Abstract
High-pressure processing (HPP) is a promising technology for increasing the shelf life of food, with minimal effects on the nutritional or sensory quality. However, there has been a concern that high-oil-content foods may protect food pathogens in HPP, and that HPP can affect the quality of lipids. We inoculated Listeria monocytogenes and Salmonella Typhimurium into 34% and 54% oil-content pesto sauce, processed them either with HPP (600 MPa, 4 min) or thermal processing (82 °C, 5 min), and analyzed bacteria counts, pH, GC-MS (Terpene compounds), the time–kill kinetic study, and lipid oxidation value for 60 days in refrigerating storage (5 ± 2 °C). Our findings show that HPP significantly reduced the number of bacteria (more than 4-log) compared to thermal processing or non-processing. Additionally, we discovered terpene compounds (highest-level terpene: L-linalool, eugenol, and 1,8-cineol) in pesto oil that exhibit antimicrobial activity. Different oil content did not have any significant effect on bacteria levels. Regarding chemical results, all samples were of acceptable quality, and the processes did not show any negative effect on lipid oxidation (Peroxide and P-Anisidine value under 10 meq per kilogram of oil). In conclusion, our study indicates that HPP is a suitable method for high-oil-content pesto sauce. In addition, functional compounds naturally present in pesto may contribute to maintaining its microbial and chemical quality.
1. Introduction
With the media giving much coverage to health, consumers today are more aware of health aspects and interested in the quality of life. Understanding has risen as to the importance of safe and quality food among consumers, to enhance their own well-being, as well as reduce the cases of diseases such as diabetes and cardiac disorders due to improper lifestyles [1]. Due to what consumers want, the quality of food now relates to food security, nutrition properties, sensory characteristics, and sustainability [2]. In this result, the connection between quality and healthiness of food products has gathered power [3]. Considering the wishes of consumers, some non-thermal methods of processing were brought, to obtain high-quality, safe, and nutritional food products [4,5]. However, Conventional thermal processing methods usually compromise the nutritional integrity, taste, and texture of foods by exposure to high temperatures. Non-thermal methods such as HPP, pulsed electric fields, and ultrasound have great potential for preserving heat-labile nutrients, extending the shelf life of food, and demonstrating higher environmental friendliness [6,7]. Non-thermal processing is a promising approach in fulfilling the requirements of advanced food safety and sustainable development, matching consumers’ needs at the present time [8,9].
HPP is a processing method that kills potential pathogens through the application of high pressure, rather than heat, to food [10]. The processing is typically carried out in sales packaging. HPP is conventionally applied to liquid and solid foods with high water content, over 87%, like ready-to-eat meals, vegetables, fruits, and dairy products [11,12]. HPP is uniform and instantaneous, unlike thermal processes [13]. The pressure transmission is independent of both mass and time, and, for this reason, the process is short, and minimal nutrient change occurs in food products. Furthermore, the scalability of this technique may be easily and safely transferred from the laboratory to the industry, because HPP is not reliant on the product geometry and size [14].
The European Food Safety Authority (EFSA) has evaluated the safety and efficacy of HPP and believes it is capable of reaching adequate reductions of harmful microorganisms without introducing other safety risks [15]. Since HPP does not denature covalent bonds, it preserves the properties of food products; hence, only the taste, texture, and nutritional value are minimally affected. The technology is active on pathogens such as Listeria spp., Salmonella spp., and Brucella spp., besides proteins associated with microbial replication [16,17]. The improvement in food safety includes the inhibition of essential enzyme synthesis, damage to the microbial cell membrane, disruption of ion exchange and permeability, and leakage of cellular content [17,18]. However, it is important to underline the fact that the efficacy of HPP should be measured in various food matrices.
HPP is currently utilized for a variety of products. In addition to deactivating microorganisms, it is crucial to maintain the stability of other compounds, particularly fats, after undergoing the process. Some studies have reported that a high lipid content in food materials, such as peanut butter and cheese, increases resistance against spoilage and pathogenic bacteria. This is due to the low water activity rendered by lipids [19,20]. More recently, lipid fractions have been the focus of research on how they respond to HPP, since harmful secondary products of oxidation can inflict very negative impacts on the final product [21]. Numerous studies have demonstrated that high-pressure processing in high-fat foods leads to increased lipid oxidation [22,23]. A study on yak fat revealed that applying 600 Mbar pressure significantly increased secondary oxidation and caused changes in the amount of fatty acids, particularly unsaturated fatty acids [24]. Additionally, various studies on lipid oxidation after high-pressure processing in food products containing fat and high fat, such as pork meat and fat [25], salmon [26], cold-smoked salmon [27] and beef muscle [28] have indicated that oxidation can occur at pressures ranging from 200 to 600 Mbar, depending on the nature of the food. The compression resulting from the HPP can increase the temperature of the food by 3 °C per 100 Mbar [29], and, in high-fat food, the increase is approximately 8 to 9 °C [30]; after the decompression, the food’s temperature returns to its initial level. This temperature rise, along with the composition of fatty acids and time of process, can influence the oxidation rate of lipids in high-fat products [21].
Pesto sauce is a flavorful sauce that is employed in variety of dishes. Its main ingredients are basil, along with cheese, oil, and nuts. The unique taste and aroma of pesto come from basil. This distinct aroma and flavor are due to terpene compounds in basil, such as methyl cinnamate, linalool, eugenol, eucalyptol, and estragole [31,32]. These compounds not only create the aroma and flavor in basil but also have beneficial properties, such as antimicrobial and antioxidant effects [33]. Several studies reported that the antioxidant and antimicrobial potential of essential oil is related to the presence of terpene and aromatic compounds [34].
The aim of the study will be to investigate the combined impact of high-pressure processing (HPP) and formulation ingredients on the microbial and chemical properties of pesto sauce with high oil content. It is important to understand whether high oil content can protect bacteria and change HPP efficacy or not, as well as how HPP can change lipid quality in pesto sauce with functional ingredients (basil).
2. Materials and Methods
2.1. Sample Preparation
The pesto sauces (Välimeren Herkut Pesto-Kastike, including rape oil, basil (41.5%), cheese, olive oil, sunflower seeds, salt, sugar, garlic, black pepper, ascorbic acid, and potassium sorbate) were processed with 34% and 54% oil content in the Välimeren Herkut factory of Nurmijärvi, Finland, and delivered to the laboratory of the university under cold-chain conditions of 5 ± 2 °C. The samples were inoculated with bacteria (Listeria monocytogenes ATCC 4342 and Salmonella enterica subsp. enterica serovar Typhimurium (Salmonella Typhimurium) ATCC 13311) (ATCC, Manassas, VA 20110, USA) and repacked in 50 mL HPP-tested PET bottles (50 g pesto sauce) for the next step, and some were repacked without bacteria to evaluate processing properties. All samples were divided according to the processing method into three groups: non-processed (NP), thermal processed (TP), and HPP (HP). The samples of the HPP-treated group were transferred to the Toripiha factory (Suonenjoki, Finland) under conditions of the cold chain (5 ± 2 °C) for the process of HPP (600 MPa, 4 min). After the process was completed, the samples were taken back to the laboratory of the university for the examination of microbial and chemical features. At the university laboratory, the water-bath thermal processing was carried out at 82 °C for 5 min. A thermometer within the paralleled samples assured that the expected temperature had been reached. Finally, all the samples were then stored under cold conditions (5 ± 2 °C) for 60 days, to evaluate their storage stability and properties.
