Aqueous Waste Containing AOXs: Critical Issues in Analytical Methods and Assessment of Possible Inhibitory Effects on Mesophilic and Thermophilic Aerobic Biomasses
Abstract
1. Introduction
2. Materials and Methods
2.1. AOX Analytical Method
- Enrichment of organic halogen compounds on activated carbon with contemporaneous isolation of unwanted organohalogen byproducts from acidified samples;
- Use of a nitrate solution to wash the activated carbon in order to remove interfering inorganic halides;
- High-temperature—at least 950 °C [1,4]—combustion in order to mineralize the adsorbed organic halogens; reaction gases are conveyed to a drier and then to the Ag-coulometric titration cell for the determination of released hydro-halogenides. Here the halogen content is calculated based on the number of electric charges using Faraday’s law. The result of the AOX analysis is expressed as µg(Cl) L−1.
- In the column method, the sample is pressed over at least two interconnected columns filled with granular activated carbon (GAC) placed inside an adsorption column;
- In the shaking (or batch) method, the sample is shaken, for a specific period, with powdered activated carbon (PAC), which is subsequently separated from the matrix to be analyzed using a polycarbonate filter.
- A concentration of inorganic chloride ions above 1000 mg L−1, which may cause interference or false-high readings;
- The presence of bacteria and algae containing chloride ions;
- The presence of inorganic bromides and iodides;
- A dissolved organic carbon (DOC) content that is too high, which can lead to an underestimation;
- A poor affinity of the halogenated compounds for activated carbon.
- The standard method is applicable to samples with concentrations of inorganic chloride ions of less than 1 g L−1; samples with higher concentrations should be diluted before enrichment on activated carbon.
- The standard method is also applicable to samples containing insoluble halides, i.e., halogens that are adsorbed on suspended solids; filtration of the sample before the analysis allows for the separate estimation of dissolved and particulate AOXs.
- The pH value for the adsorption of the sample should be ≤2 [4].
- Oxidizing agents in the sample must be rendered ineffective by adding sodium sulfite [4].
- Sample S1, which was effluent from a thermophilic oxidative biological plant;
- Sample S2, which was a mixture of aqueous waste in which the presence of organochlorine compounds was ascertained.
2.2. Oxygen Uptake Rate Tests
- Mesophilic biomass from a municipal WWTP (Wastewater Treatment Plant) with a capacity of 50,000 PE (Population Equivalent) that can receive industrial aqueous waste (B1);
- Mesophilic biomass from a municipal WWTP with a capacity of 100,000 PE (B2);
- Mesophilic biomass from a municipal WWTP with a capacity of 1500 PE (B3);
- Mesophilic biomass from a municipal WWTP with a capacity of 15,000 PE (B4);
- Thermophilic biomass from a WWTP with a capacity of 50,000 PE that treats industrial aqueous waste (B5).
2.2.1. Batch Tests
- The volatile Suspended Solid (VSS) concentration (gVSS L−1) of the tested biomass was analyzed;
- The biomass volume was put into a flask placed on a magnetic stirrer; the magnetic bar spinning at the bottom of the flask allowed the biomass (or the biomass–substrate mixture in the case of an exogenous test) to remain well mixed during the test;
- The porous stone connected to the mechanical aerator was immersed in the biomass volume to increase the DO value, together with the probe to measure the DO value;
- The biomass was aerated until saturation: when the DO value was stable, the aeration was interrupted;
- Only in the case of an exogenous test, after turning off the aeration, the volume of substrate to be tested was poured into the flask together with the biomass;
- Immediately after turning off the aeration, the flask was sealed with parafilm in order to isolate the volume and to avoid air exchange with the external environment;
- Lasty, data transfer from the probe to the computer was started. The data were recorded in an Excel spreadsheet at 5 s intervals.
2.2.2. Continuous Tests
- Possible medium–long-term toxic effects caused by the tested substrate on the biomass;
- The AOX removal by measuring the AOX concentration in the settled effluent at the end of the test.
3. Results
3.1. AOX Analytical Method
- The adopted adsorption method;
- The amount of activated carbon;
- The sample volume;
- The volume of ultra-pure water used for washing before combustion;
- Any other data used for the quantification of AOXs in the sample.
