Abstract
In this study, we decided to test the hypothesis that the fungal biostarter M. flavus used during a 21-day beef dry-aging process significantly impacts the composition of other microorganisms, the profile of volatile compounds, meat hardness characteristics, and, consequently, the sensory quality. The experiments were performed on samples derived from animals crossbred between Holstein–Fresian cows and meat breed bulls. Two groups of samples were studied, including the control group, without biostarter, and a group inoculated with the M. flavus biostarter. Both sample groups were seasoned for 21 days in the dry-aging fridge. The physicochemical parameters (pH, color parameters), the chemical composition of muscle, the determination of the shear force, the profile of volatile compounds (VOCs), and the sensory quality were evaluated after aging. During this study, classical microbiological methods were used to investigate the influence of fungal biostarters on the growth and survival of bacteria and other fungi (e.g., yeasts) during the dry-aging process of beef (DAB). The M. flavus biostarter improved the sensory quality of DAB, allowing high sensory quality to be achieved after just 21 days. This is likely due to the diverse VOCs produced by the fungus, including 1-tetradecanol, 2-nonenal, trans-2-undecenoic acid, and the following esters: formic acid hexyl ester, 10-undecenoic acid methyl ester, and 4-methylpentanoic acid methyl ester. The presence of the biostarter had no significant effect on the number of the bacteria or the survivability of the L. monocytogenes on the meat’s surface in laboratory conditions.
1. Introduction
There are two types of beef aging. The first, a conventional industrial process known as wet-aging involves keeping vacuum-packaged portions at temperatures from 1 to 4 °C for 14–28 days. The second, dry-aging, which is becoming more popular globally, refers to storing cuts at 0 to 4 °C, without protective packaging, under controlled conditions of airflow and relative humidity, for up to 60 days [1]. This process results in improved tenderness and imparts unique sensory characteristics. The appearance and flavor of the meat are altered by the growth of microorganisms [2]. Steaks made from dry-aged beef are served at high prices in steakhouses and exclusive restaurants. Specific descriptors are used to highlight dry-aged beef’s advantages over wet-aged beef, such as “buttery and rich”, “superb in taste and texture”, “superior in taste and tenderness”, “mellow and intense”, and “earthy and nutty” [3]. The dry-aging of beef allows various microorganisms to grow on the surface of the meat, among which the psychrophilic Mucoraceae fungi dominate. Representatives of this group are ubiquitous, rapidly growing, mainly saprotrophic fungi. Although the majority of them are related to plant substrates, some may develop on more protein-rich substrates, such as soybeans or meat [4]. On dry-aged beef, Mucoraceae fungi develop together with a few genera of ascomycetous yeast (e.g., psychrophilic members of Debaryomyces and Candida) and different bacterial groups [5]. The presence of Mucoraceae members is considered beneficial in dry-aged beef (DAB) because it enhances the flavor compounds, amino acids, and fatty acids due to the production of proteolytic and lipolytic enzymes. Moreover, during the extended aging process, many microbes colonize the meat’s surface, and the composition of the microbial community changes continuously. It should be emphasized that meat is a product on which microorganisms develop very easily. Microbes metabolize nutrients in the meat and produce different metabolites that affect the flavor, tenderness, and rancidity of aged meat. The rancidity aroma and flavor can influence the sensory quality in a negative way. For this reason, the continuous control of aging conditions is an important strategy to reduce the risk of meat rancid and spoilage, especially for extended aging periods [6,7]. Some research suggests that Mucoracea fungi may also limit the growth of pathogenic microorganisms.
Moreover, an aroma development in meat products is a complex process, produced by the interactions of many factors [8]. Aroma-active compounds are essential to the final sensory characteristics, so the elucidation of key aroma compounds is a major area of interest in the meat industry [9].
Kim et al. [2] studied the effect of various aging methods, including dry aging, on beef quality and characteristics, and observed high counts of LAB, Pseudomonas, yeast, and molds after 60 days. Although the fungal counts were not determined, these authors isolated fungi present on the crust and identified the dominant ones as belonging to the Mucor flavus (M. flavus) and Helicostylum pulchrum species [10]. Therefore, using microorganisms interactions can also be a natural way for limiting food spoilage. Although this fact was already reviewed in the literature [11], less is known about bacterial–fungal interactions in meat products.
On one hand, the usage of some fungal biostarters containing, e.g., M. flavus or Helicostylum pulchrum has been recently widely discussed [5,12] in dry-aged beef processing. On the other hand, meat aging was shown to be related to dynamic microbiological changes [13,14]. The dry-aging of beef occurs in the presence of various types of microorganisms that colonize raw material and affect its quality. The understanding of microorganisms’ interactions in dry-aged beef is crucial for food safety reasons, as well as for determining their impact on volatile aroma compounds, sensory quality, and the bacterial pathogens’ survival on beef after 21 days of dry-aging with biostarter containing M. flavus.
Therefore, in this study, we decided to test the hypothesis whether the fungal biostarter M. flavus used during the process of 21 days long beef dry-aging significantly impacted the profile of volatile compounds, meat hardness characteristics, and consequently, sensory quality.
2. Materials and Methods
2.1. Materials for Analysis
2.1.1. Collection of Samples
This study utilized twelve samples taken from animals resulting from the crossbreeding of Holstein–Friesian cows with meat breed bulls. The slaughter age of the animals was 18 months, with a body weight of 500–550 kg. Carcasses were classified according to the EUROP system as R (taking into account muscularity) and 2 (considering fatness). The animals were slaughtered in a slaughterhouse in the western part of Poland, following the European Union Council Regulations (EC) No 1099/2009 for the protection of animals at the time of slaughter. The carcasses were cooled for 48 h at a temperature of 2–4 °C. Samples of the Longissimus lumborum muscle were taken from both half-carcasses, i.e., left and right, along with the fat, as part of a culinary element entrecote. They were cooled and transported to the laboratory. The average weight of the samples was around 3.5 ± 0.5 kg.
