Abstract
This perspective article delves into the current state of the art pertaining to the nutritional aspects of plant-based meat and identifies future opportunities for improvement in this line of research. A comparative overview of the macro- and micronutrients of plant-based meat products vis-à-vis conventional animal meat is presented in the initial section. This article explains the differences in their nutritional profiles, highlighting the advantages (equivalent protein content, low saturated fat, source of dietary fiber) and challenges (incomplete amino acid profile, anti-nutrients, and low bioavailability of nutrients) of plant-based alternatives. Emphasis has been placed on the health challenges posed by anti-nutrients in plant-based meat and the role of phytase as a promising solution for mitigating these concerns. The latter sections of this article highlight the ability of phytase enzymes to cause a substantial reduction in phytic acid content and improve the absorption of iron and zinc from the food matrix while not affecting the textural attributes of end products. By deliberating on these critical factors, the article aims to contribute to the ongoing dialogue on the nutritional aspects of plant-based meat and the scientific strategies to mitigate the nutritional challenges currently associated with this category of alternative protein products.
1. Introduction
According to the Food and Agriculture Organization (FAO), food production needs to increase by 60% to feed a projected global population of nearly 10 billion by 2050 [1]. However, increased production does not guarantee food security. Current volumes are enough to feed everyone, but one third of the food gets wasted or lost, and the remaining is not nutritious or accessible. Approximately 735 million people suffered from chronic hunger and 2 billion did not have access to nutritious food in 2022 [2]. Minimizing waste and transitioning to a healthier diet is a way forward. A healthy diet has a balanced composition of macro- and micronutrients. Plant-based alternative proteins have emerged as a potential choice in recent years. The term “plant-based foods” refers to products made from ingredients solely derived from plants, which are alternatives to animal-based products. This includes plant-based meat, eggs, dairy and seafood. Plant-based food products, like animal-derived ones, contain essential nutrients, such as protein, fat, vitamins, and minerals, while also providing complex carbohydrates and fiber, replicating the appearance, taste, and cooking properties of traditional animal-based products.
Several studies have documented the health benefits of plant-based whole foods such as legumes, whole grains, and vegetables and their role in alleviating the risk of diet-related illness and mortality [3,4,5]. Most studies have concluded that a diet rich in plant-based foods offers a dual advantage in terms of health and environmental benefits. Moreover, such diets benefit both people and the planet [6]. These findings and consumer trends are projected to increase the Indian plant-based meat market from 12.6 USD million in 2022 to 228 million by 2030 at a 44% year-on-year growth rate [7]. A recent study found that 72% of customers who bought plant-based meat intend to buy again, citing protein content, health, and nutrition as key reasons, highlighting the importance of understanding the nutritional aspects of plant-based meat due to its increasing popularity [8]. Evidence-based claims must be established on the linkage between plant-based meat, nutrition, and health. Preliminary studies have shown that replacing conventional meat with plant-based options could reduce the risk of heart disease [9,10], improve gut health [11,12], and help maintain a healthy weight [13]. Nevertheless, the nutritional challenge associated with protein quality and the presence of anti-nutritional factors associated with ingredients in plant-based meat should be addressed. Mitigation of anti-nutritional factors and enhancing the digestibility and bioavailability of nutrients in plant-based meat are highly relevant.
Owing to the breadth and depth of the subject, this perspective article focuses on the nutritional aspects of plant-based meat products. Their nutritional quality and composition of macro- and micronutrients vis-à-vis their animal-derived counterparts will be reviewed in the initial sections of this article. The latter sections focus on the challenges of plant-based meat from a nutritional standpoint, emphasizing the anti-nutritional factors and the scientific means of alleviating the same. In this context, the application of “phytase”, a type of phosphatase enzyme, will be discussed as a solution to address the challenges associated with anti-nutrients in plant-based meat products.
2. Plant-Based Meat versus Conventional Meat: A Nutritional Stance
Nutrition is not just limited to calories. The amount of macronutrients and micronutrients and their ability to cater to the dietary requirements of consumers govern the nutritional quality of any food product. Plant-based meat is no exception to this! Indeed, this product category has emerged due to the increasing demand for protein and the consequent innovations led by the food industry [14]. Generally, under-consumption of plant-based foods and over-consumption of red and processed meat and foods high in salt, sugar, and saturated fat have been associated with diet-related illness and death. On the other hand, plant-based meat can satisfy many of the nutritional requirements met by conventional meat and often has less saturated fat and more fiber. The optimal choice of processing methods can aid in enhancing the nutrient content and digestibility of plant-based meat products [15]. The following subsections present a comparative overview of the different macro- and micronutrients and energy provided by plant-based meat and conventional meat.
