Abstract
There is currently increased interest in the use of alternatives to autoclaved culture media, in order to maintain the properties of the media, while saving energy and time. In this study, we assess a new system for culture media preparation, using a conventional microwave with a water bath and a glass bottle with a rubber cap that allows depressurization. Sterilization, using the proposed system (1000 W, 3 to 20 min), was compared with autoclaving for the preparation of tryptone soy agar (TSA), tryptone soy broth (TSB), Sabouraud 4% dextrose agar (SDA), and violet red bile glucose agar (VRBG). Microwave exposure for 7 min yielded sterile TSA plates. The productivity of both sterilization methods was assessed using the pour plate method, and significant increases in the growth of certain micro-organisms after using a microwave were observed for every culture medium, especially those that were sterilized by boiling (VRBG). The kinetics of microbial destruction showed that Escherichia coli and Bacillus subtilis spores were destroyed after 3 and 7 min in a microwave, respectively, while three decimal reductions were obtained for Geobacillus stearothermophilus spores after 15 min in an autoclave. This new sterilization method could be a feasible, rapid, and economical method to prepare microbiological media, with a quality similar to that obtained through autoclaving.
1. Introduction
In the present era of rapid methods (including molecular and serological ones), the method involving culture and isolation is still recommended by the current regulations for the microbiological analysis of food, medical products, and so on, in order to assess key characteristics of the micro-organisms, such as their antibiotic microbial sensitivities [1,2,3]. One key element is the sterilization of culture media and related materials to ensure the isolation of micro-organisms. However, autoclaving, as a sterilization method, is associated with certain drawbacks, such as high energy and equipment costs, long cycles, and the occupancy of considerable laboratory space, making it potentially unaffordable for laboratories around the world. Moreover, it is known that common autoclaving conditions can degrade key nutrients (such as peptones, agar, yeast extracts, and so on) and generate toxic compounds to which micro-organisms are sensitive [4]. The generation of reactive oxygen species (ROS) or products of Maillard reactions (such as melanoidins) has been reported as compromising the culturability of certain sensitive species [1,5,6,7,8].
A reduction in the autoclaving conditions, or the separation of agar from the rest of the components during autoclaving [6], have been demonstrated to increase the productivity of different media, particularly for key sensitive species.
There is also interest in the use of alternatives to the autoclave for sterilizing culture media, which could maintain the properties of the medium and even increase microbial growth and selectivity [6,8]. Alternative sterilization methods could include filtration, ionizing radiation, or microwave sterilization. However, filtration is not a viable option for large amounts of media and cannot eliminate viruses, while ionizing radiation (e.g., gamma radiation) is not a feasible and economical option for most laboratories [9,10], and is typically reserved for key culture media or ingredients.
Microwave technology has been gaining increasing interest as a potential alternative method for the sterilization of culture media, as it can produce high-quality media, while saving significant time and energy, and is considerably more affordable than autoclaves [11,12,13].
It is known that microwave irradiation can destroy micro-organisms though thermal and non-thermal effects [14,15,16,17,18]. However, in the current routine by microbiological laboratories, its use is limited to melting certain solid media or to regenerate media intended for anaerobic growth, such as sulfite polymyxin sulfadiazine (SPS). To date, however, few studies have explored the utility of microwaves to prepare culture media from dehydrated ingredients in comparison with autoclaves. In a previous study, the use of microwaves was demonstrated to be an efficient alternative for the preparation of Sabouraud dextrose agar (SDA) [7]. However, the additional devices required to ensure safety (for gas elimination) and to maintain the tightness of the media container during the microwave process are issues that have not yet properly been assessed. In normal conditions, common bottles do not resist the generated pressure and, due to the loosening of normal caps, leaks of the medium from the container are common.
In the present study, the use of a microwave was compared to autoclave sterilization for the production of common microbiological culture media, including tryptone soy agar (TSA), tryptone soy broth (TSB), Sabouraud 4% dextrose agar (SDA), Sabouraud dextrose broth, and violet red bile glucose agar (VRBG), through the implementation of an innovative device based on the use of a water bath made out of polycarbonate, and an easily perforated rubber cap, allowing medium depressurization. A commonly used power level (1000 W) and different exposure times (min) were assessed.
2. Materials and Methods
Manufacturing of culture media
All media (TSA, TSB, SDA, Sabouraud dextrose broth, and VRBG) were manufactured at Reactivos para Diagnóstico (RPD), S.L. (Sentmenat, Barcelona, Spain) from dehydrated ingredients, which were mixed in 250 mL of deionized water and resuspended, and sterilized using the following: (1) moist heat (121 °C for 15 min) in an autoclave (AES-150 model, Raypa, Barcelona, Spain) or by boiling (VRBG); (2) a water bath in a microwave, in a glass bottle with a special rubber cap, specially designed for this purpose (Figure 1). The cap was punctured with a 28 mm sterile needle, and capped with a sticker to allow gas elimination and to prevent contamination. For the microwave method, the bottle with the rehydrated medium was placed in a polycarbonate tube (14 cm high and 8 cm in diameter) filled with water (Figure 1), covering the whole part of the filled bottle, similar to a water bath. This tube allowed a homogeneous temperature distribution and a safety barrier to be created. The cycles were programmed, with different durations (from 3 to 20 min), at 1000 W, in a conventional microwave oven (Cecotec ProClean 5110 Inox, Valencia, España).

Figure 1.
Design of the cap on the bottle for sterilization of culture media in a microwave oven equipped with a water bath.
The only difference in the resulting culture media was the sterilization method. The reference media were sterilized using common autoclaving conditions, as specified by the manufacturer. The TSA and SDA were autoclaved at 121 °C for 15 min, and the VRBG was boiled for 1 min until completely dissolved. Using the microwave method, all culture media were sterilized in a microwave oven, as mentioned above. Solid culture media were maintained in bottles at room temperature, until being dispensed onto Petri plates (with the bacterial inoculum, using the pour plate method or without it using spiral inoculation).
All culture media were pH adjusted, if necessary (to pH values of 5.6 ± 0.2 for SDA; 7.3 ± 0.2 for TSA, and 7.4 ± 0.2 for VRBG), to achieve the physicochemical properties required for each medium.
Micro-organism strains used
The microbial strains used in the productivity and selectivity assays were obtained from a reference strain collection (American Type Culture Collection, ATCC). The reference strains were sub-cultured once to obtain stock strains, from which the stock and working cultures were prepared. The strains used (Table 1) were those recommended in ISO 11133:2014/amended 1:2018 for the quality control of TSA, TSB, SDA, Sabouraud dextrose broth, and VRBG [19].

Table 1.
Micro-organisms and culture media tested.
Inocula preparation
The stock culture strains were maintained in either TSA (bacteria) or SDA (molds and yeasts) slant tubes (RPD S.L.) under refrigeration. The inocula of the studied culture were prepared according to ISO 11133 [19]. Each strain was verified before use in non-selective media (TSA for bacteria, and SDA for molds and yeasts) and then cultured in appropriately selected and differential media, according to ISO 17025:2017 (ISO 17025) [20]. Stock dilutions were prepared in maximum recovery diluent (RPD S.L.) using turbidimetry (around 0.5 McFarland units) (DEN-1B densitometer, Grant Instruments, Cambridgeshire, UK), from which serial dilutions were prepared using the same diluent. For the productivity assays, serial dilutions were prepared to inoculate 50–120 CFU per plate. For the selectivity assays (S. aureus and E. faecalis species), serial dilutions were prepared to inoculate ≥104 CFU per plate, according to ISO 11133:2014/amended 1:2018 [19].
Productivity assays
Productivity assays were performed using the pour plate method, according to the corresponding ISO (ISO 4833-1:2014 [21]; ISO 21149:2017 [22]; ISO 16212:217 [23]; ISO 21528-2:2004 [24]) and European Pharmacopoeia (Ph. Eur., 10th ed.) [3], with the culture media required for each strain (Table 1). A microbial suspension (1 mL) containing 50–120 CFU (with a precision limit of ±20 CFU) was plated on sterile Petri dishes, and then the culture medium was poured onto the Petri dish and mixed with the inoculum.
In the productivity assays, to ensure the exact CFU were inoculated by the pour plate method in each Petri dish, a microbial suspension containing 50–120 CFU (100 µL) was also inoculated in the TSA or SDA, using the spiral plate method (Eddyjet, IULmicro, Barcelona, Spain), as a control.
Selectivity assays
Selectivity assays for VRBG were performed using the pour plate technique, according to ISO 6579 [25], ISO 21528-2:2017 [24], and ISO 11133:2014/amended 1: 2018 [19], with S. aureus (ATCC, equivalent to WDCM 00032) and E. faecalis (ATCC 19433, equivalent to WDCM 00009), inoculated at ≥104 CFU (1 mL).
Seeding method
Two different assays, quantitative (solid media) and qualitative (liquid media), were performed to compare the quality of the media sterilized using both methods with different micro-organisms (Table 1). In the quantitative assays, samples of 1 mL were plated in triplicate onto sterile Petri dishes, using the pour plate method. Then, the medium (20–25 mL), after melting in a boiling bath, was added once it was cooled to 40–45 °C, in both sterilization methods. In the qualitative assays, 1 mL (50–120 CFU) was inoculated in triplicate in broth bottles (TSB and Sabouraud dextrose broth, 250 mL) and then incubated for the required time and temperature.
The TSA plates and TSB bottles were incubated at 32.5 ± 2.5 °C for 24–48 h, the SDA plates and Sabouraud dextrose broth at 22.5 ± 2.5 °C for 2–5 days, and the VRBG plates and broth at 37 ± 2.5 °C for 24–48 h. For each medium and strain, inoculation was repeated in triplicate.