The first group consisted of the control samples (C; n = 6), while the next 6 were the biostarter samples (Mf; n = 6), which were treated with the Mucor flavus sp. biostarter (description below). Both samples were placed in a dry-aging refrigerator (DX 1000 Premium S, DryAger, Bad Saulgau, Germany) for a period of 21 days.
2.1.2. Fungal Starter Culture
The fungal strain KKP 2092p from the Institute of Evolutionary Biology, University of Warsaw’s culture collection was employed as the biostarter for this experiment. It was identified as Mucor flavus Bainier [MB#179990] based on ITS rDNA sequencing, as described by Ostrowski et al. [15]. This strain is registered under the application patent number P.443722.2.2. Fungal culture was grown on 4% Sabouraud Dextrose Agar (SDA) plates for 7 days at 21 °C and then removed from the plates to be lyophilized. Lyophilized mycelium was then suspended in the Sabouraud broth (30 g of lyophilized mycelium in 1 L of broth) and kept at 21 °C for 24 h. For 1 kg of meat, 10 mL of prepared inoculum (10 mL/1 kg) was distributed by pouring the appropriate volume of inoculum in small doses on the meat surface. After each pour, the inoculum was spread on the meat surface using a sterile silicone brush. The control samples were covered only with Sabouraud broth, in the same manner and amount.
2.1.3. Chemicals
The following reagents were used for microbiological analyses: half-Fraser broth\Fraser broth purchased from Biomaxima, Lublin, Poland, and PALCAM agar, as well as LB Broth with agar purchased from Oxoid Ltd., Basingstoke, UK. The following reagents were used to determine the content of fat, protein, and collagen: petroleum ether (boiling point 40–60 °C), sodium hydroxide, boric acid, sulfuric acid 96%, sulfuric acid 0.1 M, chloramine T, p-dimethylaminobenzoic aldehyde, analytical grade, purchased from Chempur, headquartered in Piekary Śląskie, Poland.
2.2. Research Protocols
2.2.1. Influence of Fungal Biostarter on Development of Bacteria on Dry-Aged Beef DAB
Dry-aged beef was aged for 21 days with and without (control) fungal biostarter. Six meat samples of about 50 g per each category were tested. Dry-aging was conducted at a temperature of 1.5 °C, with around 80–90% relative humidity, using a dry-aging refrigerator. Growth of bacteria was measured during the 21 days of beef dry-aging. Bacterial colony-forming units (CFUs) were counted at the start of the dry-aging process and then once every seven days using the surface spread plate method. Three pieces of meat were taken and homogenized separately in a Stomacher blender with 250 mL of saline. Homogenates were then subjected to serial solutions (10−1–10−6) with saline, and 0.1 mL of each solution was transferred on three separate LB plates (i.e., 9 plates for each solution). The plates were kept at 37 °C for 24 h. Following this incubation period, the bacterial colonies were counted (the experiment was repeated 5 times).
2.2.2. Influence of Fungal Biostarter on Development of Listeria monocytogenes on DAB
Bacterial inoculum was prepared by transferring 1–2 colonies of L. monocytogenes strain into a saline solution (RF) to obtain a bacterial suspension with a turbidity matching the 0.5 McFarland standard (approximately 1.5 × 108 cfu/mL). This suspension was subsequently diluted using a tenfold dilution method, where 0.1 mL was taken from the 10−3 dilution of the L. monocytogenes solution.
Twenty meat samples of 50 g each were inoculated with the L. monocytogenes solution by massaging with a spatula to ensure that the inoculum covered only the top and sides of the beef pieces. Next, 10 of them were additionally inoculated with M. flavus KKP 2092p fungal biostarter, following manufacturer’s instruction. The remaining 10 meat samples were treated as control. Dry-aging was carried out for 21 days at a temperature of 1.5 °C, with around 80–90% relative humidity, using a dry-aging fridge (DX 1000 Premium S, DryAger, Bad Saulgau, Germany).
After the incubation time, all the pieces of meat were taken and homogenized separately in a Stomacher blender with 225 mL Half Fraser Broth enrichment medium, intended for the isolation of Listeria spp. The samples were placed in an incubator at 37 °C for 24 h and transferred onto selective and differential media: PALCAM and Chromogenic Listeria. Plates were incubated for 24 h at 37 °C. After this time, the presence of L. monocytogenes was assessed using the protocol [16].
2.2.3. The pH Value Measurement
The pH was measured initially, the day after slaughter (before aging), and then 21 days post-slaughter, using an automatic pH meter 330i (WTW®, Weilheim, Germany) with specialized SenTix® SP Number 103645 electrodes. Calibration of the pH meter was performed with standard buffers at pH 4.0 and 7.0 at room temperature (21 °C), and the measurement was standardized automatically (in triplicate).
2.2.4. Color Analysis
Color parameters (CIE Lab*) were recorded before aging and after 21 days of aging using a CR-310 Konica Minolta® Chroma Meter (Osaka, Japan) on the meat’s cross-section. The samples were cut and allowed to bloom for 1 h at 4 °C without any surface covering. The equipment was calibrated with black and white reference tiles (X = 80.4, Y = 85.3, Z = 91.5) under illuminant D-65 with a 10° standard observer. Each sample was measured five times.
