Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Antibacterial and Antifungal Activity
2.2.1. Bacteria and Fungi
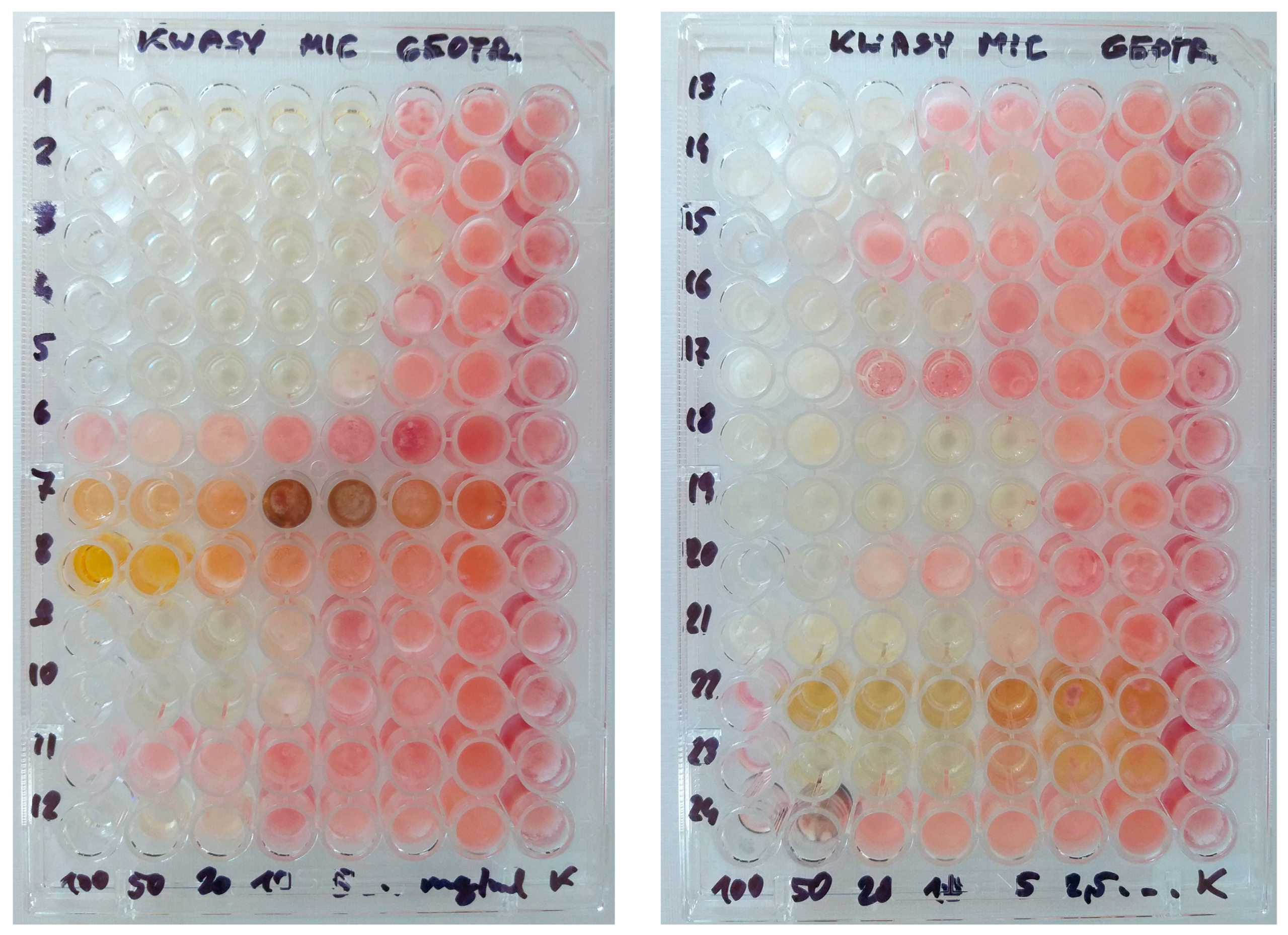
2.2.2. Minimal Inhibitory Concentrations (MIC)
2.3. In Silico Bioavailability Toxicity Prediction
3. Results
3.1. Antibacterial and Antifungal Activity
3.2. In Silico Bioavailability and Toxicity Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, J.H.; Song, M.; Cho, J.H.; Kim, S.; Kim, E.S.; Keum, G.B.; Kim, H.B.; Lee, J.-H. Evaluating the Prevalence of Foodborne Pathogens in Livestock Using Metagenomics Approach. J. Microbiol. Biotechnol. 2021, 31, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Samson, R.A. Current Taxonomy and Identification of Foodborne Fungi. Curr. Opin. Food Sci. 2017, 17, 84–88. [Google Scholar] [CrossRef]
- Benedict, K.; Chiller, T.M.; Mody, R.K. Invasive Fungal Infections Acquired from Contaminated Food or Nutritional Supplements: A Review of the Literature. Foodborne Pathog. Dis. 2016, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Skora, M.; Witalis, J.; Krzysciak, P.; Macura, A.B. Fungal genus Geotrichum: An opportunistic pathogen of humans. Postępy Mikrobiol. 2009, 48, 125–132. [Google Scholar]
- Rannikko, J.; Holmberg, V.; Karppelin, M.; Arvola, P.; Huttunen, R.; Mattila, E.; Kerttula, N.; Puhto, T.; Tamm, Ü.; Koivula, I.; et al. Fungemia and Other Fungal Infections Associated with Use of Saccharomyces boulardii Probiotic Supplements. Emerg. Infect. Dis. 2021, 27, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Enache-Angoulvant, A.; Hennequin, C. Invasive Saccharomyces Infection: A Comprehensive Review. Clin. Infect. Dis. 2005, 41, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Characteristic of Bacteriocines and Their Application. Pol. J. Microbiol. 2013, 62, 223–235. [Google Scholar] [CrossRef]
- Farid, N.; Waheed, A.; Motwani, S. Synthetic and Natural Antimicrobials as a Control against Food Borne Pathogens: A Review. Heliyon 2023, 9, e17021. