Application of Microbial-Induced Carbonate Precipitation for Disintegration Control of Granite Residual Soil
Abstract
1. Introduction
2. Materials
2.1. Sampling Conditions
2.2. Bacterial Solution and Cementation Solution
3. Methods
3.1. Disintegration Apparatus
3.2. Disintegration Test Procedure
3.2.1. Specimen Preparation
3.2.2. Test Processes
3.3. Experimental Scheme
4. Results and Discussion
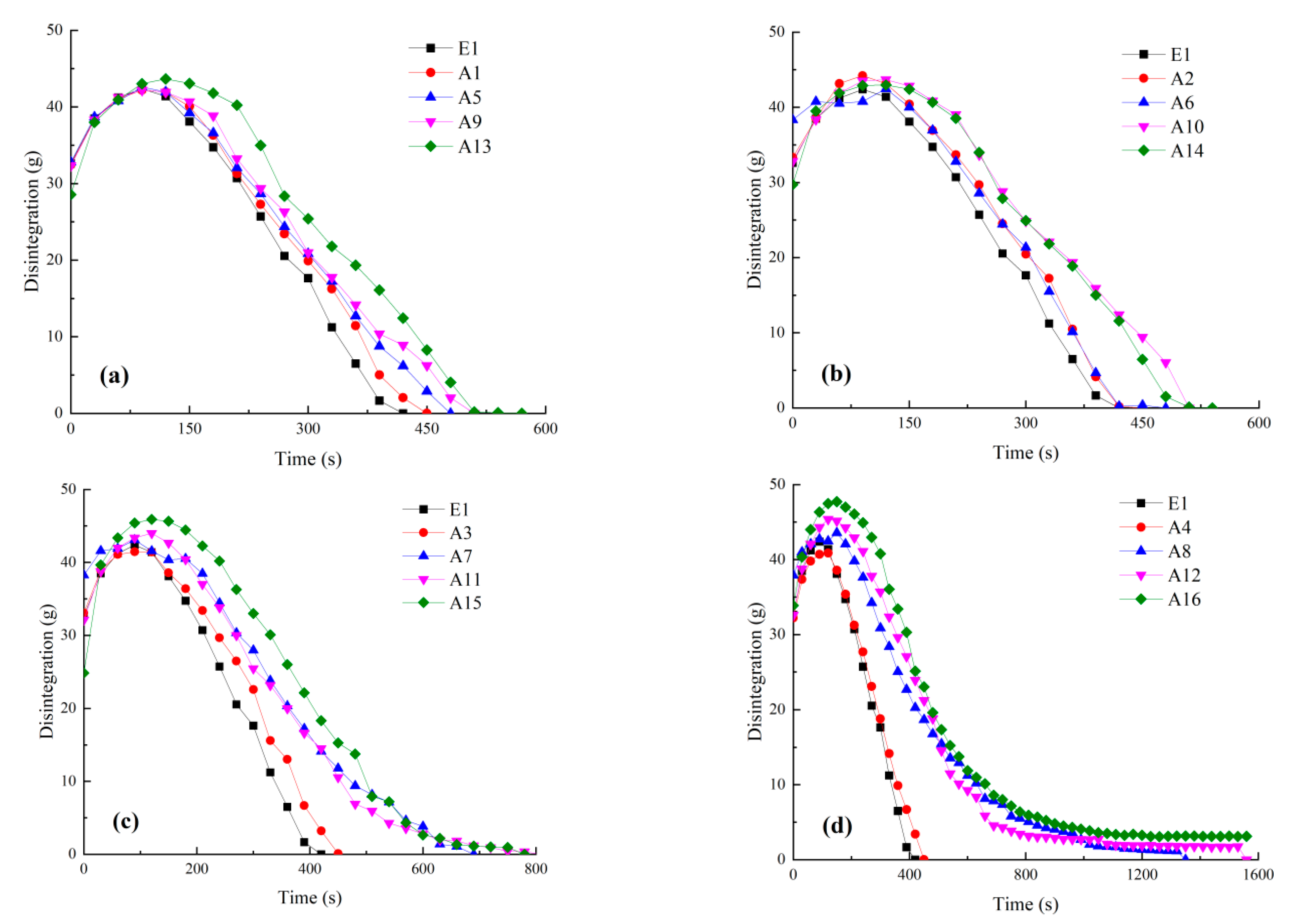
4.1. Disintegration Processes
4.2. Effect of Bacterial Solution Concentration on Disintegration
4.3. Effect of Cementation Solution Concentration on Disintegration
4.4. Effect of Treatment Cycle on Disintegration
5. Microstructure of Soil Samples
6. Conclusions
- (1)
- MICP technology with a spraying method for treating granite residual soil can effectively generate calcium carbonate (CaCO3) between soil particles. In this way, a crust layer on the surface is formed, which can significantly resist disintegration.