2.2. Bacteria Inoculation
The L. monocytogenes and S. Typhimurium were inoculated into the Tryptic Soy Broth medium (84675.0500, VWR, Avantor, Aurora, OH, USA) and then incubated (B8000, Termaks, Upplands Väsby, Sweden) for 24 h at 37 °C to activate the cells. The inoculum suspension was standardized using the 0.5 McFarland standard method with a spectrophotometer (UV-1600PC, VWR, Aurora, OH, USA) [35]. Then, the bacteria were prepared in amounts that would be added to the pesto sauces, which aimed for approximately 6–7 log CFU/g [36]. The inoculated samples were designated for making pesto into the following groups: LNP (pesto prepared with L. monocytogenes, non-processed), LTP (pesto prepared with L. monocytogenes, thermal processed), LHP (pesto prepared with L. monocytogenes, HPP processed), SNP (pesto prepared with S. Typhimurium, non-processed), STP (pesto prepared with S. Typhimurium, thermal processed), and SHP (pesto prepared with S. Typhimurium). The samples without inoculated bacteria were named NP, TP, or HP according to the processing method.
2.3. Microbiological Analysis
2.3.1. Bacteria Enumeration
To assess the microbiological quality of the pesto sauce, bacterial counts were determined by placing 10 g of pesto sauce in 90 mL of sterile 0.9% NaCl (7647-14-5, Fisher Chemical, Roskilde, Denmark) solution and mixing it for 1 min in a Stomacher blender (Stomacher 400 circulator, Seward, Easting CI, UK) at 260 rpm. After homogenization, additional decimal dilutions were prepared using 0.9% NaCl solution as the diluent. Subsequently, 100 µL of the final three dilutions was transferred to ready-to-use Petri-dish media (82.1194.500, SARSTEDT, Hanover, Germany). All counts were expressed as log CFU per gram and were obtained in duplicate. L. monocytogenes and S. Typhimurium counts were determined using Listeria Selective Agar base Ottaviani and Agosti (LSA) (84748.0500, VWR, Avantor, Aurora, OH, USA) and Xylose Lysine Deoxycholate agar (XLD) (84667.0500, VWR, Avantor, Aurora, OH, USA), respectively, with incubation at 37 °C for 48 h.
2.3.2. Time–Kill Kinetic Studies
The time–kill kinetic study [37] was carried out with 500 µL oil separated from pesto sauce (the oil was separated from the solid materials of the pesto by simply centrifuging at 4000 rpm for 5 min (5415D, Eppendorf, Hamburg, Germany)) in 10 mL Tryptic soy broth medium (84675.0500, VWR, Avantor, USA), which was inoculated (0.5 McFarland standard method, using spectrophotometer (UV-1600PC, VWR, USA)) [35] with L. monocytogenes and S. Typhimurium, separately. Samples were taken at different time points (0, 2, 4, 6, and 24 h) during the experiment. Each sample was 1 mL in volume and was mixed with a saline solution to create serial dilutions. After dilution, 100 µL from each dilution was placed onto specific mediums (LSA and XLD medium) and then kept in an incubator for 24 h at 37 °C. This process was repeated three times, to ensure accuracy. The growth rate was then calculated before and after treatment with oil, and expressed as log CFU/mL.
2.4. Chemical Analysis
2.4.1. GC/MS Analysis
Terpenes in the oil phases of pestos were monitored with gas chromatography–mass spectrometry (GC-MS). Each sample was analyzed as three replicants. The oil was separated from the solid materials of the pesto by simply centrifuging at 4000 rpm for 5 min (5415D, Eppendorf, Germany) and pipetting. Then pesto oil samples were diluted with hexane at a 1:10 ratio, the internal standard (99% 1-chlorooctane, Alfa Aesar/Thermo, Thermo Scientific Chemicals, Haverhill, Boston, MA, USA) was added to the samples at a 1:105 ratio, and the samples were filtered using Whatman Mini-UniPrep G2 Syringeless Filters (GE HealthCare, Amersham, UK). The analysis was performed with a GC-MS device consisting of GCMS-QP2010 Ultra and AOC-5000 Plus injection system (Shimadzu Scientific Instruments, Columbia, MD, USA). A 1 µL sample was injected into the injector at 280 °C temperature and split 5. Separation of compounds was performed with BP-5 column (30 m/0.25 mm/0.25 µm, Trajan Scientific and Medical, RingWood, Australia) using oven temperature 40–280 °C and helium (N60, Woikoski, Mäntyharju, Finland) as a carrier gas with linear velocity 39.5. The temperatures of ion source and interface were 200 and 290 °C, respectively, and ions were scanned at 33–400 m/z. The compounds were tentatively identified by comparing their mass spectra with data from mass spectral libraries (NIST 11 Mass Spectral library, The National Institute of Standards and Technology, USA Wiley Registry 10th Edition, John Wiley & Sons, USA and Flavour & Fragrance Natural & Synthetic Compounds GCMS library FFNSC 2, Shimadzu Corp, Kyoto, Japon) and checking their retention indexes with alkane standards (C8-C40 Alkanes Calibration Std, Supelco, Bellefonte, PA, USA). The areas of the GC-MS signal intensities of the terpenes were integrated in GCMSsolution software version 4.20 (Shimadzu, Scientific Instruments, Haverhill, Boston, USA) to check if the processes affected the levels of terpenes in pesto oils.
The coefficient of variation of the GC-MS method was monitored with the internal standard, and it was 16.0% as calculated from all the samples in the GC-MS (n = 18). Two replicant samples were excluded from the analysis, since their signal levels varied more than 30% from those of the other two replicants and were therefore considered as outliers.
2.4.2. pH
A 2 g sample of pesto sauce was mixed with 8 mL of deionized water for 2 min. The pH was then measured at room temperature using a digital pH meter (CG 842, SCHOTT, Mainz, Germany) with a probe-type combined electrode (Ingold, Amsterdam, The Netherlands) by immersing the electrode directly into the mixture.