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Mira, N.P.; Marshall, R.; Pinheiro, M.J.F.; Dieckmann, R.; Dahouk, S.A.; Skroza, N.; Rudnicka, K.; Lund, P.A.; De Biase, D.; Working Group 3 of the COST Action EuroMicropH. On the Potential Role of Naturally Occurring Carboxylic Organic Acids as Anti-Infective Agents: Opportunities and Challenges. Int. J. Infect. Dis. 2024, 140, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the Analysis of Organic Acids in Thirty-Five Species of Food and Medicinal Plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Raj, K.; Węglarz, Z.; Przybył, J.L.; Kosakowska, O.; Pawełczak, A.; Gontar, Ł.; Puchta-Jasińska, M.; Bączek, K. Chemical Diversity of Wild-Growing and Cultivated Common Valerian (Valeriana officinalis L. s.l.) Originating from Poland. Molecules 2023, 29, 112. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, J.; Yang, H.; Feng, L.; Wang, M.; Xu, G.; Shao, J.; Ma, C. Grape Tartaric Acid: Chemistry, Function, Metabolism, and Regulation. Horticulturae 2023, 9, 1173. [Google Scholar] [CrossRef]
- Ben Braïek, O.; Smaoui, S. Chemistry, Safety, and Challenges of the Use of Organic Acids and Their Derivative Salts in Meat Preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- Korbecka-Paczkowska, M.; Karpiński, T.M. In Vitro Assessment of Antifungal and Antibiofilm Efficacy of Commercial Mouthwashes against Candida albicans. Antibiotics 2024, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and Antibiofilm Activity of Selected Lamiaceae Essential Oils. Front. Biosci. (Landmark Ed.) 2023, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Rosiak, N.; Plech, T.; Karpiński, T.M.; Miklaszewski, A.; Witkowska, K.; Jaskólski, M.; Erdem, C.; Cielecka-Piontek, J. Electrospun Nanofibers Loaded with Marigold Extract Based on PVP/HPβCD and PCL/PVP Scaffolds for Wound Healing Applications. Materials 2024, 17, 1736. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; de Sá, A.G.C.; Ascher, D.B. Deep-PK: Deep Learning for Small Molecule Pharmacokinetic and Toxicity Prediction. Nucleic Acids Res 2024, 52, W469–W475. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Michalska, A.; Jurowski, K. The Prediction of Acute Toxicity (LD50) for Organophosphorus-Based Chemical Warfare Agents (V-Series) Using Toxicology in Silico Methods. Arch. Toxicol. 2024, 98, 267–275. [Google Scholar] [CrossRef]
- Tannic Acid. Available online: https://go.drugbank.com/drugs/DB09372 (accessed on 12 July 2024).
