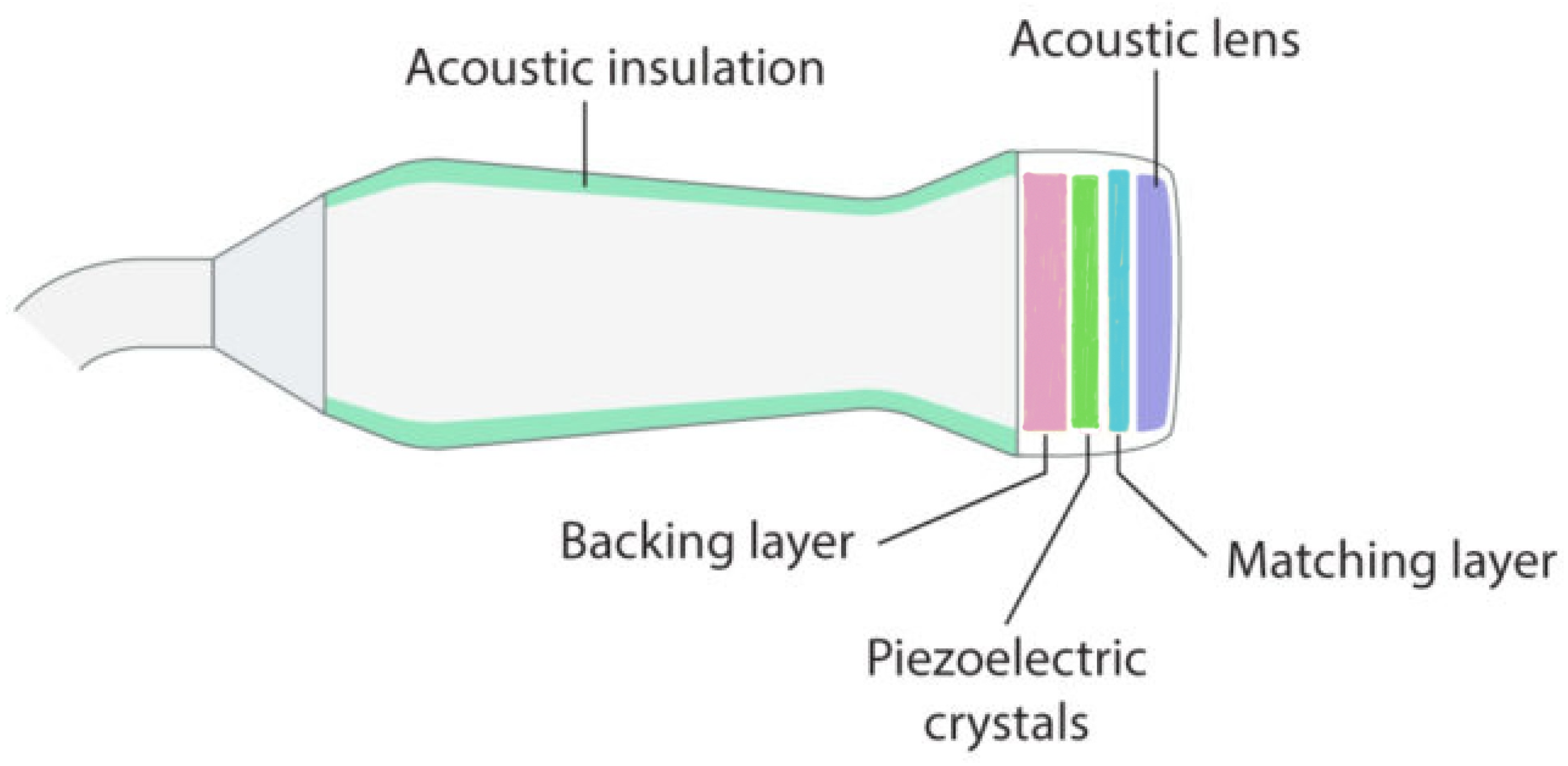
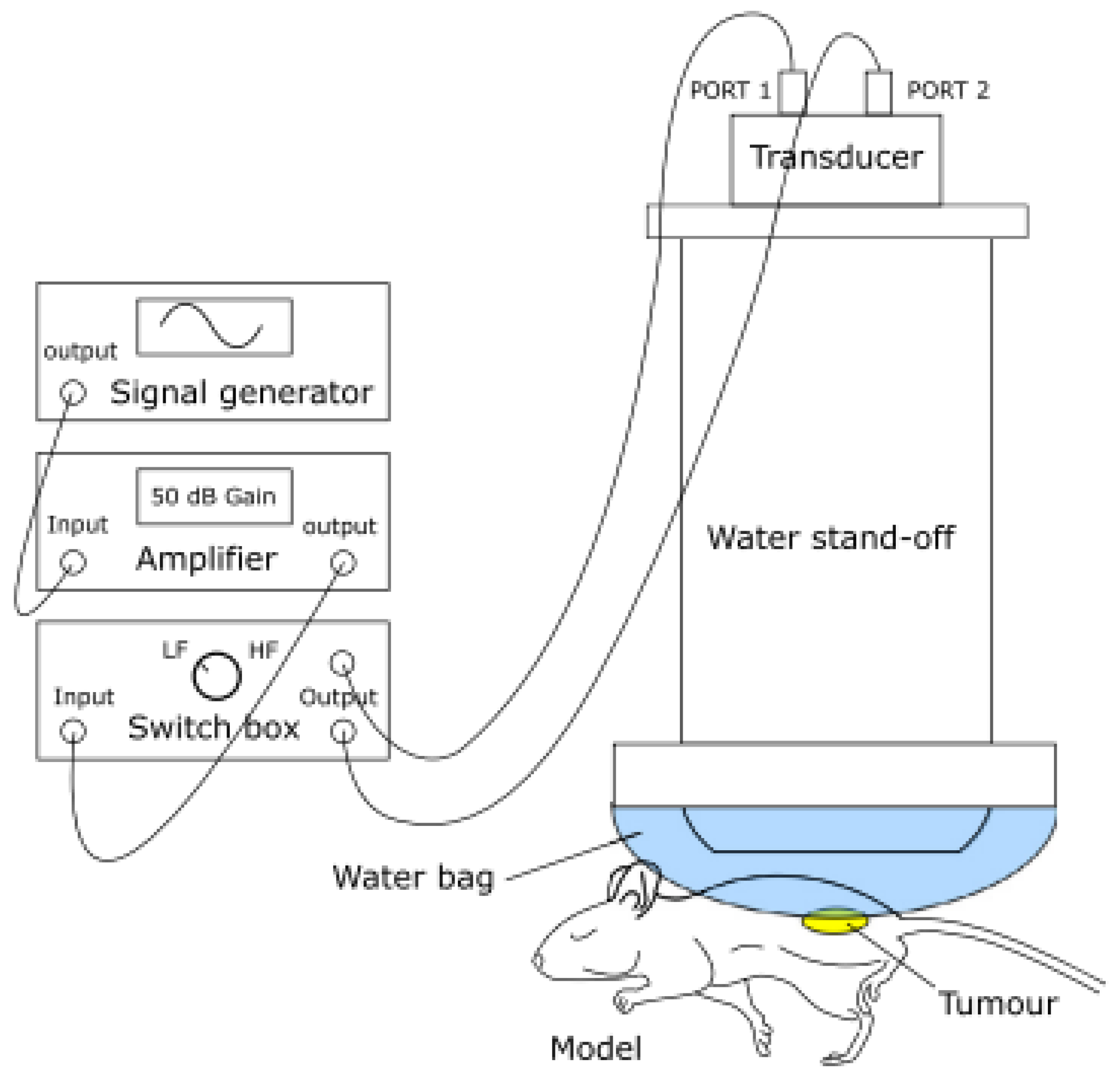
The following paragraphs list selected articles in the literature by body districts and provide a brief explanation of the content, highlighting innovative aspects.
3.2.1. Colon
In 2012, Zhang et al. conducted a study [
12] using a mouse model of human colon cancer. They employed ultrasonic cavitation with specific parameters (238 kHz, 0.5 MPa, 60-s sonication, and a 50% duty cycle) in conjunction with the anti-angiogenic drug, Endostar-MB. Endostar was attached to streptavidin-coated microbubbles, and the study focused on HT29 cells. A custom-built probe was used for ultrasonic cavitation, and contrast-enhanced ultrasound imaging assessed changes in tumor blood flow. The findings indicated that this approach significantly inhibited tumor blood flow, promoted apoptosis, and showcased promise for colon cancer treatment. Unfortunately, this study does not report enough transducer information to derive ISPTA. The Loria et al., (2019) [
13] study applied very low-intensity ultrasound (VLIUS) to tumor and normal cells, revealing that a specific VLIUS intensity (120 mW/cm
2) significantly enhanced the uptake of nanoparticles and improved the delivery of the chemotherapy drug trabectedin in cancer cells. The study potentially offers a promising strategy for targeting colon cancer cells (HT29, HCT116) in a selective way. In the case of Loria and colleagues’ study, the acoustic intensity ISPTA threshold was below the cavitational level; this is important to underline and represents an important and innovative approach to the matter. In Nittayacharn et al.’s 2019 in vivo study using a mouse model of colorectal cancer [