To provide a quality control for the qualitative assays (broth), 50 μL of each strain was inoculated in a sterile TSB bottle, sterilized in an autoclave, and incubated at 30 ± 1 °C for 24–48 h.
Colony count
The colony count was performed either manually or using an automatic colony counter (SphereFlash®, IULmicro, Barcelona, Spain).
Physicochemical properties
To check that each culture medium had the required pH values, the pH was measured with a pH meter (Crison GLP21, Barcelona, Spain), before and after sterilization (before pouring onto a sterile Petri dish). The pH was measured at different times after sterilization in a microwave oven (7 min, 10 min, 13 min, and 15 min) and after autoclaving sterilization (control).
Assessment of temperatures achieved in the microwave during sterilization
The temperature of the glass bottle was measured with an infrared thermometer (Helect, Shenzhen, China) every 10 s during the sterilization cycle, as an indicative measure of the degrees reached inside the microwave oven.
Sterility test
In order to determine the necessary duration at which the culture medium was completely sterile in the microwave oven equipped with a water bath, 250 mL bottles of TSA were produced, and several sterilization durations (3 min, 5 min, and 7 min) were tested at 1000 W, without inoculating the micro-organisms in the medium. Dehydrated culture medium was mixed with 250 mL of deionized water, after which the bottle was closed with the special cap and sticker (Figure 1) and placed in a water bath in a microwave oven for the mentioned durations. The resulting plates were incubated at 32.5 ± 2.5 °C for 24–48 h.
With the media prepared for the optimal exposure time to obtain sterile plates, a quality control of the sterility test was performed, according to ISO 11333 (ISO 11133:2014/amended 1:2018) [19], keeping the plates and broth for 7 days, as follows: the TSA plates and TSB bottles were incubated at 32.5 ± 2.5 °C, the SDA plates and Sabouraud dextrose broth at 22.5 ± 2.5 °C, and the VRBG plates at 37 ± 2.5 °C.
Kinetics of microbial destruction in the microwave oven
The bacterial strain Escherichia coli ATCC 8739 was obtained from slant tubes (RPD S.L.). Spores from Bacillus subtilis ATCC 6633 were obtained after a thermal shock at 80 °C for 10 min after 7-day cultures (maintained at 32.5 ± 2.5°C) and Geobacillus stearothermophilus ATCC 7953 spores were taken from ampoules (Sterikon® plus bioindicator, MERCK, Rahway, NJ, USA). Then, 50 μL of suspension containing 106 CFU/mL bacteria or spores was injected into a test bottle (5 × 104/bottle), which was exposed to a microwave at a power of 1000 W for periods of time ranging from 2.5 min to 20 min (depending on the destruction of each strain), with measurements taken every 30 s. The suspension was then cooled and poured into a sterile Petri dish and left to solidify. After incubation at 32.5 ± 2.5 °C for 48 h (E. coli ATCC 8739 and B. subtilis ATCC 6633) and 56 ± 2.5 °C (G. stearothermophilus ATCC 7953), the number of colonies was obtained. These tests were performed in triplicate.
Statistical Analysis
The data were analyzed using R software version 4.3.1. Generalized linear mixed models were used to model the bacterial counts (CFU), in which the linear predictor contains random effects, as well as fixed effects. Colonies (response) were modeled using Poisson’s distribution, and in the case of overdispersion, the negative binomial distribution model was used. The different media were compared pairwise through multiple comparisons, using the Tukey method.
The pH values of the different media, both reference and modified, were compared using the ANOVA test. The different media were compared pairwise, with a post hoc analysis using the Tukey test. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Sterility Test
To assess the capacity of the sterilization method using a microwave oven equipped with a water bath as a valid method similar to autoclave sterilization, it was first necessary to establish the duration at which the culture medium (TSA), without the addition of micro-organisms, was completely sterile. For this purpose, after comparing various durations (3, 5, and 7 min), in which complete dissolution of the dehydrated medium in deionized water was obtained, it was concluded that at 7 min, the medium was completely sterilized.
After exposure for 3 min in the water bath in the microwave and incubation at 32.5 ± 2.5 °C for 7 days, 66.66% of the TSA plates were contaminated, while 58.33% of the TSA plates were contaminated at 5 min, and an absence of contamination (out of 12 plates) was reported at 7 min, indicating that the culture medium was sterilized.
3.2. Temperature Analysis
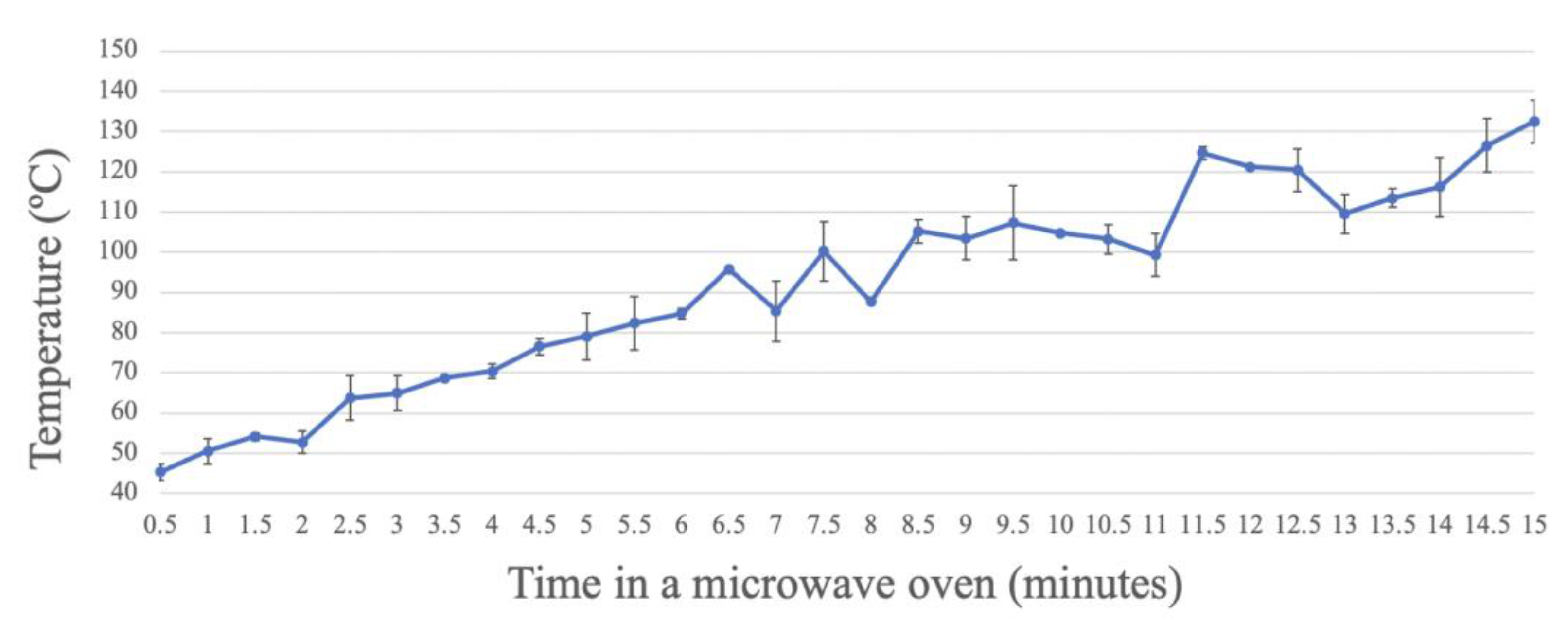
Analysis of the temperature of the glass wall of the bottle in the water bath, from 0 to 15 min, showed that the temperature increased with the exposure time of the culture medium in the microwave oven, in an exponential manner (Figure 2). When the bottle was placed in the microwave oven for 15 min, a wall temperature higher than 130 °C was reached. After exposure for the duration that allows complete sterilization, set at 7 min according to the above results, approximately 90 °C was reached, which is lower than the autoclaving temperature (usually 121 °C for 15 min).
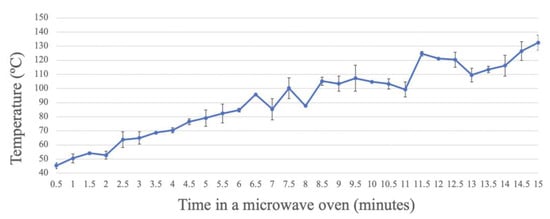
Figure 2.
Registered temperature of the glass bottle wall over time, measured with an infrared thermometer, during microwave treatment at different times.
