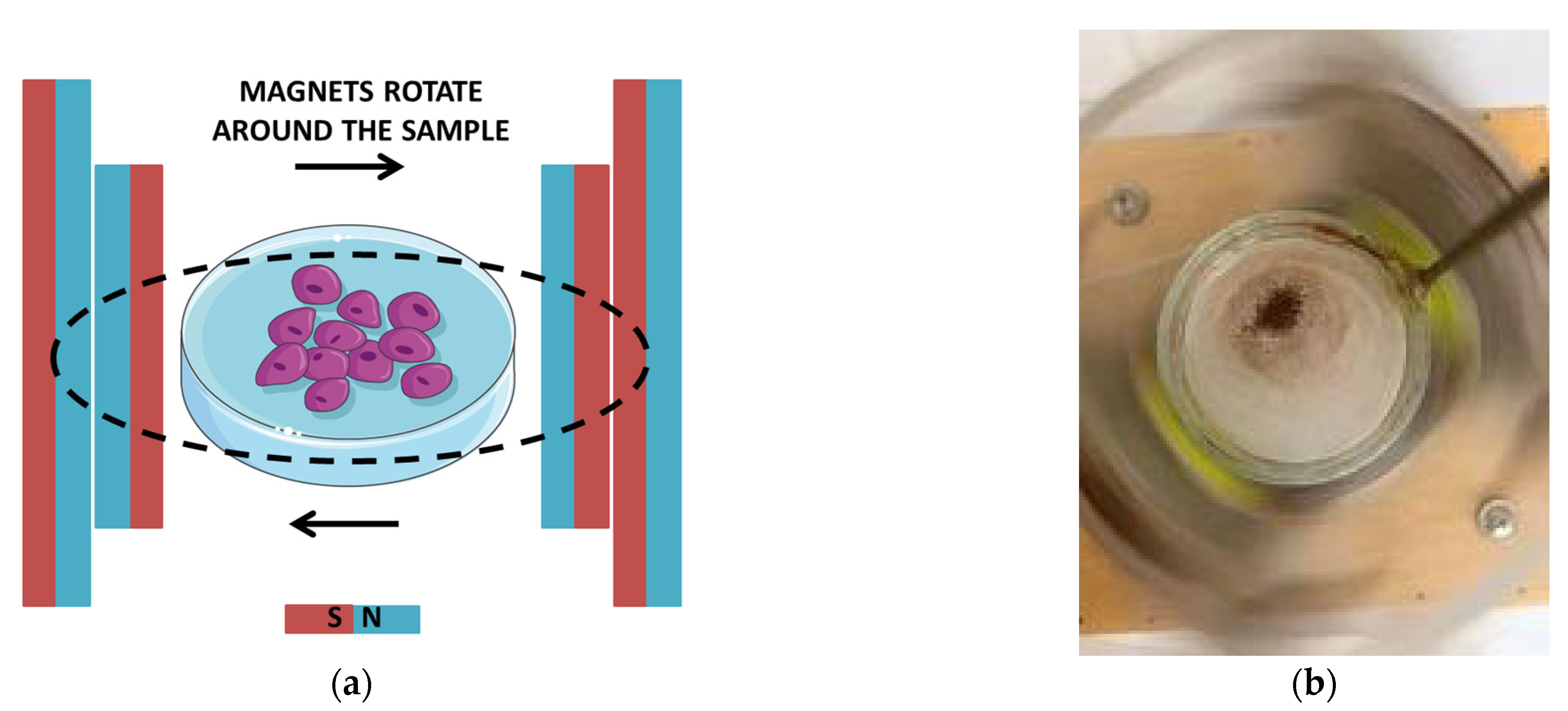
2.2.4. The Impact of a Rotating Magnetic Field on Nanoparticle Uptake by Yeast Cells
2.2.5. Microscopic Observation of Yeast Cell Proliferation
2.2.6. SEM and EDS of Yeast Cells with Fe3O4@TEMPOL Nanoparticles
2.2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benguettat-El Mokhtari, I.; Schmool, D.S. Ferromagnetic Resonance in Magnetic Oxide Nanoparticules: A Short Review of Theory and Experiment. Magnetochemistry 2023, 9, 191. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Tavares Sousa, C. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Medical Imaging. Imaging Med. 2022, 14, 1–16. [Google Scholar]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Indian Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and future perspectives of multifunctional magnetic nanoparticles based controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.-C.; Ficai, A. Smart Magnetic Drug Delivery Systems for the Treatment of Cancer. Nanomaterials 2023, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Frickenstein, A.N.; Wilhelm, S. High-throughput single-cell analysis of nanoparticle-cell interactions. Trac-Trend. Anal. Chem. 2023, 166, 117172. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.; Nordmeyer, D.; Boreham, A.; Holzhausen, C.; Mundhenk, L.; Graf, C.; Meinke, M.C.; Vogt, A.; Hadam, S.; Lademann, J.; et al. Overview about the localization of nanoparticles in tissue and cellular context by different imaging techniques. Beilstein J. Nanotech. 2015, 23, 263–280. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, L.I.; Johnston, A.P.R. It’s what’s on the inside that counts: Techniques for investigating the uptake and recycling of nanoparticles and proteins in cells. J. Colloid. Interf. Sci. 2021, 587, 64–78. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A.R. ESR method in monitoring of nanoparticle endocytosis in cancer cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef]
- Shashni, B.; Nagasaki, Y. Newly Developed Self-Assembling Antioxidants as Potential Therapeutics for the Cancers. J. Pers. Med. 2021, 11, 92. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018, 47, 2534. [Google Scholar] [CrossRef]
