Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts
Abstract
1. Introduction
2. Extraction of Phytochemicals and Volatile Compounds from Agri-Food Byproducts: An Overview of Traditional Techniques and Novel Sustainable Approaches
2.1. Traditional Approaches Involving the Use of Organic Solvents
2.1.1. Maceration
2.1.2. Soxhlet Extraction
2.1.3. Ultrasound-Assisted Extraction (UAE)
2.1.4. Extraction of Volatile Compounds: Hydrodistillation
2.2. Innovative and Sustainable Extraction Methods
2.2.1. Microwave-Assisted Extraction (MAE)
2.2.2. Pressurized Liquid Extraction (PLE)
2.2.3. Enzyme-Assisted Extraction (EAE)
2.2.4. Pulsed Electric Fields (PEF)
2.2.5. Negative Pressure Cavitation (NPC)
2.2.6. High-Pressure Homogenization (HPH)
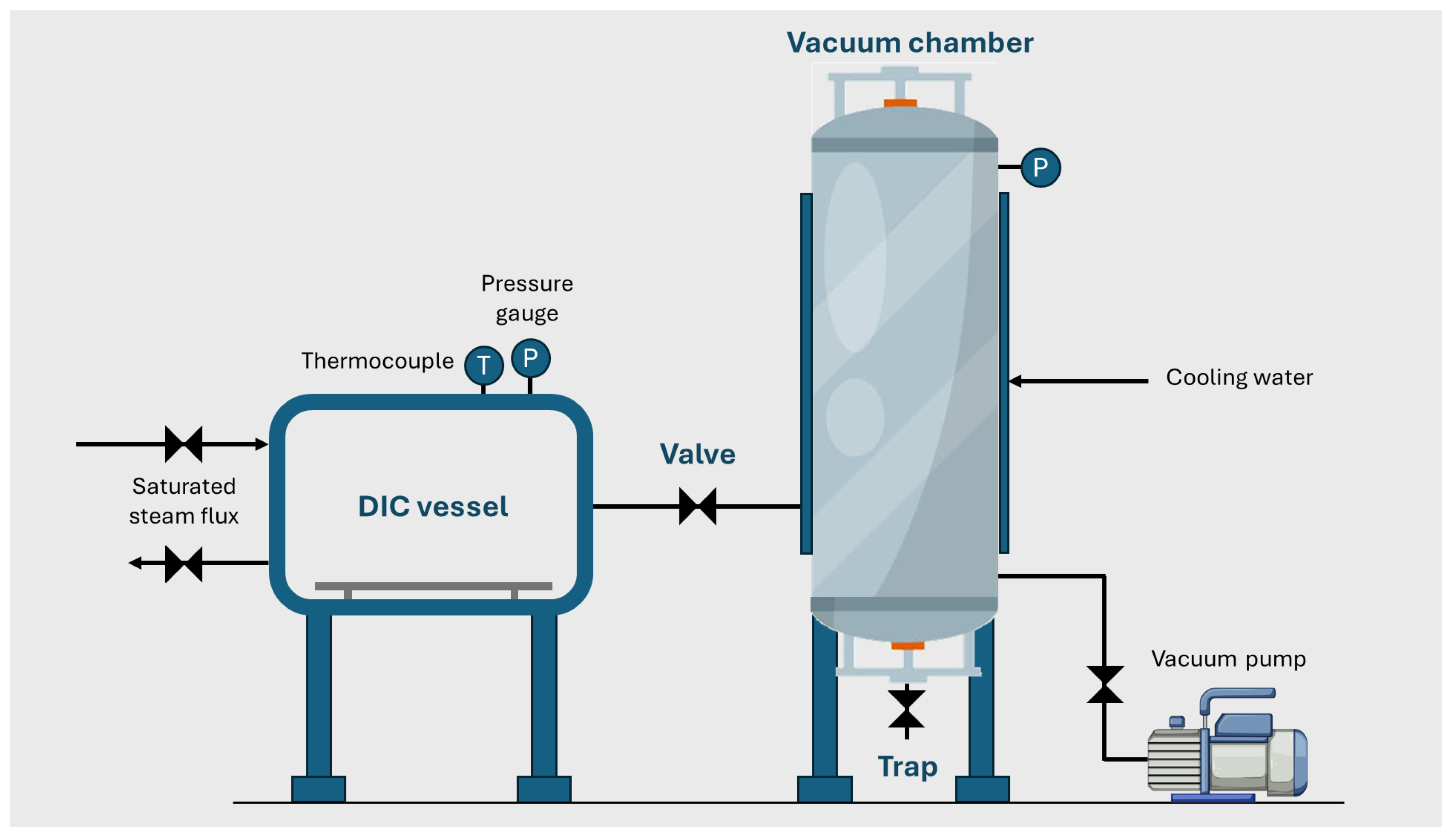
2.2.7. Instant Controlled Pressure Drop (DIC)
2.2.8. Supercritical CO2
3. Agri-Food Byproducts as Valuable Sources of Bioactive Compounds: Chemical Composition of Chicory, Honey, and Red Fruit Byproducts
3.1. Lettuce and Chicory Byproducts
Green Extraction of Bioactive Compounds from Chicory Byproducts
3.2. Honey and Other Bee Byproducts
3.3. Red Fruits and Grape
Green Extraction of Bioactive Compounds from Red Fruit and Grape Pomace
4. Valorization of Agrifood Byproducts Through Extraction and Recovery of Valuable Compounds: Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Food Waste. 2024. Available online: https://food.ec.europa.eu/food-safety/food-waste_en (accessed on 16 November 2024).
- Amicarelli, V.; Lagioia, G.; Bux, C. Global warming potential of food waste through the life cycle assessment: An analytical review. Environ. Impact Assess. Rev. 2021, 91, 106677. [Google Scholar] [CrossRef]
- Van Der Werf, P.; Gilliland, J.A. A systematic review of food losses and food waste generation in developed countries. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2017, 170, 66–77. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. United States 2030 Food Loss and Waste Reduction Goal. Available online: https://www.epa.gov/sustainable-management-food/united-states-2030-food-loss-and-waste-reduction-goal (accessed on 31 October 2024).
- Eurostat. Food Waste and Food Waste Prevention-Estimates-Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 31 October 2024).