3.2. Oxygen Uptake Rate Tests
3.2.1. Batch Tests
3.2.2. Continuous Tests
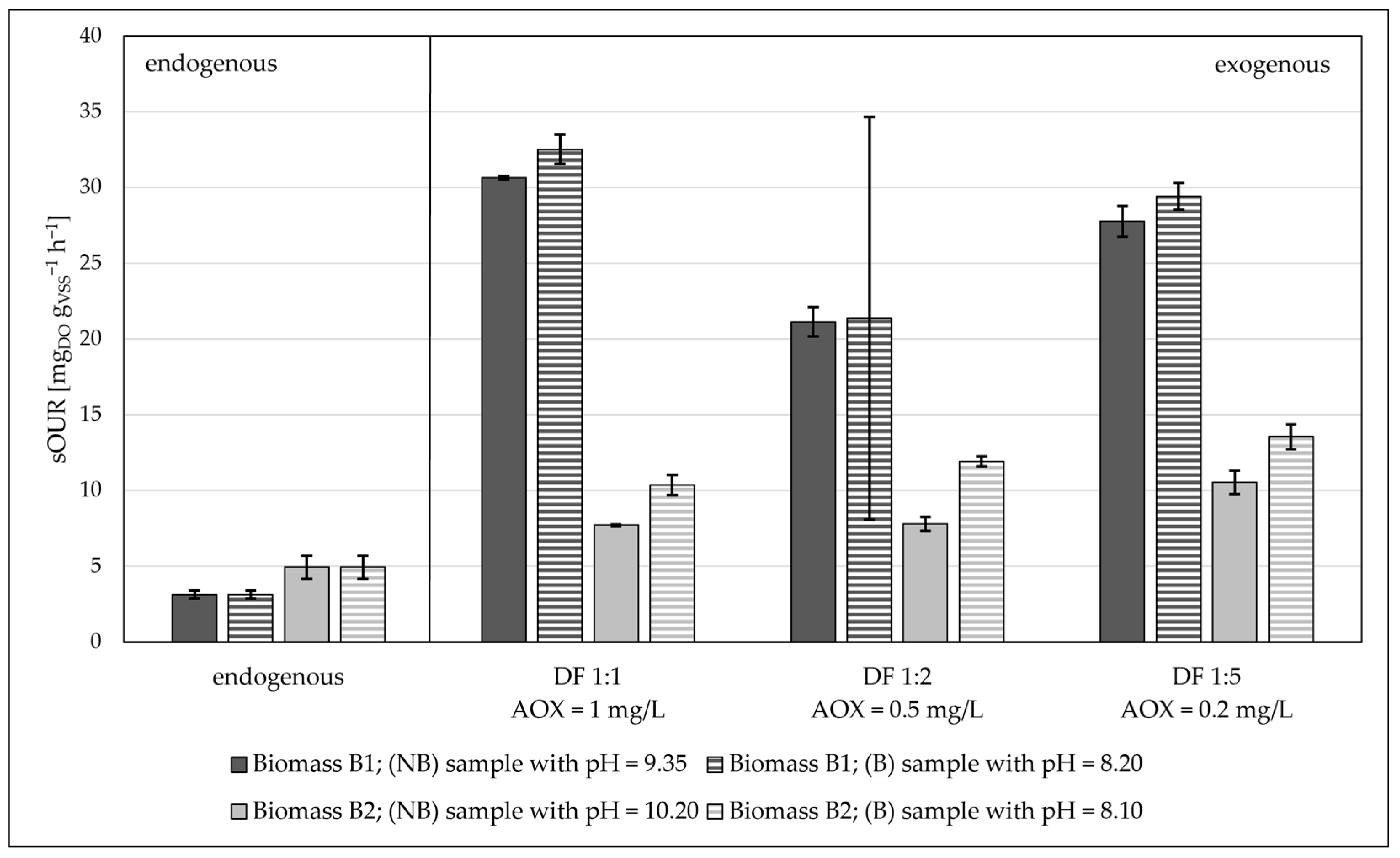
- By lowering the pH from values of about 10 to around 8, the oxygen consumption increases.
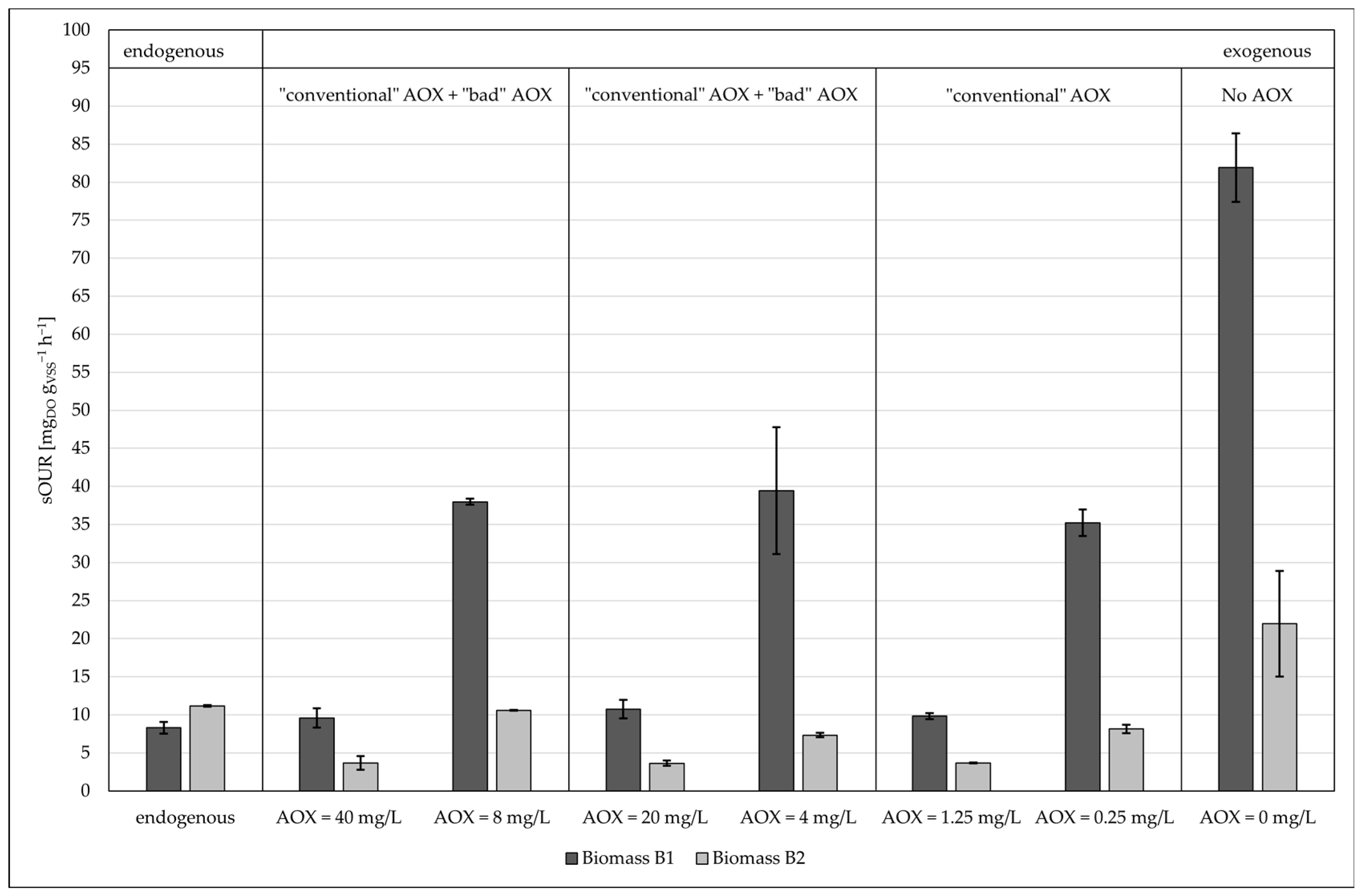
- Industrial biomass B1, which was already acclimated to industrial wastewaters, showed a higher oxygen consumption than the civil biomass.
- Under the same substrate conditions—same AOX concentration and same pH—the thermophilic biomass (B5) showed a lower oxygen consumption with respect to the industrial biomass (B1).