- Jiang, J.; Tian, S.; Wang, K.; Wang, Y.; Zang, S.; Yu, A.; Zhang, Z. Electron spin resonance spectroscopy for immunoassay using iron oxide nanoparticles as probe. Anal. Bioanal. Chem. 2018, 410, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Dogan, N.; Ozel, F.; Koten, H. Structural, Morphological, and Magnetic Characterization of Iron Oxide Nanoparticles Synthesized at Different Reaction Times via Thermal Decomposition Method. Curr. Nanosci. 2023, 19, 33–38. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Sonawane, S.H.; Bhanvase, B.A.; Ashokkumar, M.; Pimplapure, M.S.; Gogate, P.R. Synthesis of iron oxide nanoparticles in a continuous flow spiral microreactor and Corning® advanced flow™ reactor. Green Process. Synth. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Bakker, M.G.; Fowler, B.; Bowman, M.K.; Patience, G.S. Experimental methods in chemical engineering: Electron paramagnetic resonance spectroscopy-EPR/ESR. Can. J. Chem. Eng. 2020, 98, 1668–1681. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Konstantinova, E.A.; Kokorin, A.I.; Gyergyek, S.; Leitgeb, M. Structural and magnetic characteristics of carboxymethyl dextran coated magnetic nanoparticles: From characterization to immobilization application. React. Funct. Polym. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Elamin, N.Y.; Modwi, A.; El-Fattah, W.A.; Rajeh, A. Synthesis and structural of Fe3O4 magnetic nanoparticles and its effect on the structural optical, and magnetic properties of novel Poly(methyl methacrylate)/Polyaniline composite for electromagnetic and optical applications. Opt. Mater. 2023, 135, 113323. [Google Scholar] [CrossRef]
- Abele, N.; Münz, F.; Zink, F.; Gröger, M.; Hoffmann, A.; Wolfschmitt, E.-M.; Hogg, M.; Calzia, E.; Waller, C.; Radermacher, P.; et al. Relation of Plasma Catecholamine Concentrations and Myocardial Mitochondrial Respiratory Activity in Anesthetized and Mechanically Ventilated, Cardiovascular Healthy Swine. Int. J. Mol. Sci. 2023, 24, 17293. [Google Scholar] [CrossRef]
- Vanreusel, I.; Vermeulen, D.; Goovaerts, I.; Stoop, T.; Ectors, B.; Cornelis, J.; Hens, W.; de Bliek, E.; Heuten, H.; Van Craenenbroeck, E.M.; et al. Circulating Reactive Oxygen Species in Adults with Congenital Heart Disease. Antioxidants 2022, 11, 2369. [Google Scholar] [CrossRef]