3.3. Quality Control and Effect of Sterilization in a Microwave Oven vs. Autoclave on the Productivity of SDA
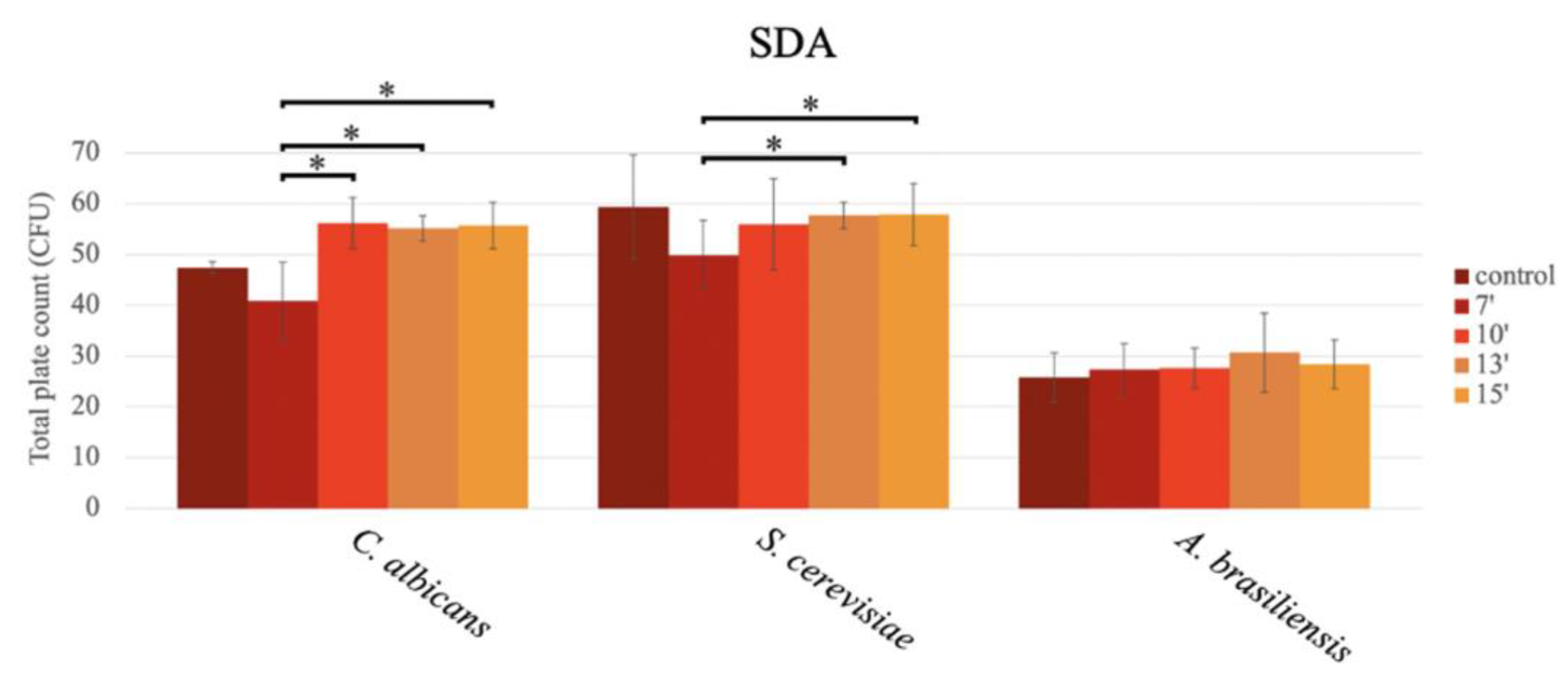
In the analysis of productivity using the pour plate method, significant differences were observed depending on the exposure time in the microwave concerning C. albicans and S. cerevisiae, while for A. brasiliensis ATCC 16404, the productivity results were similar in all media, regardless of the exposure time in the microwave oven, with CFU counts similar to those obtained for the autoclave media (Figure 3).
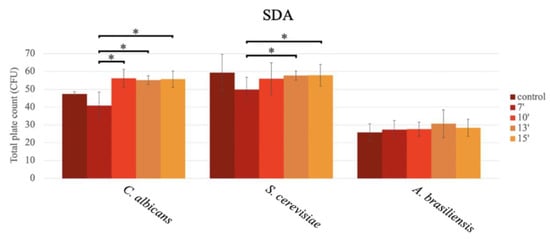
Figure 3.
Bar graph showing the mean CFU obtained in SDA prepared with conventional sterilization (control, in an autoclave (or by boiling)), and in a microwave oven for 7 min (7′), 10 min (10′), 13 min (13′), and 15 min (15′), after inoculation using the pour plate method, for C. albicans, S. cerevisiae, and A. brasiliensis.
For C. albicans ATCC 10231, significant differences in the mean number of CFU were observed among the different durations assayed (p < 0.001; GLM), specifically among the different microwave oven exposure times, as follows: 7 min vs. 10 min, 7 min vs. 13 min, and 7 min vs. 15 min (p = 0.001; Tukey) (Figure 3). The highest productivity occurred in the media sterilized for 10 min (56.20 ± 5.07 CFU), followed by 15 min (55.73 ± 4.54 CFU), 13 min (55.13 ± 2.52 CFU), and 7 min (40.80 ± 7.68 CFU). No statistically significant differences in the productivity obtained in the medium sterilized in the autoclave (121 °C, 15 min) (47.4 ± 1.14 CFU) were observed in comparison to the productivity obtained in the microwave oven at the different exposure times (Figure 3).
In S. cerevisiae ATCC 9763, a significantly higher CFU count was observed as the exposure time in the microwave increased, as follows: 7 min vs. 13 min (p = 0.019; Tukey) and 7 min vs. 15 min (p = 0.015; GLM) (Figure 3), with the highest productivity at 15 min (57.87 ± 6.13 CFU), followed by 13 min (57.66 ± 2.64 CFU), 10 min (55.93 ± 9.02 CFU), and 7 min (49.87 ± 6.77 CFU) (Figure 3). In the media sterilized through autoclaving, no significant differences were observed between the autoclaved media (59.40 ± 10.23 CFU) and any of the media exposed to the microwave (Figure 3).
3.4. Quality Control and Effect of Sterilization in a Microwave Oven vs. Autoclave on the Productivity of TSA
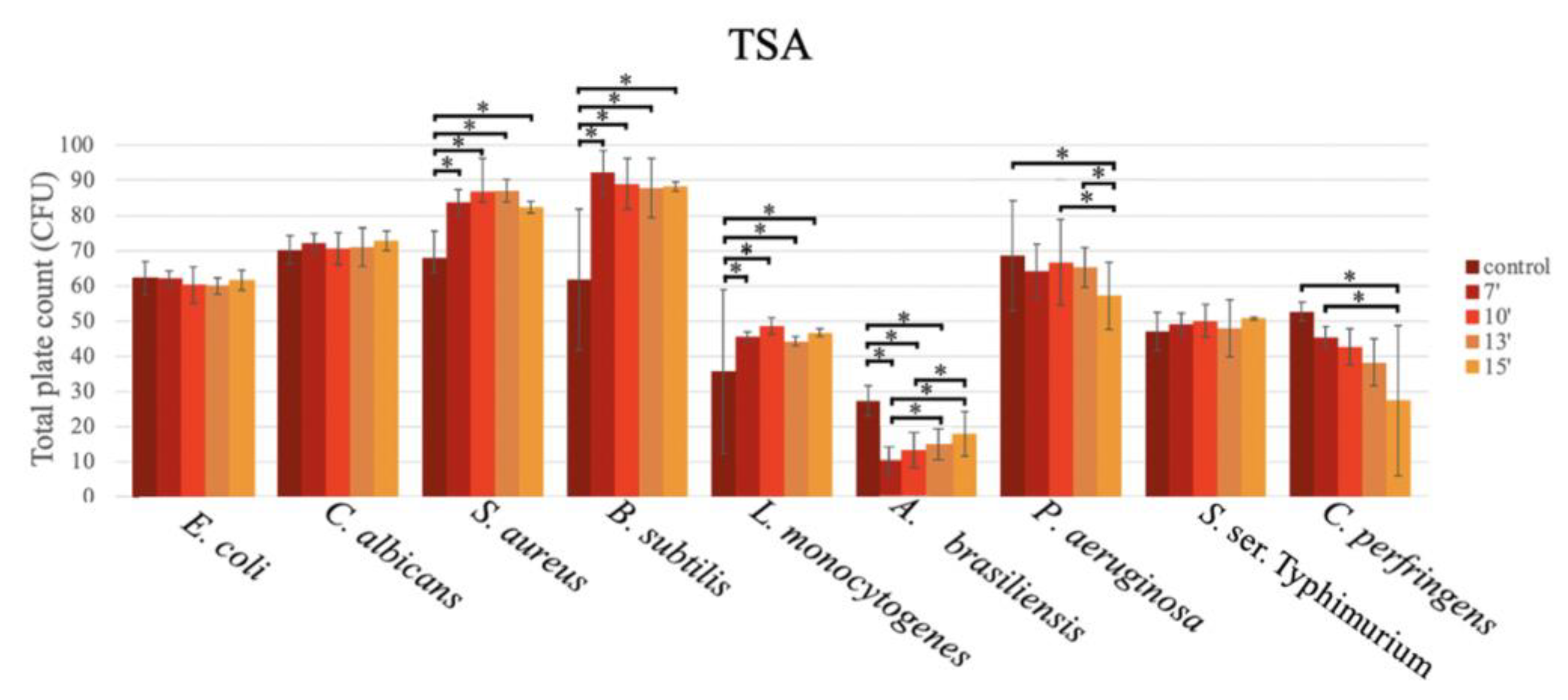
For the TSA (Figure 4), the mean number of CFU were similar between the microwave exposure time and the use of the autoclave.
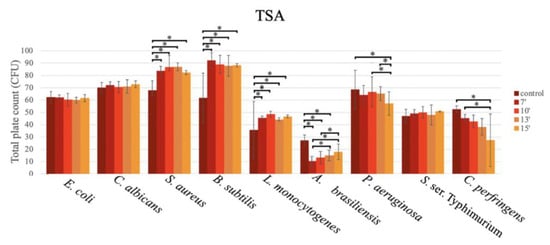
Figure 4.
Bar graph showing the mean CFU obtained in TSA prepared with conventional sterilization (control, in an autoclave (or by boiling)), and in a microwave oven for 7 min (7′), 10 min (10′), 13 min (13′), and 15 min (15′), after inoculation using the pour plate method, for E. coli, C. albicans, S. aureus, B. subtilis, L. monocytogenes, A. brasiliensis, P. aeruginosa, S. ser. Typhimurium, and C. perfringens.
As shown in Figure 4, for E. coli, C. albicans, and S. ser. Typhimurium, no statistically significant differences were observed in the productivity at the different exposure times in the microwave oven, and the productivity obtained in the autoclave was similar to that of the microwave oven, regardless of the exposure time.