14], the research explores the potential of doxorubicin-loaded nanoscale bubbles (Dox-NBs) combined with low-frequency ultrasound at 3 MHz to enhance drug delivery, demonstrating improved intracellular uptake and therapeutic efficacy, along with increased drug accumulation and distribution within tumors, offering a promising approach to improving cancer therapy.
3.2.2. Liver
In the study of Zhao et al. (2018) [
15] nanoparticles loaded with a drug called 10-hydroxycamptothecin (10-HCPT) were developed and modified with hyaluronic acid-mediated cell-penetrating peptides (HA/CPPs) to enhance their targeting abilities. In vivo and in vitro studies with different acoustic settings were conducted. In vitro, the nanoparticles were combined with low-intensity focused ultrasound with a frequency of 1.0 MHz, a duty cycle of 50%, and an acoustic intensity of 2000, 2400, and 2800 mW/cm
2, whereas in 15 mice bearing tumor xenografts, the LIFU settings were changed regarding the intensity (3200 mW/cm
2). Recently, in 2023, in the study of Chen et al. [
16], low-intensity pulsed ultrasound (frequency 1.0 MHz, duty cycle 50%, intensity 500 mW/cm
2) in combination with microbubbles (MBs) was employed to enhance the delivery of the drug HCPT. In both in vivo and in vitro studies, the researchers found that this technique effectively reduced liver fibrosis. The study suggests that mechanical forces induced by sonoporation might contribute to the observed therapeutic effects. This approach holds promise for clinical use and requires further investigation.
