Development of Sourdough Bread Made with Probiotic Lactiplantibacillus plantarum Bacteria Addition
Abstract
1. Introduction
2. Materials and Methods
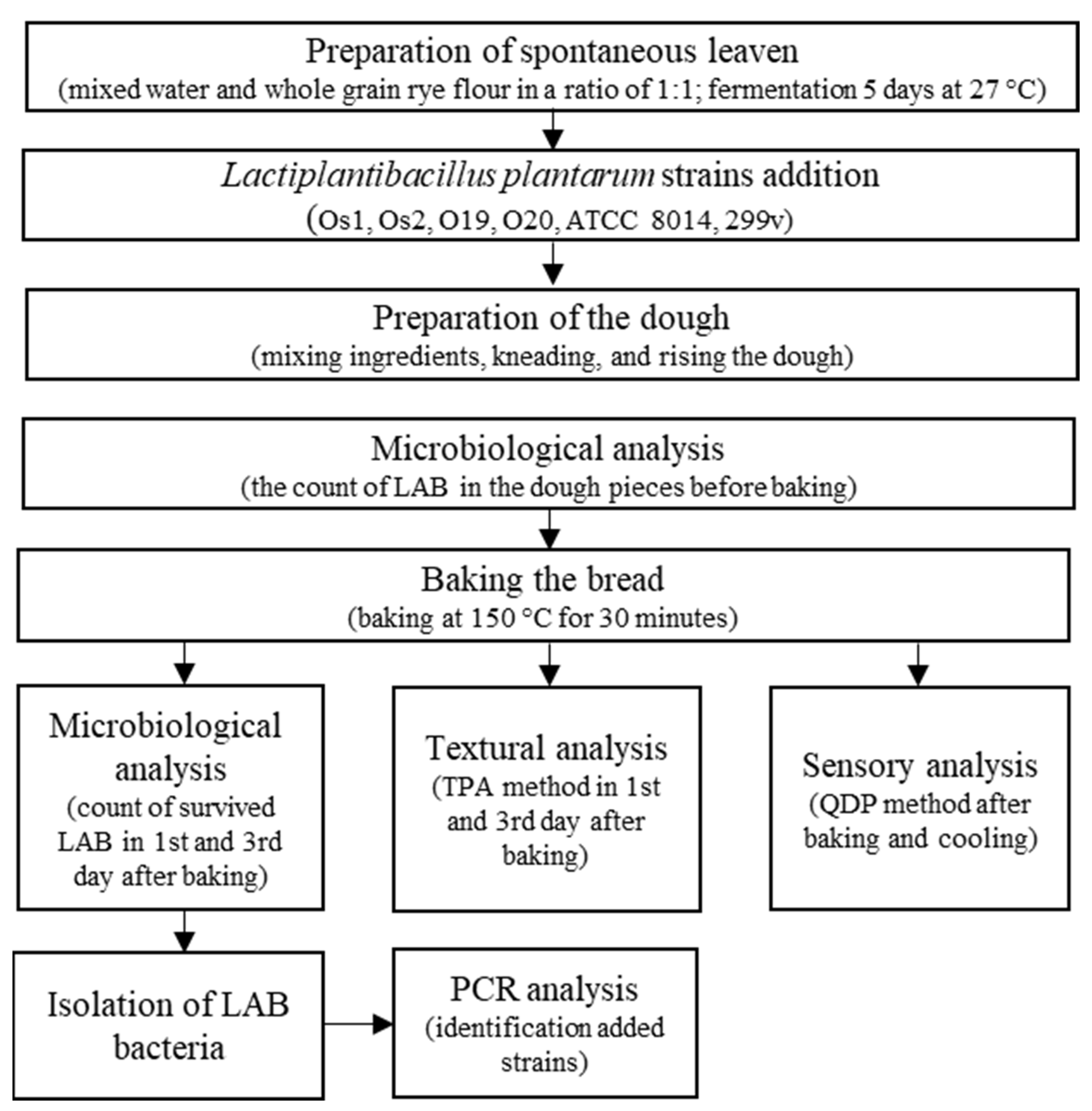
2.1. Scheme of Experiment
2.1.1. Baker’s Sourdough Preparation
2.1.2. Lactiplantibacillus plantarum Strains Preparation and Sourdough Enrichment
2.1.3. Bread Preparation
2.2. Microbiological Analysis
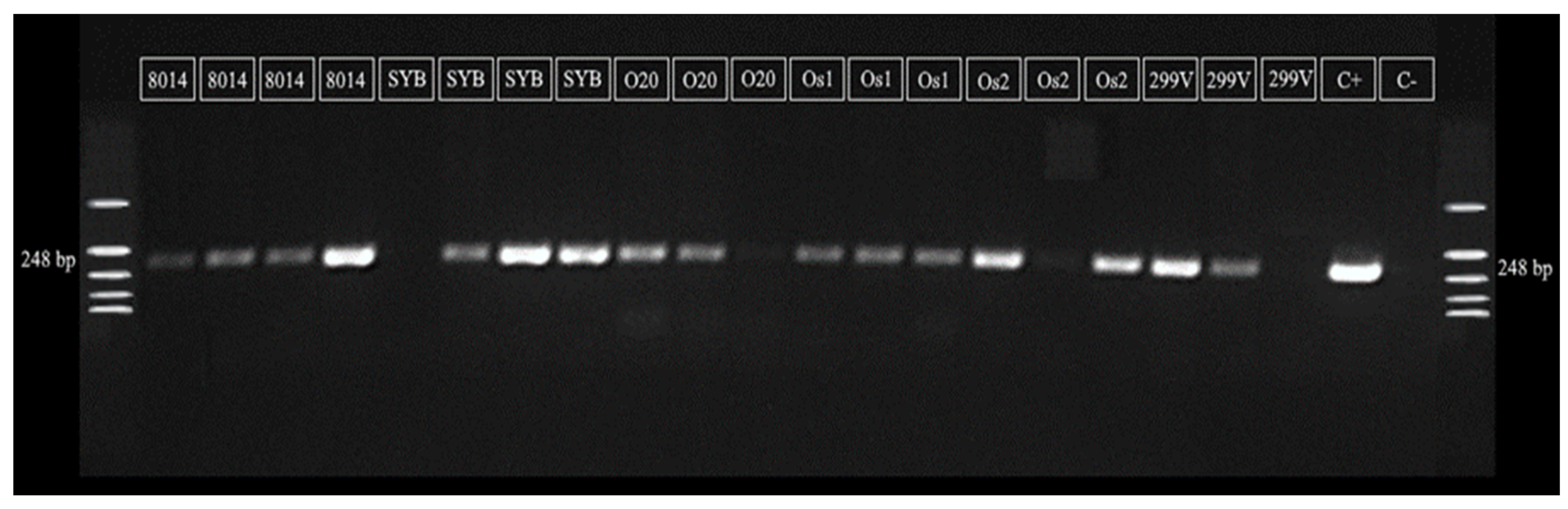
2.3. PCR Analyses
2.4. Texture Analysis
2.5. Sensory Analyses
2.6. Statistical Analyses
3. Results
3.1. Microbiological and PCR Analyses
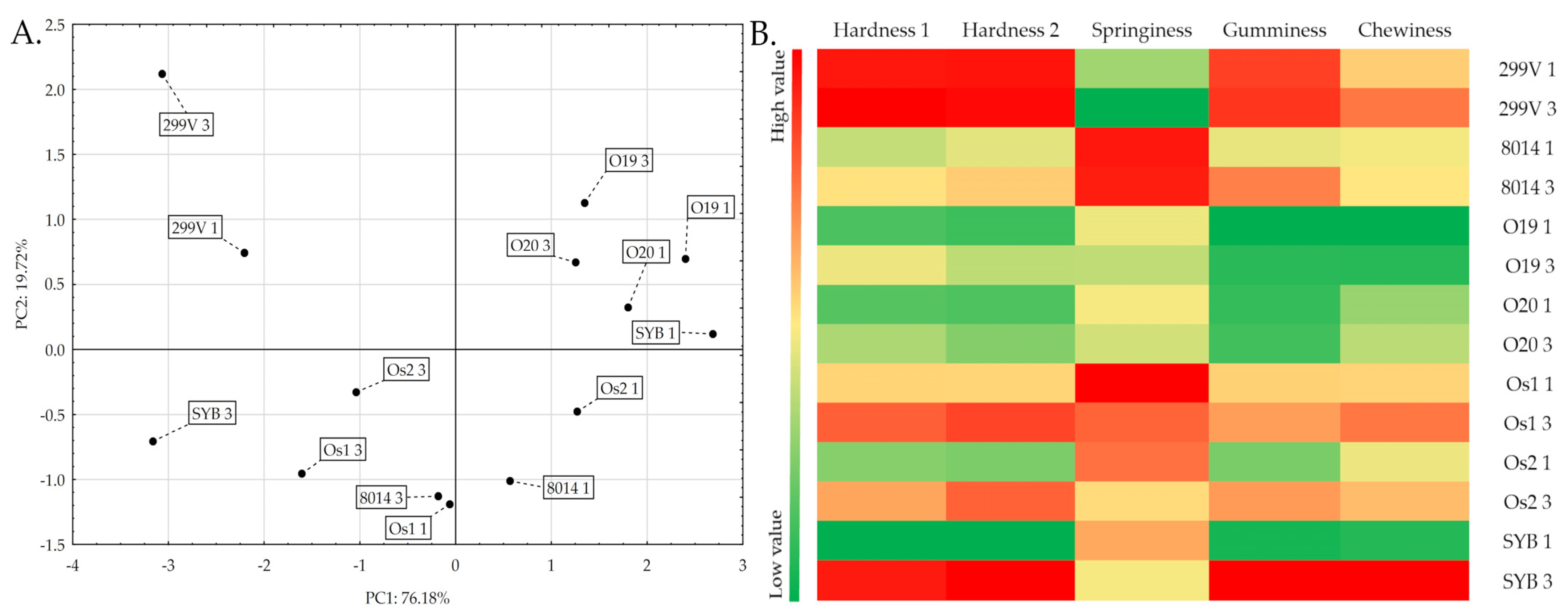
3.2. Texture Analysis
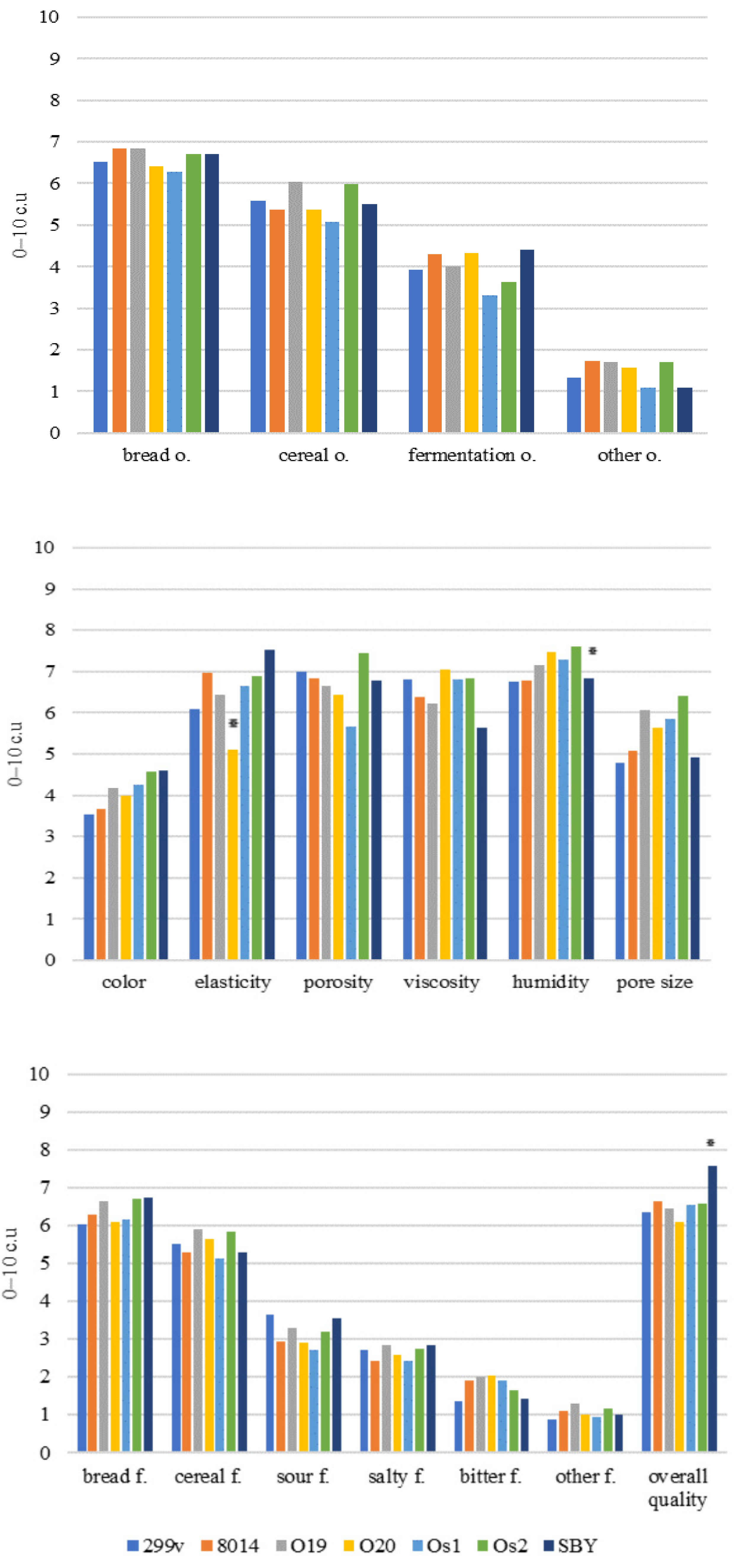
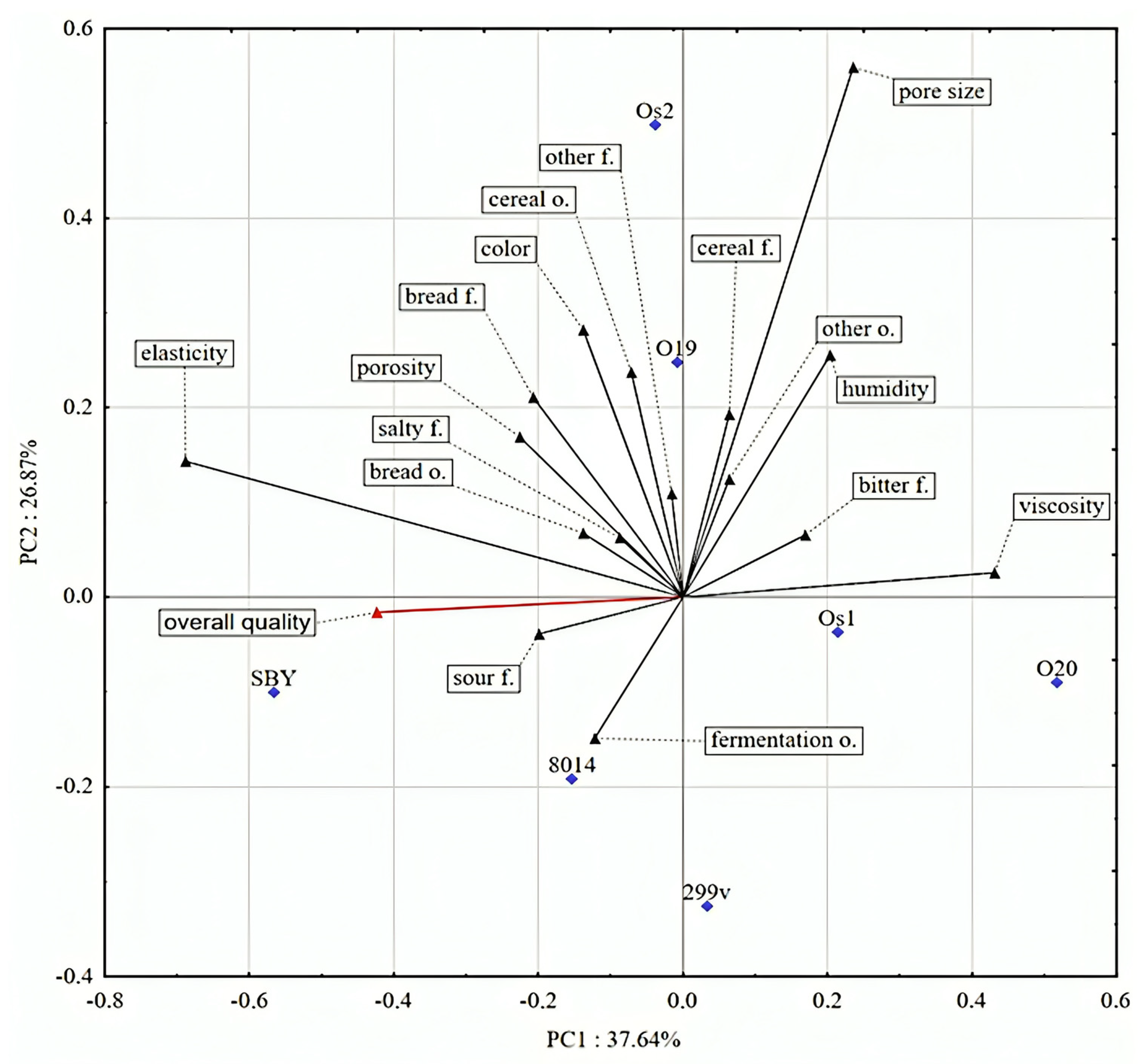
3.3. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Food Waste. Available online: https://ec.europa.eu/food/safety/food-waste_en (accessed on 20 May 2024).