For S. aureus ATCC 6538, similar growth was obtained with the different exposure times in the microwave oven, but a significantly lower number of CFU were obtained in the autoclave (67.80 ± 7.73 CFU) vs. the microwave oven, as follows: 7 min (83.73 ± 3.60 CFU; p = 0.005; Tukey), 10 min (86.87 ± 9.32 CFU; p < 0.001; Tukey), 13 min (86.93 ± 3.21 CFU; p < 0.001; Tukey), and 15 min (82.33 ± 1.70 CFU; p = 0.013; Tukey).
This trend was also observed for B. subtilis ATCC 6633, with an even bigger difference in the growth in comparison to the autoclave (61.80 ± 20.01 CFU), as follows: 7 min (92.20 ± 6.29 CFU; p < 0.001; Tukey), 10 min (88.93 ± 7.20 CFU; p < 0.001; Tukey), 13 min (87.73 ± 8.44 CFU; p < 0.001; Tukey), and 15 min (88.20 ± 1.40 p < 0.001) (Figure 4).
For L. monocytogenes ATCC 13932, although less growth in all media was reported, this trend was also observed, with significantly higher productivity in the microwave oven vs. the autoclave (35.60 ± 23.37 CFU), as follows: at 7 min (45.47 ± 1.36 CFU; p = 0.030; Tukey), 10 min (48.53 ± 2.31 CFU; p = 0.002; Tukey), 13 min (44.20 ± 1.40 CFU; p = 0.010; Tukey), and 15 min (46.60 ± 1.11 CFU; p = 0.012; Tukey) (Figure 4).
For A. brasiliensis ATCC 16404, the lowest growth was observed at 7 min in the microwave oven (9.67 ± 3.97 CFU). Significantly lower CFU counts were obtained in comparison to the autoclave (27.20 ± 4.38 CFU; p < 0.001; Tukey) at the following times: 7 min (9.67 ± 3.97 CFU), 10 min (12.53 ± 5.21 CFU), and 13 min (14.33 ± 4.50 CFU) (Figure 4).
For two bacteria, P. aeruginosa ATCC 9207 (reclassified as Pseudomonas paraeruginosa) and C. perfringens ATCC 13124, higher productivity was observed using the autoclave vs. the microwave oven. For P. aeruginosa ATCC 9207, a significantly higher CFU count was obtained in the autoclave in comparison to 15 min of exposure in the microwave oven (68.60 ± 15.61 CFU vs. 57.13 ± 9.58 CFU; p = 0.034; Tukey). The productivity in the media sterilized in the microwave oven decreased with the exposure times, as follows: 10 vs. 15 min (66.67 ± 12.23 CFU vs. 57.13 ± 9.58 CFU; p = 0.005; Tukey) and 13 vs. 15 min (65.13 ± 5.67 CFU vs. 57.13 ± 9.58 CFU; p = 0.026; GLM) (Figure 4).
For C. perfringens ATCC 13124, a gradual decrease in productivity was observed across the exposure times in the microwave oven, with statistically significant differences (p = 0.009; Tukey) between the autoclave-sterilized media (52.60 ± 2.70 CFU) and the media sterilized in the microwave oven for 15 min (27.40 ± 21.33 CFU).
3.5. Quality Control and Effect of Sterilization in a Microwave Oven vs. Boiling on the Productivity of VRBG
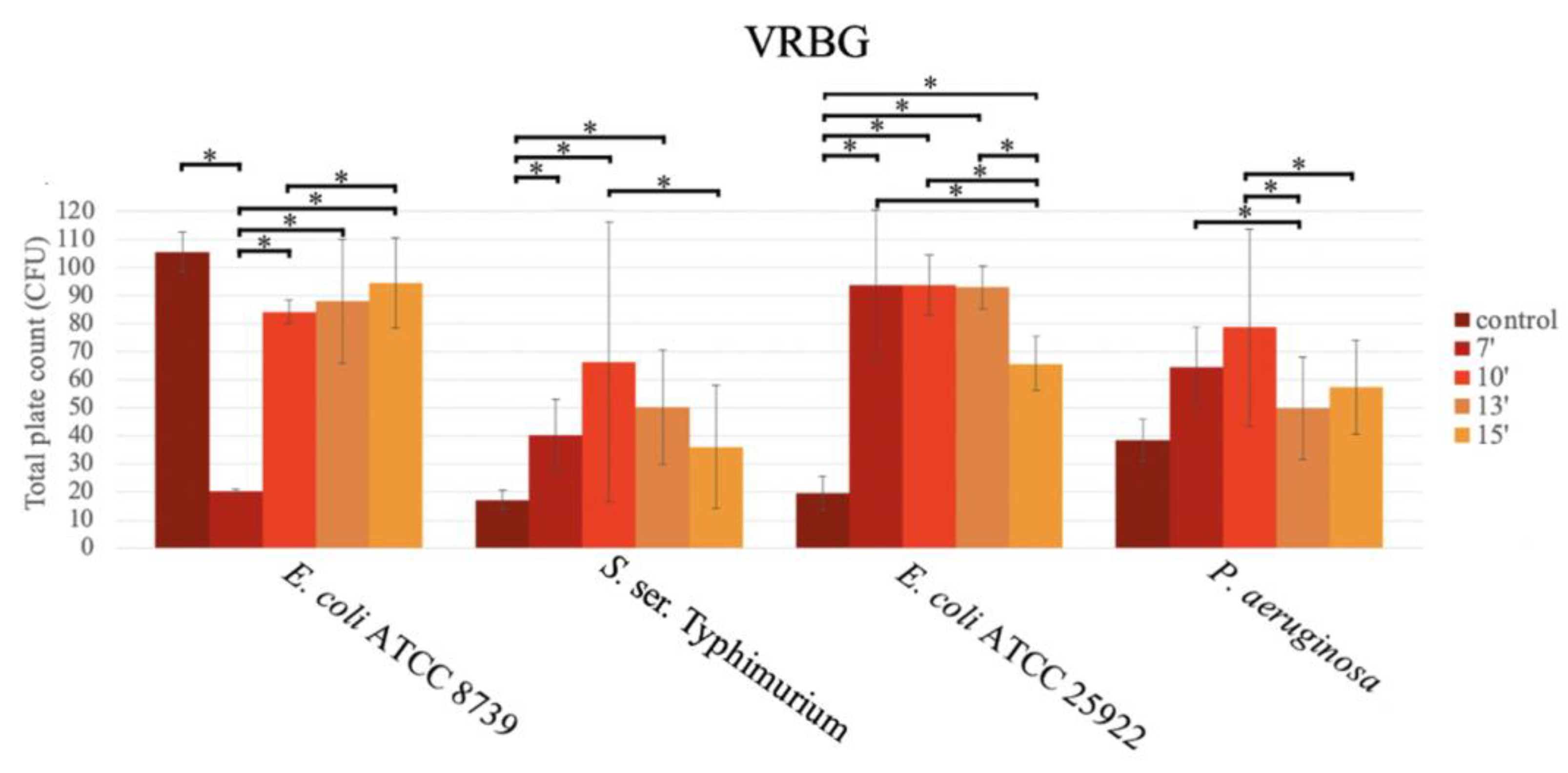
For E. coli ATCC 25922, S. ser. Typhimurium and P. aeruginosa, significantly higher growth in the medium exposed to the microwave was observed in comparison to the boiled media (Figure 5).
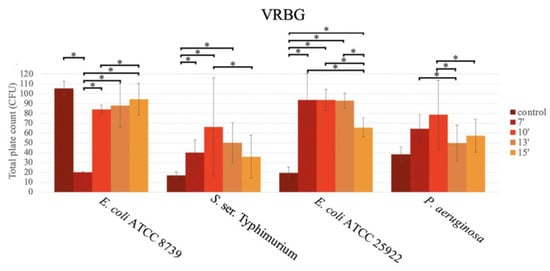
Figure 5.
Bar graph showing the mean CFU obtained in VRBG prepared with conventional sterilization (control, in an autoclave (or by boiling)), and in a microwave oven for 7 min (7′), 10 min (10′), 13 min (13′), and 15 min (15′), after inoculation using the pour plate method, for E. coli ATCC 8739, S. ser. Typhimurium, E. coli ATCC 25922, and P. aeruginosa.
For S. ser. Typhimurium ATCC 14028, the highest productivity was observed at 10 min (66.27 ± 49.98 CFU), followed by 13 min (50.27 ± 20.24 CFU), 7 min (40.27 ± 12.88 CFU), and 15 min (36.07 ± 21.99 CFU), with significant differences between the durations of 7 and 10 min (p = 0.049; Tukey), and 10 and 15 min (p = 0.009; Tukey).
For E. coli ATCC 25922, similar CFU values were obtained at 7 min (116.67 ± 26.95 CFU), 10 min (93.73 ± 10.72 CFU), and 13 min (92.93 ± 7.69 CFU), with a significant decrease at 15 min (65.80 ± 9.61 CFU) vs. the rest of the times (7 vs. 15 min; p < 0.001; 10 vs. 15 min; p = 0.002 and 13 vs. 15 min; p = 0.003; Tukey). The growth in the boiled medium was very low.
For E. coli ATCC 8739, higher productivity was obtained in the boiled medium. In this case, the growth in the media exposed for 7 min in the microwave oven was very low (20.27 ± 0.64 CFU) in comparison to the rest of the durations (p < 0.001; Tukey), as follows: 10 min (84.67 ± 4.06 CFU), 13 min (88.00 ± 22.01 CFU), and 15 min (94.60 ± 16.03 CFU) (Figure 5).
All media were equally selective against S. aureus ATCC 6538 and E. faecalis ATCC 19433, without differences according to the sterilization method or the exposure time.