4. Discussion
- By changing the pH value from about 9–10 to about 8, the kinetics of the oxygen consumption increased. Thus, the optimum pH range to ensure the best biomass respiration kinetics is close to neutrality;
- The industrial biomass B1 showed higher biological kinetics compared to the civil biomass B2 when put in contact with aqueous waste containing AOXs. The explanation for this lies in the fact that the industrial biomass was already accustomed to “unconventional” pollutants;
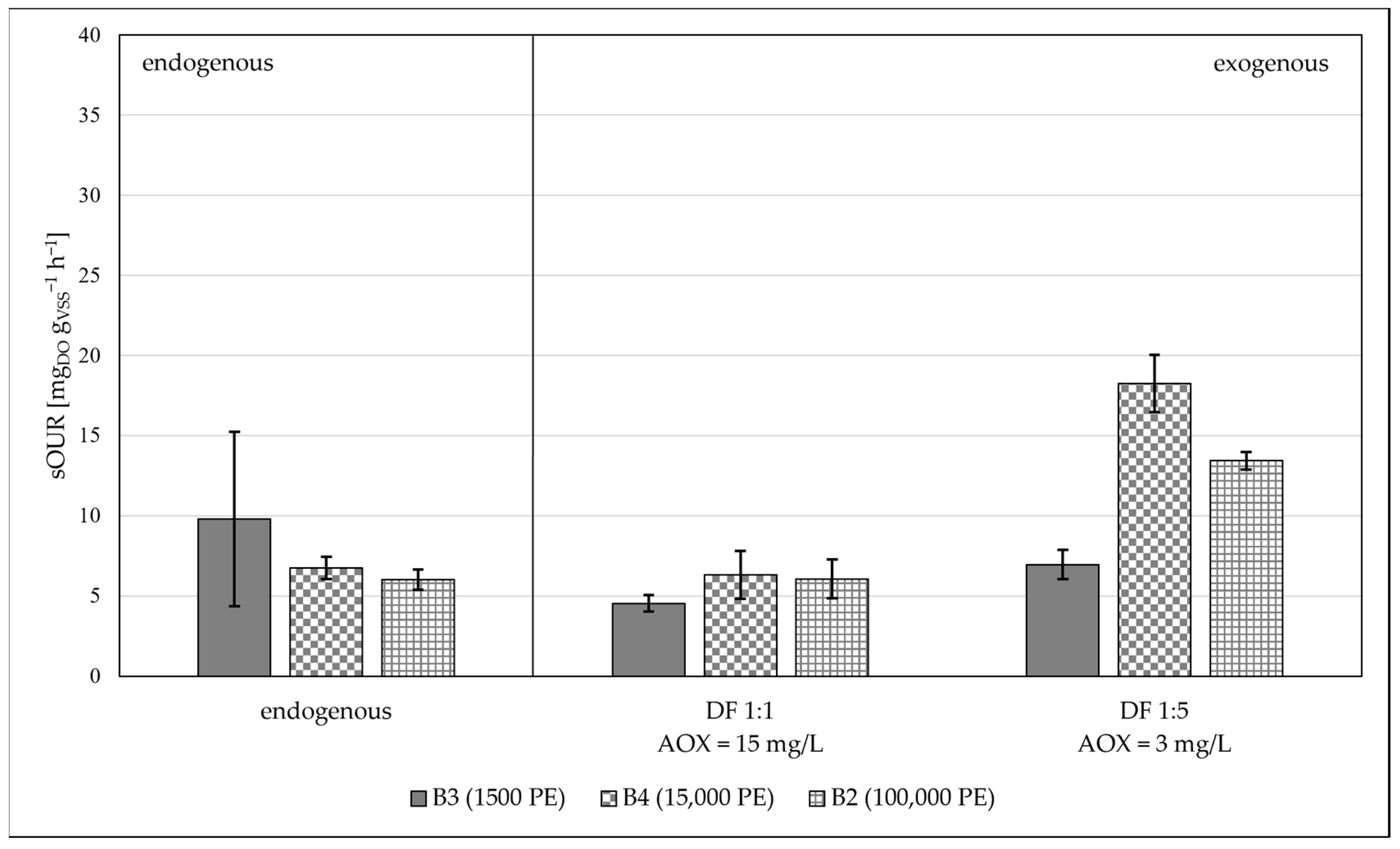