2.4.3. Total Phenolic Content (TPC)
Two mL vials containing 0.5 g of frozen pesto sauce samples were spiked with internal standards and homogenized with methanol (20864.320, VWR, Avantor, USA) (1.3 mL) for 2 min in a Tissulyzer LT homogenizer (85600, Qiagen, Venlo, The Netherlands). The obtained extracts were centrifuged at 15,000× g for 10 min at 4 °C using a 5424R centrifuge (Eppendorf, Hamburg, Germany) [38]. The supernatant collected was used to determine the TPC, based on the method by Hilma et al. (2018), but with some modifications [39]. Briefly, 100 µL of supernatant was mixed with 100 µL of 0.1 N Folin–Ciocalteu reagent (1090010100, Merck, Darmstadt, Germany) in a 2 mL vial and incubated for 1 min at room temperature in the dark. A total of 500 μL of 7% Na2CO3 solution (31432, Riedel-de Haën, Hanover, Germany) and 1.3 mL of deionized water were added to the mixture. The mixture was additionally incubated at room temperature in darkness for 120 min before taking absorbance at 750 nm. Phenolic content is obtained from the absorption against the standard curve previously developed (Supplementary S1. TPC content was expressed in milligrams of Gallic Acid (398225-100, Sigma-Aldrich, Steinheim, Germany) Equivalents (GAEs) per 100 g of pesto sauce.
2.4.4. Total Antioxidant Capacity
Antioxidant activity percentage (AA%) was evaluated according to Kulkarani and Aradhya (2005) methods, with some modification [40]. A 0.1 mL sample (same sample extract in TPC) was mixed with 0.9 mL methanol and, after that, mixed with 1 mL 2,2-diphenyl-1-picrylhydrazil (DPPH) (0.500 µM in methanol). The control sample was prepared in a similar way, and the sample was replaced with water. The solution was mixed very well and kept in dark conditions for 30 min. The absorbance of the final solution was determined at 517 nm by a spectrophotometer. The antioxidant activity was calculated using the following equation:
2.4.5. Lipid Oxidation
The present study assessed the peroxide value as an indicator of initial oxidation and the p-Anisidine value as an indicator of secondary oxidation [41]. To determine the peroxide value of pesto sauce after various processing methods, the oil part was first separated by centrifuging (5415D, Eppendorf, Germany) control samples at 4000 rpm for 5 min and keeping the oil at 4–6 °C. Chemical solutions for the titration method were prepared, including an acetic acid–chloroform solution, saturated potassium iodide (7681-11-0, Merck, Germany) solution, 0.1 M sodium thiosulfate (7772-98-7, Sigma-Aldrich, Germany) solution, 10% sodium lauryl sulfate (Riedel-de Haën, Hanover, Germany), and 1% starch (9005-84-9, Thermo Fisher, Rochester, New York, NY, USA) indicator solution. The titration technique required weighing 5 g of extracted oil, dissolving it in a mixture of acetic acid (64-19-7, VWR, Amsterdam, The Netherlands) and chloroform (64-19-7, VWR, Amsterdam, The Netherland), and adding potassium iodide solution. The mixture was then titrated using sodium thiosulfate until a color change appeared. To liberate all iodine from the chloroform layer, sodium lauryl sulfate and starch indicator were added, and then titrated until the blue color disappeared [42]. The volume of titrant used in both blank and control samples was recorded, and the peroxide value was determined using the following equation:
S = Volume of titrant used for control samples (mL)
B = Volume of titrant used for blank (mL)
M = Molarity of sodium thiosulfate (Na2S2O3) standard solution
To determine the p-anisidine content of the pesto sauce control sample, p-anisidine reagent (S8426458346, Sigma-Aldrich, Germany) was made by dissolving 0.25 g of p-anisidine in 100 mL of glacial acetic acid (64-19-7, VWR, The Netherlands), protected from light with aluminum foil. For the measurement, 4 g of the oil was diluted with isooctane in a 25 mL volumetric flask, and then 1 mL of this solution was diluted with 9 mL isooctane (540-84-1, Merck, Germany). The absorbance (Ab) of this solution was measured at 350 nm using UV–Visible spectroscopy, with isooctane serving as the blank. The oil solution and isooctane were then combined with 1 mL of p-anisidine reagent in separate test tubes, agitated, and left for 10 min. The absorbance (As) of the oil solution was measured again at 350 nm, using the isooctane mixture as the blank. The p-anisidine value was eventually obtained using the following equation [43]:
2.5. Statistical Analysis
All experiments were carried out in triplicate. One-way analysis of variance (ANOVA) and the Duncan group were used to determine the significance of differences (p < 0.05) between means. GraphPad Prism 10 was used for the statistical analysis.
3. Results
3.1. Bacteria Enumeration Results of the Pesto Sauces
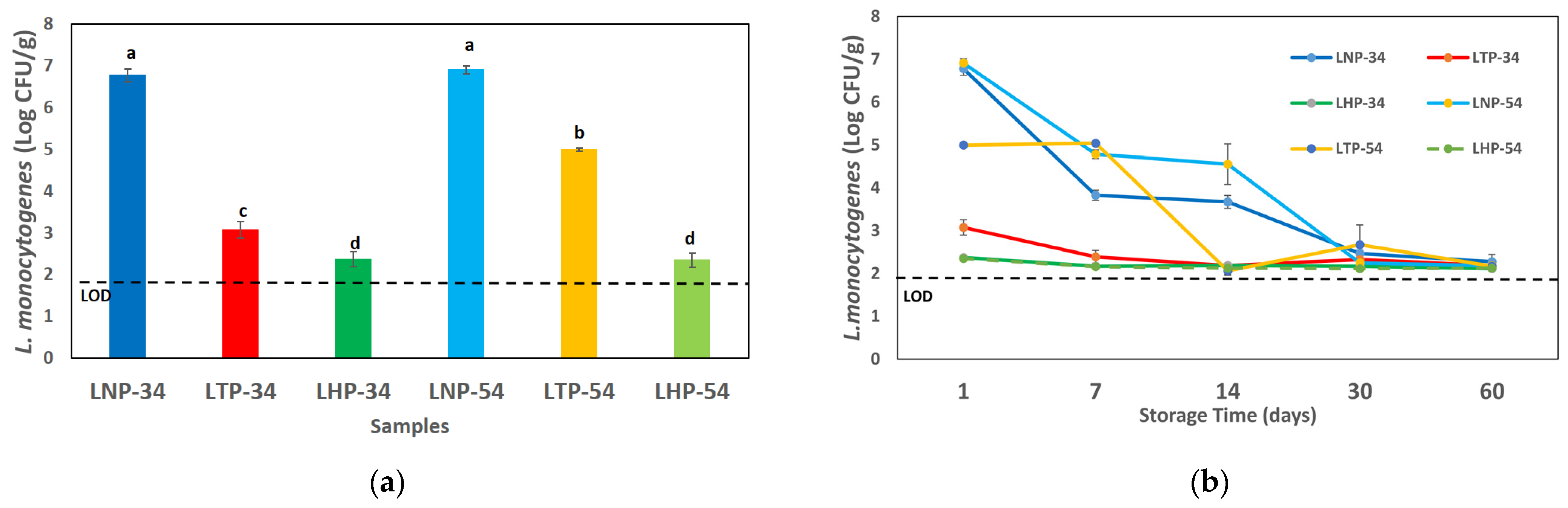
The results of the L. monocytogenes and S. Typhimurium counts are shown in Figure 1. The result of L. monocytogenes counts before and after processing is reported in Figure 1a. HPP drastically (p < 0.05) decreased the L. monocytogenes count in the LHP-34 (2.37 ± 0.18 log CFU/g) and LHP-54 (2.35 ± 0.17 log CFU/g) samples compared to the non-processed samples LNP-34 (6.78 ± 0.15 log CFU/g) and LNP-54 (6.91 ± 0.10 log CFU/g). There was no significant difference between HPP samples with different oil content in the amount of L. monocytogenes. Also, the thermal processing led to a significant decrease (p < 0.05) in L. monocytogenes counts in LTP-34 (2.90 ± 0.46 log CFU/g) and LTP-54 (4.99 ± 0.04 log CFU/g) compared to LNP samples. The oil content of pesto sauce was found to impact the survival rate of L. monocytogenes in these samples. In other words, the LTP-34 samples had a significant (p < 0.05) reduction in L. monocytogenes compared to the LTP-54 samples (Figure 1a). Figure 1b shows L. monocytogenes counts during the storage time (60 days). The results showed that L. monocytogenes drastically decreased more than 5-fold log after 30 days in non-processed samples. In contrast, LHP and LTP-34 samples revealed no significant difference during storage time (Figure 1b).