- (2)
- The bacterial solution concentration at a value of 0.75 and the cementation solution concentration at a value of 1.2 are optimal for resisting disintegration. An insufficient concentration of urease is produced by a low bacterial solution concentration, which makes it difficult to generate sufficient calcium carbonate to bond particles. An excessive bacterial solution concentration can lead to the premature formation of a dense layer on the soil surface, preventing further infiltration of bacteria and cementitious. A low cementation solution concentration cannot provide sufficient calcium ions, resulting in the insufficient generation of calcium carbonate. An excessive cementation solution concentration significantly inhibits the activity of urease.
- (3)
- Due to the formation of a sufficiently thick protective layer, five cycles of MICP treatment effectively suppresses disintegration. The thickness of the reinforcement layer generated by one or three cycles of reinforcement is not sufficient to completely wrap the surface.
- (4)
- Further research is underway to investigate the mechanical stability and ability to resist erosion by heavy rainfall. The long-term durability of cemented granite residual soil also needs to be considered to achieve the effective regulation of mineralization reaction efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Song, X.; Huang, F. Experimental study on the disintegration of granite residual soil under the combined influence of wetting–drying cycles and acid rain. Geomat. Nat. Hazards Risk 2019, 10, 1912–1927. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, J.; Zhang, D. Characteristics of slope runoff and soil water content in benggang colluvium under simulated rainfall. J. Soils Sediments 2018, 18, 39–48. [Google Scholar] [CrossRef]
- Deng, Y.; Shen, X.; Xia, D. Soil Erodibility and Physicochemical Properties of Collapsing Gully Alluvial Fans in Southern China. Pedosphere 2019, 29, 102–113. [Google Scholar] [CrossRef]
- Tang, Q.; Duan, X.; He, L. Variability and driving factors of the soil saturated hydraulic conductivity along the horizontal and vertical directions in the upper catchment of Benggang. CATENA 2023, 222, 106810. [Google Scholar] [CrossRef]
- Xu, J. Benggang erosion: The influencing factors. CATENA 1996, 27, 249–263. [Google Scholar]
- Liang, Y.; Zhang, B.; Pan, X. Current status and comprehensive control strategies of soil erosion for hilly region in the Southern China. Sci. Soil Water Conserv. 2008, 6, 22–27. [Google Scholar]
- Wei, Y.; Liu, Z.; Wu, X. Can Benggang be regarded as gully erosion? CATENA 2021, 207, 105648. [Google Scholar] [CrossRef]
- Liang, Y.; Ning, D.; Pan, X. Characteristics and management of Benggang erosion in Southern red soil region. Soil Water Conserv. China 2009, 1, 31–34. [Google Scholar]
- Liao, Y.; Zheng, M.; Li, D. Relationship of benggang number, area, and hypsometric integral values at different landform developmental stages. Land Degrad. Dev. 2020, 31, 2319–2328. [Google Scholar] [CrossRef]
- Liu, W.; Ouyang, G.; Luo, X. Moisture content, pore-water pressure and wetting front in granite residual soil during collapsing erosion with varying slope angle. Geomorphology 2020, 362, 107210. [Google Scholar] [CrossRef]
- Liu, W.; Cui, Y.; Ouyang, G. An experimental study on influence of grain-size composition on collapsing erosion of granite residual soil. CATENA 2023, 223, 106949. [Google Scholar] [CrossRef]