- Lower biological kinetics was observed for small civil plant biomasses, proving that biomasses coming from plants with a medium-low capacity need to be acclimatized;
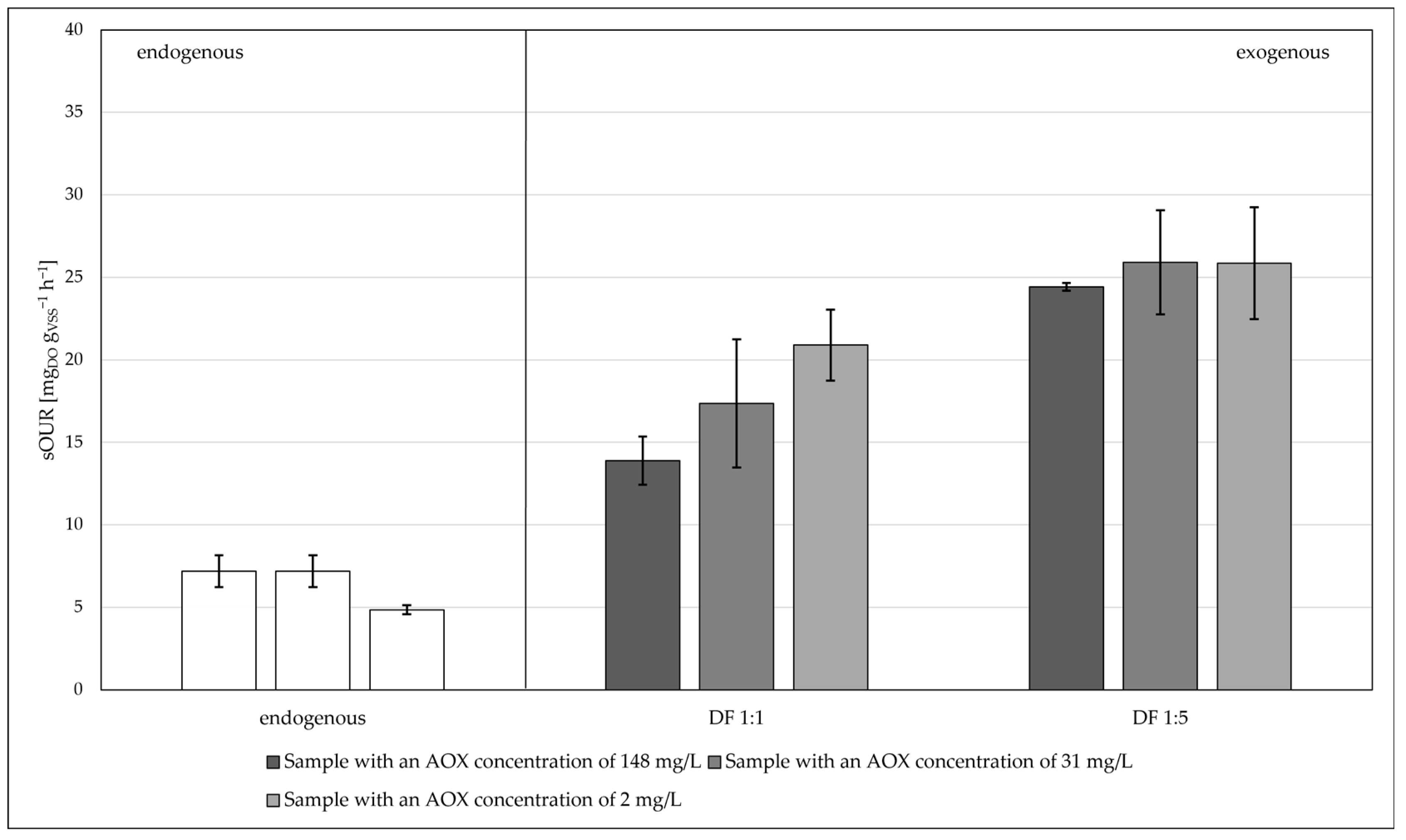
- When the substrate was more diluted, i.e., it had a lower concentration of AOXs, the kinetics of the oxygen consumption was higher;
- The type of AOX did not affect short-term oxygen consumption for both biomass B1 and biomass B2;
- There was no inhibition of the tested aerobic biomasses, either in the short term nor in the medium term. However, low oxygen consumption was noticed.