3.6. Effect of Sterilization in a Microwave Oven vs. Autoclave on Liquid Culture Media
Bottles of TSB and Sabouraud dextrose broth were inoculated with the corresponding micro-organisms (Table 1). After incubation for periods of 24 h (TSB) and 48 h (Sabouraud dextrose broth), growth (turbidity) was observed in all of them, without any differences regarding the sterilization method or the exposure time.
3.7. Kinetics of Microbial Destruction in the Microwave Oven
The kinetics of microbial destruction were evaluated for E. coli ATCC 8739, B. subtilis ATCC 6633, and G. stearothermophilus ATCC 7953, after being added to the rehydrated media.
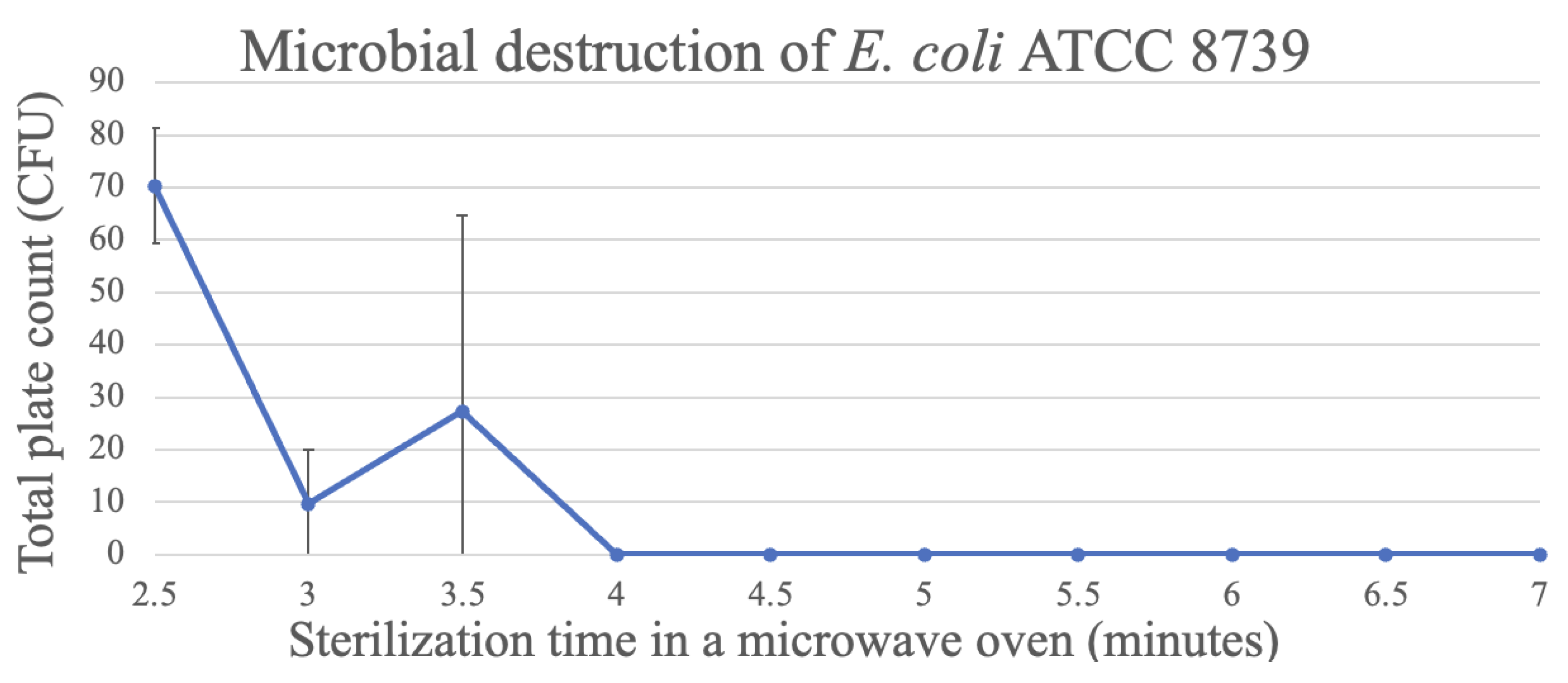
For E. coli ATCC 8739, the highest reduction in the CFU count was observed after an exposure of 3 min, while after 4 min, total destruction of the micro-organism was observed when the medium was sterilized in the microwave oven (Figure 6).
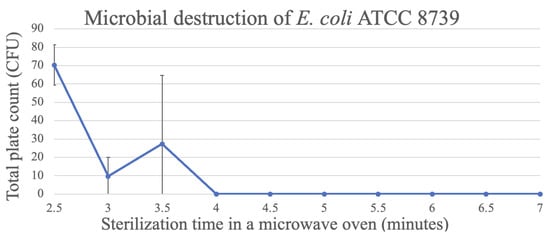
Figure 6.
Destruction of E. coli in TSA through microwave exposure at 1000 W over time (performed in triplicate, UFC count per bottle).
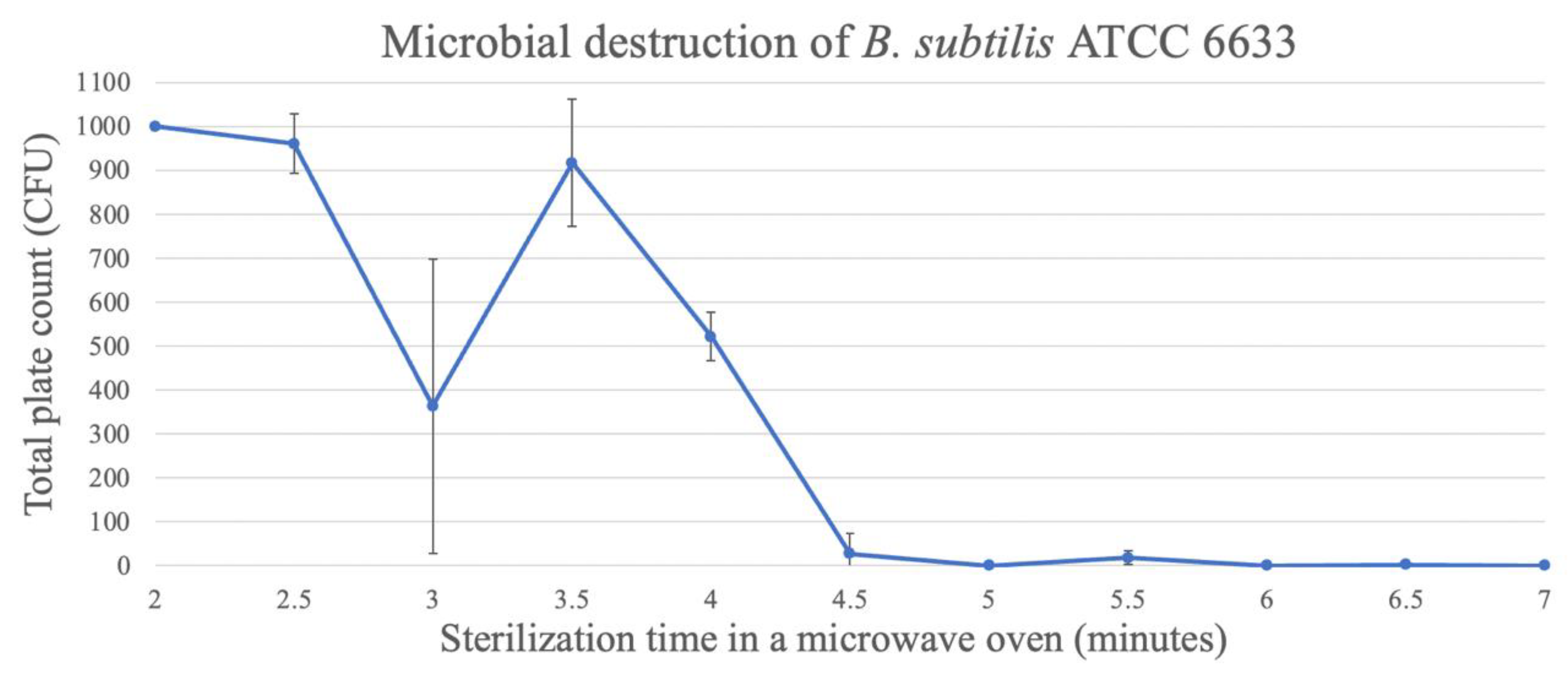
For the spores of B. subtilis ATCC 6633, the time needed to reach three decimal reductions was approximately 4.5 min, reaching 0.33 ± 0.58 CFU/bottle, when the medium was exposed for 7 min (Figure 7).
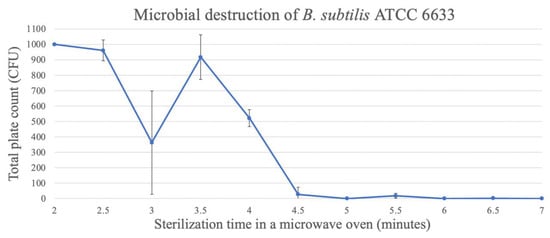
Figure 7.
Destruction of B. subtilis spores in TSA through microwave exposure at 1000 W over time (performed in triplicate, UFC count per bottle).
