Changes in Anticholinesterase and Antioxidant Activities of Fruit Products during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Fruit Preserves
2.3. Analytical Methods
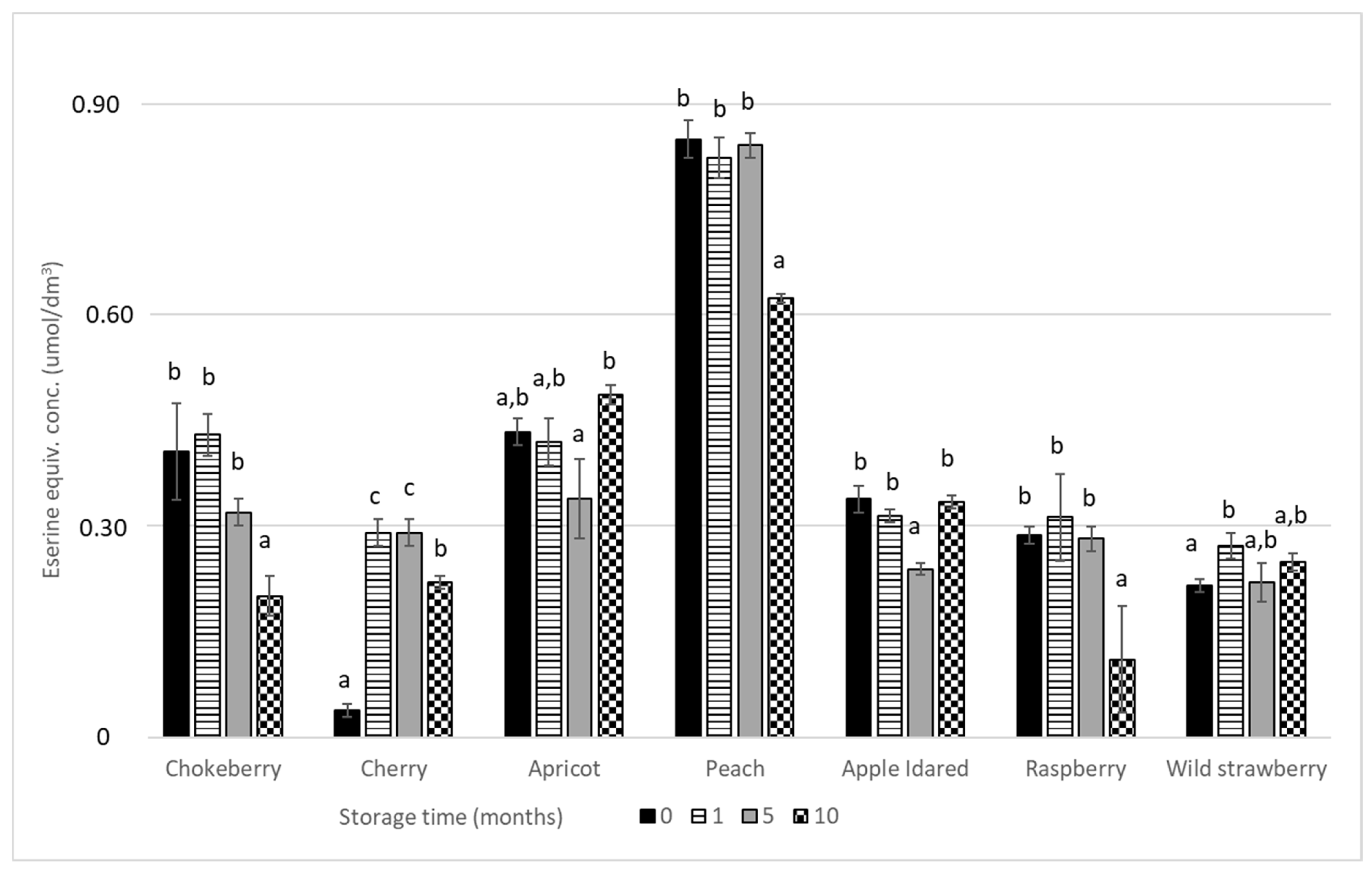
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer potential and other pharmacological properties of Prunus armeniaca L.: An updated overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Manohar, S.; Kumari, P.; Krishnan, V.; Maheshwari, C.; Narwal, S.; Bansal, N.; Dahuja, A. The role of major phenolics in apple to total antioxidant capacity. In Apple Cultivation-Recent Advances; IntechOpen: Houston, TX, USA, 2023. [Google Scholar]
- Suljević, D.; Mitrašinović-Brulić, M.; Klepo, L.; Škrijelj, R.; Fočak, M. Impact of dietary supplementation with chokeberry (Aronia melanocarpa, Michx.) on tetrachloride-induced liver injury in Wistar rats: Hematological and biochemical implication. Cell Biochem. Funct. 2023, 41, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Yurt, B.; Celik, I. Hepatoprotective effect and antioxidant role of sun, sulphited-dried apricot (Prunus armeniaca L.) and its kernel against ethanol-induced oxidative stress in rats. Food Chem. Toxicol. 2011, 49, 508–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, X.; Chen, X.; Ding, C.; Dong, L.; Zhang, J.; Sun, S.; Ding, Q.; Khatoom, S.; et al. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An overview. J. Future Foods 2021, 1, 168–178. [Google Scholar] [CrossRef]
- Bento, C.; Goncalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev. Int. 2022, 38, 1703–1734. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods Hum. Nutr. 2008, 63, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Larrosa, M.; García-Cantalejo, J.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Transcriptional changes in human Caco-2 colon cancer cells following exposure to a recurrent non-toxic dose of polyphenol-rich chokeberry juice. In Genes & Nutrition; Springer: Berlin/Heidelberg, Germany, 2007; Volume 2, pp. 111–113. [Google Scholar]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Cosić, Z.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Meza, E.I.; Yanez, J.A.; Remsberg, C.M.; Takemoto, J.K.; Davies, N.M.; Rasco, B.; Clary, C. Effect of dehydration on raspberries: Polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J. Food Sci. 2010, 75, H5–H12. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Z.; Zhu, M.; Wan, X.; Zhang, L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr. Opin. Food Sci. 2023, 51, 101014. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Lebedeva, T.N.; Vidyagina, E.O.; Sorokopudov, V.N.; Popova, A.A.; Shestibratov, K.A. Relationship between phenolic compounds and antioxidant activity in berries and leaves of raspberry genotypes and their genotyping by SSR markers. Antioxidants 2022, 11, 1961. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Roussos, P.A.; Sefferou, V.; Denaxa, N.