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, G. Sense or no-sense of the sum parameter for water soluble “adsorbable organic halogens” (AOX) and “absorbed organic halogens” (AOX-S18) for the assessment of organohalogens in sludges and sediments. Chemosphere 2003, 52, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xie, Y.; Tian, J.; Chen, L. Adsorbable organic halogens in contaminated water environment: A review of sources and removal technologies. J. Clean. Prod. 2021, 283, 124645. [Google Scholar] [CrossRef]
- UNI EN ISO 9562:2004; Water Quality–Determination of Adsorbable Organically Bound Halogens (AOX). ISO: Geneva, Switzerland, 2004.
- Analytik-jena. Available online: https://www.analytik-jena.com/products/chemical-analysis/combustion-elemental-analysis/aox (accessed on 21 June 2024).
- Murányi, R.; Cserfalvi, T. High Performance Microcoulometry. Mater. Sci. Forum 2007, 537–538, 687–694. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Marques, C.C.; Portugal, I.; Nunes, M.I. Fenton processes for AOX removal from a kraft pulp bleaching industrial wastewater: Optimisation of operating conditions and cost assessment. J. Environ. Chem. Eng. 2020, 8, 104032. [Google Scholar] [CrossRef]
- Gupta, S.; Charabarti, S.; Singh, S. Effect of Varying Load of Organochlorine Compounds on Activated Sludge Process. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2011, 23, 175–179. [Google Scholar]
- Kamenická, B.; Weidlich, T. A Comparison of Different Reagents Applicable for Destroying Halogenated Anionic Textile Dye Mordant Blue 9 in Polluted Aqueous Streams. Catalysts 2023, 13, 460. [Google Scholar] [CrossRef]
- Naumczyk, J.; Kucharska, M.; Ładyńska, J.; Wojewódka, D. Electrochemical oxidation process in application to raw and biologically pre-treated tannery wastewater. Desalination Water Treat. 2019, 162, 166–175. [Google Scholar] [CrossRef]
- Toth, A.J.; Ladanyi, R.; Thi, H.T.D.; Szilagyi, B.; Haaz, E. Sustainable Technology Solutions for Reuse of Process Wastewaters from Fine Chemical Industries. Chem. Eng. Trans. 2022, 91, 187–192. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, P.; Ye, L.; Chen, H.; Xu, X.; Zhu, L. Biological performance and fouling mitigation in the biochar-amended anaerobic membrane bioreactor (AnMBR) treating pharmaceutical wastewater. Bioresour. Technol. 2020, 302, 122805. [Google Scholar] [CrossRef]
- Murat Hocaoğlu, S.; Dursun, M.; Baştürk, İ.; Partal, R. Treatment-based hospital wastewater characterization and fractionation of pollutants. J. Water Process Eng. 2021, 43, 102205. [Google Scholar] [CrossRef]
- Noma, Y.; Yamane, S.; Kida, A. Adsorbable organic halides (AOX), AOX formation potential, and PCDDs/DFs in landfill leachate and their removal in water treatment processes. J. Mater. Cycles Waste Manag. 2001, 3, 126–134. [Google Scholar]
- Xie, Y.W.; Chen, L.J.; Liu, R. Oxidation of AOX and organic compounds in pharmaceutical wastewater in RSM-optimized-Fenton system. Chemosphere 2016, 155, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Decision (EU) 2018/1147 of 10 August 2018 Establishing Best Available Techniques (BAT) Conclusions for Waste Treatment, under Directive 2010/75/EU of the European Parliament and of the Council. Off. J. Eur. Union 2018, 38–87. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D1147 (accessed on 13 March 2024).
- Yang, J.; Li, J.; Tan, X.; Li, J.; Croué, J.-P.; Chen, B. Insights into adsorbable organic halogen analysis: Two overlooked factors impacting water quality assessment. Sci. Total Environ. 2024, 928, 172429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Shang, C. Effect of reductive property of activated carbon on total organic halogen analysis. Environ. Sci. Technol. 2010, 44, 2105–2111. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Determination of TOCl, TOBr and TOI in drinking water by pyrolysis and off-line ion chromatography. Anal. Bioanal. Chem. 2006, 384, 495–504. [Google Scholar] [CrossRef]
- Jekel, M.R.; Roberts, P.V. Total organic halogen as a parameter for the characterization of reclaimed waters: Measurement, occurrence, formation, and removal. Environ. Sci. Technol. 1980, 14, 970–975. [Google Scholar] [CrossRef]
- Kristiana, I.; McDonald, S.; Tan, J.; Joll, C.; Heitz, A. Analysis of halogen-specifc TOX revisited: Method improvement and application. Talanta 2015, 139, 104–110. [Google Scholar] [CrossRef]
- Kußmaul, H.; Simon, H.; Sietz, M. Impact of halide ions on the determination of adsorbable organically bound halogens (AOX). Fresen. J. Chem. 1988, 330, 532. [Google Scholar] [CrossRef]
- Grøn, C.; Dybdahl, H.P. Determination of total organic halogens (TOX); bias from a non-halogenated organic compound. Environ. Int. 1996, 22, 325–329. [Google Scholar] [CrossRef]
- Novák, L.; Larrea, L.; Wanner, J.; Garcia-Heras, J.L. Non-filamentous activated sludge bulking in a laboratory scale system. Water Res. 1993, 27, 1339–1346. [Google Scholar] [CrossRef]
- Technologies for Quality S.r.l. Available online: https://www.tqsrl.com/tecniche_analitiche/analisi-elementare/aox-200/ (accessed on 26 June 2024).