- Klampfl, C.W.; Buchberger, W.; Haddad, P.R. Determination of Organic Acids in Food Samples by Capillary Zone Electrophoresis. J. Chromatogr. A 2000, 881, 357–364. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Mai, T.L. Preservatives|Traditional Preservatives—Organic Acids. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 119–130. ISBN 978-0-12-384733-1. [Google Scholar]
- Beier, R.C.; Harvey, R.B.; Poole, T.L.; Hume, M.E.; Crippen, T.L.; Highfield, L.D.; Alali, W.Q.; Andrews, K.; Anderson, R.C.; Nisbet, D.J. Interactions of Organic Acids with Vancomycin-Resistant Enterococcus faecium Isolated from Community Wastewater in Texas. J. Appl. Microbiol. 2019, 126, 480–488. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Caldwell, D.; Andrews, K.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Inhibition and Interactions of Campylobacter jejuni from Broiler Chicken Houses with Organic Acids. Microorganisms 2019, 7, 223. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Zahli, R.; Abrini, J. Evaluation of Antimicrobial Activity of Four Organic Acids Used in Chicks Feed to Control Salmonella typhimurium: Suggestion of Amendment in the Search Standard. Int. J. Microbiol. 2018, 2018, 7352593. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Boopathy, R. Effect of Organic Acids on Shrimp Pathogen, Vibrio harveyi. Curr. Microbiol. 2011, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Štempelová, L.; Kubašová, I.; Bujňáková, D.; Karahutová, L.; Gálová, J.; Kužma, E.; Strompfová, V. Antimicrobial Activity of Organic Acids against Canine Skin Bacteria. Vet. Res. Commun. 2023, 47, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lues, J.F.R.; Theron, M.M. Comparing Organic Acids and Salt Derivatives as Antimicrobials against Selected Poultry-Borne Listeria monocytogenes Strains in Vitro. Foodborne Pathog. Dis. 2012, 9, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Akbas, M.Y.; Cag, S. Use of Organic Acids for Prevention and Removal of Bacillus subtilis Biofilms on Food Contact Surfaces. Food Sci. Technol. Int. 2016, 22, 587–597. [Google Scholar] [CrossRef]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tabaran, A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. UASVM Vet. Med. 2017, 74, 212–217. [Google Scholar] [CrossRef][Green Version]
- Castro, V.S.; da Silva Mutz, Y.; Rosario, D.K.A.; Cunha-Neto, A.; de Souza Figueiredo, E.E.; Conte-Junior, C.A. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens 2020, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Dubal, Z.B.; Paturkar, A.M.; Waskar, V.S.; Zende, R.J.; Latha, C.; Rawool, D.B.; Kadam, M.M. Effect of Food Grade Organic Acids on Inoculated S. aureus, L. monocytogenes, E. coli and S. typhimurium in Sheep/Goat Meat Stored at Refrigeration Temperature. Meat Sci. 2004, 66, 817–821. [Google Scholar] [CrossRef]
- Albuquerque, G.N.; Costa, R.G.; Barba, F.J.; Gómez, B.; Ribeiro, N.L.; Beltrão Filho, E.M.; Sousa, S.; Santos, J.G.; Lorenzo, J.M. Effect of Organic Acids on the Quality of Sheep “Buchada”: From Food Safety to Physicochemical, Nutritional, and Sensorial Evaluation. J. Food Process. Preserv. 2019, 43, e13877. [Google Scholar] [CrossRef]