2.2.5. Water Holding Capacity
The water holding capacity (WHC) was assessed before aging to indicate the free water content in the meat, following the Grau–Hamm method, as detailed by Przybylski et al. [17]. A 0.3 g sample of minced meat (3 mm) was placed on filter paper (Whatman 1) between two glass plates and subjected to a uniform load of 2 kg for 5 min (repeated 5 times). The surface area was analyzed using a computer image analysis program.
2.2.6. Fat, Protein, and Collagen Content
The protein content determination method was described in ISO 166634 [18]. An extraction method was employed to determine the free fat content of the meat, following the procedure outlined in ISO 1444 [19]. The principle of the method is extraction with petroleum ether, the drying of the residue, and finally, the weighing of the fat extracted. The total collagen content of the samples was determined by the hydrolysis of the samples with sulfuric acid and the measurement of absorbance, as described in ISO-3496 [20]. Collagen was calculated by dilutions and a factor of 8. The content of protein, fat, and collagen was determined in raw meat before aging, as well as after 21 days of aging. Each analysis was repeated three times.
2.2.7. Shear Force Determination
The shear force was determined using the ZWICKI 1120 apparatus (Zwick–Roell GmbH & Co. KG, Ulm, Germany). From the grilled meat samples, a cross-section of 1 × 1 cm was taken, oriented with the longitudinal arrangement of muscle fibers (five individual measurements). The maximum cutting force of the sample was measured using a Warner–Bratzler attachment fitted with a flat knife, along with the knife’s penetration depth, until the sample was fully cut. The measurements were performed on samples at ambient temperature. The test was conducted at a speed of 50 mm/min with an initial force of 0.5 N, using a measuring head with a range of 2–1000 N [21].
2.2.8. Sensory Evaluation of the Quality of Dry-Aged Beef
Sample preparation. Before heat treatment, the meat pieces, 2.0 cm thick steaks (cut using an automatic cutter, Ma-Ga, 250 W; 50 Hz, Bydgoszcz, Poland), were allowed to sit until they reached room temperature. They were then grilled on a hot contact grill (PK2745E, 3000 W, 60 Hz, Potis GmbH, Goettingen, Germany) at 250 °C for 3 min to achieve a medium-rare level of doneness. After grilling, the steaks were left to rest, and 4.0 cm × 2.5 cm sections were cut perpendicular to the muscle fibers for sensory evaluation. The meat samples were placed in odorless, disposable plastic containers with lids, each marked with a unique three-digit code.
2.2.9. Sensory Consumer Study
The examination took place within a controlled laboratory environment. A total of 40 consumers, organized into 4 groups, were invited to take part in this study at the Institute of Human Nutrition, Warsaw University of Life Sciences. This study involved participants who identified themselves as regular beef consumers. The consumer study comprised two stages. Initially, participants evaluated the aroma of raw steaks. They were presented with three sets of two steak samples each: a control and one treated with M. flavus. Consumers assessed their preference for the aroma of these steaks before they were subjected to the thermal process. Then, the next step of this study involved evaluating the samples after grilling. The evaluated attributes were aroma, tenderness, juiciness, flavor, and overall liking. Consumers rated each meat sample using a 9-point hedonic scale, with endpoints ranging from 1 (extremely dislike) to 9 (extremely like) [22]. These coded samples were evaluated after following grilling, with the samples averaging 60 °C. The evaluation and condition mode were established based on the procedures described by Meilgaard et al. [23] and by Baryłko-Pikielna and Matuszewska [22]. The sensory and consumer study was conducted as described by the Helsinki Declaration [24] and with the regulation and approval of the Ethical Commission No 15/2021.
2.2.10. Determination of Volatile Compounds
About 5 g of meat sample (cut into pieces about 2 cm) was placed in 20 mL vials and sealed. The absorption time in SPME sampling, using a DVB/CAR/PDMS fiber (Merck KGAA, Darmstadt, Germany) (1 cm long), was 50 min at 70 °C. After injecting the fiber into the GC/MS apparatus (GCMS-QP2010, Shimadzu Corporation, Kyoto, Japan), the process of desorption lasted 120 s (splitless mode). A polyethylene glycol column (30 m × 0.25 mm × 0.25 µm, Restek GmbH, Bad Homburg, Gremany) was used to separate the volatile compounds. The column flow of helium was 0.64 mL/min. The temperature program of separation was as follows: 40 °C (10 min.); next, 5 °C/min to 200 °C (2 min.); and 220–250 °C at a rate of 20 °C/min, holding time at 250 °C (10 min.). The interface, ion source, and injector were working at 250 °C [25]. The mass spectra of the tested compounds were registered in the range of 40–450 m/z, with an ionization energy of 70 eV. The identification of volatile compounds was performed based on the analysis of mass spectral databases, e.g., NIST or Wiley. Each sample was analyzed twice. The analytical results were presented as a percentage of the total pool of volatile compounds.
2.2.11. Statistical Analysis
The results of the experiment were computed by using the statistical analysis system STATISTICA version 13.3 (TIBCO Software Inc. 2017, Palo Alto, CA, USA, Statistica data analysis software system, version 13, http://tibco.com, accessed on 31 July 2023). The normality of the distribution of variables was tested using the Shapiro–Wilk test. Two-way ANOVA was used to assess the significance of the effects of aging time and biostarter on data, such as pH, color parameters, protein, fat, and collagen content. Fisher’s least significant difference post hoc test was used to determine differences between means. The classic Student’s t-test was used to determine the significance of differences between the means for other traits, i.e., the study group (with biostarter) and the control group (without biostarter). The significance of the differences was tested at the probability level of p < 0.05.