- Liao, Y.; Changyuan, T.; Zaijian, Y. Research Progress on Benggang Erosion and Its Prevention Measure in Red Soil Region of Southern China. Acta Pedol. Sin. 2018, 55, 1297–1312. [Google Scholar]
- Zhu, X.; Liang, Y.; Qu, L. Characteristics of runoff and sediment yield for two typical erodible soils in southern China. Int. J. Sediment Res. 2022, 37, 653–661. [Google Scholar] [CrossRef]
- Bullock, A.; King, B. Evaluating China’s Slope Land Conversion Program as sustainable management in Tianquan and Wuqi Counties. J. Environ. Manag. 2011, 92, 1916–1922. [Google Scholar] [CrossRef]
- van Paassen Leon, A.; Ghose, R.; van der Linden Thomas, J.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Portugal, C.R.M.e.; Fonyo, C.; Machado, C.C. Microbiologically Induced Calcite Precipitation biocementation, green alternative for roads—Is this the breakthrough? A critical review. J. Clean. Prod. 2020, 262, 121372. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef]
- Tang, C.S.; Zhu, C.; Cheng, Q. Desiccation cracking of soils: A review of investigation approaches, underlying mechanisms, and influencing factors. Earth-Sci. Rev. 2021, 216, 103586. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yao, D. Feasibility of microbially induced carbonate precipitation and straw checkerboard barriers on desertification control and ecological restoration. Ecol. Eng. 2020, 152, 105883. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Yu, J. Effectiveness of the anti-erosion of an MICP coating on the surfaces of ancient clay roof tiles. Constr. Build. Mater. 2020, 243, 118202. [Google Scholar] [CrossRef]
- Çanakçi, H.; Sidik, W.; Kiliç, İ.H. Effect of bacterial calcium carbonate precipitation on compressibility and shear strength of organic soil. Soils Found. 2015, 55, 1211–1221. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Tang, C.S.; Pan, X.H. Application of microbial induced carbonate precipitation for loess surface erosion control. Eng. Geol. 2021, 294, 106387. [Google Scholar] [CrossRef]
- Jiang, N.J.; Tang, C.S.; Yin, L.Y. Applicability of Microbial Calcification Method for Sandy-Slope Surface Erosion Control. J. Mater. Civ. Eng. 2019, 31, 04019250. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, J.; Liu, S. Experimental study to improve the mechanical properties of iron tailings sand by using MICP at low pH. Constr. Build. Mater. 2021, 273, 121729. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Wang, L.; Ren, Z.; Wang, H. Microstructure-property relationships in cement mortar with surface treatment of microbial induced carbonate precipitation. Compos. Part B Eng. 2022, 239, 109986. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C. Factors Affecting Improvement of Engineering Properties of MICP-Treated Soil Catalyzed by Bacteria and Urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Tang, C.S.; Yin, L.Y.; Jiang, N.J. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Choi, S.G.; Chang, I.; Lee, M. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Fajardo, M.; McBratney, A.B.; Field, D.J. Soil slaking assessment using image recognition. Soil Tillage Res. 2016, 163, 119–129. [Google Scholar] [CrossRef]
- Ze, Z.; Vadim, P.; Svetlana, N. Disintegration characteristics of a cryolithogenic clay loam with different water content: Moscow covering loam (prQIII), case study. Eng. Geol. 2019, 258, 105159. [Google Scholar] [CrossRef]