3.2.3. Pancreas
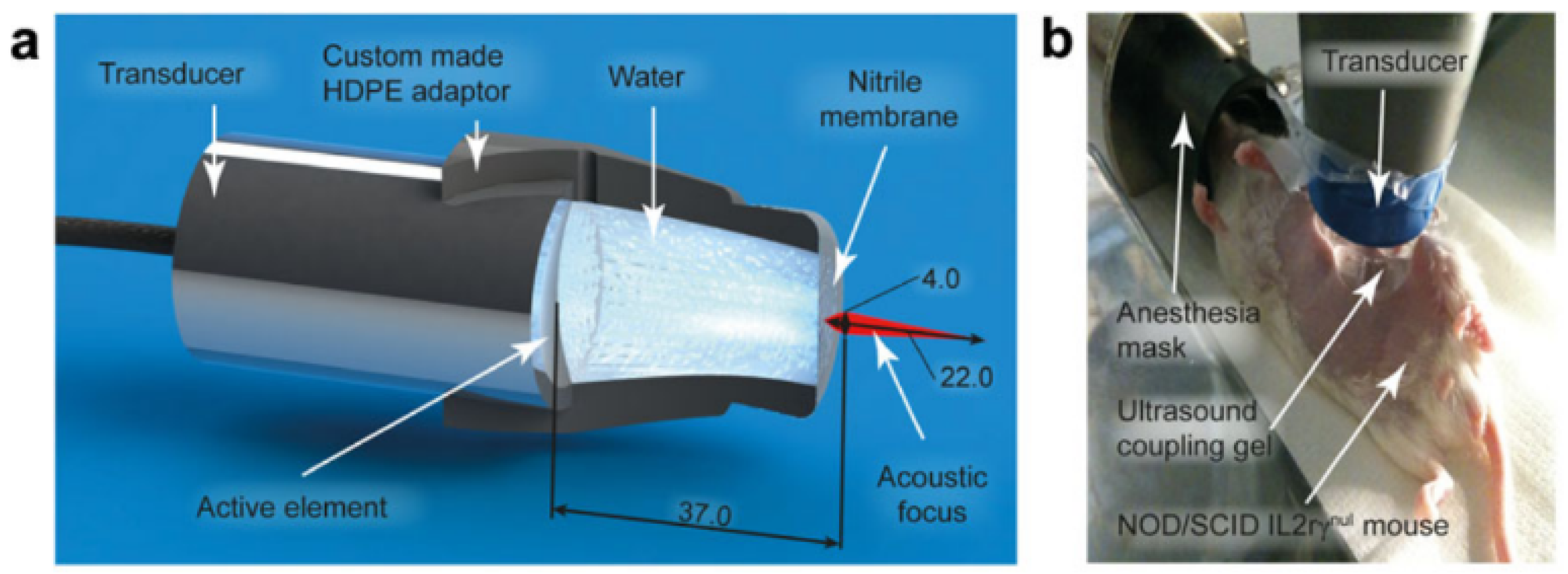
In their 2014 study, Kotopoulis and colleagues [
17] aimed to enhance the delivery of gemcitabine to pancreatic tumors by investigating the effect of sonoporation via ultrasound (frequency 1.0 MHz, duty cycle 40%) in an orthotopic xenograft mouse model of pancreatic cancer.
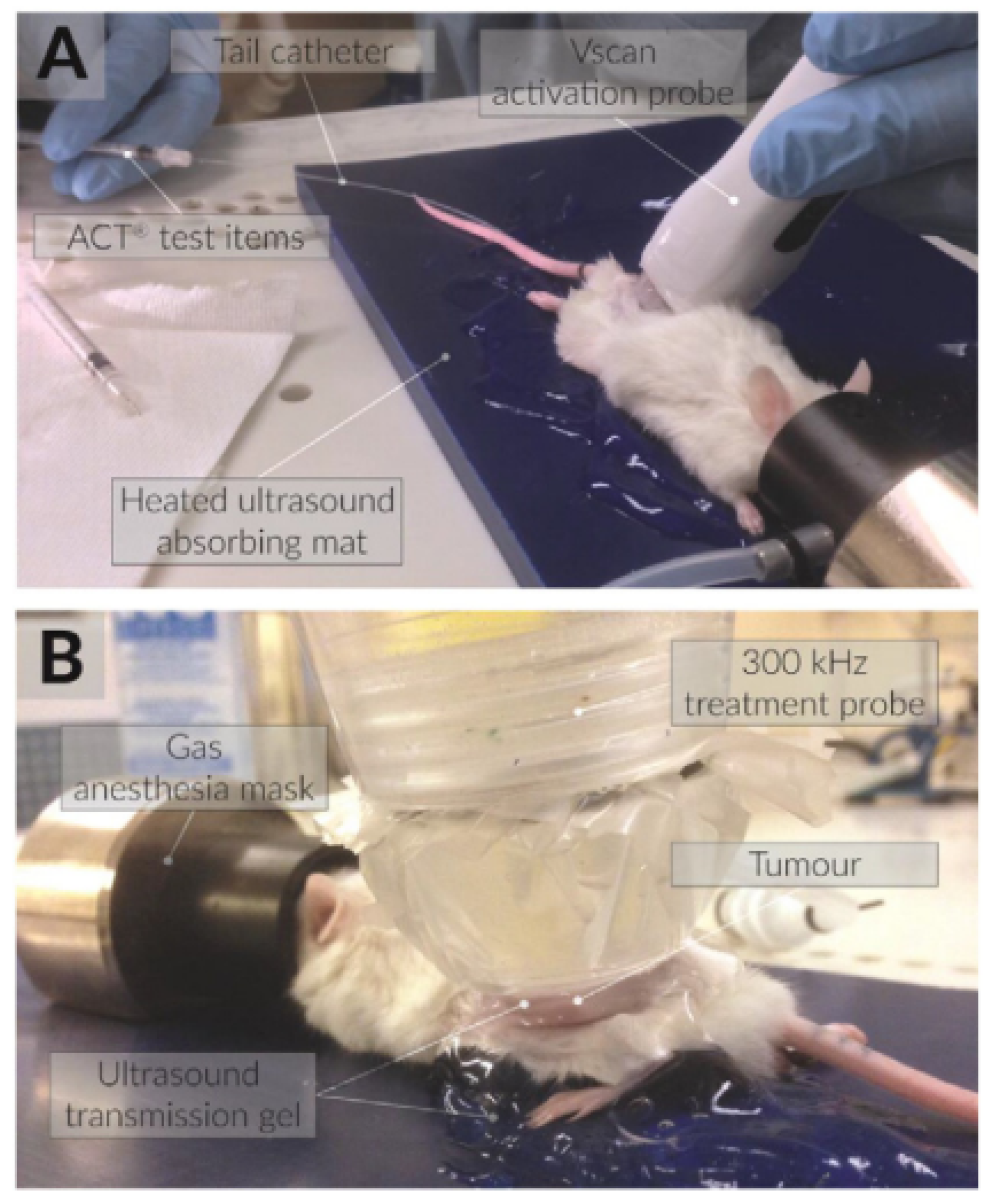
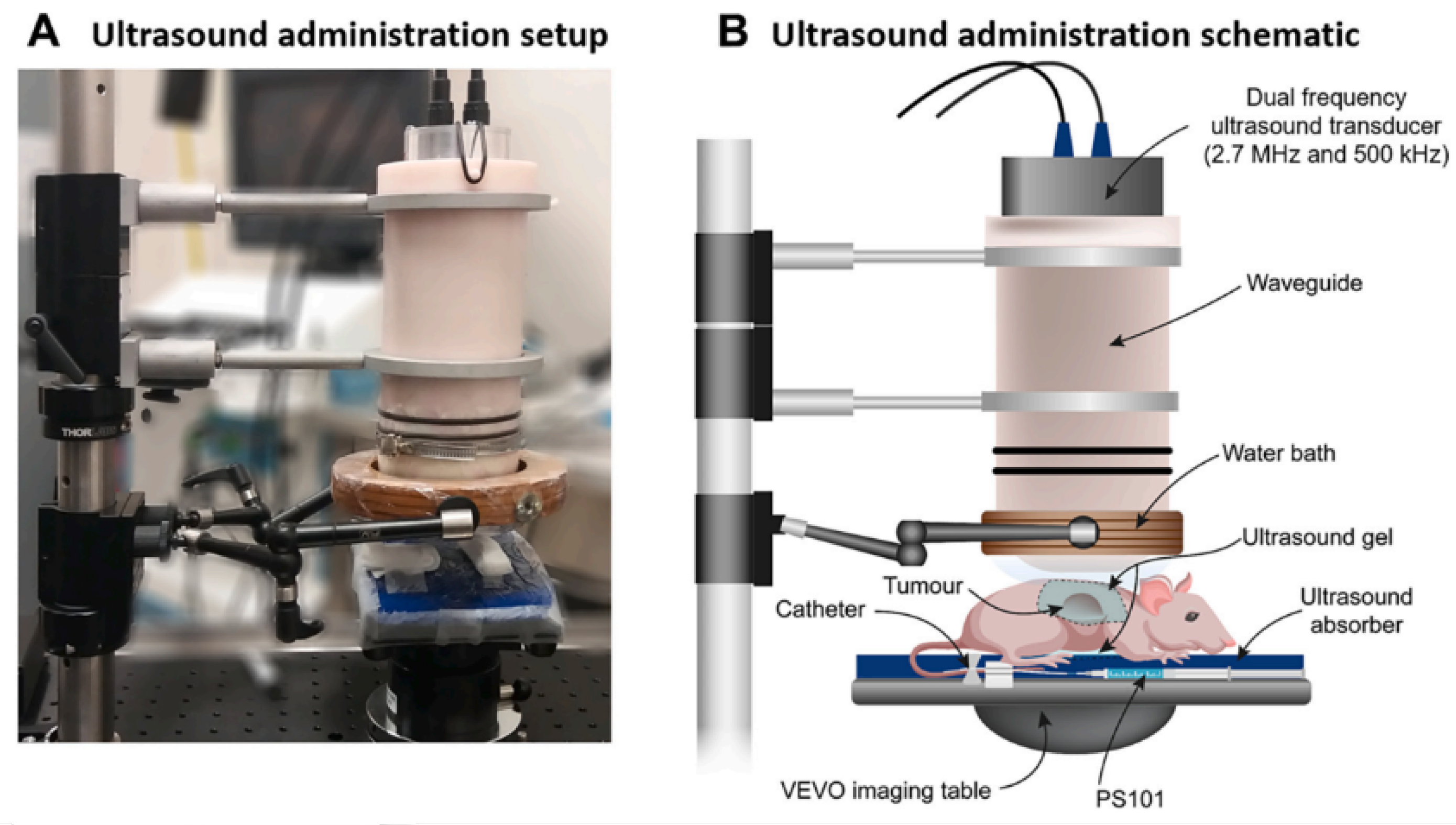
Figure 4 demonstrates how, in the experimental setup under examination, the transducer was adapted to the body of the mouse by means of an adapter designed to contain an aqueous medium that conforms to the acoustic intensity and the focal length. The results demonstrated that combining sonoporation with gemcitabine therapy significantly reduced primary tumor volumes and increased median survival by up to 10% when compared to control groups. Building on this work, in 2017, the same research team [
18] explored the use of acoustic cluster therapy (ACT
®, a patented microbubble system that can be loaded with drugs that can be activated via ultrasound stimulation) in combination with paclitaxel to improve the therapeutic efficacy in a pre-clinical pancreatic ductal adenocarcinoma (PDAC) mouse model. The experimental setup is visible in
Figure 5, where the ecoguided injection of the drug is also taken. The results showed that ACT
® with paclitaxel led to transient reductions in tumor volume and a potential mechanism for enhanced therapy. In this second article, it is underscored that the acoustic parameters utilized for ultrasonic stimulation and the formulation of the drug warrant further optimization. This optimization process has the potential to yield even more promising results in the context of enhancing therapeutic outcomes. These studies collectively offer promising approaches for enhancing the treatment of pancreatic cancer, which is known for its high mortality rates and limited treatment options, especially in advanced stages. Finally, in 2022, Ng et al.’s research (
Figure 6) provides an overview of the results on PDAC xenograft mouse models with the ACT
® technique combined with paclitaxel, which provided significant reductions in tumor volume in PDAC cell-line-based mouse xenograft models.