3. Results and Discussion
3.1. Meat Quality Traits
No difference in meat quality traits between the control and study samples was observed after 21 days of the dry-aging process. Table 1 summarizes the pH value and color parameters of raw meat before and after 21 days of dry-aging. After 21 days of aging, the samples from both groups did not differ significantly in the same parameters or in terms of chemical composition. Similar results were previously obtained by Przybylski et al. [17] for pH and color parameters and by Hanagasaki and Asato [12] for other meat quality traits. However, the results show that significant changes occur already during 21 days of dry-aging of beef for both groups together. The pH value increased form 5.67 to 5.80 (p < 0.05), the protein content changed significantly from 17.57% to 22.37% (p < 0.05), the fat content from 17.92% to 21.55%, and the collagen content from 1.12% to 1.54% (p < 0.05). The significant changes in protein, fat, and collagen content were the result of moisture loss during aging. Similar results were reported in the research of Hanagasaki and Asato [12]. In case of color parameters the results are varied, and both an increase and a decrease in the values of parameters characterizing meat color can be observed [26,27]. Among the factors that may influence these changes are, for example, the counted fat content (Table 1) or changes in mitochondrial activity [27,28].

Table 1.
Beef characteristics before and after 21 days of dry-aging.
The pH changes during aging are in agreement with results presented in the study of Oh et al. [29] and Sha et al. [30], but in contrast to the research of Iida et al. [31]. Obuz et al. [32] stated that the increase in pH during aging can be attributed to nitrogenous compounds deriving from protein hydrolysis. It should be emphasized that an increase in pH during meat aging may favor the growth of microorganisms. Coton et al. [10] showed that Pseudomonas spp. was the dominant bacterial group in dry-aged beef samples. The obtained showed a correlation between bacterial counts and surface pH (r = 0.7–0.8; p < 0.01). The study by Przybylski et al. [17] also showed that the bacterial community of samples aged without biostarter was more variable than those aged with the M. flavus. It was also shown that the increase in the pH value during beef aging was also associated with the degradation of myofibrillar proteins, the increase in meat tenderness, and an improvement in sensory meat quality. The proteolysis of muscle protein during aging, as a result of the activity of endogenous muscle endopeptidase and microbial proteases, has already been confirmed in other studies [33].
The results showed that the beef dry-aged for 21 days with M. flavus biostarter had less shear force after grilling, with significant differences (p < 0.05) (Table 2). Similar results were obtained by Colle et al. [26]. The lower shear force after heat treatment in meat aged with biostarter corresponds to the higher juiciness of the meat. In addition, similar sensory quality results were obtained over 21 days of aging in the study by Colle et al. [26].

Table 2.
Instrumental assessment of beef hardness after 21 days of aging.
In addition, the samples with the biostarter were characterized by significantly higher flavor liking and better overall acceptability (p < 0.05) compared to the non-biostarter samples (Figure 1). Hanagasaki and Asato [12] claimed that an isolated mold strain, identified as Mucor flavus, gradually decreased the hardness of the beef, so the described tenderness was higher. Hanagasaki and Asato [12] and Dashdorj et al. [34] stated that Mucor flavus growing on dry-aged beef emits a pleasant aroma note. These findings emphasize the significant influence of M. flavus on enhancing or altering the flavor of beef. In contrast, Campbell et al. [35] observed that the palatability of beef aged in different ways did not differ, possibly due to the lack of visible fungal growth on the beef cuts.
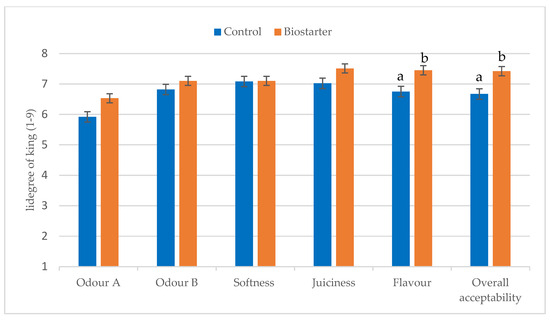
Figure 1.
Comparison of sensory liking (40 consumers) control and biostarter samples after 21 days of dry aging. Odor A—raw material (after 21 days of dry-aging; before heat treatment), Odor B—after heat treatment. Different letters (a, b) indicate means that are significantly different at the level of p < 0.05.
3.2. Volatile Aroma Compounds Characteristic
There is no doubt that volatile compounds, namely their quality and quantity, determine the sensory quality of the product. The results show that the samples aged with biostarter are characterized by significantly higher shear of aldehydes and acids compared to the control ones (Table 3, Figure 2). The samples aged with the biostarter are also characterized by a lower shear of alcohols, ketones, and hydrocarbons. The most abundant volatile compounds in the profile of samples aged with Mf fungal biostarter are nonanal (43.9%), octanal (12.5%), heptanal (10.2%), hexanal (8.7%), and 1-octanol (6%), representing about 80% of the shear of all determined compounds (Table 3). The control samples are characterized by high amounts of aldehydes and alcohols. Moreover, ketones, acids, and hydrocarbons represented other groups of the most abundant chemical substances found in the profile. The most abundant volatiles determined in the beef control samples were nonanal (43.8%), octanal (11.1%), 1-octanol (7.1%), heptanal (6.8%), and 1-decanol (5.8%) (Table 3). The table shows that aldehydes are the most important group of volatiles found in beef samples with biostarters (83.8%) and control beef samples (70%) (Figure 2).

Table 3.
Comparison of the most abundant volatile aroma compounds [%] extracted from the beef control samples and the beef samples with biostarter (M. flavus).