- Kasmerchak, C.S.; Mason, J.A.; Liang, M. Laser diffraction analysis of aggregate stability and disintegration in forest and grassland soils of northern Minnesota, USA. Geoderma 2019, 338, 430–444. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Kong, L. Disintegration of granite residual soils with varying degrees of weathering. Eng. Geol. 2022, 305, 106723. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Xu, H.S. Influences of different modifiers on the disintegration of improved granite residual soil under wet and dry cycles. Int. J. Min. Sci. Technol. 2022, 32, 831–845. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, H.M. Experimental study of disintegration mechanism for unsaturated granite residual soil. Rock Soil Mech. 2013, 34, 1668–1674. [Google Scholar]
- Meyer, F.; Bang, S.; Min, S.; Stetler, L.; Bang, S. Microbiologically-induced soil stabilization: Application of Sporosarcina pasteurii for fugitive dust control. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 4002–4011. [Google Scholar]
- Wang, R.; Tang, C.S.; Pan, X.; Shen, Z.; Liu, Y.; Lu, X. A biotechnological approach for suspended solids removal in biogas slurry via microbially induced calcite precipitation (MICP). J. Clean. Prod. 2024, 459, 142537. [Google Scholar] [CrossRef]
- Katebi, H.; Fahmi, A.; Ouria, A.; Babaeian Amini, A.; Kafil, H.S. Microbial surface treatment of sand with Sporosarcina pasteurii to Improve the wind erosion resistance in urmia lake. Appl. Environ. Soil Sci. 2021, 8893115. [Google Scholar] [CrossRef]
- Bruce Donald, A. Glossary of Grouting Terminology. J. Geotech. Geoenviron. Eng. 2005, 131, 1534–1542. [Google Scholar] [CrossRef]




















| Sample Number | Bacterial Solution Concentration (OD600) | Cementation Solution Concentration (mol/L) | Treatment Cycle |
|---|---|---|---|
| A1~A4 | 0.25 | 0.4, 0.8, 1.2, 1.6 | 1 |
| A5~A8 | 0.5 | 0.4, 0.8, 1.2, 1.6 | 1 |
| A9~A12 | 0.75 | 0.4, 0.8, 1.2, 1.6 | 1 |
| A13~A16 | 1.0 | 0.4, 0.8, 1.2, 1.6 | 1 |
| B1~B4 | 0.25 | 0.4, 0.8, 1.2, 1.6 | 3 |
| B5~B8 | 0.5 | 0.4, 0.8, 1.2, 1.6 | 3 |
| B9~B12 | 0.75 | 0.4, 0.8, 1.2, 1.6 | 3 |
| B13~B16 | 1.0 | 0.4, 0.8, 1.2, 1.6 | 3 |
| C1~C4 | 0.25 | 0.4, 0.8, 1.2, 1.6 | 5 |
| C5~C8 | 0.5 | 0.4, 0.8, 1.2, 1.6 | 5 |
| C9~C12 | 0.75 | 0.4, 0.8, 1.2, 1.6 | 5 |
| C13~C16 | 1.0 | 0.4, 0.8, 1.2, 1.6 | 5 |
| D1~D4 | 0.25 | 0.4, 0.8, 1.2, 1.6 | 7 |
| D5~D8 | 0.5 | 0.4, 0.8, 1.2, 1.6 | 7 |
| D9~D12 | 0.75 | 0.4, 0.8, 1.2, 1.6 | 7 |
| D13~D16 | 1.0 | 0.4, 0.8, 1.2, 1.6 | 7 |
| E1~E4 | 0 | 0 | 1, 3, 5, 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Feng, Y.; Li, C.; Liu, W. Application of Microbial-Induced Carbonate Precipitation for Disintegration Control of Granite Residual Soil. Appl. Sci. 2024, 14, 6343. https://doi.org/10.3390/app14146343
Luo X, Feng Y, Li C, Liu W. Application of Microbial-Induced Carbonate Precipitation for Disintegration Control of Granite Residual Soil. Applied Sciences. 2024; 14(14):6343. https://doi.org/10.3390/app14146343
Chicago/Turabian StyleLuo, Xiaoyan, Yingqi Feng, Chunjun Li, and Weiping Liu. 2024. "Application of Microbial-Induced Carbonate Precipitation for Disintegration Control of Granite Residual Soil" Applied Sciences 14, no. 14: 6343. https://doi.org/10.3390/app14146343
APA StyleLuo, X., Feng, Y., Li, C., & Liu, W. (2024). Application of Microbial-Induced Carbonate Precipitation for Disintegration Control of Granite Residual Soil. Applied Sciences, 14(14), 6343. https://doi.org/10.3390/app14146343