- WRAP. Food Surplus and Waste in the UK—Key Facts; The Waste and Resources Action Programme: Banbury, UK, 2021. Available online: www.wrap.org.uk/food-drink (accessed on 20 May 2024).
- Ghosh, R.; Eriksson, M. Food waste due to retail power in supply chains: Evidence from Sweden. Glob. Food Secur. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Brancoli, P.; Bolton, K.; Eriksson, M. Environmental impacts of waste management and valorisation pathways for surplus bread in Sweden. Waste Manag. 2020, 117, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, M.; Bilska, B.; Kołożyn-Krajewska, D. Food Waste in Catering Establishments—An Analysis of Causes and Consequences. Eur. J. Sustain. Dev. 2021, 10, 365. [Google Scholar] [CrossRef]
- Immonen, M.; Maina, N.H.; Wang, Y.; Coda, R.; Katina, K. Waste bread recycling as a baking ingredient by tailored lactic acid fermentation. Int. J. Food Microbiol. 2020, 327, 108652. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Chouliaras, Y.; Tsakalidou, E.; Kalantzopoulos, G. Application of selected starter cultures for the production of wheat sourdough bread using a traditional three-stage procedure. Process Biochem. 2005, 40, 2813–2819. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology—A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S. The diversity and function of sourdough starter microbiomes. eLife 2021, 10, e61644. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Comasio, A.; Harth, H.; De Vuyst, L. Impact of starter culture, ingredients, and flour type on sourdough bread volatiles as monitored by selected ion flow tube-mass spectrometry. Food Res. Int. 2018, 106, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Møllgaard, H. Production of probiotic cultures and their addition in fermented foods. In Chapter 19: Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2008; pp. 513–532. [Google Scholar]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. Biomed. Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Rzepkowska, A.; Radawska, A.; Zieliński, K. In Vitro Screening of Selected Probiotic Properties of Lactobacillus Strains Isolated from Traditional Fermented Cabbage and Cucumber. Curr. Microbiol. 2015, 70, 183–194. [Google Scholar] [CrossRef]
- Zielińska, D.; Długosz, E.; Zawistowska-Deniziak, A. Functional Properties of Food Origin Lactobacillus in the Gastrointestinal Ecosystem-in Vitro Study. Probiotics Antimicrob. Proteins 2019, 11, 820–829. [Google Scholar] [CrossRef]
- Zielińska, D.; Łepecka, A.; Ołdak, A.; Długosz, E.; Kołożyn-Krajewska, D. Growth and adhesion inhibition of pathogenic bacteria by live and heat-killed food-origin Lactobacillus strains or their supernatants. FEMS Microbiol. Lett. 2021, 368, fnab024. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Kruk, M.; Ścibisz, I.; Zielińska, D. The Novel Strain of Gluconobacter oxydans H32 Isolated from Kombucha as a Proposition of a Starter Culture for Sour Ale Craft Beer Production. Appl. Sci. 2022, 12, 3047. [Google Scholar] [CrossRef]
- Kruk, M.; Wójcik, T.; Trząskowska, M. Application of Kombucha tea brew and SCOBY symbiotic culture to produce fermented milk beverage. Żywność. Nauka Technol. Jakość 2019, 120, 97–108. [Google Scholar] [CrossRef]
- Song, Y.L.; Kato, N.; Liu, C.X.; Matsumiya, Y.; Kato, H.; Watanabe, K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 2000, 187, 167–173. [Google Scholar] [CrossRef]
- Zięć, G. Właściwości teksturalne miękiszu i jakość chlebów pszenno-owsianych. Żywność Nauka Technol. Jakość 2016, 106, 102–117. [Google Scholar] [CrossRef]
- ISO 13299:2016; Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2003.
- Piasecka-Jozwiak, K.; Chablowska, B.; Stefanska, I. Kultury starterowe w piekarstwie—możliwość odzwierciedlenia tradycyjnego procesu fermentacji poprzez kształtowanie mikrobiota zakwasów piekarskich w przemysłowej produkcji pieczywa. Postępy Mikrobiol. 2016, 55, 268–278. [Google Scholar]
- De Angelis, M.; Di Cagno, R.; Huet, C.; Crecchio, C.; Fox, P.F.; Gobbetti, M. Heat Shock Response in Lactobacillus plantarum. Appl. Environ. Microbiol. 2004, 70, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Turkarslan, S.; Beer, K.D.; Lo, F.Y.; Baliga, N.S. Adaptation of cells to new environments. Wiley Interdiscip Rev. Syst. Biol. Med. 2011, 3, 544–561. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.G.; Rinker, D.C. The genomics of microbial domestication in the fermented food environment. Curr. Opin. Genet. Dev. 2015, 35, 1–8. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: World Health Organization, FAO Food and Nutrition Paper. 2006. Available online: https://catalogue.nla.gov.au/catalog/3788914 (accessed on 20 May 2024).