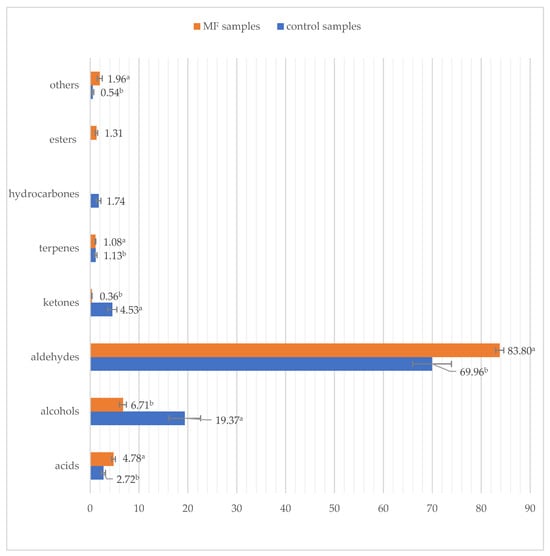
Figure 2.
Volatile aroma compound groups [%] found in dry-aged beef samples with biostarter (M. Flavus) and without it (control beef samples) over 21 days. a.b—values within a bar of a specific compound group with different letters are significantly different (p ≤ 0.05).
Among alcohols, 1-octanol was found in the samples with the biostarter corresponding, respectively, to spicy notes [36], nutty, pop-corn like [37], citrus, aromatic [38]. Altogether, the obtained results suggest that dry-aging with M. flavus biostarter significantly enhances the levels of particular aldehydes and esters, such as hexanal (fresh, green, fatty), heptanal (fatty, floral), pentanal (fermented, bread, fruity, nutty odor), hexyl ester of formic acid (fruity odor), and trans-2-undecenoic acid (mild odor), which represent compounds formed during lipid oxidation [8]. These flavors have often been used to describe dry-aged beef: beefy, buttery, nutty, and earthy [34]. Wang et al. [39] reported that nonanal and hexanal are derived from the oxidation of linoleic acid and linolenic acid, and very high amounts of hexanal can lead to an off-flavor. Among the compounds detected in the current study, 2-nonenal, trans-2-undecenoic acid, 1,2-dimethyl-cyclopentane, and the following esters: formic acid hexyl ester, 4-methyl-pentanoic acid methyl ester, 10-undecenoic acid methyl ester, were found only in the samples aged with biostarters. According to Flores [8], the most important aroma compounds in fermented sausages are aldehydes, especially hexanal (being the most significant aldehyde), followed by pentanal, octanal, and 2-nonenal (grass, green, herbal odors). In addition, Mikami et al. [5] described the volatile profile of dry and wet-aged beef. These authors reported that, in all samples, the highest amounts of volatile compounds were accounted for by hexanal, 1-octen-3-ol, and also 2-nonenal. However, other aldehydes were also found. However, it is worth mentioning that Mikami et al. [5] performed the GC analysis using a different chromatographic column (nonpolar InertCap I column) than the one we used (Stabilwax column). 1,2-dimethyl-cyclopentane was extracted from endophytic fungi isolated from Bruguiera gymnorhiza [40] and is also known as a volatile compound produced by Mucor in meat products [2]. Trans-2-undecenoic acid gives an earthy, fungal, fatty odor in meat samples and was found in different types of Korean dry-fermented sausages. Kim et al. [2] reported a very strong correlation between the concentration of L-limonene in Mucor fermented sausages, which also supports the results of our study [2,39]. Specific aroma compounds of fermented pork sausages were detected in traditional Chinese fermented foods prepared with the addition of different biostarters (Sx YCC3 strain and Lp MSZ2 strain). It was also described that the aroma profile of fermented sausage, with an addition of Sx YCC3 strain, contained, for example, trans-2-undecenoic acid, but they did not provide information on whether the Sx YCC3 starter contained Mucor representatives; they mentioned only Staphylococcus and Acinetobacter representatives [2].
The are two classes of meat flavor precursors: the first one represents water-soluble components and the second one lipids. Aroma compounds during cooking are mainly formed due to the thermal degradation or oxidation of lipids and the Maillard reactions between reducing sugars and amino acids. The following substances, including sugars, amino acids, peptides, nucleotides, other nitrogenous components, and their other derivatives, are known as aroma compound precursors [41,42]. Cooked meat has been found to contain many volatile compounds resulting from lipid degradation, including aldehydes, aliphatic hydrocarbons, ketones, carboxylic acids, alcohols, and esters [42]. Moreover, other volatiles, such as oxygenated heterocyclic compounds, e.g., lactones and alkyl furans, have been identified in cooked meat samples [41]. As for the odor attributes, in the study by Watanabe et al. [41] as well as in that by Yang et al. [42], the authors claimed that, with a longer period of wet aging, the content of aroma compounds was higher, mentioning aldehydes and furans as aroma representatives. In our study, furans were not found. This finding might have been caused by the very low amounts of such compounds in our samples, but also by the chromatographic column that was used for the separation of the volatile compounds (Stabilwax). Tsao et al. [43] suggest that using non-polar phase for the separation of furans is better. In addition, Haganasaki and Asato [12] underlined that M. flavus growing on dry-aged beef gives off a nutty odor, while Camppell et al. [35] observed that, possibly due to the lack of visible mold growth on cuts of beef aged in different ways, palatability did not differ significantly. This study may confirm the significant effect which fungal microorganisms can have on enhancing or changing the flavor of beef. According to Lee et al. [44], volatile compounds are substances of low molecular weight that are formed during lipid oxidation and Maillard reactions. Other researchers also indicate the importance of the interactions between Maillard reactions and lipid oxidation products and the thermal degradation of thiamine in developing the appropriate aroma [45]. On the other hand, another study [30] reported that the largest group of compounds was the group of hydrocarbons, followed by alcohols and aldehydes. A higher amount of heptane and esters and unclassified compounds was found in dry-aged meat. The high index of heptane and hexanal in meat and their exposure to oxygen molecules became suitable factors for the auto-oxidation and/or thermal degradation of fatty acids, e.g., oleic acid, linoleic acid. This study indicates that the fungal strain used in the maturation process significantly influences the meat odor notes. Moreover, it is stated that the aroma profile of dry-aged beef with mold was significantly different from the meat samples that were dry-aged without it [3]. A total of 75 peaks were identified in the aroma profile of dry-aged beef with the fungal culture (within 30 min of chromatographic analysis), and only 67 peaks were identified in the samples without fungus (within 20 min of chromatographic analysis). They found that there is a high probability that M. flavus could produce compounds with higher polarity in beef [3]. To conclude, the obtained results suggest that lower level of ketones and alcohols, as well as higher levels of acids and aldehydes, were related to higher acceptance of odor notes and improved the palatability of the meat samples with biostarters in significant ways. Based on the analyses of the volatile compound profiles occurring in the meat samples, several compounds were present only in the meat samples aged with the addition of M. flavus, e.g., 1-tetradecanol, 2-nonenal, and trans-2 undecenoic acid, as well as the following esters: formic acid, hexyl ester, 10-Undecenoic acid, methyl ester, 4-methyl-pentanoic acid, methyl ester. This may indicate that they are a specific marker of meat dry-aged with the addition of M. flavus. However, further research on the dry-aging of other types of meat is needed to confirm this observation.