- Chatti, C.B.; Hassen, T.B.; El Bilali, H. Closing the Loop: Exploring Food Waste Management in the Near East and North Africa (NENA) Region During the COVID-19 Pandemic. Sustainability 2024, 16, 3772. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food Losses and Waste in Latin America and the Caribbean. Newsletter #3 February 2016. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/5569e764-205c-4165-9251-1cbc78f7e0fb/content (accessed on 5 November 2024).
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Chapter 33—Food Waste and By-Product Valorization as an Integrated Approach with Zero Waste: Future Challenges; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 569–596. ISBN 978-0-323-91001-9. [Google Scholar]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato pomace food waste from different variants as a high antioxidant potential resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple pomace, a bioresource of functional and nutritional components with potential of utilization in different food formulations: A review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products Within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Ingle, A.P.; Franco Marcelino, P.R.; Silva, G.M.; Barbosa, F.G.; dos Santos, J.C.; da Silva, S.S. Agroindustrial Byproducts for the Generation of Biobased Products: Alternatives for Sustainable Biorefineries. Front. Energy Res. 2020, 8, 152. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Teshome, E.; Teka, T.A.; Nandasiri, R.; Rout, J.R.; Harouna, D.V.; Astatkie, T.; Urugo, M.M. Fruit By-Products and Their Industrial Applications for Nutritional Benefits and Health Promotion: A Comprehensive Review. Sustainability 2023, 15, 7840. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Ioannidou, S.M.; Filippi, K.; Kookos, I.K.; Koutinas, A.; Ladakis, D. Techno-economic evaluation and life cycle assessment of a biorefinery using winery waste streams for the production of succinic acid and value-added co-products. Bioresour. Technol. 2022, 348, 126295. [Google Scholar] [CrossRef]
- Ente Nazionale per la Meccanizzazione Agricola (ENAMA). Biomasse ed Energia & Disponibilità delle Biomasse. 2008. Available online: https://www.progettobiomasse.it/it/pdf/studio/p1c2.pdf (accessed on 5 November 2024).