- Wendel, U. Assessing viability and stress tolerance of probiotics—A review. Front. Microbiol. 2022, 12, 4351. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, R.; Scheirlinck, I.; Van Schoor, A.; Huys, G.; Vancanneyt, M.; Vandamme, P.; De Vuyst, L. Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Appl. Environ. Microbiol 2007, 73, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A.I. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. Food Sci. Technol. = Lebensm. -Wiss. Und Technol. 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Penhasi, A.; Reuveni, A.; Baluashvili, I. Microencapsulation May Preserve the Viability of Probiotic Bacteria During a Baking Process and Digestion: A Case Study with Bifidobacterium animalis Subsp. lactis in Bread. Curr. Microbiol. 2021, 78, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Seyedain-Ardabili, M.; Sharifan, A.; Tarzi, B.G. The Production of Synbiotic Bread by Microencapsulation. Food Technol. Biotechnol. 2016, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Lopes Neto JH, P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT-Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Tamani, R.J.; Goh, K.; Brennan, C. Physico-Chemical Properties of Sourdough Bread Production Using Selected Lactobacilli Starter Cultures. J. Food Qual. 2013, 36. [Google Scholar] [CrossRef]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread Staling: Updating the View. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J. Anim. Sci. Biotechnol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Acosta-Martînez, V.; Tabatabai, M.A. Arylamidase activity in soils: Effect of trace elements and relationships to soil properties and activities of amidohydrolases. Soil Biol. Biochem. 2001, 33, 17–23. [Google Scholar] [CrossRef]
- Karimi, N.; Zeynali, F.; Bari, M.R.; Nikoo, M.; Mohtarami, F.; Kadivar, M. Amaranth selective hydrolyzed protein influence on sourdough fermentation and wheat bread quality. Food Sci. Nutr. 2021, 9, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Sandoval, N.G.; Valencia-Tapia, M.Y.; Calderón de la Barca, A.M.; Islas-Rubio, A.R. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality. Foods 2016, 5, 59. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Analisis of Food Basics—Methods—Applications; Wydawnictwo Naukowe PTTŻ: Kraków, Poland, 2014. [Google Scholar]
- Pejcz, E. Biotechnological Approach of Technological Advancements for Sustainable Probiotic Bread Production. Sustainability 2024, 16, 3275. [Google Scholar] [CrossRef]

| Dough Ingredients [g 100 g−1] | ||||||
|---|---|---|---|---|---|---|
| Water | Wheat Flour 1 | Wheat Flour 2 | Rye Flour | Salt | Sourdough | Bakery Yeast |
| 41.90 | 39.70 | 5.70 | 5.70 | 0.90 | 5.70 | 0.40 |
| Sample | LAB [log CFU g−1] | AAB [log CFU g−1] | Yeast [log CFU g−1] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Days of Storage | |||||||||
| 0 | 1st | 3th | 0 | 1st | 3th | 0 | 1st | 3th | |
| SBY | 7.99 aA | 3.07 aB | 3.52 aB | 7.84 aA | 2.91 aB | 2.03 aB | 6.58 aA | 1.53 B | 1.59 aB |
| 299V | 8.17 aA | 2.39 aB | 1.84aB | 7.70 aA | 1.03 aB | 1.69 aB | 6.39 aA | nd | 1.53 aB |