-K.; Tsantili, E.; Stathis, V. Apricot (Prunus armeniaca L.) fruit quality attributes and phytochemicals under different crop load. Sci. Hortic. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Pliszka, B. Content and correlation of polyphenolic compounds, bioelements and antiradical activity in black elder berries (Sambucus nigra L.). J. Elem. 2020, 25, 595–605. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Zhang, J.; Zhang, Y.T.; Sun, J.Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Cyuńczyk, M.; Zujko, M.E. Antioxidant Properties of Chokeberry Products—Assessment of the Composition of Juices and Fibers. Foods 2023, 12, 4029. [Google Scholar] [CrossRef]
- Catană, L.; Catană, M.; Iorga, E.; Asănică, A.C.; Lazăr, A.G.; Lazăr, M.A.; Belc, N. Vitamin C and total polyphenol content and antioxidant capacity of fresh and processed fruits of Aronia melanocarpa. Sci. Papers. Ser. B. Hortic. 2017, 61, 433–440. [Google Scholar]
- Cairone, F.; Simonetti, G.; Orekhova, A.; Casadei, M.A.; Zengin, G.; Cesa, S. Health potential of celery strawberries: Enzymatic inhibition and anti-Candida activity evaluation. Molecules 2021, 26, 1731. [Google Scholar] [CrossRef]
- Biondo, E.; Corrêa, A.P.F.; Brandelli, A.; Sant’Anna, V. Wild strawberries (Rubus rosifolius SM.) from Southern Brazil: Centesimal and mineral composition, total polyphenols, antioxidant, antibacterial and anti-hypertensive activities. Rev. Ciência Agrícola 2021, 19, 71–78. [Google Scholar] [CrossRef]
- Huneif, M.A.; Alqahtani, S.M.; Abdulwahab, A.; Almedhesh, S.A.; Mahnashi, M.H.; Riaz, M.; Ur-Rahman, N.; Jan, M.S.; Ullah, F.; Aasim, M.; et al. α-glucosidase, α-amylase and antioxidant evaluations of isolated bioactives from wild strawberry. Molecules 2022, 27, 3444. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Hussain, L.; Shahid, F.; Anwar, F.; Chauhdary, Z.; Zafar, A. Pharmacological Potential of the Standardized Methanolic Extract of Prunus armeniaca L. in the Haloperidol-Induced Parkinsonism Rat Model. Evid. -Based Complement. Alternative. Med. 2022, 2022, 3697522. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Zapp, J. Activity-guided isolation of cholinesterase inhibitors quercetin, rutin and kaempferol from Prunus persica fruit. Z. Für Naturforschung C 2020, 75, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Desseva, I.; Popova, A.; Dincheva, I.; Vrancheva, R.; Lante, A.; Krastanov, A. GC-MS metabolic profile and α-glucosidase-, α-amylase-, lipase-, and acetylcholinesterase-inhibitory activities of eight peach varieties. Molecules 2021, 26, 4183. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.E.; Perez, R.G. Anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Nexus of cholinergic and nerve growth factor dysfunction. Curr. Alzheimer Res. 2021, 18, 1010. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Asghari, K.; Niknam, Z.; Mohammadpour-Asl, S.; Chodari, L. Cellular junction dynamics and Alzheimer’s disease: A comprehensive review. Mol. Biol. Rep. 2024, 51, 273. [Google Scholar] [CrossRef]