3.2.7. Ovaries
In the study of Suzuki et al. (2010) [
25], a combination of ultrasound and innovative ultrasound-sensitive liposomes was employed to enhance interleukin-12 (IL-12) gene therapy for cancer, as in [
26]. An impactful and noteworthy study by Pu et al. (2014) [
27] developed LHRH receptor-targeted and paclitaxel-loaded lipid microbubbles (TPLMBs) to enhance chemotherapy delivery in ovarian cancer treatment. In an ovarian cancer model, TPLMBs effectively bound to tumor cells, and ultrasound-mediated destruction of these microbubbles resulted in significantly improved therapeutic outcomes, including increased tumor apoptosis and reduced angiogenesis. The findings indicate that ultrasound-mediated intraperitoneal administration of targeted drug-loaded microbubbles holds promise for ovarian cancer treatment. Furthermore, it is worth noting that the stimulation in this case occurred at low frequencies, approximately 300 KHz. All the parameters obtained from the bibliography are reported in summary tables (
Table 1,
Table 2,
Table 3,
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8). One important parameter in US is the acoustic intensity, which, in this review, is expressed as ISPTA, as in most of the scientific literature on sonoporation. Compared to the application of diagnostic imaging, which applies ultrasound at intensities ranging from 0.05 to 0.5 W/cm
2, the application of ultrasound in surgery involves high intensities of 0.2–100 W/cm
2 and up to 10,000 W/cm