Figure 1.
Listeria monocytogenes counts in the pesto samples having 34 or 54% oil content and different processing methods on day 1 (a) and during 60 days of storage (b). Data represent the mean ± standard deviation of three independent replicates; different superscript letters in each column indicate significant differences (p < 0.05). LNP: non-processed samples with L. monocytogenes, LTP: thermal-processed samples with L. monocytogenes, LHP: HPP samples with L. monocytogenes. LOD: limit of detection (2 log CFU/g).
S. Typhimurium counts after exposure to HPP and thermal processing were below the limit of detection (LOD, 2 log CFU/g), being significantly different compared to non-processed samples (SNP-34: 5.07 ± 0.35, SNP-54: 5.06 ± 0.18 Log CFU/g) on day 1. Also, the results showed that S. Typhimurium counts were under LOD for 60 days in processed samples. Surprisingly, the S. Typhimurium counts decreased under the LOD after 7 days, also in non-processed samples. The oil content did not affect the survival rate of S. Typhimurium.
3.2. Time–Kill Kinetic Study Results of the Pesto Sauces
In Table 1, the results of the time–kill study of L. monocytogenes are presented. The results indicated that there was no statistically significant (p < 0.05) difference between the samples control, NPO-34, and NPO-54 at 0 h, and this finding persisted at 2 and 4 h. However, at 4 h, the growth rate of L. monocytogenes in NPO-34 (6.66 ± 0.23 log CFU/g) was significantly lower than in the control (7.62 ± 0.12 log CFU/g) and NPO-54 (7.49 ± 0.19 log CFU/g). As the incubation time increased to 24 h, no significant difference was observed among the samples. Also, the same trend (p < 0.05) was observed in TPO and HPO groups, and the samples containing oil from pesto sauce with 34% oil showed less growth at the fourth point of the day compared to their peers, but, with the continuation of the incubation process (24 h), no significant (p < 0.05) difference was observed.

Table 1.
Time–kill kinetic study results of oils from different pesto sauces against L. monocytogenes.
The results of S. Typhimurium growth are presented in Table 2. The data indicate that, initially, there is no significant difference in the growth of S. Typhimurium in the control, TPO, and HPO samples. However, after 2 h of incubation, there is a notable (p < 0.05) decrease in the growth rate of S. Typhimurium in the samples containing oil from pesto sauce with 34% oil, compared to the other samples. Subsequently, there were no significant differences observed in the growth rates among the different samples over the continued duration of incubation.

Table 2.
Time–kill kinetic study results of oils from different pesto sauces against S. Typhimurium.
3.3. GC-MS Results of the Pesto Oils
A total of 12 terpene compounds were identified in the pesto oil samples with GC-MS (Table 3). Specifically, L-linalool, eugenol, and 1,8-cineol had the highest levels of terpenes in the samples. Interestingly, it was found that the HPP or thermal processes did not affect the levels of terpenes in the pesto oils. Additionally, the terpene levels were lower in the 54% oil samples compared to the 34% oil samples. This could be due to the higher oil content in the 54% pesto, which diluted the terpenes more, compared to the 34% oil content.

Table 3.
Identified terpenes and their levels in studied basil pesto oils.
3.4. pH Changes in the Pesto Sauces
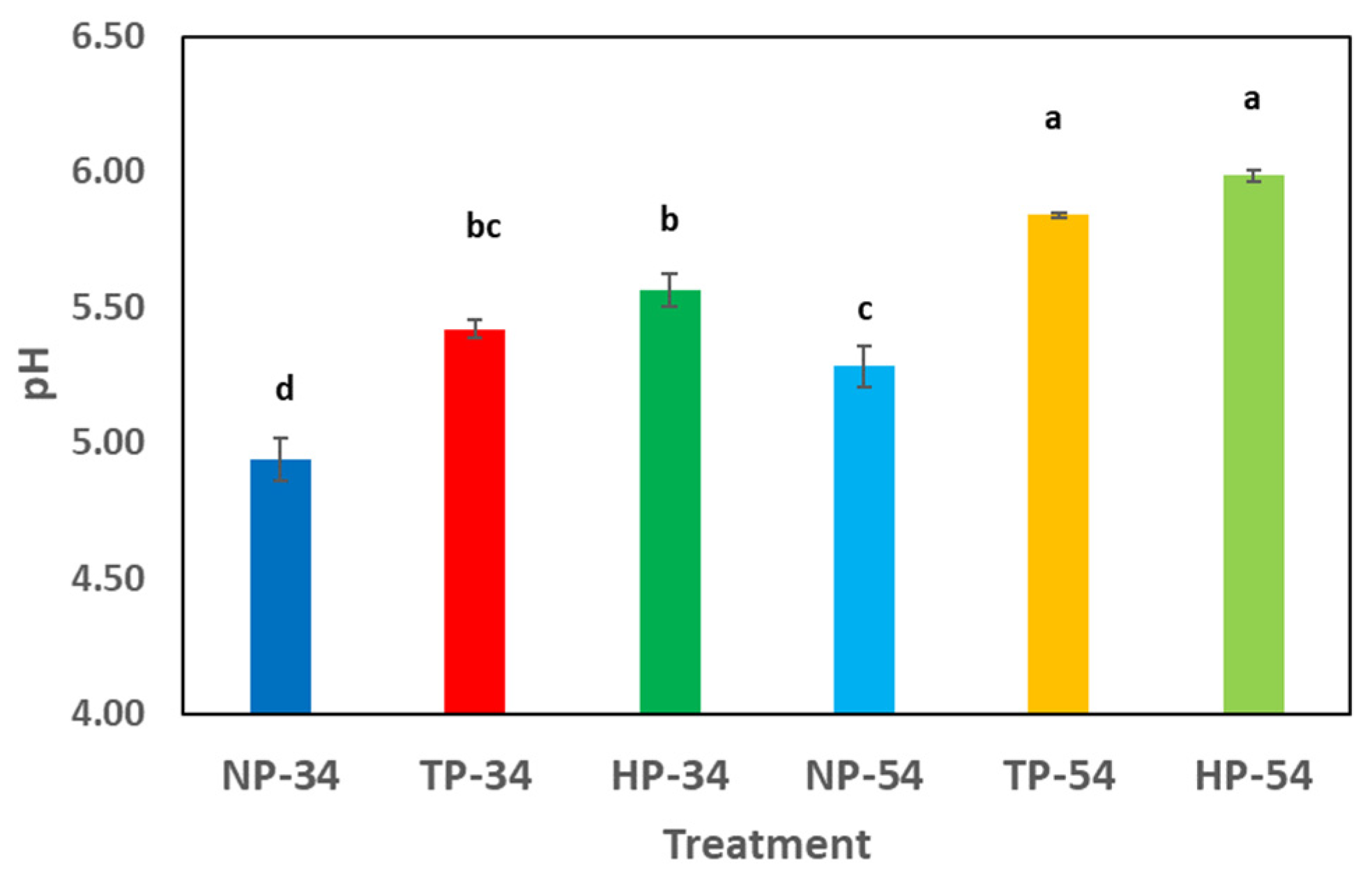
Figure 2 illustrates the changes in pH before and after processing pesto sauces. The results indicate a significant increase in pH levels when HPP and thermal processes are used, compared to the non-processed samples.