- Barcaccia, G.; Ghedina, A.; Lucchin, M. Current Advances in Genomics and Breeding of Leaf Chicory (Cichorium intybus L.). Agriculture 2016, 6, 50. [Google Scholar] [CrossRef]
- Kagkli, D.M.; Corich, V.; Bovo, B.; Lante, A.; Giacomini, A. Antiradical and antimicrobial properties of fermented red chicory (Cichorium intybus L.) by-products. Ann. Microbiol. 2016, 66, 1377–1386. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Raba, D.; Viorica-Mirela, P. The effect of long-term frozen storage on the nutraceutical compounds, antioxidant properties and color indices of different kinds of berries. J. Food Agric. Environ. 2010, 8, 54–58. [Google Scholar]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria x ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef]
- Jensen, W. The Origin of the Soxhlet Extractor. J. Chem. Educ. 2007, 84, 1913. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Hui, X.; Wu, G.; Han, D.; Gong, X.; Stipkovits, L.; Wu, X.; Tang, S.; Brennan, M.A.; Brennan, C.S. Bioactive compounds from blueberry and blackcurrant powder alter the physicochemical and hypoglycaemic properties of oat bran paste. LWT 2021, 143, 111167. [Google Scholar] [CrossRef]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical Study and Antibacterial and Antibiotic Modulation Activity of Punica granatum (Pomegranate) Leaves. Scientifica 2020, 2020, 8271203. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from blackberry (Rubus fruticosus) growing in Poland. J. Sci. Food Agric. 2017, 97, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, S. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Agnieszka, M.; Michał, S.; Robert, K. Selection of Conditions of Ultrasound-Assisted, Three-Step Extraction of Ellagitannins from Selected Berry Fruit of the Rosaceae Family Using the Response Surface Methodology. Food Anal. Methods 2020, 13, 1650–1665. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Kirillov, V.; Stikhareva, T.; Atazhanova, G.; Makubayeva, A.; Serafimovich, M.; Kabanova, S.; Rakhimzhanov, A.; Adekenov, S. Composition of essential oil of leaves and fruits of green strawberry (Fragaria viridis Weston) growing wild in Northern Kazakhstan. J. Appl. Bot. Food Qual. 2019, 92, 39–48. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.; Harizanis, P.; Polissiou, M. Evaluation of four isolation techniques for honey aroma compounds. J. Sci. Food Agric. 2005, 85, 91–97. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Pozzoli, C.; Terno, M.; Canilli, L.; Angarano, M.; Maranta, N.; Dell’Agli, M.; et al. Unveiling the Ability of Witch Hazel (Hamamelis virginiana L.) Bark Extract to Impair Keratinocyte Inflammatory Cascade Typical of Atopic Eczema. Int. J. Mol. Sci. 2022, 23, 9279. [Google Scholar] [CrossRef] [PubMed]
- Mielnik, M.B.; Sem, S.; Egelandsdal, B.; Skrede, G. By-products from herbs essential oil production as ingredient in marinade for turkey thighs. LWT-Food Sci. Technol. 2008, 41, 93–100. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted Extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 113–156. ISBN 978-1-84973-606-0. [Google Scholar]
- Zheng, X.; Xu, X.; Liu, C.; Sun, Y.; Lin, Z.; Liu, H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep. Purif. Technol. 2013, 104, 17–25. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Pereira, A.G.; Cruz, L.; Cassani, L.; Chamorro, F.; Lourenço-Lopes, C.; Freitas, V.; Otero, P.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J.; et al. Comparative Study of Microwave-Assisted Extraction and Ultrasound-Assisted Extraction Techniques (MAE vs. UAE) for the Optimized Production of Enriched Extracts in Phenolic Compounds of Camellia japonica var Eugenia de Montijo. Eng. Proc. 2023, 37, 124. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Ahmed, M.N.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.; Maggi, F.; Caprioli, G.; Sakar, E.H.; et al. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Višnjevec, A.M.; Barp, L.; Lucci, P.; Moret, S. Pressurized liquid extraction for the determination of bioactive compounds in plants with emphasis on phenolics. TrAC Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Nardi, T.; Zocca, F.; Giacomini, A.; Corich, V. Evaluation of Red Chicory Extract as a Natural Antioxidant by Pure Lipid Oxidation and Yeast Oxidative Stress Response as Model Systems. J. Agric. Food Chem. 2011, 59, 5318–5324. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Guo, P.; Chen, H.; Ma, J.; Zhang, Y.; Chen, H.; Wei, T.; Gao, D.; Li, J. Enzyme-assisted extraction, characterization, and in vitro antioxidant activity of polysaccharides from Potentilla anserina L. Front. Nutr. 2023, 10, 1216572. [Google Scholar] [CrossRef]
- Wang, D.; Wang, B.; Jin, X.; Peng, Y.; Zhao, J.; Zhang, M.; Wei, Y.; Long, Z.; Chen, Q. Microwave-enzyme-assisted extraction of pectin from feijoa (Acca sellowiana) fruit: Extraction optimization, physicochemical and functional properties. LWT 2024, 204, 116445. [Google Scholar] [CrossRef]
- Amulya, P.R.; ul Islam, R. Optimization of enzyme-assisted extraction of anthocyanins from eggplant (Solanum melongena L.) peel. Food Chem. X 2023, 18, 100643. [Google Scholar] [CrossRef]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, G.N.; Coelho, M.A.Z.; Marrucho, I.M.; Ribeiro, B.D. Enzyme-assisted extraction of carotenoids and phenolic compounds from sunflower wastes using green solvents. 3 Biotech 2020, 10, 405. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Vergara-Barberán, M.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Use of an enzyme-assisted method to improve protein extraction from olive leaves. Food Chem. 2015, 169, 28–33. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Basegmez, H.I.O.; Povilaitis, D.; Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Alasalvar, C.; Venskutonis, P.R. Biorefining of blackcurrant pomace into high value functional ingredients using supercritical CO2, pressurized liquid and enzyme assisted extractions. J. Supercrit. Fluids 2017, 124, 10–19. [Google Scholar] [CrossRef]
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-Assisted Extraction of Bioactive Compounds from Raspberry (Rubus idaeus L.) Pomace. J. Food Sci. 2019, 84, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Blikra, M.J.; Skipnes, D.; Skåra, T. On the use of pulsed electric field technology as a pretreatment to reduce the content of potentially toxic elements in dried Saccharina latissima. LWT 2022, 169, 114033. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of Pulsed Electric Field Treatment for the Extraction of Bioactive Compounds from Blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G. V Changes in Quality Characteristics of Strawberry Juice After Equivalent High Pressure, Ultrasound, and Pulsed Electric Fields Processes. Food Eng. Rev. 2021, 13, 601–612. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Greiner, R.; Orlien, V.; Lebovka, N.I. Negative pressure cavitation extraction: A novel method for extraction of food bioactive compounds from plant materials. Trends Food Sci. Technol. 2016, 52, 98–108. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.-X.; Kou, P.; Zhao, C.-J.; Fu, Y.-J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Wang, T.; Xu, W.-J.; Wang, S.-X.; Kou, P.; Wang, P.; Wang, X.-Q.; Fu, Y.-J. Integrated and sustainable separation of chlorogenic acid from blueberry leaves by deep eutectic solvents coupled with aqueous two-phase system. Food Bioprod. Process. 2017, 105, 205–214. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Chandel, A.; Smitha, P.M.; Semwal, A.D. High pressure homogenization and retention of bioactive compounds in fruits and vegetables products. Food Humanit. 2023, 1, 1559–1569. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Moscovici Joubran, A.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The effect of pressure level and cycling in high-pressure homogenization on physicochemical, structural and functional properties of filtered and non-filtered strawberry nectar. Innov. Food Sci. Emerg. Technol. 2019, 57, 102203. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef]
- Pech-Almeida, J.L.; Téllez-Pérez, C.; Alonzo-Macías, M.; Teresa-Martínez, G.D.; Allaf, K.; Allaf, T.; Cardador-Martínez, A. An Overview on Food Applications of the Instant Controlled Pressure-Drop Technology, an Innovative High Pressure-Short Time Process. Molecules 2021, 26, 6519. [Google Scholar] [CrossRef]
- Ranjbar, N.; Eikani, M.H.; Javanmard, M.; Golmohammad, F. Impact of instant controlled pressure drop on phenolic compounds extraction from pomegranate peel. Innov. Food Sci. Emerg. Technol. 2016, 37, 177–183. [Google Scholar] [CrossRef]
- Rahnemoon, P.; Sarabi-Jamab, M.; Javanmard, M.; Bostan, A.; Safari, O. Comparison of two methods of solvent extraction of phenolic compounds from pomegranate (Punica granatum L.) peels. J. Agric. Sci. Technol. 2018, 20, 939–952. [Google Scholar]
- Gandhi, K.; Arora, S.; Kumar, A.; Kamal Gandhi, C. Industrial applications of supercritical fluid extraction: A review. Int. J. Chem. Stud. 2017, 5, 235–243. [Google Scholar]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- RAMSEY, E.; SUN, Q.; ZHANG, Z.; ZHANG, C.; GOU, W. Mini-Review: Green sustainable processes using supercritical fluid carbon dioxide. J. Environ. Sci. 2009, 21, 720–726. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Liu, H.; Chen, B.-Q.; Pan, Y.-J.; Fu, C.-P.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Role of supercritical carbon dioxide (scCO2) in fabrication of inorganic-based materials: A green and unique route. Sci. Technol. Adv. Mater. 2021, 22, 695–717. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; Carvalho Junior, R.N. de From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Gondo, T.F.; Jönsson, M.; Karlsson, E.N.; Sandahl, M.; Turner, C. Extractability, selectivity, and comprehensiveness in supercritical fluid extraction of seaweed using ternary mixtures of carbon dioxide, ethanol, and water. J. Chromatogr. A 2023, 1706, 464267. [Google Scholar] [CrossRef]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Marques, M.P.; Brito, M.S.C.A.; Negro, C.; Monte, M.C.; Manrique, Y.A.; Santos, R.J.; Blanco, A. Valorization of Vegetable Waste from Leek, Lettuce, and Artichoke to Produce Highly Concentrated Lignocellulose Micro- and Nanofibril Suspensions. Nanomaterials 2022, 12, 4499. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Manoni, M.; Fumagalli, F.; Rovere, N.; Luciano, A.; Ottoboni, M.; Ferrari, L.; Cheli, F.; Djuragic, O. Reduce, Reuse, Recycle for Food Waste: A Second Life for Fresh-Cut Leafy Salad Crops in Animal Diets. Animals 2020, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Lettuce and Chicory Byproducts as a Source of Antioxidant Phenolic Extracts. J. Agric. Food Chem. 2004, 52, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Manco, M.A.; D’Antuono, L.F. Variation of sesquiterpene lactones and phenolics in chicory and endive germplasm. J. Food Compos. Anal. 2015, 39, 77–86. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, N. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef]