3.3. Influence of Fungal Biostarter on Bacterial Growth on DAB
The bacterial growth (as a CFU number) was tested over 21 days of dry-aging (at the start—0 days—and then once per week, throughout the experiment) in order to describe how the presence of fungal strains can affect bacterial development on DAB. No significant differences between the samples inoculated with the fungal biostarter and the control were observed after 21 d of seasoning (Figure 3). The highest overall number of CFUs (10,200,000 units per sample) was observed in both cases after 7 days of aging, and then it started to decrease. However, the drop in the bacteria colonies counts was more explicit and faster in the biostarter-aged samples compared to the control ones.
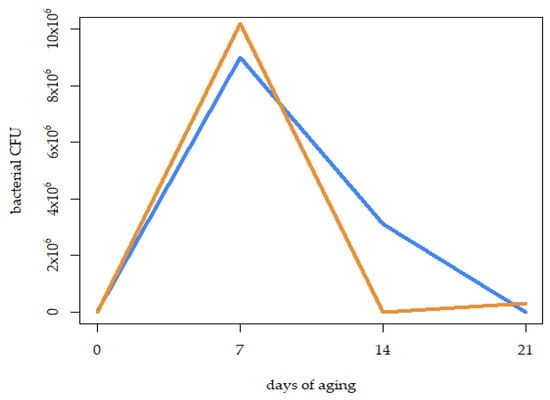
Figure 3.
Mean number of bacterial colony-forming units (CFUs) developed on the surface of dry-aged beef over time, during the 21 days of the dry-aging process, when seasoned with M. flavus biostarter (Mf) and without it (control).
3.4. Inoculated Listeria monocytogenes Development on DAB
No difference was observed between the studied and control samples inoculated with L. monocytogenes. The bacterial cells survived 21 days of dry-aging in all of the studied and control samples, except for one replicate in both variants (Mf, control) on the PALCAM medium (Table 4). Although recent studies show that Mucoraceae fungi can facilitate the spread and contribute to the survival of flagellated bacteria [46,47], in this study, the promotion of Listeria growth was not observed.

Table 4.
Survival of Listeria monocytogenes ATCC13932 on Chromogenic Listeria and PALCAM media (in % of plates on which the growth was observed).
We observed that in all replicates of Chromogenic Listeria medium and most of the PALCAM medium, L. monocytogenes colonies were present (Table 4). Compared to cheesemaking, where extensive studies have been conducted on the impact of microorganisms [11], the impact of microorganisms during the process of beef dry-aging is still poorly understood [48]. Further studies are required in order to understand the interplay between bacteria and fungi. This would help to evaluate their contribution to increasing consumer safety during the dry-aging process.
4. Conclusions
The obtained results show that significant changes occurred during the 21 days of dry-aging of beef for both groups. The pH value increased from 5.67 to 5.80, the protein content changed significantly, from 17.57% to 22.37%, the fat content from 17.92% to 21.55%, and the collagen content from 1.12% to 1.54%. The significant changes in protein, fat, and collagen content were due to moisture loss during aging. The results also showed that the beef dry-aged for 21 days with M. flavus biostarter were characterized by lower shear force after grilling, with significant differences. The samples with biostarter were characterized by significantly higher flavor liking and better overall acceptability.
Based on the analyzes carried out regarding the profile of volatile compounds occurring in meat samples, several compounds were present only in the meat samples aged with the addition of M. flavus, e.g., 1-tetradecanol, 2-nonenal, trans-2 undecenoic acid, and the following esters: formic acid, hexyl ester, 10-Undecenoic acid, methyl ester, 4-methyl-pentanoic acid, and methyl ester. This may indicate that they are a specific marker of dry-aged meat with the addition of M. flavus. However, further research on the dry-aging of other types of meat is needed to confirm this observation.
Finally, the presence of the biostarter had no significant effect on the number of bacteria or the survivability of L.monocytogenes on the meat’s surface in laboratory conditions.