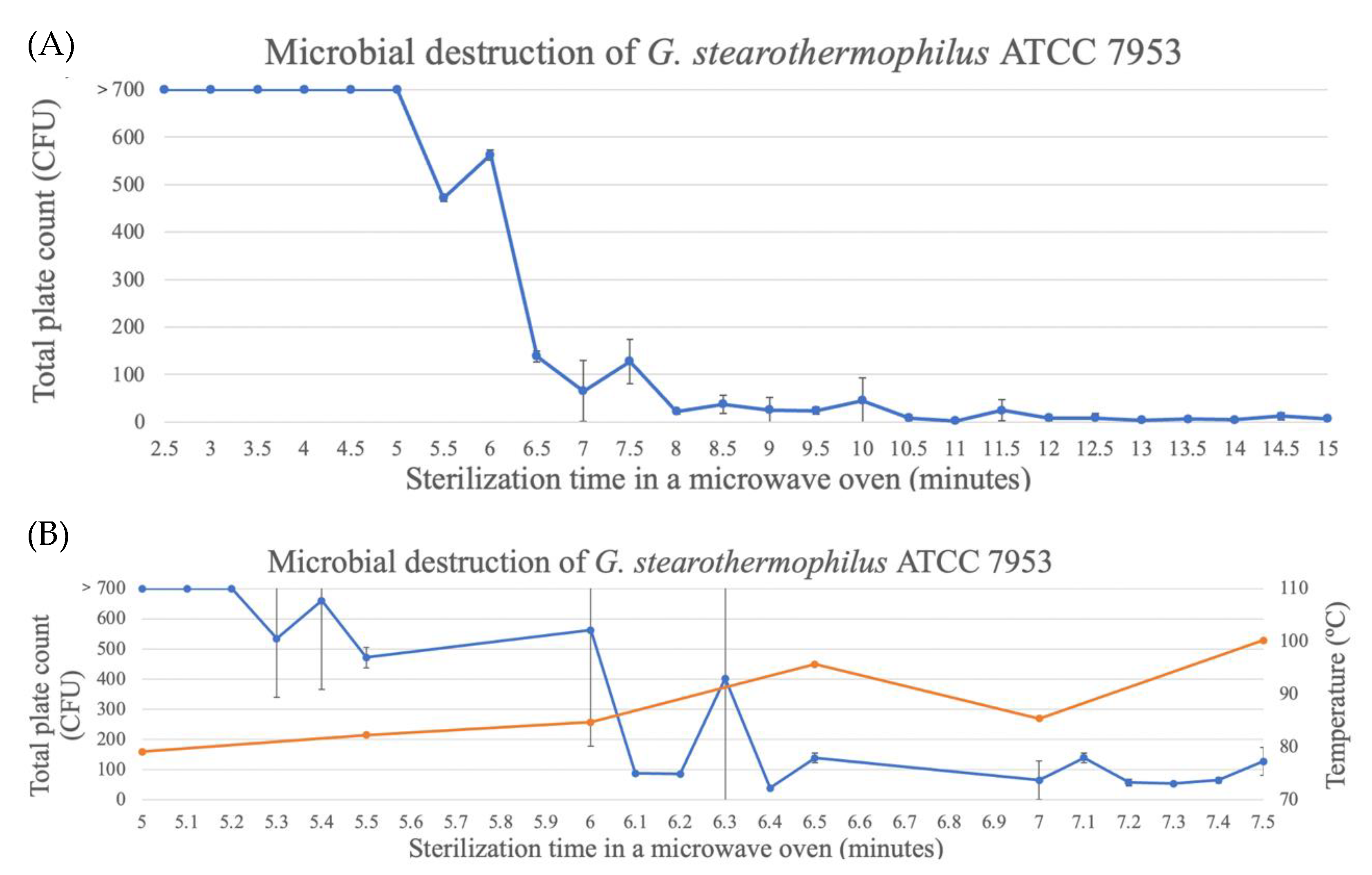
For the spores of G. stearothermophilus ATCC7953, the time to reach three decimal reductions was about 10.5 min (Figure 8). At this time, the temperature of the glass bottle was 100–110 °C, as reported above (Figure 2). Although the spores were not completely eliminated, as shown in Figure 6, spore destruction started at around 5 min, and between 5 and 7 min, the highest destruction occurred, with temperatures of around 90 °C.
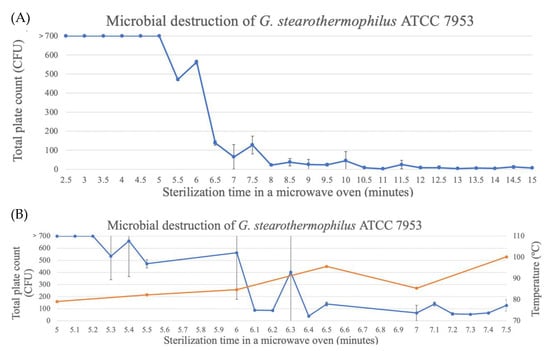
Figure 8.
Destruction of G. stearothermophilus spores in TSA through microwave exposure at 1000 W over time (performed in triplicate, UFC count per bottle). (A) Full assay for the complete destruction period. (B) Assay of the destruction period between 5 and 7.5 min being the blue line the CFU count and the orange line the temperature (°C).
3.8. Effect of Sterilization in a Microwave Oven on Medium pH
The pH of the medium was measured after sterilization in all cases, before pouring it onto sterile Petri dishes without microbial inocula.
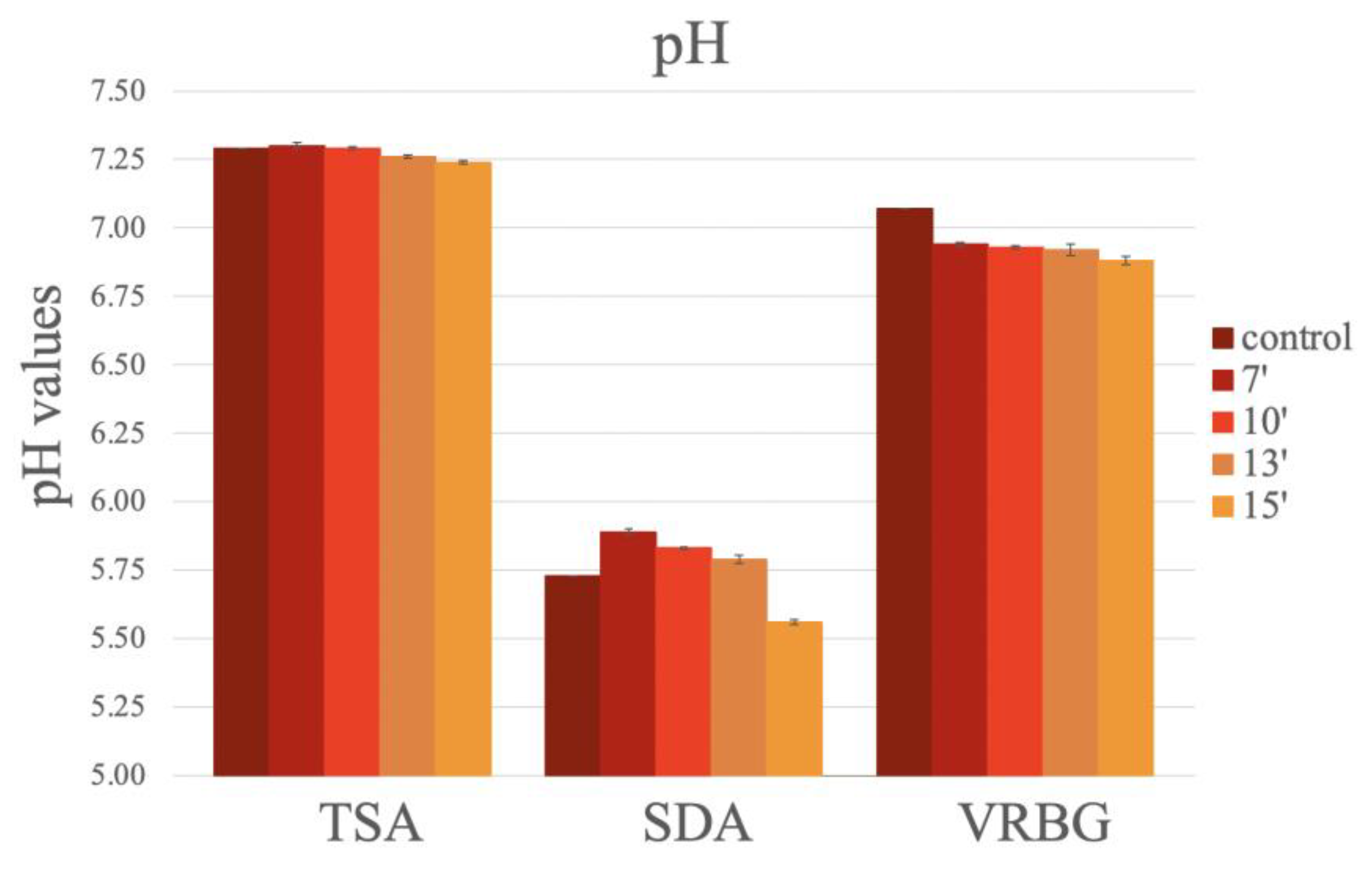
No significant differences in the pH values were observed in the different media, according to the sterilization method or the exposure times (Figure 9). Only a slight decrease in pH value was observed over the exposure times in the microwave oven, with a tendency to acidify the culture medium. For the TSA, similar values were obtained in all media. For the SDA, lower pH values were reported in the autoclaved media and in the media exposed for 15 min in the microwave oven. For the VRBG, higher pH values were obtained in the autoclaved media, and similar pH values were observed in all microwaved media (Figure 9).
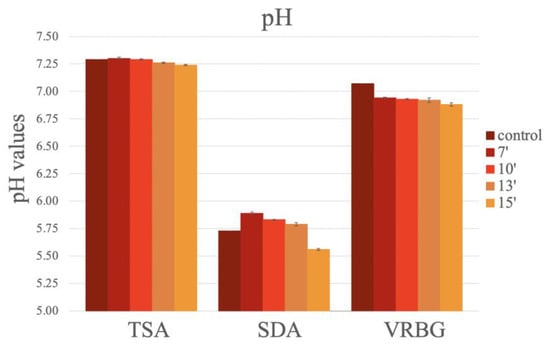
Figure 9.