- Velayutham, M.; Poncelet, M.; Eubank, T.D.; Driesschaert, B.; Khramtsov, V.V. Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe. Molecules 2021, 26, 2781. [Google Scholar] [CrossRef]
- Gertsenshteyn, I.; Giurcanu, M.; Vaupel, P.; Halpern, H. Biological validation of electron paramagnetic resonance (EPR) image oxygen thresholds in tissue. J. Physiol. 2021, 599, 1759–1767. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kucińska, M.; Murias, M.; Schroeder, G.; Kurczewska, J. The spin probes as scavengers of free radicals in cells. Appl. Sci. 2022, 12, 7999. [Google Scholar] [CrossRef]
- Nibbe, P.; Schleusener, J.; Siebert, S.; Borgart, R.; Brandt, D.; Westphalen, R.; Schüler, N.; Berger, B.; Peters, E.M.J.; Meinke, M.C.; et al. Oxidative stress coping capacity (OSC) value: Development and validation of an in vitro measurement method for blood plasma using electron paramagnetic resonance spectroscopy (EPR) and vitamin C. Free Radic. Biol. Med. 2023, 194, 230–244. [Google Scholar] [CrossRef]
- Vesković, A.; Nakarada, Đ.; Pavićević, A.; Prokić, B.; Perović, M.; Kanazir, S.; Popović-Bijelić, A.; Mojović, M. In Vivo/Ex Vivo EPR Investigation of the Brain Redox Status and Blood-Brain Barrier Integrity in the 5xFAD Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 18, 25–34. [Google Scholar] [CrossRef]
- Jakubowska, M.A.; Pyka, J.; Michalczyk-Wetula, D.; Baczyński, K.; Cieśla, M.; Susz, A.; Ferdek, P.E.; Płonka, B.K.; Fiedor, L.; Płonka, P.M. Electron paramagnetic resonance spectroscopy reveals alterations in the redox state of endogenous copper and iron complexes in photodynamic stress-induced ischemic mouse liver. Redox Biol. 2020, 34, 101566. [Google Scholar] [CrossRef]
- Tzivaki, M.; Hassan, A.; Waller, E. Electron paramagnetic resonance spectroscopy for the detection of radiation exposure in dreissenid mussels. Radiat. Prot. Dosim. 2023, 199, 1626–1631. [Google Scholar] [CrossRef]
- Karmakar, P.; Mishra, L.; Mishra, M. Electron spin resonance spectroscopy: A tool for dating mollusc shells, corals, and other materials. In Spectroscopic and Microscopy Techniques for Archaeological and Cultural Heritage Research, 2nd ed.; IOP Publishing Ltd.: Bristol, UK, 2023; pp. 9-1–9-11. [Google Scholar]