- Aluko, R.E. Food-derived Acetylcholinesterase Inhibitors as Potential Agents against Alzheimer’s Disease. Efood 2021, 2, 49–58. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-Mahasneh, M.A.; Kilani, I.; Yang, W.; Alhamad, M.N.; Ereifej, K.; Al-u’datt, M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J. Sci. Food Agric. 2011, 91, 1096–1102. [Google Scholar] [CrossRef]
- Arancibia-Avila, P.; Namiestnik, J.; Toledo, F.; Werner, E.; Martinez-Alaya, A.L.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gorinstein, S. The influence of different time durations of thermal processing on berries quality. Food Control 2012, 26, 585–587. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Salazar-Orbea, G.L.; García-Villalba, R.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. High–pressure processing vs. thermal treatment: Effect on the stability of polyphenols in strawberry and apple products. Foods 2021, 10, 2919. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Mariutti, L.R.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Banaś, A.; Korus, A.; Korus, J. Texture, color, and sensory features of low-sugar gooseberry jams enriched with plant ingredients with prohealth properties. J. Food Qual. 2018, 2018, 1646894. [Google Scholar] [CrossRef]
- Rahman, M.M.; Moshiur, A. Preparation of strawberry jam and estimation of its nutritive value during storage. J. Postharvest Technol. 2018, 6, 41–56. [Google Scholar]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-derived compounds and extracts as biologically active substances with anticancer and neuroprotective properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Lourtney, D.K.; Andres, V.; Gmelin, G. A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2003, 14, 127–131. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K. Screening for cholinesterase inhibitors in selected fruits and vegetables. Electron. J. Pol. Agric. Univ. 2012, 15, 6. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss.U. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wojcik, E.; Borowiec, K. Phenolic acids exert anticholinesterase and cognition-improving effects. Curr. Alzheimer Res. 2018, 15, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and phenylpropanoids as acetyl-and butyrylcholinesterase inhibitors: A comparative study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kazazic, M.; Mehic, E.; Djapo-Lavic, M. Phenolic content and bioactivity of two sour cherry cultivars and their products. Bull. Chem. Technol. Bosnia Herzeg. 2022, 58, 1–6. [Google Scholar]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant property and storage stability of quince juice phenolic compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef]
- Vukoja, J.; Pichler, A.; Kopjar, M. Stability of anthocyanins, phenolics and color of tart cherry jams. Foods 2019, 8, 255. [Google Scholar] [CrossRef]