- D′evoli, L.; Morroni, F.; Lombardi-Boccia, G.; Lucarini, M.; Hrelia, P.; Cantelli-Forti, G.; Tarozzi, A. Red Chicory (Cichorium intybus L. cultivar) as a Potential Source of Antioxidant Anthocyanins for Intestinal Health. Oxid. Med. Cell. Longev. 2013, 2013, 704310. [Google Scholar] [CrossRef]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Vieira do Carmo, M.A.; Azevedo, L.; Marques, M.B.; Granato, D.; Rosso, N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef]
- Sinkovič, L.; Demšar, L.; Žnidarčič, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef]
- Baiano, A.; Bevilacqua, L.; Terracone, C.; Contò, F.; Del Nobile, M.A. Single and interactive effects of process variables on microwave-assisted and conventional extractions of antioxidants from vegetable solid wastes. J. Food Eng. 2014, 120, 135–145. [Google Scholar] [CrossRef]
- Cova, C.M.; Boffa, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules 2019, 24, 2681. [Google Scholar] [CrossRef] [PubMed]
- Baixinho, J.P.; Anastácio, J.D.; Ivasiv, V.; Cankar, K.; Bosch, D.; Menezes, R.; de Roode, M.; dos Santos, C.N.; Matias, A.A.; Fernández, N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules 2021, 26, 2583. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Aamer, R.A.; Ali, A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar] [CrossRef]
- Stökle, K.; Jung, D.; Kruse, A. Acid-assisted extraction and hydrolysis of inulin from chicory roots to obtain fructose-enriched extracts. Biomass Convers. Biorefinery 2023, 13, 159–170. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Wojtczuk, M.; Roszko, M.; Bryła, M.; Trajkovska Petkoska, A. Recent advances and opportunities related to the use of bee products in food processing. Food Sci. Nutr. 2023, 11, 4372–4397. [Google Scholar] [CrossRef]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Ávila, S.; Hornung, P.S.; Teixeira, G.L.; Malunga, L.N.; Apea-Bah, F.B.; Beux, M.R.; Beta, T.; Ribani, R.H. Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin. Food Res. Int. 2019, 123, 1–10. [Google Scholar] [CrossRef]
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Reports 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Yung An, C.; Rao, P.V.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of Phenolic Acids and Flavonoids in Monofloral Honey from Bangladesh by High Performance Liquid Chromatography: Determination of Antioxidant Capacity. Biomed Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P. Analysis of 22 free amino acids in honey from Eastern Europe and Central Asia using LC-MS/MS technique without derivatization step. J. Food Compos. Anal. 2021, 98, 103837. [Google Scholar] [CrossRef]
- Hermosín, I.; Chicón, R.M.; Dolores Cabezudo, M. Free amino acid composition and botanical origin of honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of Organic Acids in Honey by Liquid Chromatography with Tandem Mass Spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production of d-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, M.; Wang, K.; Yang, E.; Su, J.; Wang, Q.; Cheng, N.; Xue, X.; Wu, L.; Cao, W. Identification and quantitation of bioactive components from honeycomb (Nidus vespae). Food Chem. 2020, 314, 126052. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Orantes-Bermejo, F.J.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Sánchez-González, C.; Llopis, J.; Rivas-García, L.; Afrin, S.; Varela-López, A.; et al. Are by-products from beeswax recycling process a new promising source of bioactive compounds with biomedical properties? Food Chem. Toxicol. 2018, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Carmo dos Santos, N.A.; Ferrarese, I.; Rizzo, F.; Bernabè, G.; Paccagnella, M.; Panozzo, M.; Francescato, S.; Castagliuolo, I.; Dall’Acqua, S.; et al. The beeswax processing by-product: A potential antibacterial ingredient for food and nutraceutical applications. Int. J. Food Sci. Technol. 