- Timar-Gabor, A.; Kabacińska, Z.; Constantin, D.; Dave, A.K.; Buylaert, J.-P. Reconstructing dust provenance from quartz optically stimulated luminescence (OSL) and electron spin resonance (ESR) signals: Preliminary results on loess from around the world. Radiat. Phys. Chem. 2023, 212, 111138. [Google Scholar] [CrossRef]
- Ghimire, L.; Waller, E. Electron Paramagnetic Resonance Measurements of Lifetime Doses in Teeth of Durham Region Residents, Ontario. Health Phys. 2023, 124, 175–191. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Z.; Yang, Y.; Li, S.; Lee, C.-S. Organic radical materials in biomedical applications: State of the art and perspectives. Exploration 2022, 2, 20210264. [Google Scholar] [CrossRef]
- Feliciano, C.P.; Nagasaki, Y. Antioxidant Nanomedicine Protects against Ionizing Radiation-Induced Life-Shortening in C57BL/6J Mice. ACS Biomater. Sci. Eng. 2019, 5, 5631–5636. [Google Scholar] [CrossRef]
- Mołoń, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two faces of TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl)—An antioxidant or a toxin? BBA–Mol. Cell Res. 2023, 1870, 119412. [Google Scholar] [CrossRef]
- Feliciano, C.P.; Cammas-Marion, S.; Nagasaki, Y. Recent advances in self-assembling redox nanoparticles as a radiation protective agent. AIMS Mol. Sci. 2023, 10, 52–69. [Google Scholar] [CrossRef]
- Matsumoto, K.-I.; Nakanishi, I.; Zhelev, Z.; Bakalova, R.; Aoki, I. Nitroxyl Radical as a Theranostic Contrast Agent in Magnetic Resonance Redox Imaging. Antioxid. Redox Signal. 2022, 36, 95–121. [Google Scholar] [CrossRef]
- Azuma, R.; Yamasaki, T.; Emoto, M.C.; Sato-Akaba, H.; Sano, K.; Munekane, M.; Fujii, H.G.; Mukai, T. Effect of relative configuration of TEMPO-type nitroxides on ascorbate reduction. Free Radic. Biol. Med. 2023, 194, 114–122. [Google Scholar] [CrossRef]
- Nakamura, H.; Watano, S. Direct Permeation of Nanoparticles Across Cell Membrane: A Review. Kona Powder Part. J. 2018, 35, 49–65. [Google Scholar] [CrossRef]