- Dias, F.S.; da Silva Ávila, C.L.; Schwan, R.F. In Situ Inhibition of Escherichia coli Isolated from Fresh Pork Sausage by Organic Acids. J. Food Sci. 2011, 76, M605–M610. [Google Scholar] [CrossRef]
- Moro, C.B.; Lemos, J.G.; Gasperini, A.M.; Stefanello, A.; Garcia, M.V.; Copetti, M.V. Efficacy of Weak Acid Preservatives on Spoilage Fungi of Bakery Products. Int. J. Food Microbiol. 2022, 374, 109723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal Activity and Mechanism of Action of Tannic Acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Maimaitiyiming, R.; Yang, Y.; Mulati, A.; Aihaiti, A.; Wang, J. The Use of Ultraviolet Irradiation to Improve the Efficacy of Acids That Are Generally Recognized as Safe for Disinfecting Fresh Produce in the Ready-to-Eat Stage. Foods 2024, 13, 1723. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.C.; Bhunia, F.; Kaviraj, A. Comparative Toxicity of Three Organic Acids to Freshwater Organisms and Their Impact on Aquatic Ecosystems. Hum. Ecol. Risk Assess. 2006, 12, 192–202. [Google Scholar] [CrossRef]
- Türkoğlu, Ş. Genotoxicity of Five Food Preservatives Tested on Root Tips of Allium cepa L. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 626, 4–14. [Google Scholar] [CrossRef]
| Organic Acid | Molecular Formula | pH of Prepared Solutions | Exemplary Natural Occurrence |
|---|---|---|---|
| Acetic acid | C2H4O2 | 2.4 | Apples, grapes, and blackberries |
| Aminoacetic acid | C2H5NO2 | 6.2 | Common amino acid |
| Ascorbic acid | C6H8O6 | 2.5 | Fruits and vegetables |
| Benzoic acid | C7H6O2 | 3.8 | Cranberries, mushrooms, anise, cherries, raspberries, and food additive (as a preservative) |
| Caproic acid | C6H12O2 | 2.7 | Vegetable oils |
| Chlorogenic acid | C16H18O9 | 4.4 | Apples, pears, carrots, tomatoes, sweet potatoes, coffee, and tea |
| Citric acid | C6H8O7 | 2.9 | Fruits |
| Formic acid | CH2O2 | 2.3 | Stinging hairs of nettles |
| Fumaric acid | C4H4O4 | 4.5 | Mosses and mushrooms |
| Glutamic acid | C5H9NO4 | 2.9 | Sunflower seeds, flax seeds, peanut, pistachio, almond, broad bean, Brussels sprout, and lentil |
| Malic acid | C4H6O5 | 2.0 | Fruits |
| Nicotinic acid | C6H5NO2 | 4.0 | Common in plants |
| Oleic acid | C18H34O2 | 2.8 | Olive oil and grape seed oil |
| Oxalic acid | C2H2O4 | 1.8 | Fruits |
| Palmitic acid | C16H32O2 | 4.5 | Seeds of beans, sunflowers, and cotton |
| Rosmarinic acid | C18H16O8 | 5.8 | Rosemary, sage, Spanish sage, basil, oregano, thyme, spearmint, and perilla |
| Salicylic acid | C7H6O3 | 3.0 | Common in plants |
| Succinic acid | C4H6O4 | 2.4 | Fruits and vegetables |
| Tannic acid | C76H52O46 | 2.6 | Bark of oak, beech, American chestnut, spruce, willow, witch hazel, walnut, blackberry, raspberry leaves, blueberries, sloes, rhizome of cinquefoil, hen’s weed, and snakeweed |
| Tartaric acid | C4H6O6 | 1.9 | Peaches, apples, grapes, cherries, and strawberries |
| Valeric acid | C5H10O2 | 2.8 | Valerian rhizome and angelica root |