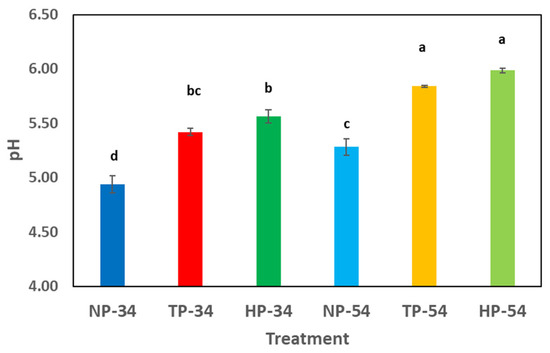
Figure 2.
Effect of HPP and thermal processing on pH in pesto sauces. Data represent the mean ± standard deviation of three independent replicates; different superscript letters in each column indicate significant differences (p < 0.05). NP-34: 34% oil-content non-processed sample, HPP-34: 34% oil-content HPP sample, TP-34: 34% oil-content thermal-processed sample, NP-54: 54% oil-content non-processed sample, HPP-54: 54% oil-content HPP sample, TP-54: 54% oil-content thermal-processed sample.
3.5. Total Phenolic Content Results of the Pesto Sauces
The TPC results presented in Table 4 show an increase in TPC concentration in the HPP samples compared to the non-processed samples. Additionally, it was observed that the level of TPC in the HPP sample was significantly higher than in the thermal-processed sample, while no significant difference was found between the control and thermal samples. This trend was consistent for both oil content.

Table 4.
Effect of HPP and thermal processing on total phenolic content (TPC) and Antioxidant capacity (AA%) in pesto sauces.
3.6. Total Antioxidant Capacity of Pesto Sauces
The result of antioxidant capacity is shown in Table 4. The result illustrated that there is no significant difference (p < 0.05) among treatments, and HPP and thermal processing did not influence the antioxidant activities potential of pesto samples.
3.7. Lipid Oxidation of Pesto Sauces
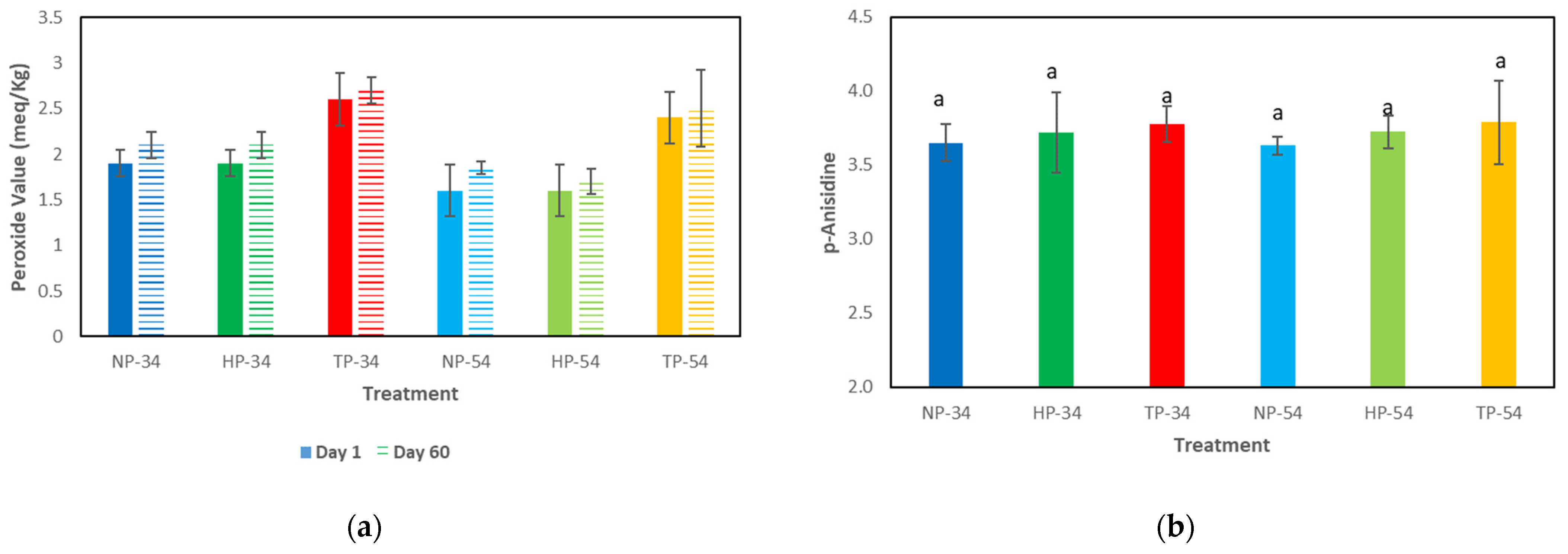
The peroxide and anisidine values are shown in Figure 3. The peroxide value results indicated that the amount of peroxide in all samples remained lower than the standard (10 milliequivalents of active oxygen per kilogram of oil) during the storage period. However, the peroxide levels in the TP samples increased significantly, compared to the HP and NP samples in 34% and 54% oil content. The analysis of anisidine values showed no noticeable differences among the samples during storage time (60 days) in the cold room.