2. The use of therapeutic ultrasound ranges from high to low intensities. Thermal effects predominate in the former, and non-thermal effects, such as cavitation and transfer of drug, in the latter. Low-intensity US can be further subdivided into pulsed and continuous US, depending on the duty cycle. Pulsed US is characterized by ON/OFF cycles, while continuous US has a duty cycle of 100%. A new frontier to improve delivery to tumor cells could be the use of very low-intensity ultrasound (VLIUS), at intensities lower than 120 mW/cm
2 [
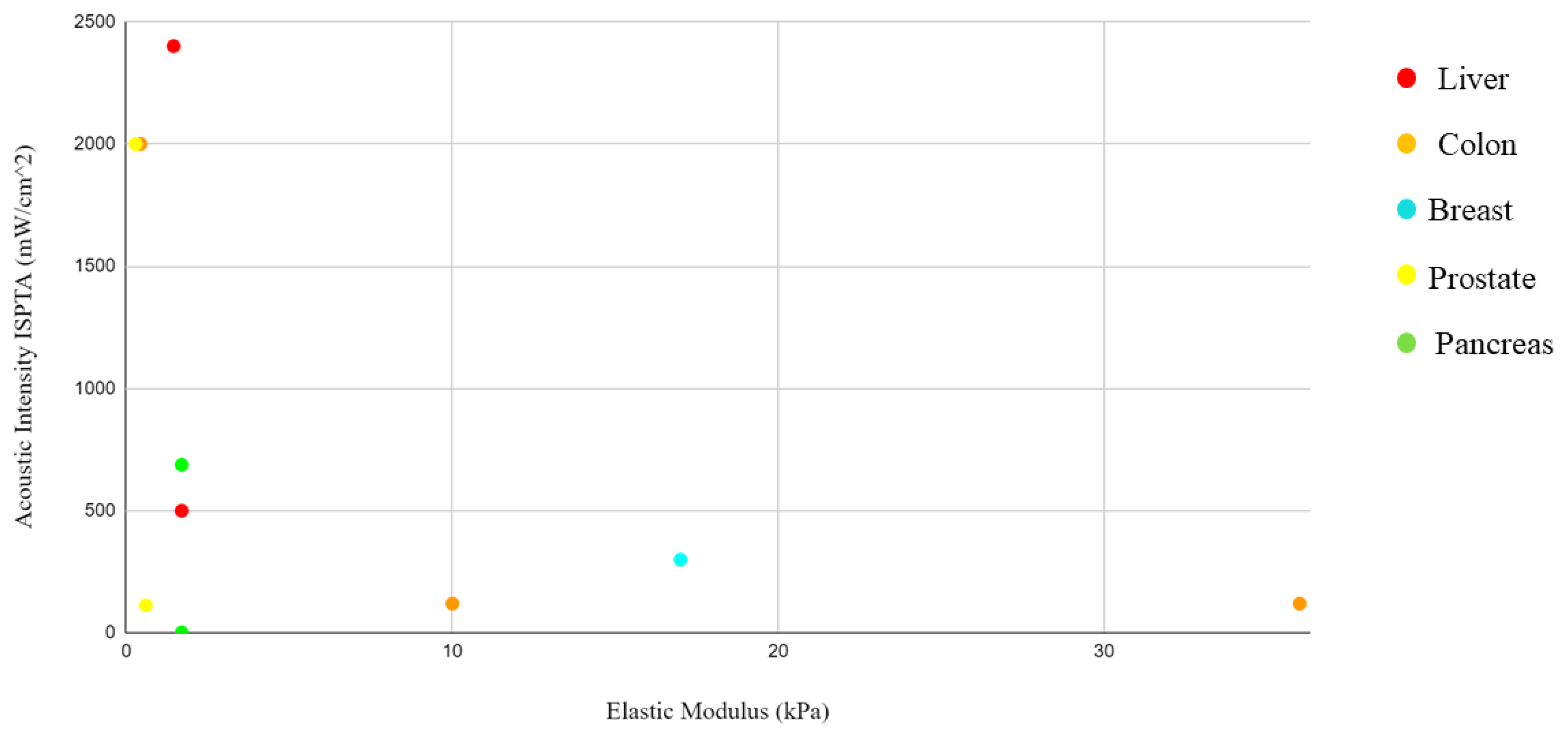
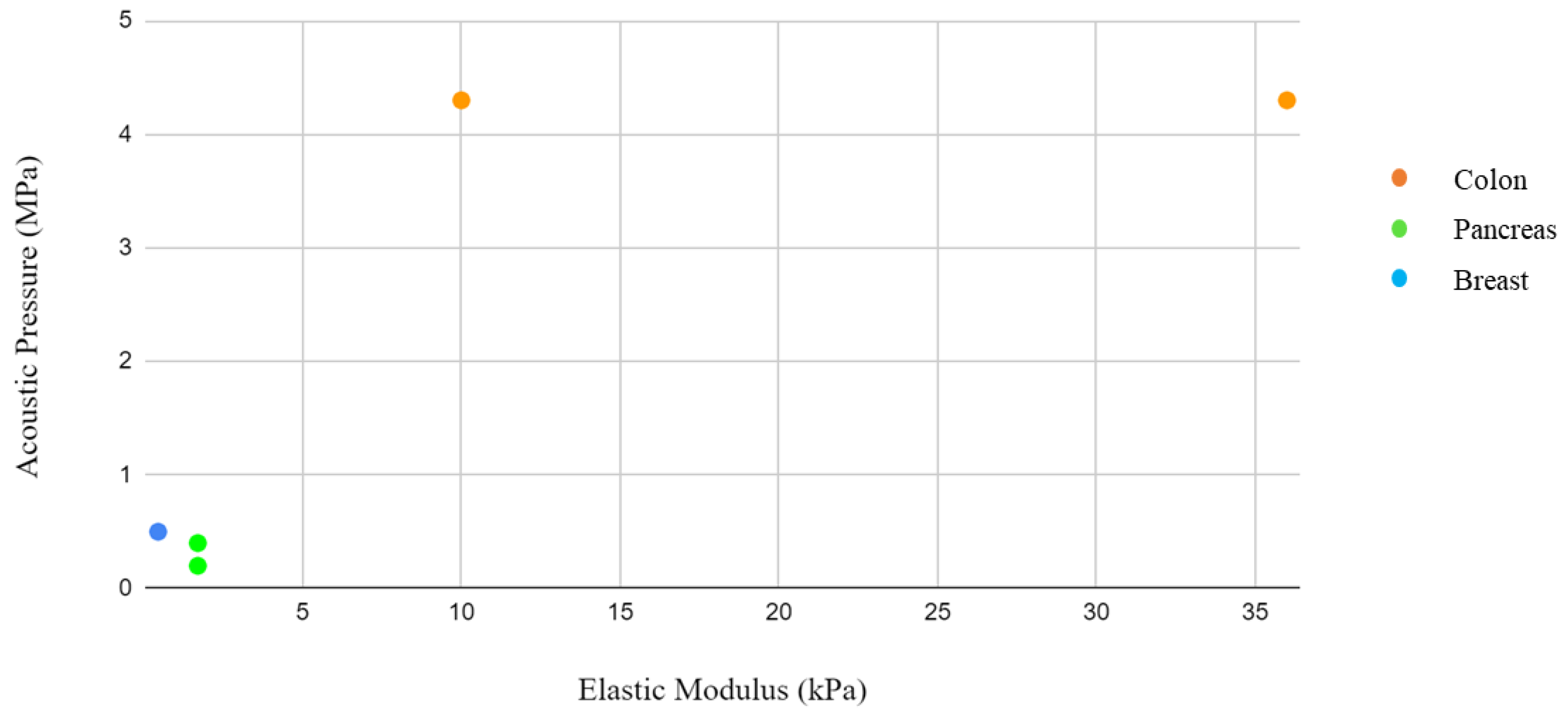
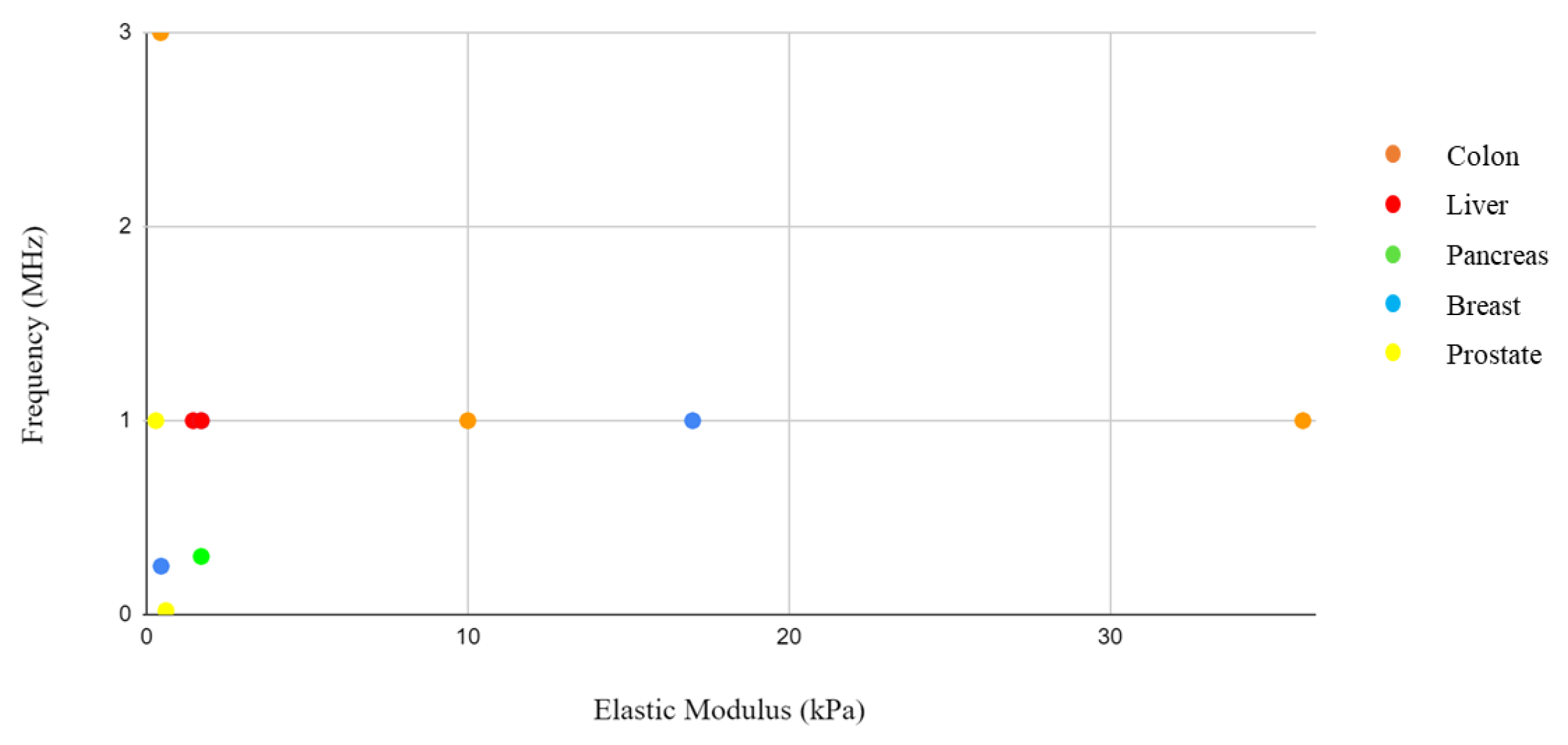
13]. These would further reduce the generation of significant amounts of heat that would damage tissues and cells. By integrating the information from the elastic modulus and acoustic pressure tables, where both values were present, it was not possible to identify a correlation. However, this could be linked to the different study approaches and sample selection. Solid tumors present interesting characteristics that differentiate them from their surrounding environment. Palpation often serves as the initial diagnostic method, as tumors exhibit greater rigidity than their surroundings.
At the cellular level, individual cancer cells appear to be softer and even more deformable than their healthy counterparts. Cancer cells undergo changes throughout the progression of the disease, acquiring biological properties not present in normal cells, such as the ability to replicate indefinitely [
28]. One of the most remarkable phenomena associated with cancer is metastatic spreading. This leads to the formation of secondary tumors in distant organs, far away from the primary tumor site. The process involves cancer cells invading the bloodstream (intravasion) and then exiting the blood vessels to establish new tumors (extravasion). Research has revealed an essential aspect of tumor development: the role of stroma stiffening. Stiffening of the surrounding tissue induces tumor rigidity, which, in turn, contributes to tumor progression [