- Oleksy-Frenzel, J.; Wischnack, S.; Jekel, M. Application of ion-chromatography for the determination of the organic-group parameters AOCl, AOBr and AOI in water. Fresenius' J. Anal. Chem. 2020, 366, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kinani, A.; Sa lhi, H.; Bouchonnet, S.; Kinani, S. Determination of adsorbable organic halogens in surface water samples by combustion–microcoulometry versus combustion–ion chromatography titration. J. Chromatogr. A. 2018, 1539, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; La, J.; Jansen, C. Oxygen uptake rate measurements for application at wastewater treatment plants. Vatten 2007, 63, 131–138. [Google Scholar]
- Andreottola, G.; Foladori, P.; Ziglio, G.; Cantaloni, C.; Bruni, L.; Cadonna, M. Methods for Toxicity Testing of Xenobiotics in Wastewater Treatment Plants and in Receiving Water Bodies. In Dangerous Pollutants (Xenobiotics) in Urban Water Cycle; Springer: Dordrecht, The Netherlands, 2007; pp. 191–206. [Google Scholar]
- Borzooei, S.; Simonetti, M.; Scibilia, G.; Chiara, M. Journal of Environmental Chemical Engineering. Critical evaluation of respirometric and physicochemical methods for characterization of municipal wastewater during wet-weather events. J. Environ. Chem. Eng. 2021, 9, 105238. [Google Scholar] [CrossRef]
- Capodici, M.; Fabio, S.; Di, F.; Di, D.; Torregrossa, M. An innovative respirometric method to assess the autotrophic active fraction: Application to an alternate oxic–anoxic MBR pilot plant. Chem. Eng. J. 2016, 300, 367–375. [Google Scholar] [CrossRef]
- Yankovych, H.; Vaclavikova, M.; Melnyk, I. A Review on Adsorbable Organic Halogens Treatment Technologies: Approaches and Application. Sustainability 2023, 15, 9601. [Google Scholar] [CrossRef]
- LaPara, T.M.; Alleman, J.E. Thermophilic aerobic biological wastewater treatment. Water Res. 1999, 33, 895–908. [Google Scholar] [CrossRef]
| Name | Optimal Operating Temperature [°C] | Type of Wastewater Treated | VSS Concentration [gVSS L−1] | Plant Capacity [PE] |
|---|---|---|---|---|
| B1 | 20 | Municipal wastewater mixed with aqueous waste | 3.2–4.3 | 50,000 |
| B2 | 20 | Municipal wastewater | 2.5–2.8 | 100,000 |
| B3 | 20 | Municipal wastewater | 3.0 | 1500 |
| B4 | 20 | Municipal wastewater | 3.0 | 15,000 |
| B5 | 50 | Aqueous waste | 26.4–27.2 | 50,000 |
| Substrate | AOX Concentration [mg L−1] | pH (NB) [-] | pH (B) [-] | Tested DFs | Tested Biomass(es) |
|---|---|---|---|---|---|
| Sub1 (real) | 2.00 ± 0.01 | 10.20 | 8.10 | 1:1/1:2/1:5 | B1/B2 |
| Sub2 (real) | 148.00 ± 0.01 | 8.00 | - | 1:1/1:5 | B1 |