5. Patents
The research solution, describing the application of the Mucor flavus KKP 2092p for beef dry-aging, has been filed at the Patent Office of the Republic of Poland and has been assigned an application number: P.443722.
Author Contributions
Conceptualization, W.P. and D.J.; methodology, W.P., D.J. and D.D.; formal analysis, W.P., D.J., J.P. and M.P.; investigation, W.P., D.J., D.K., L.A., U.S., P.K. and G.O.; writing—original draft preparation, W.P., D.J., J.P., D.D., M.P. and G.O.; writing—review and editing, J.P., W.P., D.J. and D.D.; visualization, D.D., P.K. and G.O.; supervision, W.P., D.J. and J.P.; project administration, J.P.; funding acquisition, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The National Centre for Research and Development (Poland) grant no TANGO-IV-C/0005/2019-00.
Institutional Review Board Statement
The sensory and consumer study was conducted as described by the Helsinki Declaration [24] and with the regulation and approval of the Ethical Commission, No 15/2021 (Date of consent: 7 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original data presented in this study are openly available in the Zenodo repository at DOI: https://doi.org/10.5281/zenodo.13268279.
Acknowledgments
The authors would like to thank Zakłady Mięsne Biernacki in Golina (Poland) for making the material available for research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rezende-de-Souza, J.H.; Cardello, F.A.B.; de Paula, A.P.M.; Ribeiro, F.A.; Calkins, C.R.; Pflanzer, S.B. Profile of producers and production of dryaged beef in Brazil. Foods 2021, 10, 2447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, E.-S.; Kim, B.-M.; Oh, M.-H. Potential Correlation between Microbial Diversity and Volatile Flavor Compounds in Different Types of Korean Dry-Fermented Sausages. Foods 2022, 11, 3182. [Google Scholar] [CrossRef] [PubMed]
- Hanagasaki, T.; Asato, N. Effect of dry-ageing with Mucor flavus on beef taste and aroma. Food Res. 2023, 7, 224–229. [Google Scholar] [CrossRef]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.L.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G.S. DNA barcoding in Mucorales: An invetory of biodiversity. Persoonia 2013, 30, 11–47. [Google Scholar] [CrossRef]
- Mikami, N.; Toyotomi, T.; Yamashiro, Y.; Sugo, K.; Yoshitomi, K.; Takaya, M.; Han, K.H.; Fukushima, M.; Shimada, K. Dry-aged beef manufactured in Japan: Microbiota identification and their effects on product characteristics. Food Res. Int. 2021, 140, 110020. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Liu, S.Q. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Coton, E.; Dubée, M.; Pawtowski, A.; Denoyelle, C.; Mounier, J. Microbiota associated with commercial dry-aged beef in France. Food Res. Int. 2024, 181, 114118. [Google Scholar] [CrossRef]
- Irlinger, F.; Mounier, J. Microbial interactions in cheese: Implications for cheese quality and safety. Curr. Opin. Biotechnol. 2009, 20, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hanagasaki, T.; Asato, N. Changes in free amino acid content and hardness of beef while dry-aging with Mucor flavus. J. Anim. Sci. Technol. 2018, 60, 19. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Park, M.R.; Maburutse, B.E.; Lee, W.J.; Park, D.J.; Cho, S.; Hwang, I.; Oh, S.; Kim, Y. Diversity and characteristics of the meat microbiological community on dry aged beef. J. Microbiol. Biotechnol. 2018, 28, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Shin, M.; Cho, S.; Hwang, I.; Kim, Y.; Oh, S. Molecular characterization of microbial and fungal communities on dry-aged beef of hanwoo using metagenomic analysis. Foods 2020, 9, 1571. [Google Scholar] [CrossRef]
- Ostrowski, G.; Jaworska, D.; Płecha, M.; Przybylski, W.; Sałek, P.; Sawicki, K.; Pawłowska, K. Cold adapted and closely related mucoraceae species colonise dry-aged beef (DAB). Fungal Biol. 2023, 127, 1397–1404. [Google Scholar] [CrossRef]
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- Przybylski, W.; Jaworska, D.; Płecha, M.; Dukaczewska, K.; Ostrowski, G.; Sałek, P.; Sawicki, K.; Pawłowska, J. Fungal Biostarter Effect on the Quality of Dry-Aged Beef. Foods 2023, 12, 1330. [Google Scholar] [CrossRef]
- ISO 1871. 2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. ISO: Geneva, Switzerland, 2009.
- PN-ISO 1444:2000; Meat and Meat Products–Determination of free Fat Content. Polish Committee for Standardization: Warsaw, Poland, 2013.
- ISO 3496:1994; Meat and Meat Products—Determination of Hydroxyproline Content. 2nd ed. ISO: Geneva, Switzerland, 1994.