| Organic Acid | Staphylococcus aureus | Escherichia coli | Listeria monocytogenes | Salmonella Typhimurium | Mean MIC ± SD for All Bacteria |
|---|---|---|---|---|---|
| Acetic acid | 1.25 | 1.25–2.5 | 1.25 | 2.5 | 1.72 ± 0.65 |
| Aminoacetic acid | 100 | 50 | 50 | 50–100 | 70.83 ± 25.75 |
| Ascorbic acid | 1.25 | 1.25–2.5 | 2.5 | 5 | 2.66 ± 1.56 |
| Benzoic acid | 0.63 | 0.31–0.63 | 0.31–0.63 | 0.63–1.25 | 0.63 ± 0.29 |
| Caproic acid | 5 | 2.5 | 2.5 | 5 | 3.75 ± 1.34 |
| Chlorogenic acid | 1.25–2.5 | 1.25 | 1.25–2.5 | 1.25–2.5 | 1.72 ± 0.65 |
| Citric acid | 5 | 2.5 | 1.25–2.5 | 2.5 | 2.97 ± 1.33 |
| Formic acid | 1.25 | 1.25 | 1.25 | 2.5 | 1.56 ± 0.58 |
| Fumaric acid | 1.25–2.5 | 2.5 | 2.5–5 | 2.5 | 2.66 ± 1.04 |
| Glutamic acid | 5 | 5 | 10 | 10 | 7.50 ± 2.67 |
| Malic acid | 2.5 | 0.63 | 0.63–1.25 | 1.25 | 1.33 ± 0.78 |
| Nicotinic acid | 1.25–2.5 | 0.63–1.25 | 0.63–1.25 | 1.25 | 1.25 ± 0.56 |
| Oleic acid | 5 | 10 | 5 | 10 | 7.50 ± 2.67 |
| Oxalic acid | 2.5–5 | 0.63–1.25 | 1.25–2.5 | 2.5 | 2.27 ± 1.33 |
| Palmitic acid | 20–50 | 20–50 | 20 | 20–50 | 27.5 ± 13.57 |
| Rosmarinic acid | 2.5 | 1.25 | 1.25–2.5 | 1.25–2.5 | 1.88 ± 0.67 |
| Salicylic acid | 0.63–1.25 | 0.31–0.63 | 0.63 | 0.63 | 0.67 ± 0.26 |
| Succinic acid | 2.5–5 | 0.63–1.25 | 1.25–2.5 | 2.5 | 2.27 ± 1.33 |
| Tannic acid | 2.5–5 | 1.25–2.5 | 0.63–1.25 | 2.5 | 2.27 ± 1.33 |
| Tartaric acid | 5 | 1.25–2.5 | 2.5 | 2.5 | 2.97 ± 1.33 |
| Valeric acid | 5 | 1.25–2.5 | 2.5 | 2.5 | 2.97 ± 1.33 |
| Octenidine dihydrochloride | 0.00004–0.00008 (0.04–0.08 µg/mL) | 0.00008–0.00016 (0.08–0.16 µg/mL) | 0.00008–0.00016 (0.08–0.16 µg/mL) | 0.00008–0.00016 (0.08–0.16 µg/mL) | 0.00016–0.00005 (0.11 ± 0.05 µg/mL) |
| Organic Acid | Geotrichum candidum | Penicillium candidum | Mean MIC ± SD for Both Fungi |
|---|---|---|---|
| Acetic acid | 5 | 10 | 7.5 ± 2.74 |
| Aminoacetic acid | >100 | >100 | >100 |
| Ascorbic acid | 50–100 | 100 | 83.33 ± 25.82 |
| Benzoic acid | 2.5–5 | 10 | 7.08 ± 3.32 |
| Caproic acid | 5 | 10 | 7.5 ± 2.74 |
| Chlorogenic acid | 1.25–5 | 5–10 | 5.63 ± 3.69 |
| Citric acid | 5–10 | 10 | 8.33 ± 2.58 |
| Formic acid | 5 | 10 | 7.5 ± 2.74 |
| Fumaric acid | 5–10 | 20 | 14.17 ± 6.65 |
| Glutamic acid | >100 | >100 | >100 |
| Malic acid | 20–50 | 50 | 45 ± 12.25 |
| Nicotinic acid | 10 | 20 | 15 ± 5.48 |
| Oleic acid | 50 | 50 | 50 ± 0.0 |
| Oxalic acid | 10–20 | 20 | 16.67 ± 5.16 |
| Palmitic acid | 20–50 | 20–50 | 30 ± 15.49 |
| Rosmarinic acid | 1.25–5 | 10 | 6.46 ± 4.06 |
| Salicylic acid | 2.5–5 | 10 | 7.08 ± 3.32 |
| Succinic acid | 50–100 | 100 | 83.33 ± 25.82 |
| Tannic acid | 10–20 | 5–20 | 14.17 ± 6.65 |
| Tartaric acid | 20–50 | 100 | 70 ± 34.64 |
| Valeric acid | 5 | 5 | 5 ± 0.0 |
| Octenidine dihydrochloride | 0.00008–0.00016 (0.08–0.16 µg/mL) | 0.00008–0.00032 (0.08–0.32 µg/mL) | 0.00016 ± 0.00009 (0.16 ± 0.09 µg/mL) |
| Organic Acid | Gastro-Intestinal Tract Absorption | Human Oral Bioavailability 20% | Predicted LD50 [mg/kg] | Toxicity Class | Carcinogenicity | Hepatotoxicity | Neurotoxicity | Nephrotoxicity | Skin Sensitization | Eye Irritation |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | High | Yes | 333 | 1 | No | No | No | No | Yes | Yes |