| O19 | 8.15 aA | 1.00 aB | nd | 7.48 aA | nd | nd | 6.19 aA | nd | nd |
| O20 | 8.18 aA | 1.80 aB | nd | 8.15 bA | 1.43 aB | 0.94 aB | 6.33 aA | nd | nd |
| Os1 | 8.17 aA | 0.63 aB | 1.56 aB | 7.98 aA | nd | 0.66 aB | 5.92 aA | nd | 0.83 aB |
| Os2 | 8.74 aA | nd | 1.43 aB | 7.93 aA | 2.57 aB | nd | 6.33 aA | nd | nd |
| 8014 | 8.03 aA | 2.74 aB | 1.26 aB | 7.71 aA | 0.66 aB | 1.53 aB | 6.33 aA | nd | nd |
| Sample (S) | Days of Storage (D) | Hardness 1 [N] | Hardness 2 [N] | Springiness [mm] | Gumminess [N] | Chewiness [mJ] |
|---|---|---|---|---|---|---|
| SYB | 1 | 5.59 ± 1.10 a | 4.95 ± 0.89 a | 8.63 ± 0.48 a | 3.79 ± 0.67 a | 31.49 ± 5.38 a |
| 3 | 11.44 ± 2.66 b | 10.38 ± 1.83 b | 8.51 ± 0.42 a | 6.48 ± 1.3 b | 56.09 ± 9.16 b | |
| 299V | 1 | 11.51 ± 1.5 a | 10.16 ± 1.33 a | 8.23 ± 0.48 a | 6.15 ± 0.81 a | 41.83 ± 4.93 a |
| 3 | 11.85 ± 2.86 a | 10.29 ± 2.83 a | 7.71 ± 1.09 a | 6.21 ± 1.32 a | 47.79 ± 6.44 a | |
| O19 | 1 | 6.33 ± 0.95 a | 5.58 ± 0.84 a | 8.48 ± 0.56 a | 3.65 ± 0.5 a | 30.09 ± 4.19 a |
| 3 | 7.93 ± 1.56 a | 6.89 ± 1.38 a | 8.33 ± 0.49 a | 3.91 ± 0.51 a | 31.58 ± 5.04 a | |
| O20 | 1 | 6.44 ± 1.33 a | 5.76 ± 1.11 a | 8.51 ± 0.42 a | 3.99 ± 0.77 a | 35.91 ± 5.87 a |
| 3 | 7.29 ± 0.72 a | 6.29 ± 0.66 a | 8.39 ± 0.27 a | 4.07 ± 0.5 a | 37.23 ± 4.77 a | |
| Os1 | 1 | 8.48 ± 1.69 a | 7.82 ± 2.43 a | 8.87 ± 0.25 a | 5.4 ± 1.62 a | 41.51 ± 5.49 a |
| 3 | 10.34 ± 2.55 a | 9.56 ± 1.96 a | 8.73 ± 0.22 a | 5.66 ± 1.32 a | 47.87 ± 6.30 a | |
| Os2 | 1 | 6.92 ± 1.31 a | 6.22 ± 1.18 a | 8.71 ± 0.32 a | 4.42 ± 0.67 a | 39.14 ± 6.21 a |
| 3 | 9.23 ± 0.94 a | 9.20 ± 1.94 a | 8.56 ± 0.27 a | 5.68 ± 1.22 a | 43.00 ± 8.57 a | |
| 8014 | 1 | 7.54 ± 0.97 a | 7.27 ± 1.25 a | 8.84 ± 0.11 a | 5.12 ± 0.88 a | 39.41 ± 6.45 a |
| 3 | 8.28 ± 0.94 a | 7.92 ± 1.04 a | 8.83 ± 0.17 a | 5.82 ± 0.71 a | 40.21 ± 5.55 a |
| Effect | Hardness 1 | Hardness 2 | Springiness | Gumminess | Chewiness |
|---|---|---|---|---|---|
| p-Value | |||||
| Sample (S) | 0.000000 | 0.000000 | 0.000002 | 0.000001 | 0.000000 |
| Days of storage (D) | 0.000000 | 0.000000 | 0.095694 | 0.092957 | 0.000406 |
| Sample × Days of storage (S × D) | 0.000000 | 0.000000 | 0.697849 | 0.000000 | 0.000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Kostrzewska, A. Development of Sourdough Bread Made with Probiotic Lactiplantibacillus plantarum Bacteria Addition. Appl. Sci. 2024, 14, 6155. https://doi.org/10.3390/app14146155
Zielińska D, Kostrzewska A. Development of Sourdough Bread Made with Probiotic Lactiplantibacillus plantarum Bacteria Addition. Applied Sciences. 2024; 14(14):6155. https://doi.org/10.3390/app14146155
Chicago/Turabian StyleZielińska, Dorota, and Aleksandra Kostrzewska. 2024. "Development of Sourdough Bread Made with Probiotic Lactiplantibacillus plantarum Bacteria Addition" Applied Sciences 14, no. 14: 6155. https://doi.org/10.3390/app14146155
APA StyleZielińska, D., & Kostrzewska, A. (2024). Development of Sourdough Bread Made with Probiotic Lactiplantibacillus plantarum Bacteria Addition. Applied Sciences, 14(14), 6155. https://doi.org/10.3390/app14146155