- Nollet, L.M.L.; Toldrá, F. Handbook of Muscle Foods Analysis; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Food Research; Wydawnictwo Naukowe PTTZ: Kraków, Poland, 2014; ISBN 978-83-935421-3-0. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; Taylor & Francis: Abingdon, UK, 2006; ISBN 9780849338397. [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Xing, J.; Xing, W.; Tang, C.; Rao, Z.; Zhang, J. Untargeted profiling and differentiation of volatiles in varietes of meat using GC orbitrap MS. Foods 2022, 11, 3997. [Google Scholar] [CrossRef]
- Colle, M.J.; Richard, R.P.; Killinger, K.M.; Bohlscheid, J.C.; Gray, A.R.; Loucks, W.I.; Doumit, M.E. Influence of extended aging on beef quality characteristics and sensory perception of steaks from the biceps femoris and semimembranosus. Meat Sci. 2016, 119, 110–117. [Google Scholar] [CrossRef]
- Passetti, R.A.C.; Macedo, F.D.A.F.D.; Santos, G.R.D.A.; Bonin, E.; Vital, A.C.P.; Ramos, T.R.; Gomes Passetti, L.C.; Ornaghi, M.G.; Almeida Costa, I.C. Sensorial, color, lipid oxidation, and visual acceptability of dry-aged beef from young bulls with different fat thickness. Anim. Sci. J. 2019, 91, e13498. [Google Scholar] [CrossRef]
- Mancini, R.A.; Ramanathan, R. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 2014, 98, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, H.J.; Yoon, J.W.; Choe, J.; Jo, C. Electrical resistance and mold distribution on beef surface as indicators of dry aging. J. Food Process Eng. 2019, 42, e13122. [Google Scholar] [CrossRef]
- Sha, K.; Lang, Y.M.; Sun, B.Z.; Su, H.W.; Li, H.P.; Zhang, L.; Lei, Y.H.; Li, H.B.; Zhang, Y. Changes in lipid oxidation, fatty acid profile and volatile compounds of traditional Kazakh dry-cured beef during processing and storage. J. Food Process. Preserv. 2017, 41, e13059. [Google Scholar] [CrossRef]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- Obuz, E.; Akkaya, L.; Gók, V.; Dikeman, M.E. Effects of blade tenderization, aging method and aging time on meat quality characteristics of longissimus lumborum steaks from cull Holstein cows. Meat Sci. 2014, 96, 1227–1232. [Google Scholar] [CrossRef]
- Lee, Y.E.; Lee, H.J.; Kim, C.H.; Ryu, S.; Kim, Y.; Jo, C. Effect of Penicillium candidum and Penicillium nalgiovense and their combination on the physicochemical and sensory quality of dry-aged beef. Food Microbiol. 2022, 107, 104083. [Google Scholar] [CrossRef]
- Dashdorj, D.; Tripathi, V.K.; Cho, S.; Kim, S.; Hwang, I. Dry aging of beef; Review. J. Anim. Sci. Technol. 2016, 58, 20. [Google Scholar] [CrossRef]
- Campbell, R.E.; Hunt, M.C.; Levis, P.; Chambers, E. Dry-Aging Effects on Palatability of Beef Longissimus Muscle. J. Food Sci. 2001, 66, 196–199. [Google Scholar] [CrossRef]
- Wrona, M.; Vera, P.; Pezo, D.; Nerín, C. Identification and quantification of odours from oxobiodegradable polyethylene oxidised under a free radical flow by headspace solid-phase microextraction followed by gas chromatography, olfactometry-mass spectrometry. Talanta 2017, 172, 37–44. [Google Scholar] [CrossRef]
- Migita, K.; Iiduka, T.; Tsukamoto, K.; Sugiura, S.; Tanaka, G.; Sakamaki, G.; Matsuishi, M. Retort beef aroma that gives preferable properties to canned beef products and its aroma components. Anim. Sci. J. 2017, 88, 2050–2056. [Google Scholar] [CrossRef]
- Feng, T.; Shui, M.; Song, S.; Zhuang, H.; Sun, M.; Yao, L. Characterization of the key aroma compounds in three truffle varieties from China by flavoromics approach. Molecules 2019, 24, 3305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, J.; Zhang, X.; Hu, J.; Yu, Z.; Zhu, Y. Improving the Flavor of Fermented Sausage by Increasing Its Bacterial Quality via Inoculation with Lactobacillus plantarum MSZ2 and Staphylococcus xylosus YCC3. Foods 2022, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, S.I.; Izzati, F.; Yadi, S.; Bustanussalam, E.; Simanjuntak, P. Antioxidant Activities of Mangrove Fruits Endophytic Fungus from Segara Anakan Lagoon, Indonesia. Earth Environ. Sci. 2020, 439, 012034. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Yang, J.; Dashdorj, D.; Hwang, I. Volatile flavor components as a function of electrical stimulation and chiller aging for m. longissimus and biceps femoris of Hanwoo beef. Food Sci. Anim. Resour. 2019, 39, 474–493. [Google Scholar] [CrossRef]
- Tsao, W.X.; Chen, B.H.; Lin, P.; You, S.H.; Kao, T.H. Analysis of Furan and Its Derivatives in Food Matrices Using Solid Phase Extraction Coupled with Gas Chromatography-Tandem Mass Spectrometry. Molecules 2023, 28, 1639. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.J.; Yoon, J.W.; Kim, M.; Jo, C. Effect of Different Aging Methods on the Formation of Aroma Volatiles in Beef Strip Loins. Foods 2021, 10, 146. [Google Scholar] [CrossRef]
- Domaradzki, P.; Florek, M.; Litwińczuk, Z. Dry Ageing of Beef—Technological Aspects. Żywność Nauka Technol. Jakość 2016, 26, 17–37. [Google Scholar]
- Zhang, Y.; Kastman, E.K.; Guasto, J.S.; Wolfe, B.E. Fungal networks shape dynamics of bacterial dispersal and community assembly in cheese rind microbiomes. Nat. Commun. 2018, 9, 336. [Google Scholar] [CrossRef]
- Pion, M.; Bshary, R.; Bindschedler, S.; Filippidou, S.; Wick, L.Y.; Job, D.; Junier, P. Gains of bacterial flagellar motility in a fungal world. Appl. Environ. Microbiol. 2013, 79, 6862–6867. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Tyc, O.; de Boer, W.; Peereboom, N.; Debets, F.; Zaagman, N.; Janssens, T.K.S.; Garbeva, P. Fungus-associated bacteriome in charge of their host behavior. Fungal Genet. Biol. 2017, 102, 38–48. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).