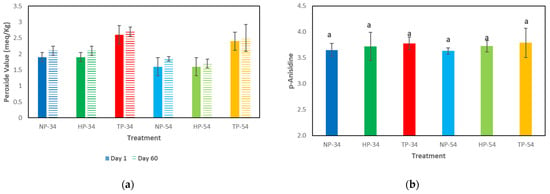
Figure 3.
Effect of HPP and thermal processing on lipid oxidation in pesto sauce. (a) Peroxide value results in day 1 (solid fill) and 60 (pattern fill) of storage time, (b) p-anisidine value results after 60 days storage time. Data represent the mean ± standard deviation of three independent replicates; different superscript letters in each column indicate significant differences (p < 0.05, Same letters mean no significant differences). NP-34: 34% oil-content non-processed sample, HPP-34: 34% oil-content HPP sample, TP-34: 34% oil-content thermal-processed sample, NP-54: 54% oil-content non-processed sample, HPP-54: 54% oil-content HPP sample, TP-54: 54% oil-content thermal-processed sample.
4. Discussion
HPP is a potential technique for producing high-quality food items with an extended shelf life. However, the applicability of the technique should be tested with various food products. There have been issues with high-oil products that can form a protective barrier against spoilage and pathogenic bacteria during thermal [44,45] and non-thermal processes [46], reducing the efficiency of these procedures in eradicating microorganisms. Furthermore, the high oil concentration might speed up the oxidation of lipids and reduce product quality. This study sought to evaluate the microbiological and chemical aspects of pesto sauce, a high-oil food.
The microbiological study demonstrated that the HPP efficiently reduced the levels of L. monocytogenes and S. Typhimurium, with no significant influence of oil content on bacterial survival. However, the findings indicated that the presence of oil may alter the efficacy of thermal processing, potentially allowing L. monocytogenes bacteria to survive. According to studies, high oil content can reduce the efficacy of thermal processing by lowering water activity (aw). This decrease in aw can lead to L. monocytogenes bacteria more resistant to thermal and non-thermal processes [45]. A study by D’Souza et al. (2014) reported that peanut butter formulations could reduce the impact of HPP on Salmonella bacteria [47]. The researchers adjusted the aw by adding oil and water, and the results showed that increasing the amount of oil (and therefore lowering aw) dramatically reduced the impact of HPP on Salmonella bacteria. Morales et al. (2006) found that the aw of cheese significantly affected the pressure resistance of L. monocytogenes and proposed that fat improved microorganism resistance to HPP destruction in a similar way as thermal inactivation [19]. Cream presents a challenge in removing bacteria, due to its high fat content and reduced aw. Previous study has demonstrated that the microbial load of cream with 35% fat can be significantly reduced with HPP. For example, Listeria innocua can be inactivated using HPP at 450 MPa and 25 °C for 10–30 min. It has been suggested that HPP could help extend the refrigerated shelf life of dairy creams [48]. These findings confirm the HPP process as a safe way of producing high-oil-content foods. Evert-Arriagada et al. (2018) found that Listeria inactivation increased with higher applied pressure, showing statistical differences based on the strains used (L. innocua, L. monocytogenes CECT 4031 and L. monocytogenes Scott A), inoculum levels (4–6 Log CFU/g), and sublethal injury. All three examined strains had the maximum lethality values at 600 MPa; however, L. innocua and L. monocytogenes CECT 4031 also had high lethality at 500 MPa. Following treatment, the counts of L. innocua and L. monocytogenes CECT 4031 in fresh cheese gradually increased during cold storage [49]. Another study determined that 6000 bar is the highest effective pressure for inactivating L. monocytogenes [10]. Furthermore, in our investigation, all samples containing bacteria showed a significant decrease in Listeria and Salmonella bacteria during storage. This decrease may be attributable to the antimicrobial compounds extracted from basil during pesto sauce production, and our GC/MS results show that there are 12 different terpene compounds in oils, with linalool being the most abundant. Notably, the oil included terpene compounds with antibacterial characteristics that are naturally found in basil. Basil, a member of the Lamiaceae plant family, is utilized not only as a spice, but is also known for its medical capabilities as a powerful antibacterial, antimutagenic, and chemopreventive agent. According to a study, basil essential oil comprises 65 compounds, with linalool (31.6%) and methyl chavicol (23.8%) being the most prominent [33]. Basil also includes phenolic chemicals, including rosmarinic, chicoric, ferulic, and caffeic acid, which are predominantly phenolic acids. Several studies have demonstrated that basil extract has a high concentration of natural antioxidants with a variety of health advantages, including antibacterial, anti-inflammatory, and antidiabetic activities, due to the synergistic antioxidant impact of these components [50,51].
The findings of the time–kill investigation indicated that these compounds had antimicrobial properties, resulting in a drop in bacteria counts in unprocessed samples throughout storage time. Furthermore, it was shown that the oil content impacted the rate of bacteria reduction, with 34% oil-content samples showing a greater reduction compared to 54% oil-content samples, possibly due to the higher concentration of terpene compounds detected in the GC-MS analysis. Therefore, the higher oil content might have diluted the antimicrobial capabilities of the compounds.
The pH results showed that the pH level mildly increased when thermal and high-pressure processes were applied. This rise can be attributed, for example, to the release of amino from the cheese in the formulation. When thermal and non-thermal processes are used, the protein molecules are broken down and amino compounds are released, which increases the pH level of the products. Ramirez-Suarez et al. (2006) demonstrated that HPP increased the pH in the tuna samples compared to the control. It can occur that pressure, which causes structural changes associated with protein denaturation, can unfold proteins, expose more basic amino acids to the medium, and raise the pH. Several investigations have found that after HPP treatment, the pH of food product samples might rise considerably compared to controls [52,53]. The lipid oxidation analysis in pesto sauce samples revealed that the peroxide and p-anisidine levels were within the standard range. According to the Centre for Food Safety in Hong Kong, the recommended maximum peroxide value in edible fats and oils is 10 meq per kilogram of oil, and the p-anisidine value should be less than 10 meq/kg. After 60 days of cold storage, the oil was found to be of excellent grade. Furthermore, contrary to prior investigations, high pressure and thermal processes did not significantly accelerate lipid oxidation.
The presence of antioxidant-rich phenolic components in pesto sauce inhibits oil oxidation. TPC analysis showed that using basil in the formulation increased the total TPC of all samples [38,54]. Furthermore, the HPP method, in comparison to the thermal process, resulted in increased TPC levels in the samples. This increase in TPC levels may be connected to the improved extractability of some antioxidant components, following HPP [55].