29]. Understanding these unique characteristics of cancer cells and the mechanisms behind tumor progression is crucial in developing targeted and effective treatments to combat this disease. A further distinction between healthy and tumor cells is their stiffness, as reported in
Table 8. Tumor cells, unlike their healthy counterparts, show a significantly lower elastic rigidity. This specific characteristic is closely linked to the ability of cancer cells to metastasize or spread throughout the body. To determine the elastic modulus of normal and cancer cells and observe how they react to applied forces, researchers often use an atomic force microscope (AFM). With this technique, the mechanical properties of cell surfaces are deformed and analyzed [
30]. Interestingly, the elastic moduli of benign cells show a wider variation, following a log-normal distribution. On the other hand, malignant cells show a much narrower log-normal distribution of elastic rigidity.
Table 1.
Colon cancer cells.
Table 1.
Colon cancer cells.
| Parameters | Colon (HT29): Colorectal Adenocarcinoma [12] | Colon (HT29, HCT116): Colorectal Adenocarcinoma [13] | Colon (LS-174T): Colorectal Adenocarcinoma [14] |
|---|
| Probe | Transducer from Haiying Medical Electronic Instrument Company | US transducer 10–100 V | Linear US Probe |
| Frequency (MHz) | 0.238 | 1 | 3 |
| Acoustic Pressure (MPa) | 0.5 | 4.3 1 | - |
| ISPTA (mW/cm2) | - | 120 | 2000 |
| Duty Cycle | 0.50 | - | 0.20 |
Table 2.
Liver cancer cells.