- Escobar, J.F.; Vaca-González, J.J.; Guevara, J.M.; Garzón-Alvarado, D.A. Effect of magnetic and electric fields on plasma membrane of single cells: A computational approach. Eng. Rep. 2020, 2, e12125. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interfac. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, C.; Teng, Z.; Jin, Q.; Liao, Z.; Zhu, X.; Huo, S. Ultrasound meets the cell membrane: For enhanced endocytosis and drug delivery. Nanoscale 2023, 15, 13532. [Google Scholar] [CrossRef]
- Zablotskii, V.; Syrovets, T.; Schmidt, Z.W.; Dejneka, A.; Simmet, T. Modulation of monocytic leukemia cell function and survival by high gradient magnetic fields and mathematical modeling studies. Biomaterials 2014, 35, 3164–3171. [Google Scholar] [CrossRef]
- Min, K.A.; Shin, M.C.; Yu, F.; Yang, M.; David, A.E.; Yang, V.C.; Rosania, G.R. Pulsed magnetic field improves the transport of iron oxide nanoparticles through cell barriers. ACS Nano 2013, 7, 2161–2171. [Google Scholar] [CrossRef]
- Soheilian, R.; Choi, Y.S.; David, A.E.; Abdi, H.; Maloney, C.E.; Erb, R.M. Toward Accumulation of Magnetic Nanoparticles into Tissues of Small Porosity. Langmuir 2015, 31, 8267–8274. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Kubinova, S.; Polyakova, T.; Sykova, E.; Dejneka, A. Effects of high-gradient magnetic fields on living cell machinery. J. Phys. D Appl. Phys. 2016, 49, 493003. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Dejneka, A.; Jastrabik, L.; Polyakova, T.; Syrovets, T.; Simmet, T. Nanomechanics of magnetically driven cellular endocytosis. Appl. Phys. Lett. 2011, 99, 183701. [Google Scholar] [CrossRef]
- Uzhytchak, M.; Lynnyk, A.; Zablotskii, V.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Lunova, M.; Jirsa, M.; Kubinová, Š.; Lunov, O.; et al. The use of pulsed magnetic fields to increase the uptake of iron oxide nanoparticles by living cells. Appl. Phys. Lett. 2017, 111, 243703. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef]
- van der Laan, K.J.; Morita, A.; Perona-Martinez, F.P.; Schirhagl, R. Evaluation of the Oxidative Stress Response of Aging Yeast Cells in Response to Internalization of Fluorescent Nanodiamond Biosensors. Nanomaterials 2020, 10, 372. [Google Scholar] [CrossRef]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.-I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef]