5. Conclusions
Our study demonstrated that HPP improves the microbial and chemical quality of high-oil-content pesto sauce compared with thermal processing. The HPP significantly reduces Listeria monocytogenes and Salmonella Typhimurium counts (more than 4-log reduction) without being significantly affected by the oil content. Furthermore, the lipids are well preserved by HPP, as shown by low peroxide and p-anisidine values (under 10 meq/Kg oil). This activity increases the total phenolic content, indicating higher antioxidant properties than those observed before the treatment. The presence of functional compounds, particularly phenolics, including terpenes with antimicrobial properties found in basil, likely contributes to the prolonged shelf life and maintains the quality of the pesto sauce. These ingredients—in addition to enhancing the nutritional profile, taste, and texture of the food—help boost the efficacy of this non-thermal preservation tool for high-oil-content foods, further solidifying HPP as an effective method for controlling spoilage organisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14209425/s1, Figure S1: Gallic Acid Standard Curve; Table S1. Preparation of gallic acid standards for calibration curve.
Author Contributions
Conceptualization, J.K., E.S. and K.R.; methodology, E.S., K.R., J.K. and S.P.; validation, E.S., K.R. and J.K.; formal analysis, E.S.; investigation, E.S., K.R., S.P., L.I.C., K.P.T. and H.H.: writing—original draft preparation, E.S., K.R., S.P., L.I.C. and K.P.T.: writing—review and editing, J.K. and M.H.; supervision, J.K. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the European Union, with the European Regional Development Fund A80382, admitted by the Regional Council of Pohjois-Savo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We want to acknowledge the European Regional Development Fund and the Regional Council of Pohjois-Savo, via which the regional ELTUVA project (A80382, 2023–2025) has enhanced research and innovation capacities and the uptake of advanced technologies for developing high-quality and safe food. We want to thank Company Ca-Sa Välimeren Herkut Oy, especially Susanna Työppönen and Petri Klemetti, for preparing and supplying the pesto sauces and being supportive of this study, as well as Toripiha Oy for HPP processing. We thank SIB Labs at Department of Technical Physics in the University of Eastern Finland for providing GC-MS facilities. We are also thankful for Jaana Kapustamäki and Jesse Ojala from Development Company SavoGrow Ltd. for kind cooperation in the ELTUVA project supporting this study. Moreover, we wish to thank our skillful laboratory personnel, Kristiina Kinnunen and Ida Tikkanen, for their assistance in maintaining and developing the laboratory methodology.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The companies CA-SA Välimeren Herkut and Tori-piha Oy were participating in the ELTUVA project but had no role in the design of the study, analyses, interpretation of data, or writing of the manuscript.
References
- Rana, J.; Paul, J. Consumer Behavior and Purchase Intention for Organic Food: A Review and Research Agenda. J. Retail. Consum. 2017, 38, 157–165. [Google Scholar] [CrossRef]
- Hosni, H.; Periklis, D.; Baourakis, G. Consumers Attitude towards Healthy Food:“Organic and Functional Foods”. Int. J. Food Beverage Manuf. Bus. Models 2017, 2, 85–99. [Google Scholar] [CrossRef]
- Savelli, E.; Murmura, F.; Bravi, L. Healthy and Quality Food Attitudes and Lifestyle: A Generational Cohort Comparison. TQM J. 2023. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Nowacka, M.; Colussi, R.; Frasson, S.F.; Demirkesen, I.; Mert, B.; Singha, P.; Singh, S.K.; Falsafi, S.R. Impact of Emerging Non-Thermal Processing Treatments on Major Food Macromolecules: Starch, Protein, and Lipid. Trends Food Sci. Technol. 2023, 141, 104208. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; de Alba, M.; Sun, D.-W.; Tiwari, B. Principles and Recent Applications of Novel Non-Thermal Processing Technologies for the Fish Industry—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.A.A.; Mir, N.A.; Gul, K. Recent Advances in Non-Thermal Processing Technologies for Enhancing Shelf Life and Improving Food Safety. Appl. Food Res. 2023, 3, 100258. [Google Scholar] [CrossRef]
- Chakka, A.K.; Sriraksha, M.; Ravishankar, C. Sustainability of Emerging Green Non-Thermal Technologies in the Food Industry with Food Safety Perspective: A Review. LWT-Food Sci. Technol. 2021, 151, 112140. [Google Scholar] [CrossRef]
- Riekkinen, K.; Martikainen, K.; Korhonen, J. Effectiveness of High-Pressure Processing Treatment for Inactivation of Listeria Monocytogenes in Cold-Smoked and Warm-Smoked Rainbow Trout. Appl. Sci. 2023, 13, 3735. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of Novel Technologies on Polyphenols during Food Processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Pou, K.J.; Raghavan, V. Recent Advances in the Application of High Pressure Processing-Based Hurdle Approach for Enhancement of Food Safety and Quality. J. Biosyst. Eng. 2020, 45, 175–187. [Google Scholar] [CrossRef]
- Velazquez, G.; Vázquez, P.; Vázquez, M.; Torres, J.A. Avances En El Procesado de Alimentos Por Alta Presión Advances in the Food Processing by High Pressure Avances No Procesado de Alimentos Por Alta Presión. CYTA-J. Food. 2005, 4, 353–367. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, J.A. High-Pressure Processing Technologies for the Pasteurization and Sterilization of Foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ Panel); Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F. The Efficacy and Safety of High-pressure Processing of Food. EFSA J. 2022, 20, e07128. [Google Scholar]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological Food Safety Assessment of High Hydrostatic Pressure Processing: A Review. LWT-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Impact of High-Pressure Processing (HPP) on Listeria Monocytogenes—An Overview of Challenges and Responses. Foods 2023, 13, 14. [Google Scholar] [CrossRef]
- Milani, E.; Silva, F.V. Comparing High Pressure Thermal Processing and Thermosonication with Thermal Processing for the Inactivation of Bacteria, Moulds, and Yeasts Spores in Foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar]