| Aminoacetic acid | High | Yes | 3340 | 5 | No | No | No | No | Yes | Yes |
| Ascorbic acid | High | No | 3367 | 5 | No | No | No | No | Yes | Yes |
| Benzoic acid | High | Yes | 290 | 3 | No | Yes | No | Yes | Yes | Yes |
| Caproic acid | High | Yes | 94 | 3 | No | No | No | No | Yes | Yes |
| Chlorogenic acid | Low | No | 5000 | 5 | No | No | No | Yes | Yes | No |
| Citric acid | Low | No | 80 | 3 | No | No | No | No | No | Yes |
| Formic acid | High | Yes | 162 | 3 | No | No | No | Yes | Yes | Yes |
| Fumaric acid | High | Yes | 1350 | 4 | No | No | No | Yes | Yes | Yes |
| Glutamic acid | High | Yes | 4500 | 5 | No | No | No | No | Yes | Yes |
| Malic acid | High | Yes | 2497 | 5 | No | No | No | Yes | Yes | Yes |
| Nicotinic acid | High | Yes | 3720 | 5 | No | Yes | Yes | Yes | Yes | Yes |
| Oleic acid | High | No | 48 | 2 | No | No | No | No | Yes | Yes |
| Oxalic acid | High | Yes | 660 | 4 | No | No | No | Yes | Yes | Yes |
| Palmitic acid | High | No | 990 | 4 | No | No | No | No | Yes | Yes |
| Rosmarinic acid | Low | No | 5000 | 5 | No | No | No | Yes | Yes | Yes |
| Salicylic acid | High | Yes | 1190 | 4 | No | Yes | Yes | No | Yes | No |
| Succinic acid | High | Yes | 2260 | 5 | No | No | No | Yes | Yes | Yes |
| Tannic acid | nd | * Low | 2260 | 5 | No | No | No | Yes | Yes | Yes |
| Tartaric acid | Low | Yes | 2497 | 5 | No | No | No | Yes | Yes | Yes |
| Valeric acid | High | Yes | 134 | 3 | No | No | No | No | Yes | Yes |
| Octenidine | Low | No | 300 | 3 | No | No | Yes | No | Yes | No |
| Organic Acid | Predicted LD50 [mg/kg] | Mean MIC [mg/mL] against Bacteria/Fungi | Density [g/mL] | Mean MIC in 1 kg of Food Product [mg/kg] against Bacteria/Fungi | Safe Use as a Food Preservative (MIC < LD50) against Bacteria/Fungi |
|---|---|---|---|---|---|
| Acetic acid | 333 | 1.72/7.5 | 1.05 | 1638/7142 | No/No |
| Aminoacetic acid | 3340 | 70.83/>100 | 1.61 | 43,994/>62,112 | No/No |
| Ascorbic acid | 3367 | 2.66/83.33 | 1.65 | 1612/50,503 | Yes/No |
| Benzoic acid | 290 | 0.63/7.08 | 1.27 | 496/5574 | No/No |
| Caproic acid | 94 | 3.75/7.5 | 0.93 | 4032/8065 | No/No |
| Chlorogenic acid | 5000 | 1.72/5.63 | 1.28 | 1344/4398 | Yes/Yes |
| Citric acid | 80 | 2.97/8.33 | 1.66 | 1789/5018 | No/No |
| Formic acid | 162 | 1.56/7.5 | 1.22 | 1279/6148 | No/No |
| Fumaric acid | 1350 | 2.66/14.17 | 1.64 | 1622/8640 | No/No |
| Glutamic acid | 4500 | 7.50/>100 | 1.46 | 5137/>68,493 | No/No |
| Malic acid | 2497 | 1.33/50 | 1.61 | 826/31,055 | Yes/No |
| Nicotinic acid | 3720 | 1.25/15 | 1.47 | 850/10,204 | Yes/No |
| Oleic acid | 48 | 7.50/50 | 0.895 | 8380/55,866 | No/No |
| Oxalic acid | 660 | 2.27/16.67 | 1.9 | 1195/8774 | No/No |
| Palmitic acid | 990 | 27.5/30 | 0.85 | 32,706/35,294 | No/No |
| Rosmarinic acid | 5000 | 1.88/6.46 | 1.55 | 1213/4168 | Yes/Yes |
| Salicylic acid | 1190 | 0.67/7.08 | 1.44 | 465/4917 | Yes/No |
| Succinic acid | 2260 | 2.27/83.33 | 1.56 | 1455/53,417 | Yes/No |