| Sub3 (real) | 31.00 ± 0.01 | 8.00 | - | 1:1/1:5 | B1 |
| Sub4 (real) | 2.50 ± 0.01 | 8.00 | - | 1:1/1:5 | B1 |
| Sub5 (real) | 1.81 ± 0.01 | - | 7.80 | 1:1/1:2/1:5 | B1/B2/B5 |
| Sub6 (real) | 30.80 ± 0.01 | 8.00 | - | 1:1/1:5 | B1/B2/B3/B4/B5 |
| Sub7 (synthetic) | 80.00 ± 0.01 | 8.00 | - | 1:1/1:5 | B1/B2 |
| Sub8 (synthetic) | 40.00 ± 0.01 | 8.00 | - | 1:1/1:5 | B1/B2 |
| Sub9 (synthetic) | 2.50 ± 0.01 | 8.00 | - | 1:1/1:5 | B1/B2 |
| Sub10 (real) | <0.01 | 8.00 | - | 1:1 | B1/B2 |
| Sub11 (real) | 1.81 ± 0.01 | 9.35 | 8.20 | 1:1/1:2/1:5 | B1 |
| Substrate | AOX [mg L−1] | pH (NB) [-] | pH (B) [-] | Tested Biomass(es) |
|---|---|---|---|---|
| Sub1 (real) | 2.00 ± 0.01 | 10.20 | 8.10 | B1/B2 |
| Sub1 (real) diluted 1:5 | 0.40 ± 0.01 | - | 7.80 | B1 |
| Sub1 (real) diluted 1:10 | 0.20 ± 0.01 | - | 7.80 | B1 |
| Sub6 (real) | 30.80 ± 0.01 | 8.00 | - | B1/B5 |
| Sub7 (synthetic) | 80.00 ± 0.01 | 8.00 | - | B1 |
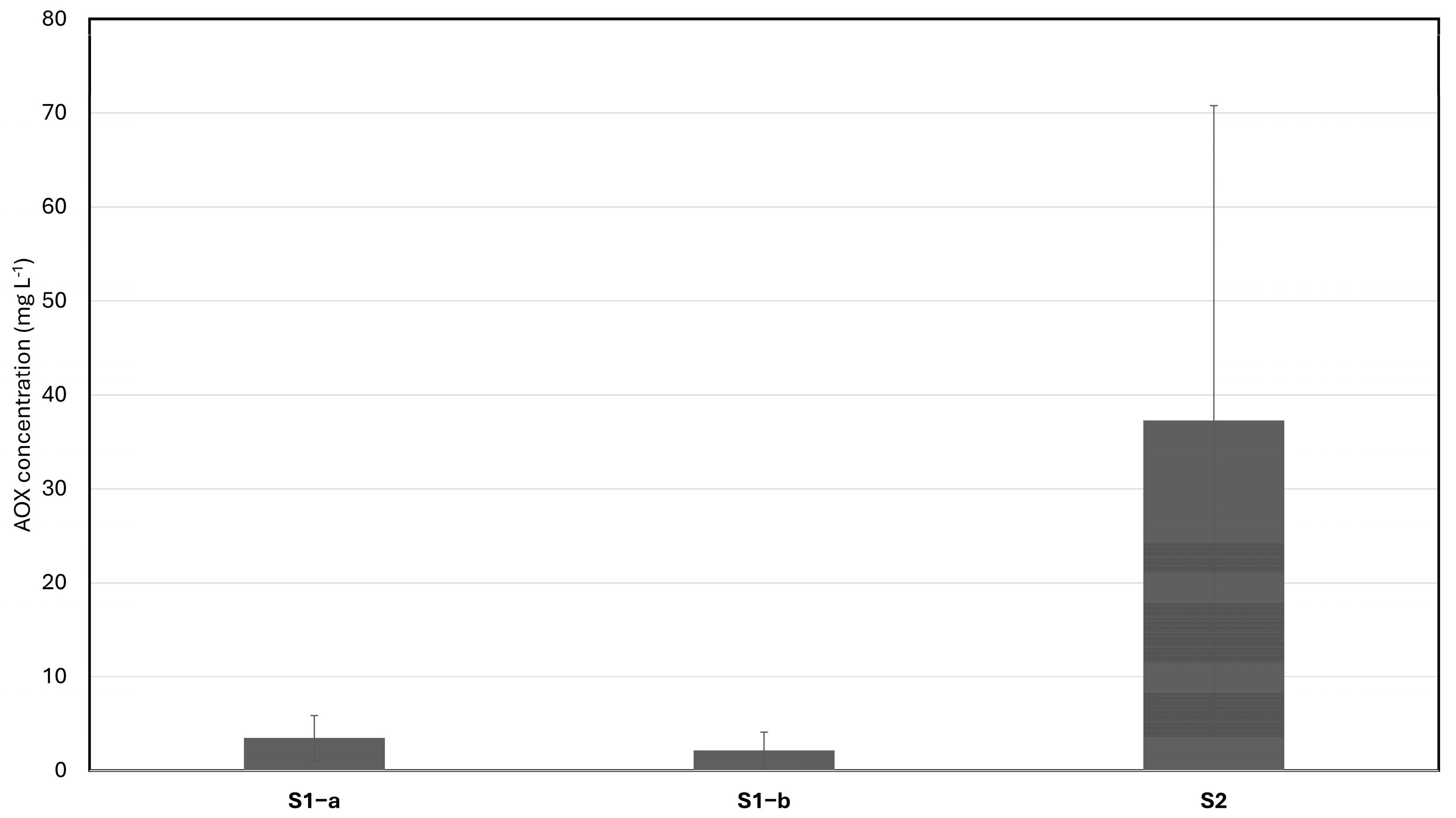
| Sample | Laboratory | AOX Concentration [mg L−1] |
|---|---|---|
| S1 | Lab-1 | 2.00 ± 0.01 |
| Lab-2 | 6.02 ± 0.76 | |
| Lab-3 | 1.55 ± 0.36 | |
| Lab-4 | 0.60 ± 0.20 | |
| S2 | Lab-1 | 30.80 ± 0.01 |
| Lab-2 | 86.50 ± 0.01 | |
| Lab-3 | 23.80 ± 5.50 | |
| Lab-4 | 8.00 ± 3.00 |
| Parameter | Lab-1 | Lab-2 | Lab-3 | Lab-4 |
|---|---|---|---|---|
| Adsorption procedure: frits (batch), column | Frits (shaking procedure paragraphs 9.3.2 of the ISO 9562 method) | 100 mL of sample (diluted based on expected concentration) + 5 mL of nitrate solution (NaNO3 + HNO3), agitated with a rocking agitator for 1 h | Column procedure from ISO 9562 method | Column method (paragraphs 7.1.3 and 9.3.4 of the ISO 9562 method) |
| Mass of activated carbon used | ~50 mg | 50 mg of activated carbon | Analytik Jena vials containing ~50 mg each; 2 vials used per sample | Specific cartridges provided by supplier; quantity was approx. 50–200 mg |
| Volume of sample used for adsorption or contact with the frit | 100 mL | 100 mL (diluted as needed) | 100 mL of sample used as is or diluted | 100 mL per method |
| Volume of ultrapure water used for washing before combustion | 25 mL of NaNO3 washing solution | 5–10 mL of diluted nitrate solution used for washing, additional 5 mL for rinsing | Washed with sodium nitrate and nitric acid solution as per supplier method | 25 mL of washing solution, prepared by diluting stock solution |
| Any other data for AOX quantification | Blank counts: ~0.2 µg; dilution factor: 100; formula: (sample counts—blank counts) (Dil/100) | Calibration curve: 10–250 ppb; control charts for blanks; adsorption capacity checks; 0.01 M HCl titration check | n.a. | Initial 100 mL sample volume; dilution factor considered; results in µg/L |