- Morales, P.; Calzada, J.; Rodríguez, B.; De Paz, M.; Gaya, P.; Nuñez, M. Effect of Cheese Water Activity and Carbohydrate Content on the Barotolerance of Listeria Monocytogenes Scott A. J. Food Prot. 2006, 69, 1328–1333. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Ter Kuile, B.H. Low--Water Activity Foods: Increased Concern as Vehicles of Foodborne Pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of High Pressure Processing on Lipid Oxidation: A Review. Innov. Food Sci. Emerg. Technol. 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and Lipid Oxidation in Meat: A Review with Emphasis on High-Pressure Treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Andrés, A.I.; Cava, R.; Ventanas, J.; Muriel, E.; Ruiz, J. Lipid Oxidative Changes throughout the Ripening of Dry-Cured Iberian Hams with Different Salt Contents and Processing Conditions. Food Chem. 2004, 84, 375–381. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Ren, Y.; Fan, E.; Chang, H.; Wu, H. Effects of High Pressure Treatment and Temperature on Lipid Oxidation and Fatty Acid Composition of Yak (Poephagus grunniens) Body Fat. Meat Sci. 2013, 94, 489–494. [Google Scholar] [CrossRef]
- Cheah, P.; Ledward, D. Catalytic Mechanism of Lipid Oxidation Following High Pressure Treatment in Pork Fat and Meat. J. Food Sci. 1997, 62, 1135–1139. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Tabilo-Munizaga, G.; Reyes, J.E.; Rodríguez, A.; Pérez-Won, M. Effect of High-pressure Treatment on Microbial Activity and Lipid Oxidation in Chilled Coho Salmon. Eur. J. Lipid Sci. Technol. 2010, 112, 362–372. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Miskin, D.; Piggott, J.R. Quality of Vacuum Packed Cold-smoked Salmon during Refrigerated Storage as Affected by High-pressure Processing. J. Sci. Food Agric. 2005, 85, 655–661. [Google Scholar] [CrossRef]
- Ma, H.; Ledward, D.; Zamri, A.; Frazier, R.; Zhou, G. Effects of High Pressure/Thermal Treatment on Lipid Oxidation in Beef and Chicken Muscle. Food Chem. 2007, 104, 1575–1579. [Google Scholar] [CrossRef]
- Butz, P.; Tauscher, B. Emerging Technologies: Chemical Aspects. Food Res. Int. 2002, 35, 279–284. [Google Scholar] [CrossRef]
- Rasanayagam, V.; Balasubramaniam, V.; Ting, E.; Sizer, C.; Bush, C.; Anderson, C. Compression Heating of Selected Fatty Food Materials during High-pressure Processing. J. Food Sci. 2003, 68, 254–259. [Google Scholar] [CrossRef]
- Amadei, G.; Ross, B.M. Quantification of Character-impacting Compounds in Ocimum basilicum and’Pesto Alla Genovese’with Selected Ion Flow Tube Mass Spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Klimankova, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma Profiles of Five Basil (Ocimum basilicum L.) Cultivars Grown under Conventional and Organic Conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical Composition, Antioxidant and Antimicrobial Activity of Basil (Ocimum basilicum L.) Essential Oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Arshad, H.M.; Mohiuddin, O.A.; Azmi, M.B. Comparative in Vitro Antibacterial Analysis of Different Brands of Cefixime against Clinical Isolates of Staphylococcus Aureus and Escherichia coli. J. Appl. Pharm. Sci. 2012, 2, 109–113. [Google Scholar]
- Usaga, J.; Acosta, Ó.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Evaluation of High Pressure Processing (HPP) Inactivation of Escherichia coli O157: H7, Salmonella Enterica, and Listeria Monocytogenes in Acid and Acidified Juices and Beverages. Int. J. Food Microbiol. 2021, 339, 109034. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, E.; Razmjooei, M.; Shad, E.; Eskandari, M.H. Antibacterial Mechanisms of Zataria Multiflora Boiss. Essential Oil against Lactobacillus curvatus. LWT-Food Sci. Technol. 2018, 87, 406–412. [Google Scholar] [CrossRef]
- Klug, T.V.; Collado, E.; Martínez-Sánchez, A.; Gómez, P.A.; Aguayo, E.; Otón, M.; Artés, F.; Artés-Hernandez, F. Innovative Quality Improvement by Continuous Microwave Processing of a Faba Beans Pesto Sauce. Food Bioprocess Technol. 2018, 11, 561–571. [Google Scholar] [CrossRef]
- Hilma, R.; Herliani, H.; Almurdani, M. Determination of Total Phenolic, Flavonoid Content Andfree Radical Scavenging Activity of Etanol Extract Sawo Stem Bark (Manilkara zapota (L.)). Pros. CELSciTech 2018, 3, 62–68. [Google Scholar]
- Kulkarni, A.P.; Aradhya, S.M. Chemical Changes and Antioxidant Activity in Pomegranate Arils during Fruit Development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Bekdeşer, B.; Çelik, S.E.; Bener, M.; Dondurmacıoğlu, F.; Yıldırım, E.; Yavuz, E.N.; Apak, R. Determination of Primary and Secondary Oxidation Products in Vegetable Oils with Gold Nanoparticle Based Fluorometric Turn-on Nanosensor: A New Total Oxidation Value. Food Chem. 2024, 434, 137426. [Google Scholar] [CrossRef] [PubMed]
- Hortwitz, W. AOAC Official Method 965.33, Peroxide Value of Oils and Fats. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC Int.: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Tompkins, C.; Perkins, E.G. The Evaluation of Frying Oils with the P-anisidine Value. J. Am. Oil Chem. Soc. 1999, 76, 945–947. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Lombardo, S.P.; Ganjyal, G.M.; Tang, J. Desiccation in Oil Protects Bacteria in Thermal Processing. Food Res. Int. 2020, 137, 109519. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xie, Y.; Lombardo, S.P.; Tang, J. Oil Protects Bacteria from Humid Heat in Thermal Processing. Food Control 2021, 123, 107690. [Google Scholar] [CrossRef]
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of Microorganisms by High Isostatic Pressure Processing in Complex Matrices: A Review. Innov. Food Sci. Emerg. Technol. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- D’Souza, T.; Karwe, M.; Schaffner, D.W. Effect of High Hydrostatic Pressure on Salmonella Inoculated into Creamy Peanut Butter with Modified Composition. J. Food Prot. 2014, 77, 1664–1668. [Google Scholar] [CrossRef]
- Raffalli, J.; Rosec, J.; Carlez, A.; Dumay, E.; Richard, N.; Cheftel, J. High Pressure Stress and Inactivation of Listeria Innocua in Inoculated Dairy Cream. Sci. Aliments 1994, 14, 349–358. [Google Scholar]
- Evert-Arriagada, K.; Trujillo, A.; Amador-Espejo, G.; Hernández-Herrero, M. High Pressure Processing Effect on Different Listeria Spp. in a Commercial Starter-Free Fresh Cheese. Food Microbiol. 2018, 76, 481–486. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the Antioxidative Systems of Ocimum basilicum L. (Cv. Fine) under Different Sodium Salts. Acta Physiol. Plant. 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- Şayin Sert, T.; Coşkun, F. The Effects of High-Pressure Processing on pH, Thiobarbituric Acid Value, Color and Texture Properties of Frozen and Unfrozen Beef Mince. Molecules 2022, 27, 3974. [Google Scholar] [CrossRef] [PubMed]
- Okpala, C.O.; Piggott, J.R.; Schaschke, C.J. Influence of High-Pressure Processing (HPP) on Physico-Chemical Properties of Fresh Cheese. Innov. Food Sci. Emerg. Technol. 2010, 11, 61–67. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and Successive Harvests Interaction Affects Phenolic Acids and Aroma Profile of Genovese Basil for Pesto Sauce Production. Foods 2021, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Kaşikçi, M.B.; Bağdatlioğlu, N. High Hydrostatic Pressure Treatment of Fruit, Fruit Products and Fruit Juices: A Review on Phenolic Compounds. Food Health 2016, 2, 27–39. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).