- Eigenfeld, M.; Wittmann, L.; Kerpes, R.; Schwaminger, S.; Becker, T. Quantifcation methods of determining brewer’s and pharmaceutical yeast cell viability: Accuracy and impact of nanoparticles. Anal. Bioanal. Chem. 2023, 415, 3201–3213. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Pasieczna-Patkowska, S.; Schroeder, G. Photoacoustic Spectroscopy of Surface-Functionalized Fe3O4-SiO2 Nanoparticles. Appl. Spectrosc. 2020, 74, 712–719. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Frański, R.; Cegłowski, M.; Schroeder, G. Mass spectrometric investigation of organo-functionalized magnetic nanoparticles binding properties toward chalcones. Materials 2021, 14, 4705. [Google Scholar] [CrossRef]
- Baghdadi, Y.N.; Youssef, L.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. Thermal and mechanical properties of epoxy resin reinforced with modified iron oxide nanoparticles. J. Appl. Polym. Sci. 2021, 138, 50533. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. The principles of a new method, MNF-3D, for concentration of magnetic particles in three-dimensional space. Measurement 2017, 112, 137–140. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. Focusing of Fe3O4 nanoparticles using a rotating magnetic field in various environments. Phys. Lett. A 2018, 382, 3192–3196. [Google Scholar] [CrossRef]
- Dobosz, B.; Schroeder, G.; Kurczewska, J. Comments on “The principles of a new method, MNF-3D, for concentration of magnetic particles in three-dimensional space”. Measurement 2023, 118, 113146. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Helekar, S.A. Rotating Magnetic Fields Inhibit Mitochondrial Respiration, Promote Oxidative Stress and Produce Loss of Mitochondrial Integrity in Cancer Cells. Front. Oncol. 2021, 10, 768758. [Google Scholar] [CrossRef]
- Sládičeková, K.H.; Bereta, M.; Misek, J.; Parizek, D.; Jakuš, J. Biological Effects of a Low-Frequency Electromagnetic Field on Yeast Cells of the Genus Saccharomyces Cerevisiae. Acta Med. Martiniana 2021, 21, 34–41. [Google Scholar] [CrossRef]
- Peng, Q.; Huo, D.; Li, H.; Zhang, B.; Li, Y.; Liang, A.; Wang, H.; Yu, Q.; Li, M. ROS independent toxicity of Fe3O4 nanoparticles to yeast cells: Involvement of mitochondrial dysfunction. Chem. Biol. Interact. 2018, 1, 20–26. [Google Scholar] [CrossRef]