The pH values obtained after the preparation of the media (TSA, SDA, and VRBG) using conventional methods (autoclaving/boiling) or using microwave exposure for different durations.
4. Discussion
The sterilization of microbiological culture media using a conventional microwave could become a simple and affordable method for most laboratories. However, when establishing a new protocol for the sterilization of culture media, several parameters must be considered, particularly exposure times and safety issues, as microwaves are not specifically designed for this purpose. Most of the studies carried out to study the sterilization capacity of microwave ovens have been performed on food samples, for which the maintenance of sterility is not necessary [26,27], or on surgical materials [28]. Previous studies have described the effects on micro-organisms (either live or in their sporulated form) when they are sterilized in a microwave oven, being thermal and non-thermal [7,11,12]. Only the study performed by Bazana et al. assessed the utility of a microwave for the preparation of SDA, adding a UV treatment during the gelling process [7].
In our study, for the first time, an innovative and simple system for the preparation of culture media was developed, based on the use of a water bath made from polycarbonate covering the rehydrated media, in order to ensure the homogeneous distribution of the temperature and to act as safety containment to avoid any possible event related to high temperatures and possible container breakage.
To evaluate the new sterilization system, we assessed the microbiological properties of media prepared in a microwave (for different exposure times) in comparison with those of autoclaved media.
We started by first establishing the minimum power and time required for the complete sterilization of the culture medium (TSA), while measuring the temperature of the culture medium as it was exposed to the microwave oven.
We established that an exposure duration of 7 min at a power of 1000 W was sufficient to sterilize the culture medium. Based on the improvement observed in the growth of an important number of species in media exposed to a microwave, in comparison to the same autoclaved media, we can suggest that these conditions are softer than the autoclaving conditions and, thus, less degradation of nutrients and less generation of toxic compounds, such as reactive oxygen species (ROS), would occur under this microwave treatment.
Due to contamination risk and the bottle design, temperature measurement was only possible on the glass bottle, being an indirect measure of the temperature reached in the culture medium. Glass was the selected material for the bottle as it maintains the temperature of the culture medium during the sterilization and resists pressure increases.
Regarding the temperature measurement, a sustained exponential increase was observed over time. At the minimum sterilization time (7 min), the temperature of the glass bottle was around 90 °C, thus indicating a higher temperature of the culture media at this time.
When comparing the temperature curves of the autoclave and microwave oven, a higher temperature was reached in both at 15–16 min [29]. However, the whole sterilization process was drastically reduced in the microwave method as, after 7 min, sterilization was achieved and the process of gas elimination was quicker, whereas the whole process in the autoclave, including the temperature increase, sterilization (commonly 21 min), and pressure decrease, may take up to 1.5 h [29]. Moreover, in the autoclave, higher pressures are likely to be reached than in the microwave system, due to the larger size of the autoclave and the autoclaving conditions. This is the most notable difference between the two devices, making sterilization in a microwave oven a faster and safer process, without the inconvenience of needing to wait for the pressure to decrease. Further studies are needed to assess the pressure and temperature variations inside the microwave system, including the culture medium, similar to the existing measurements of these parameters in autoclaves [29,30,31].
A comparison of media productivity using both systems was performed with culture media (solids and liquids) widely used in laboratories for the microbiological quality analysis of food, cosmetics, and pharmaceutical products. In addition, the media exposed for the different exposure times in the microwave were assessed, with an overall view of how the sterilization method and sterilization times affected the microbiological properties of the culture media.
The tendency of yeasts in an SDA medium to grow better in a microwave-sterilized medium than in an autoclaved medium could be attributed to the generation of toxic compounds; for example, due to Maillard reactions, the culture media darkens, being produced after the long exposure of the medium to high temperatures [4,5,12], which can inhibit the growth of certain micro-organisms [7,32,33]. Culture media containing simple sugars, such as SDA, are more likely to generate these Maillard reaction compounds than other culture media, and this process could be more pronounced in the autoclave than in the microwave system [7]. For SDA, a correlation of medium browning with the exposure time in the microwave was observed. Exposures of 7–10 min rendered a clearer medium than the rest of the exposure times and the autoclave method. This could explain the increased growth, in certain cases, in media exposed to the microwave versus the autoclave.
For both yeasts (i.e., C. albicans and S. cerevisiae), increased growth was observed with longer exposure times in the microwave oven, possibly due to their tolerance to pH variations [34]. On the other hand, the growth of A. brasiliensis in SDA was very similar in all conditions assayed. Growth in regard to SDA sterilized with this system deserves further research, as increased productivity could speed up detection in microbiological controls, food, and medicinal products that are characterized as requiring prolonged periods of incubation.
Improvements in the growth of some micro-organisms were also observed in the TSA sterilized in the microwave, particularly S. aureus, B. subtilis, and L. monocytogenes, probably due to the lower generation of toxic compounds, such as ROS, the oxidation of certain compounds produced to a greater extent at higher temperatures, and the Maillard reaction between the agar and peptones [6]. The increased growth of S. aureus and L. monocytogenes could be particularly relevant for detection in food and clinical samples, where the usefulness of the microwave system should be tested with respect to the corresponding selective media.
In the cases of P. aeruginosa, C. perfringens, and A. brasiliensis, lower growth was observed in the TSA sterilized in the microwave compared to the autoclaved media. TSA is not the optimum medium for the growth of A. brasiliensis and, as reported above, growth in regard to SDA prepared with the new system yielded similar growth to the autoclaved SDA. In the case of C. perfringens, in routine practice, selective culture media are used, such as SPS or tryptose sulfite cycloserine (TSC), which could also be assessed using this system.
In the case of P. aeruginosa, the lower growth detected in the microwave media could also be an advantage for the manufacturing of certain selective media, in which the inhibition of Pseudomonas often constitutes a challenge, as it concerns samples used for the analysis of water samples, where Pseudomonas is often the main interfering micro-organism [8].
Finally, for the selective media VRBG, which is commonly prepared by boiling, relevant growth increases were observed, particularly for E. coli ATCC 25922, S. ser. Typhimurium, and P. aeruginosa. This could be attributed to the fact that, in the conventional method, it is necessary to wait until all the components are well dissolved in the medium, after which it is boiled. In contrast, in the novel microwave oven sterilization method, the medium is heated only during the microwave oven exposure time. Furthermore, it was observed that the exposure time with the best microbial growth results was 10 min for S. ser. Typhimurium, E. coli ATCC 25922, and P. aeruginosa. In this medium, the darkening of the medium was also correlated with the exposure time in the microwave. In a previous study, the properties of this medium also improved with the separation of the components (agar vs. the rest of ingredients) during the autoclaving process [6].
Using the microwave method, liquid media (TSB and Sabouraud dextrose broth) were also analyzed, as they are routinely used in microbiological quality laboratories. All media sterilized in the microwave oven performed in the same way as the media sterilized in the autoclave for all micro-organisms tested. As both media contain glucose, in future studies, it is necessary to quantify the microbial growth obtained, as a lower degradation of glucose could have occurred in the media prepared in the microwave, thus favoring microbial growth. Improvement of the enrichment media is relevant, for example, in the analysis of food. In the case of the detection of L. monocytogenes in food, at present, there is interest in finding alternatives to the Fraser medium. Identification of the best method for medium sterilization could also contribute to obtaining higher recovery rates.
Regarding pH values, we reported that SDA particularly increased its acidification after 15 min of exposure, likely due to the thermal degradation of glucose, which is in agreement with other previous studies [7].
Regarding the study of microbial destruction using the microwave system, live bacteria (E. coli) and bacterial endospores (from B. subtilis and G. stearothermophilus) were used. In the case of the spores of G. stearothermophilus, added at 5 × 104 spores/bottle, complete destruction was not obtained at 7 min. It should be noted that these endospores are highly thermoresistant and used as a biological sterilization indicator [35] during autoclaving cycles. However, they are not commonly found in dehydrated culture media and germinate at temperatures higher than 45 °C. For these reasons, their presence at a low concentration in dehydration media would not indicate interference during the most common microbiological analysis. However, more studies should be carried out to optimize their complete elimination using this system.
When the medium was contaminated with the endospores of B. subtilis ATCC 6633, the time needed to reach three decimal reductions was approximately 4.5 min, with a count of 0.33 ± 0.58 CFU remaining when the medium was sterilized in the microwave oven for 7 min. Considering that dehydrated media would not contain high concentrations of Bacillus endospores, these results should not represent an issue, although more studies should be performed to optimize this destruction.
Finally, as expected, complete destruction was obtained for E. coli ATCC 8739 with an exposure time of 4 min, in agreement with previous studies [17]. It should be noted that, according to our experience, Pseudomonas could also be found in dehydrated media. Therefore, the inclusion of this bacteria in future microbial destruction studies would be of interest.
An important aspect of the microwave system is safety. The puncture of the rubber cap and the application of the sticker allows for depressurization, prevents medium contamination, and avoids medium leakage. To increase safety, other safety strategies could be added, such as the delayed opening of the door, allowing for depressurization before opening, and a polycarbonate cover for the water bath. Other safety issues to take into account include avoiding the introduction of metallic materials and certain types of plastic.
With the optimized microwave system, validation studies should be performed in the same way as autoclave sterilization validation studies to assess the efficiency of the new method, according to validation guidelines (ISO 11133:2014/amended 1:2018 (ISO 11133)) [19]. This system also deserves to be included in the applicable regulations.
In summary, in comparison to classical autoclave sterilization, the work performed with a microwave offers different advantages, such as time saving, as well as immediacy, increased sterilization capacity, and better microbiological properties of certain media, likely due to the lower generation of toxic compounds. Other advantages can be foreseen, such as energy and cost savings, that require assessment in future work. This system could become a common sterilization method for most microbiological media, although more studies should be carried out to confirm this.