| Fruit | Compote | Juice | Jam | |
|---|---|---|---|---|
| Chokeberry | 0.51 ± 0.02 a,b | 0.45 ± 0.04 a | 0.88 ± 0.03 c | 0.58 ± 0.03 b |
| Cherry | 0.44 ± 0.00 c | 0.38 ± 0.03 b,c | 0.35 ± 0.01 b | 0.20 ± 0.04 a |
| Apricot | 0.38 ± 0.02 b | 0.52 ± 0.06 c | 0.24 ± 0.03 a | 0.37 ± 0.01 b |
| Peach | 0.11 ± 0.01 b,c | 0.05 ± 0.02 a | 0.09 ± 0.01 b | 0.13 ± 0.01 c |
| Apple Idared | 0.51 ± 0.05 b | 0.44 ± 0.03 b | 0.53 ± 0.04 b | 0.09 ± 0.01 a |
| Apple Champion | 0.08 ± 0.01 a | 0.08 ± 0.02 a | n.p. | n.p. |
| Wild strawberry | 0.54 ± 0.06 b | 0.49 ± 0.07 b | n.p. | 0.22 ± 0.03 a |
| Raspberry | 0.41 ± 0.01 c | 0.24 ± 0.02 a | 0.35 ± 0.03 b | 0.22 ± 0.02 a |
| BChE | Fruit | Compote | Juice | Jam |
|---|---|---|---|---|
| Chokeberry | 0.65 ± 0.02 b | 0.40 ± 0.06 a | 1.04 ± 0.12 c | 0.41 ± 0.07 a |
| Cherry | 0.55 ± 0.01 c | 0.46 ± 0.02 c | 0.42 ± 0.01 b | 0.04 ± 0.01 a |
| Apricot | 0.45 ± 0.05 a | 0.82 ± 0.09 b | 0.42 ± 0.03 a | 0.43 ± 0.02 a |
| Peach | 0.21 ± 0.01 a | 0.72 ± 0.03 c | 0.31 ± 0.02 b | 0.85 ± 0.03 d |
| Apple Idared | 0.16 ± 0.02 a | 0.62 ± 0.10 c | 0.37 ± 0.03 b | 0.34 ± 0.02 b |
| Apple Champion | 0.46 ± 0.08 b | 0.32 ± 0.03 a | n.p. | n.p. |
| Wild strawberry | 0.49 ± 0.03 b | 0.79 ± 0.08 c | n.p. | 0.21 ± 0.01 a |
| Raspberry | 0.38 ± 0.05 a | 0.37 ± 0.09 a | 0.55 ± 0.01 b | 0.29 ± 0.01 a |
| DPPH | Fruit | Compote | Juice | Jam |
|---|---|---|---|---|
| Chokeberry | 1.21 ± 0.11 c | 0.90 ± 0.03 a,b | 0.82 ± 0.04 a | 1.00 ± 0.05 b |
| Cherry | 0.31 ± 0.03 c | 0.25 ± 0.00 b | 0.27 ± 0.02 b,c | 0.13 ± 0.01 a |
| Apricot | 0.22 ± 0.00 a | 0.49 ± 0.01 d | 0.27 ± 0.02 b | 0.35 ± 0.02 c |
| Peach | 0.04 ± 0.00 a | 0.09 ± 0.00 b | 0.14 ± 0.01 c | 0.17 ± 0.02 d |
| Apple Idared | 0.39 ± 0.02 c | 0.39 ± 0.01 c | 0.32 ± 0.01 b | 0.03 ± 0.01 a |
| Apple Champion | 0.18 ± 0.02 b | 0.03 ± 0.01 a | n.p. | n.p. |
| Wild strawberry | 0.28 ± 0.01 b | 0.05 ± 0.01 a | n.p. | 0.47 ± 0.02 c |
| Raspberry | 0.39 ± 0.02 a | 0.37 ± 0.01 a | 0.34 ± 0.04 a | 0.34 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajowniczek-Ałasa, D.; Baranowska-Wójcik, E.; Szwajgier, D. Changes in Anticholinesterase and Antioxidant Activities of Fruit Products during Storage. Appl. Sci. 2024, 14, 6187. https://doi.org/10.3390/app14146187
Gajowniczek-Ałasa D, Baranowska-Wójcik E, Szwajgier D. Changes in Anticholinesterase and Antioxidant Activities of Fruit Products during Storage. Applied Sciences. 2024; 14(14):6187. https://doi.org/10.3390/app14146187
Chicago/Turabian StyleGajowniczek-Ałasa, Dorota, Ewa Baranowska-Wójcik, and Dominik Szwajgier. 2024. "Changes in Anticholinesterase and Antioxidant Activities of Fruit Products during Storage" Applied Sciences 14, no. 14: 6187. https://doi.org/10.3390/app14146187
APA StyleGajowniczek-Ałasa, D., Baranowska-Wójcik, E., & Szwajgier, D. (2024). Changes in Anticholinesterase and Antioxidant Activities of Fruit Products during Storage. Applied Sciences, 14(14), 6187. https://doi.org/10.3390/app14146187