2023, 58, 5549–5556. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, bioavailability, bioactivity, nutritional aspects and human health benefits: A review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ahuja, J.K.C.; Burton-Freeman, B.M. Characterization of the nutrient profile of processed red raspberries for use in nutrition labeling and promoting healthy food choices. Nutr. Health Aging 2019, 5, 225–236. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; Sotelo-González, A.M.; Patiño-Ortiz, P.; Rocha-Guzmán, N.E.; Pérez-Ramírez, I.F. Berry by-products obtained from a decoction process are a rich source of low- and high-molecular weight extractable and non-extractable polyphenols. Food Bioprod. Process. 2021, 127, 371–387. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Polyphenol Extraction from Food (by) Products by Pulsed Electric Field: A Review. Int. J. Mol. Sci. 2023, 24, 15914. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef]
- Kitrytė, V.; Laurinavičienė, A.; Syrpas, M.; Pukalskas, A.; Venskutonis, P.R. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 6046074. [Google Scholar] [CrossRef]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, M.; Fernández-Prior, Á.; Bermúdez Oria, A.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT 2020, 130, 109645. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Silva, J.T.; Borges, M.H.; de Souza, C.A.; Fávaro-Trindade, C.S.; Sobral, P.J.; de Oliveira, A.L.; Martelli-Tosi, M. Grape Pomace Rich-Phenolics and Anthocyanins Extract: Production by Pressurized Liquid Extraction in Intermittent Process and Encapsulation by Spray-Drying. Foods 2024, 13, 279. [Google Scholar] [CrossRef]
- da Rocha, C.B.; Noreña, C.P.Z. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Viskelis, P.; Jasutiene, I.; Viskeliene, R.; Bobinas, C. Impact of various factors on the composition and stability of black currant anthocyanins. Food Res. Int. 2005, 38, 867–871. [Google Scholar] [CrossRef]
- Yüksekkaya, Ş.; Başyiğit, B.; Sağlam, H.; Pekmez, H.; Cansu, Ü.; Karaaslan, A.; Karaaslan, M. Valorization of fruit processing by-products: Free, esterified, and insoluble bound phytochemical extraction from cherry (Prunus avium) tissues and their biological activities. J. Food Meas. Charact. 2021, 15, 1092–1107. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Sady, S.; Ligaj, M.; Pachołek, B.; Błaszczyk, A.; Płaczek, Z.; Dłużniewska, N.; Kawałek, P.; Pakuła, K.; Konopelski, A.; Gołaszewski, E. Designing the Quality Characteristics of Berry Processing Byproducts Using Fermentation. Appl. Sci. 2024, 14, 3110. [Google Scholar] [CrossRef]
- Krivokapić, S.; Vlaović, M.; Damjanović Vratnica, B.; Perović, A.; Perović, S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef]


| Extraction Techniques | Advantages | Disadvantages |
|---|---|---|
| Traditional techniques | ||
| Maceration (solvent extraction) |
|
|
| Soxhlet extraction |
|
|
| Ultrasound-assisted extraction (UAE) |
|
|
| Hydro-distillation |
|
|
| Innovative techniques | ||
| Microwave-assisted extraction (MAE) |
|
|
| Pressurized liquid extraction (PLE) |
|
|
| Supercritical CO2 extraction (SFE-CO2) |
|
|
| Enzyme-assisted extraction (EAE) |
|
|
| Pulsed electric fields (PEF) |
|
|
| Negative pressure Cavitation (NPC) |
|
|
| High pressure homogenization (HPH) |
|
|
| Instant controlled pressure drop (DIC) |
|
|
| Byproduct (Pomace) | Compound(s) | Extraction Method | Yield | Extraction Conditions | Proposed Use of Extracts | Ref. |
|---|---|---|---|---|---|---|
| Grape | Polyphenols, anthocyanins, tannins. | EAE, MWE, UAE, Soxhlet. | Up to 2.98 mg/g total phenolics. | Cellulase is used as enzyme. Solvent: ethanol (50% water). | Antioxidant and antibacterial agents in food packaging and products. | [152] |