5. Conclusions
Although future assays should test the chemical properties that occur in the culture medium after sterilization in a microwave oven, this study about the sterilization capacity of a microwave oven and the maintenance and improvement of productivity in some micro-organisms is a good starting point for further studies on this novel sterilization method.
In conclusion, the results from this study have demonstrated that common media prepared in a microwave, equipped with a polycarbonate water bath and the use of a bottle with rehydrated media, a perforable rubber cap, and a sticker, were sterile after an exposure time of 7 min and had comparable or better microbiological properties than autoclaved media. This system could serve as a basis for developing a more affordable and rapid method to prepare culture media in routine laboratory practices.
Author Contributions
Conceptualization, I.T.-F. and D.A.; methodology, I.T.-F., A.L. and S.P.; software, I.T.-F. and L.R.-D.-L.; validation, I.T.-F., D.A., A.L. and N.P.; formal analysis, I.T.-F., L.R.-D.-L. and P.J.G.-M.; investigation, I.T.-F. and P.J.G.-M.; resources, A.L., D.A. and S.P.; data curation, I.T.-F., R.C. and N.P.; writing—original draft preparation, I.T.-F.; writing—review and editing, I.T.-F., N.P., P.J.G.-M. and R.C.; supervision, P.J.G.-M. and R.C.; project administration, P.J.G.-M. and R.C.; funding acquisition, P.J.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This project was possible thanks to a grant from Doctorats Industrials (2021 DI 42) from the Generalitat de Catalunya. The APC was funded by Doctorats Industrials from the Generalitat de Catalunya.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study and part of a PhD thesis within the scope of the company’s business.
Acknowledgments
The authors would like to acknowledge the Generalitat de Catalunya for providing the necessary support to the research groups IAFARG (https://iafarg.upc.edu, accessed on 1 February 2024) and CATMech (https://catmech.upc.edu/home, accessed on 1 February 2024), and thanks are is extended to the INSA-UB Maria de Maeztu Unit of Excellence (Grant CEX2021-001234-M) funded by MICIN/AEI/FEDER, UE.
Conflicts of Interest
N.P. declares no conflicts of interest. A.L., S.P., I.T.-F. and D.A. are employees of RPD, SL. This study was performed in the context of an industrial doctorate with the collaboration of a university and a company (UPC-RPD, SL).
References
- Einarsson, H.; Snygg, B.G.; Eriksson, C. Inhibition of bacterial growth by Maillard reaction products. J. Agric. Food Chem. 1983, 31, 1043–1047. [Google Scholar] [CrossRef]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect. 2020, 34, 100622. [Google Scholar] [CrossRef]
- Council of Europe. Microbiological examination of non-sterile products: Test for specified microorganisms. In European Pharmacopoeia 10.0, 10th ed.; Harmonized Method, EDQM, § 2.6.13.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Bhattacharjee, M.K.; Mehta, B.S.; Akukwe, B. Maillard reaction products inhibit the periodontal pathogen Aggregatibacter actinomycetemcomitans by chelating iron. Arch. Oral Biol. 2021, 122, 104989. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.B. Inhibitory effect of Maillard reaction products on growth of the aerobic marine hyperthermophilic archaeon Aeropyrum pernix. Appl. Environ. Microbiol. 2003, 69, 4325–4328. [Google Scholar] [CrossRef]
- Terrones-Fernandez, I.; Casino, P.; López, A.; Peiró, S.; Ríos, S.; Nardi-Ricart, A.; García-Montoya, E.; Asensio, D.; Marqués, A.M.; Castilla, R.; et al. Improvement of the Pour Plate Method by Separate Sterilization of Agar and Other Medium Components and Reduction of the Agar Concentration. Microbiol. Spectr. 2023, 11, e0316122. [Google Scholar] [CrossRef]
- Bazana, L.C.G.; Carvalho, Â.R.; Mace, M.; Fuentefria, A.M. The influence of the microwave oven on the production of solid culture medium and quality of microbial growth. An. Acad. Bras. Cienc. 2022, 94, e20211104. [Google Scholar] [CrossRef] [PubMed]
- Casino, P.; López, A.; Peiró, S.; Ríos, M.; Ríos, S.; Porta, A.; Agustí, G.; Asensio, D.; Marqués, A.M.; Piqué, N. GVPC Medium Manufactured without Oxygen Improves the Growth of Legionella spp. and Exhibits Enhanced Selectivity Properties. Microbiol. Spectr. 2022, 10, e02401-21. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.E.; Meltzer, T.H. Critical influences of particles, pores, and prefilters in sterilizing filtration. PDA J. Pharm. Sci. Technol. 2009, 63, 240–244. [Google Scholar] [PubMed]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of Using Sterilization by Gamma Radiation: The Other Side of the Coin. Int. J. Med. Sci. 2018, 15, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Shareef, S.A.; Hamasaeed, P.A.; Ismaeil, A.S. Sterilization of culture media for microorganisms using a microwave oven instead of autoclave. Rafidain J. Sci. 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Kothari, V.; Patadia, M.; Trivedi, N. Microwave sterilized media supports better microbial growth than autoclaved media. Res. Biotechnol. 2011, 2, 5. [Google Scholar]
- Iacoviello, M.P.; Rubin, S.A. Sterile preparation of antibiotic-selective LB agar plates using a microwave oven. BioTechniques 2001, 30, 963–965. [Google Scholar] [CrossRef]
- Shamis, Y.; Taube, A.; Mitik-Dineva, N.; Croft, R.; Crawford, R.J.; Ivanova, E.P. Specific electromagnetic effects of microwave radiation on Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3017–3022. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Hu, Y.; Wang, L. Microwaves, a potential treatment for bacteria: A review. Front. Microbiol. 2022, 13, 888266. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, H.; Ushioda, H.; Kudo, Y. Kinetics of Escherichia coli destruction by microwave irradiation. Appl. Environ. Microbiol. 1992, 58, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Woo, I.S.; Rhee, I.K.; Park, H.D. Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl. Environ. Microbiol. 2000, 66, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- ISO 11133:2014; Microbiology of Food, Animal Feed and Water-Preparation, Production, Storage and Performance Testing of Culture Media. International Standards Organization: Geneva, Switzerland, 2014.
- ISO 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Standards Organization: Geneva, Switzerland, 2017.
- ISO 4833-1:2014; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Standards Organization: Geneva, Switzerland, 2014.
- ISO 21149:2017; Cosmetics—Microbiology—Enumeration and Detection of Aerobic Mesophilic Bacteria. International Standards Organization: Geneva, Switzerland, 2017.
- ISO 16212:2017; Cosmetics—Microbiology—Enumeration of Yeast and Mould. International Standards Organization: Geneva, Switzerland, 2017.
- ISO 21528-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Standards Organization: Geneva, Switzerland, 2004.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Standards Organization: Geneva, Switzerland, 2017.
- Fung, D.Y.C.; Cunningham, F.E. Effect of Microwaves on Microorganisms in Foods 1,2. J. Food Prot. 1980, 43, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, H.; Huang, S.; Yang, M.; Wu, J.; Ci, Z.; He, Y.; Wu, Z.; Han, L.; Zhang, D. Insight into the incredible effects of microwave heating: Driving changes in the structure, properties and functions of macromolecular nutrients in novel food. Front. Nutr. 2022, 9, 941527. [Google Scholar] [CrossRef] [PubMed]
- Gröning, R.; Janske, U. Keimzahlreduzierung durch Mikrowellen--mikrowellenspezifische Effekte [Germ reduction by microwaves--microwave specific effects]. Pharmazie 1987, 42, 167–168. [Google Scholar] [PubMed]
- Feurhuber, M.; Burian, P.; Magno, M.; Miranda, M.; Hochenauer, C. Development of a spatially and timely resolved CFD model of a steam sterilizer to predict the load temperature and the theoretical inactivation of bacteria based on sterilization parameters. Phys. Med. 2019, 8, 100020. [Google Scholar] [CrossRef]
- Feurhuber, M.; Cattide, A.; Magno, M.; Miranda, M.; Prieler, R.; Hochenauer, C. Prediction of the fluid flow, heat transfer and inactivation of microorganism at medical devices in modern steam sterilizers using computational fluid dynamics. Appl. Therm. Eng. 2017, 127, 1391–1403. [Google Scholar] [CrossRef]
- Lau, W.L.; Reizes, J.; Timchenko, V.; Kara, S.; Kornfeld, B. Numerical modelling of an industrial steam–air sterilisation process with experimental validation. Appl. Therm. Eng. 2015, 75, 2–134. [Google Scholar] [CrossRef]
- Rufian–Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins. J. Food Qual. 2007, 30, 160–168. [Google Scholar] [CrossRef]
- Rufian–Henares, J.A.; De la Cueva, S.P. Antimicrobial Activity of Coffee Melanoidins–A Study of Their Metal-Chelating Properties. J. Agric. Food Chem. 2009, 2, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Heaney, H.; Laing, J.; Paterson, L.; Walker, A.W.; Gow, N.A.R.; Johnson, E.M.; MacCallum, D.M.; Brown, A.J.P. The environmental stress sensitivities of pathogenic Candida species, including Candida auris, and implications for their spread in the hospital setting. Med. Mycol. 2020, 58, 744–755. [Google Scholar] [CrossRef]
- Sasaki, K.; Shintani, H.; Itoh, J.; Kamogawa, T.; Kajihara, Y. Effect of calcium in assay medium on D value of Bacillus stearothermophilus ATCC 7953 spores. Appl. Environ. Microbiol. 2000, 66, 5509–5513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).