| Test-Day | AOX Concentration in the Aqueous Waste Tested [mgAOX L–1] | sOUR [mgDO gVSS–1 h–1] | |||
|---|---|---|---|---|---|
| Endogenous | DF 1:1 | DF 1:2 | DF 1:5 | ||
| A | 1.81 | 0.30 | 0.29 | 0.36 | 0.44 |
| B | 1.81 | 0.29 | 0.79 | - | 0.51 |
| C | 30.80 | 0.29 | 0.42 | - | 0.41 |
| Biomass | pH | AOX Concentration [mg L–1] | Exogenous ΔO2 [mgDO gVSS–1] | Overall ΔO2 [mgDO gVSS–1] | |
|---|---|---|---|---|---|
| Effect of the type of biomass (industrial vs. civil) and of the substrate’s pH | B1 | 10.20 | 2.00 | 15.10 | 35.70 |
| B2 | 10.20 | 2.00 | 7.20 | 24.50 | |
| B1 | 7.80 | 2.00 | 46.20 | 67.90 | |
| B2 | 7.80 | 2.00 | 22.50 | 39.60 | |
| Effect of the type of biomass (mesophilic vs. thermophilic biomass) | B1 | 8.00 | 30.80 | 9.80 | 15.75 |
| B5 | 8.00 | 30.80 | 1.72 | 1.96 |
| Biomass | pH | AOX Concentration [mg L–1] | Exogenous ΔO2 [mgDO gVSS–1] | Overall ΔO2 [mgDO gVSS–1] | |
|---|---|---|---|---|---|
| Effect of the substrate’s AOX concentration | B1 | 8.00 | 30.80 | 9.80 | 15.75 |
| B1 | 7.80 | 2.00 | 46.20 | 67.90 | |
| B1 | 7.80 | 0.40 | 71.05 | 84.90 | |
| B1 | 7.80 | 0.20 | 21.80 | 52.70 | |
| Medium-term toxicity | B1 | 8.01 | 80.00 | 549.59 | 755.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Bellazzi, S.; Grecchi, G.; Baldi, M.; Abbà, A. Aqueous Waste Containing AOXs: Critical Issues in Analytical Methods and Assessment of Possible Inhibitory Effects on Mesophilic and Thermophilic Aerobic Biomasses. Appl. Sci. 2024, 14, 11546. https://doi.org/10.3390/app142411546
Collivignarelli MC, Bellazzi S, Grecchi G, Baldi M, Abbà A. Aqueous Waste Containing AOXs: Critical Issues in Analytical Methods and Assessment of Possible Inhibitory Effects on Mesophilic and Thermophilic Aerobic Biomasses. Applied Sciences. 2024; 14(24):11546. https://doi.org/10.3390/app142411546
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Stefano Bellazzi, Giorgia Grecchi, Marco Baldi, and Alessandro Abbà. 2024. "Aqueous Waste Containing AOXs: Critical Issues in Analytical Methods and Assessment of Possible Inhibitory Effects on Mesophilic and Thermophilic Aerobic Biomasses" Applied Sciences 14, no. 24: 11546. https://doi.org/10.3390/app142411546
APA StyleCollivignarelli, M. C., Bellazzi, S., Grecchi, G., Baldi, M., & Abbà, A. (2024). Aqueous Waste Containing AOXs: Critical Issues in Analytical Methods and Assessment of Possible Inhibitory Effects on Mesophilic and Thermophilic Aerobic Biomasses. Applied Sciences, 14(24), 11546. https://doi.org/10.3390/app142411546