| Tannic acid | 2260 | 2.27/52.08 | 2.12 | 1071/24,566 | Yes/No |
| Tartaric acid | 2497 | 2.97/75 | 1.79 | 1659/41,899 | Yes/No |
| Valeric acid | 134 | 2.97/5.0 | 0.94 | 3160/5319 | No/No |
| Organic Acid | Tested Microorganism | MIC Values from Reference | Our MICs [mg/mL] | Reference |
|---|---|---|---|---|
| Acetic acid | Enterococcus faecium | 2 mg/mL | 1.25–2.5 | [31] |
| Campylobacter jejuni | 0.5–4.1 mg/mL | [32] | ||
| Salmonella Typhimurium | 0.312% (3.1 mg/mL) | [33] | ||
| Vibrio harveyi | 0.05–0.1% (0.5–1 mg/mL) | [34] | ||
| Staphylococcus aureus, E. faecium, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa | 0.5–2.0 mg/mL | [35] | ||
| Listeria monocytogenes | 0.5–32 mM (30–1920 mg/mL) | [36] | ||
| Salmonella Enteritidis, E. coli, L. monocytogenes | 1–3% (10–30 mg/mL) | [38] | ||
| non-typhoidal Salmonella, E. coli | 4.1 mg/mL | [39] | ||
| S. aureus, L. monocytogenes, E. coli, S. Typhimurium | 1.5% (15 mg/mL) acetic + 1.5% propionic acid | [40] | ||
| E. coli | 4 M (240 mg/mL) | [42] | ||
| Penicillium sp. | 200–800 mM (12,010–48,040 mg/mL) | [43] | ||
| Ascorbic acid | S. aureus, E. faecium, B. cereus, E. coli, P. aeruginosa | 4.0–16.0 mg/mL | 1.25–5.0 | [35] |
| Citric acid | E. faecium | 1–4.1 mg/mL | 1.25–5.0 | [31] |
| Campylobacter jejuni | 0.26–4.1 mg/mL | [32] | ||
| S. Typhimurium | 0.625% (6.3 mg/mL) | [33] | ||
| S. aureus, E. faecium, B. cereus, E. coli, P. aeruginosa | 1.0–4.0 mg/mL | [35] | ||
| L. monocytogenes | 0.5–16 mM (96–3072 mg/mL) | [36] | ||
| B. subtilis | 1% (10 mg/mL) | [37] | ||
| S. Enteritidis, E. coli, L. monocytogenes | 1–3% (10–30 mg/mL) | [38] | ||
| non-typhoidal Salmonella, E. coli | 4.1 mg/mL | [39] | ||
| Salmonella spp., Staphylococcus spp., and thermotolerant coliforms | 1% (10 mg/mL) | [41] | ||
| E. coli | 1.29 M (247 mg/mL) | [42] | ||
| Formic acid | E. faecium | 1 mg/mL | 1.25–2.5 | [31] |
| C. jejuni | 0.5–4.1 mg/mL | [32] | ||
| V. harveyi | 0.025–0.05% (0.25–0.5 mg/mL) | [34] | ||
| Malic acid | L. monocytogenes | 0.5–32 mM (67–4288 mg/mL) | 0.63–2.5 | [36] |
| B. subtilis | 2% (20 mg/mL) | [37] | ||
| Succinic acid | S. aureus, E. faecium, B. cereus, E. coli, P. aeruginosa | 0.8–4.0 mg/mL | 0.63–5.0 | [35] |
| Tannic acid | Penicillium digitatum | 1 mg/mL | [38] | |
| Tartaric acid | S. Typhimurium | 0.312% (3.1 mg/mL) | 1.25–5.0 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, T.M.; Ożarowski, M. Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens. Appl. Sci. 2024, 14, 6340. https://doi.org/10.3390/app14146340
Karpiński TM, Ożarowski M. Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens. Applied Sciences. 2024; 14(14):6340. https://doi.org/10.3390/app14146340
Chicago/Turabian StyleKarpiński, Tomasz M., and Marcin Ożarowski. 2024. "Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens" Applied Sciences 14, no. 14: 6340. https://doi.org/10.3390/app14146340
APA StyleKarpiński, T. M., & Ożarowski, M. (2024). Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens. Applied Sciences, 14(14), 6340. https://doi.org/10.3390/app14146340