| Grape | Catechin, gallic acid, flavan-3-ols. | EAE with tannase, UAE. | 439 g/kg GAE total phenolics, 43.5 g/kg catechin, 4.5 g/kg quercetin. | Enzyme: tannase; solvent: water. | Enhanced antioxidant activity. | [153] |
| Grape | Flavanols and anthocyanins. | PLE using an intermittent process. | Total phenolic content: 97.4 GAE/g dry basis. | Solvent: 40% ethanol. | Antioxidant for applications in food, cosmetics, and pharmaceuticals. | [154] |
| Grape | Total phenolics, anthocyanins. | UAE, MAE. | Higher yields with MAE (at 1000 W for 10 min). | UAE: 450 W, 15 min; MAE: 1000 W, 10 min. | Antioxidant potential, food industries. | [155] |
| Grape | Polyphenols, anthocyanins. | Accelerated Solvent Extraction (ASE), SFE. | ASE at 50:50 ethanol/water at 80–140 °C yielded highest procyanidins. | ASE: 80–140 °C, ethanol/water (50:50), SFE: 2000 psi. | Nutraceuticals, food additives. | [156] |
| Blackcurrant | Anthocyanins, phenolic acids. | Freeze-drying, Soxhlet extraction. | Not specified. | Water and alcohol (60:40). | Antioxidant and prebiotic. | [157] |
| Blackcurrant | Anthocyanins, flavonoids. | MAE, solid-liquid extraction (SLE). | Total phenolics: 18.45 mg/g. | Solvent: methanol (40%); conditions: 70–80 °C, 15 min. | Antioxidant products. | [158] |
| Cranberry | Anthocyanins, flavonols, procyanidins. | Alcohol extraction, freeze-drying. | Not specified. | Ethanol: water (70:30). | Antibacterial effects against Salmonella in food. | [157] |
| Strawberry | Quercetin-3-glucuronide, ellagic acid, malic acid, p-coumaric acid. | SLE, PLE. | Quercetin: 15.60 mg/g, Total phenolics: 15.34 mg/g. | Solvent: ethanol (50%); conditions: 60 °C, 90 min. | Nutraceuticals, food additives. | [159] |
| Blueberry | Anthocyanins, flavonoids (e.g., chlorogenic acid). | UAE, SFE. | Anthocyanins: 72.27 mg/g, Polyphenols: 900–1300 mg/g. | Solvent: ethanol, scCO2; conditions: 20 °C, 2000 psi for SFE. | Functional foods, dietary supplements. | [160] |
| Chokeberry | Polyphenols (e.g., chlorogenic acid, flavonoids), ascorbic acid. | Fermentation, UAE. | Not specified. | Solvent: ethanol (50%); fermentation with yeast cultures. | Fortified foods, natural colorants. | [161] |
| Raspberry | Gallic, p-coumaric, caffeic, quercitrin, chlorogenic, ellagic acids, total phenolics, flavonoids, anthocyanins. | UAE, maceration. | TPC: 27.79 mg GAE/L, TFC: 8.02 mg QE/g, TAC: 7.13 mg C3G Eq/L. | UAE: 450 W for 15 min; maceration: conventional organic solvent extraction. | Antioxidants, dietary supplements. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron, G.; Ferrarese, I.; Carmo Dos Santos, N.; Rizzo, F.; Gargari, G.; Bertoli, N.; Gobbi, E.; Perosa, A.; Selva, M.; Dall’Acqua, S. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Appl. Sci. 2024, 14, 10785. https://doi.org/10.3390/app142310785
Peron G, Ferrarese I, Carmo Dos Santos N, Rizzo F, Gargari G, Bertoli N, Gobbi E, Perosa A, Selva M, Dall’Acqua S. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Applied Sciences. 2024; 14(23):10785. https://doi.org/10.3390/app142310785
Chicago/Turabian StylePeron, Gregorio, Irene Ferrarese, Nadia Carmo Dos Santos, Filippo Rizzo, Giorgio Gargari, Noemi Bertoli, Emanuela Gobbi, Alvise Perosa, Maurizio Selva, and Stefano Dall’Acqua. 2024. "Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts" Applied Sciences 14, no. 23: 10785. https://doi.org/10.3390/app142310785
APA StylePeron, G., Ferrarese, I., Carmo Dos Santos, N., Rizzo, F., Gargari, G., Bertoli, N., Gobbi, E., Perosa, A., Selva, M., & Dall’Acqua, S. (2024). Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Applied Sciences, 14(23), 10785. https://doi.org/10.3390/app142310785