Table 2.
Liver cancer cells.
| Parameters | Liver (SMMC-7721): Hepatocarcinoma [15] | Liver (Hepatic Fibrosis) [16] |
|---|
| Probe | Linear Array Probe | Vevo 2100, MS250 transducer |
| Frequency (MHz) | 1 | 1 |
| Acoustic Pressure (MPa) | - | - |
| ISPTA (mW/cm2) | 2400 | 500 |
| Duty Cycle | 0.50 | 0.50 |
Table 3.
Pancreas cancer cells.
Table 3.
Pancreas cancer cells.
| Parameters | PDAC (MIA PaCa-2): Pancreatic Ductal Adenocarcinoma [17] | PDAC (MIA PaCa-2): Pancreatic Ductal Adenocarcinoma [18] | PDAC: Pancreatic Ductal Adenocarcinoma [19] |
|---|
| Probe | Vevo 2100, MS250 transducer | Vevo 2100, MS250 transducer | Custom-made transducer (Relab, Horten, Norway) |
| Frequency (MHz) | 1 | 0.3 | 0.5–2.7 MHz |
| Acoustic Pressure (MPa) | 0.2 | 0.4 | - |
| ISPTA (mW/cm2) | 688 | 2.39 | - |
| Duty Cycle | 0.40 | 0.73 | - |
Table 4.
Breast cancer cells.
Table 4.
Breast cancer cells.
| Parameters | 4T1: Mammary Carcinoma [22] | MDA-MB-435: Derived from Melanoma Cell Line [20] | MDA-MB-231-Luc TNBC: Derived from Melanoma Cell Line [21] |
|---|
| Probe | Spherically focused single element transducer | CZT-8A sonicator, Xinzhen Company, ShenYang, China | Aplio XG ultrasound scanner (Toshiba Medical Systems Corporation, Tochigi, Japan), 1204BT linear array ultrasound probe |
| Frequency (MHz) | 0.25 | 1 | 0.3 |
| Acoustic Pressure (MPa) | 0.5 | - | - |
| ISPTA (mW/cm2) | - | 300 | - |
| Duty Cycle | - | 0.5 | - |
Table 5.
Prostate cancer cells.
Table 5.
Prostate cancer cells.
| Parameters | DU145: Prostate Adenocarcinoma Origin [23] | LNCaP: Prostate Carcinoma [26] |
|---|
| Probe | FS450 Ultrasonic homogenizer sonicator | UltraMax, XLTEK |
| Frequency (MHz) | 0.021 | 1 |
| Acoustic Pressure (MPa) | - | - |
| ISPTA (mW/cm2) | 113 | 2000 |
| Duty Cycle | 0.7 | 0.3 |
Table 6.
Bone (treatment for osteoporosis).
Table 6.
Bone (treatment for osteoporosis).
| Parameters | Stimulation of Osteoclast [24] |
|---|
| Probe | Fisherbrand Model 120 Sonic Dismembrator |
| Frequency (MHz) | 1 |
| Acoustic Pressure (MPa) | - |
| ISPTA (mW/cm2) | 3000 |
| Duty Cycle | 0.5–1 |
Table 7.
Ovarian cancer cells.
Table 7.
Ovarian cancer cells.
| Parameters | OV-HM: Ovarian Cancer Cells [25] | A2780/DDP: Ovarian Cancer Cells [27] |
|---|
| Probe | - | Model CGZZ, Ultrasonographic Image Research Institute, Chongqing Medical University, China |
| Frequency (MHz) | 1 | 0.3 |
| Acoustic Pressure (MPa) | - | - |
| ISPTA (mW/cm2) | 700 | 1000 |
| Duty Cycle | - | 0.5 |
Table 8.
Elastic modulus of the main cell lines mentioned. The variability of values depends on the amount of collagen developed by the tumor.
Table 8.
Elastic modulus of the main cell lines mentioned. The variability of values depends on the amount of collagen developed by the tumor.
| Cellular Line | Elastic Modulus (kPa) |
|---|
| Colon (HT29) [31] | [5–15] |
| Colon (HCT116) [31] | [2–70] |
| Colon (LS174-T) [32] | [0.302–0.566] |
| Liver (SMMC-7721) [33] | [1.2–1.7] |
| Liver (hepatic fibrosis) [34] | 1.7 |
| Pancreas (MIA PaCa-2) [35] | [0.7–2.7] |
| Pancreas (PANC-1) [35] | [1.3–3.4] |
| Breast cancer cell (4T1) [36] | 0.45 |
| Breast cancer cell (MDA-MB-435) [37] | [12–22] |
| Prostate cancer cell (DU145) [38] | [0.2–0.8] |
| Prostate cancer cell (LNCaP) [39] | [0.235–0.339] |
| Bone (osteoclast) | - |
| Ovarian cancer cell (OV-HM) | - |
| Ovarian cancer cell (A2780/DDP) | - |
By integrating the information from the tables of elastic moduli and acoustic intensities, pressure, and frequency, a graph was drawn with the concentration of the items by body district. The reason for this grouping is related to the need to identify in which body districts the parameters are concentrated in order to give a guide to those who would like to approach this type of research and experimentation in the future with a background of what has been performed on that organ in the past. However, a close correlation is not expected due to the different study approaches and sample selection, considering the difference between a monolayer tissue and a tumor spheroid, where drug penetration is slowed down by aggregation between cells. In addition, some of these studies were conducted in vivo. In these cases, the treatment of the tumor mass in vivo follows the growth of an inoculated tumor mass in the animal model.
Nevertheless, it is conceivable that an effort in the direction of data standardization could give further impetus to research.