- Pahlevan, M.; Toivakka, M.; Alam, P. Mechanical properties of TEMPO-oxidised bacterial cellulose-amino acid biomaterials. Eur. Polym. J. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Wang, P.; Song, T. Mechanisms of Cellular Effects Directly Induced by Magnetic Nanoparticles under Magnetic Fields. Hindawi J. Nanomater. 2017, 2017, 1564634. [Google Scholar] [CrossRef]
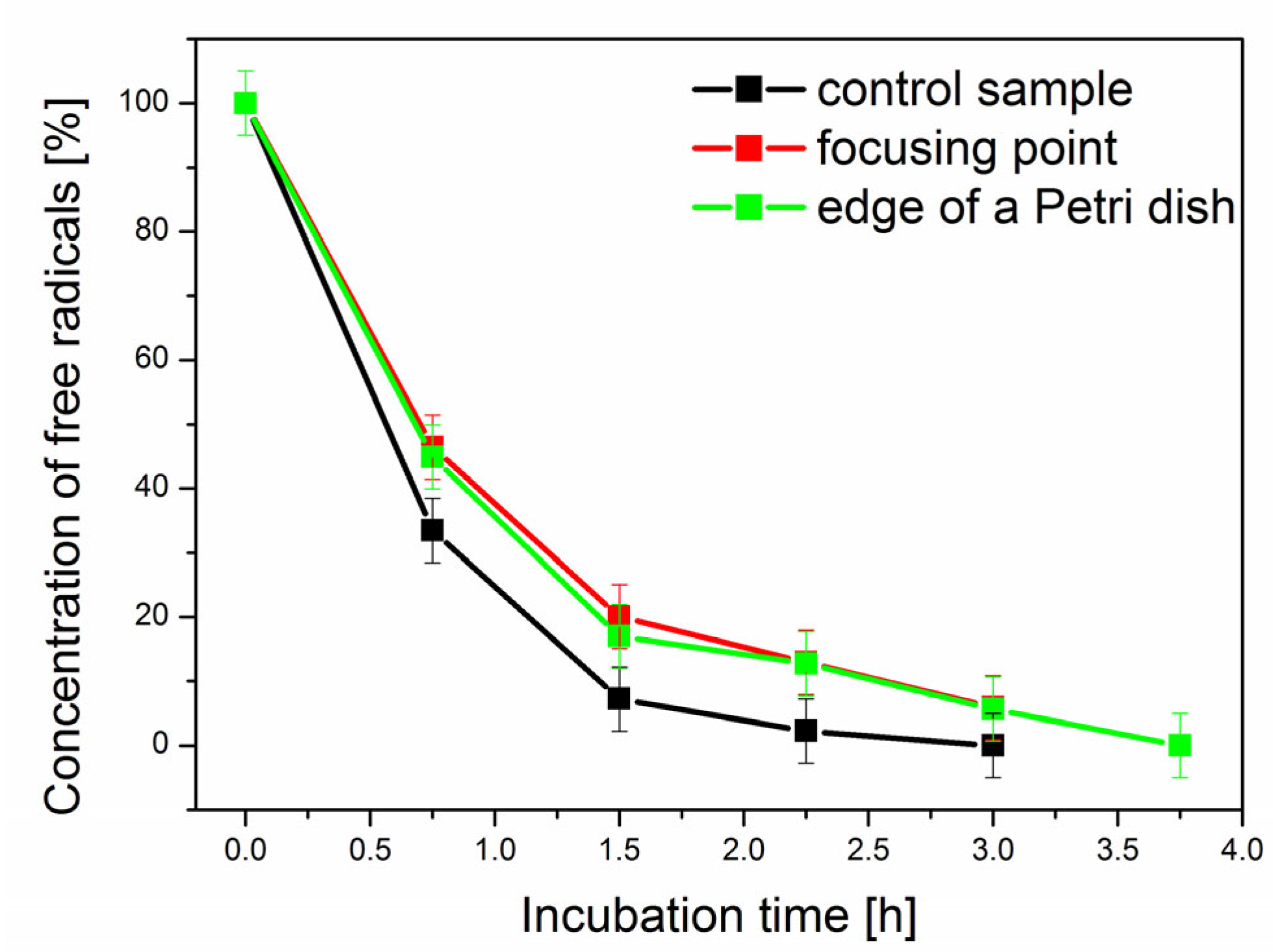


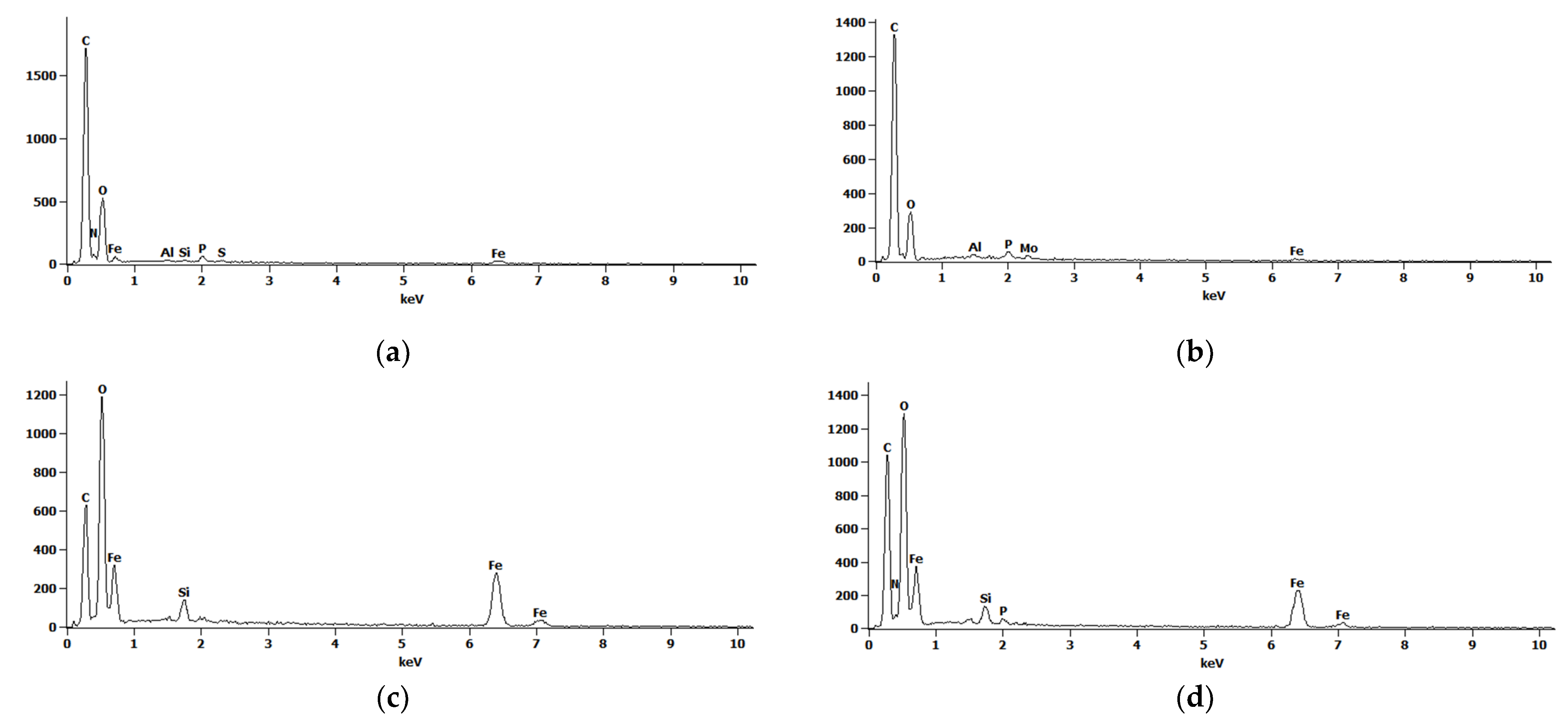
| Time (h) | Control Sample (×106/mL) | YC + MNPs 1 (×106/mL) | YC + MNPs + NM 2 (×106/mL) |
|---|---|---|---|
| 0 N | 305 ± 46 | 255 ± 38 | 282 ± 42 |
| 0.75 N | 349 ± 52 | 327 ± 49 | 262 ± 39 |
| 1.5 N | 355 ± 53 | 274 ± 41 | 373 ± 56 |
| 2.25 N | 269 ± 40 | 390 ± 59 | 383 ± 58 |
| 3 N | 343 ± 52 | 380 ± 57 | 363 ± 55 |
| 3.75 N | 326 ± 49 | 385 ± 58 | 339 ± 51 |
| Time (h) | Control Sample (×106/mL) | YC + MNPs 1 (×106/mL) | YC + MNPs + 15 2 (×106/mL) | YC + MNPs + 30 3 (×106/mL) |
|---|---|---|---|---|
| 0 N | 359 ± 54 | 332 ± 49 | 336 ± 50 | 270 ± 40 |
| 0.75 Y | 354 ± 53 | 424 ± 64 | 271 ± 41 | 273 ± 40 |
| 1.5 Y | 391 ± 59 | 396 ± 59 | 396 ± 61 | 260 ± 39 |
| 2.25 N | 332 ± 50 | 360 ± 54 | 332 ± 50 | 322 ± 48 |
| 3 N | 400 ± 60 | 352 ± 53 | 357 ± 54 | 347 ± 52 |
| 3.75 N | 376 ± 56 | 321 ± 48 | 452 ± 68 | 340 ± 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobosz, B.; Gunia, E.; Kotarska, K.; Schroeder, G.; Kurczewska, J. The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells. Appl. Sci. 2024, 14, 1343. https://doi.org/10.3390/app14041343
Dobosz B, Gunia E, Kotarska K, Schroeder G, Kurczewska J. The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells. Applied Sciences. 2024; 14(4):1343. https://doi.org/10.3390/app14041343
Chicago/Turabian StyleDobosz, Bernadeta, Eliza Gunia, Klaudia Kotarska, Grzegorz Schroeder, and Joanna Kurczewska. 2024. "The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells" Applied Sciences 14, no. 4: 1343. https://doi.org/10.3390/app14041343
APA StyleDobosz, B., Gunia, E., Kotarska, K., Schroeder, G., & Kurczewska, J. (2024). The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells. Applied Sciences, 14(4), 1343. https://doi.org/10.3390/app14041343