Protein content (as a percentage) is prioritized among the macronutrients in plant-based meat products. This is relevant, as scientists working in alternative proteins and amino acids are investigating strategies to comply with the FAO’s requirements for meat [16]. A recent report by the Good Food Institute (GFI) Europe [17] showed that the protein content per 100 g of most plant-based meat products in the European market (comprising the UK, Sweden, Germany, and The Netherlands) was slightly lower than conventional meat. However, nutritious protein sources are not just determined by the protein content by weight but also in terms of the percentage of calories provided by protein content (at least 20% of the calories), rather than carbohydrates or fat in a food product. Hence, the report concluded that plant-based foods are more similar to conventional meat based on the energy provided by their protein content.
Differences are observed in the proportion of essential amino acids between different plant-based meat products as a function of their protein source and product format [18]. For instance, a nutritional label analysis study conducted on 269 plant-based meat products across different formats (steaks, patties, meatballs, cutlets, and cured meats) sold on the Italian market showed that only plant-based patties and meatballs had a lower protein content than their conventional meat counterparts [19]. The other plant-based meat products that had more protein than their animal-derived equivalents contained cereals and legumes and a combination of plant protein sources, which could have contributed to their enhanced protein content. Legumes (15.0 g/100 g) offer significantly higher protein content than a combination of legumes and vegetables (10.1 g/100 g) or cereals and vegetables (5.4 g/100 g) [20]. The dietary fat content and source of fat are also crucial in assessing nutrition, and plant-based products are often associated with foods with a lower fat content. A recent study found lower saturated fat levels and total fat content (on average 30%) in plant-based patties relative to patties of animal origin (40%) [21]. Generally, the carbohydrate content of plant-based meat products is comparable to that of conventional meat. However, it varies with the product type, depending on its ingredients. Some plant-based meat products may also contain added carbohydrates in the form of fillers, binders, or flavorings.
Reducing the risk of cardiovascular disease and death by high-fiber intake is well supported by the literature [22]. The development of a healthy gut and microbiota and a decrease in inflammation are associated with high fiber consumption [23]. Contrary to conventional meat, plant-based meat is regarded as a source of fiber. Added plant-based components provide the small amount of fiber in animal-derived meat products. Fiber is an essential component of a balanced diet. High fiber intake has been associated with a lower risk of major diseases like pancreatic cancer, coronary artery disease, cardiovascular disease, and all-cause mortality [22]. With respect to energy, plant-based meat products generally provide fewer calories per serving compared to traditional animal meat counterparts that have higher fat content, especially saturated fat. This is particularly true if the plant-based meat is made with leaner ingredients and lower fat content. Plant-based meat may contain fiber from plant sources, contributing to a feeling of fullness and satiety without adding many calories. Table 1 shows comparative data on the nutritional composition of plant-based meat and conventional meat.

Table 1.
Median values for nutrients per 100 g of plant-based meat versus conventional meat (adapted and reproduced with permission from [24]).
The micronutrient content of plant-based meat products compared to conventional animal meat can vary significantly based on their ingredients and fortification. Legumes, grains, vegetables, and seeds used in plant-based meats can contribute to vitamin C, vitamin A, folate, and minerals like magnesium and potassium. Animal meats, especially red meats like beef, are high in heme iron, which is more readily absorbed by the body than non-heme iron found in plant-based sources. However, some plant-based meat products are fortified with iron to enhance their nutritional value. Vitamin B12 is primarily found in animal products, including meat, fish, eggs, and dairy. Plant-based meat substitutes typically do not naturally contain vitamin B12 unless fortified with this nutrient. Both plant-based and animal-based sources can provide zinc, but the bioavailability of zinc from plant sources may be lower due to anti-nutritional factors like phytates that can inhibit its absorption.
3. Nutritional Challenges in Plant-Based Meat Products
Compared to conventional meat, the nutritional quality of plant-based meat is mainly limited by its lower protein quality due to the incomplete amino acid profile caused by the lack of one or more essential amino acids that the human body cannot synthesize on its own. Animal proteins derived from meat, dairy, and eggs generally contain all essential amino acids in sufficient amounts, making them complete proteins. The incomplete amino acid profile and presence of anti-nutritional factors can reduce the digestibility and bioavailability of amino acids available in plant-based meat. Anti-nutritional factors hinder the absorption of minerals and other nutrients. The subsequent sections elaborate on these challenges and the associated consequences.
3.1. Protein Quality
The nutritional adequacy of any food product is governed by its protein quality. Protein quality can be defined as the minimum intake of high-quality protein containing all the essential amino acids that supports the maintenance of an appropriate body composition and promotes growth at a normal rate for age, assuming normal physical activity [25]. According to the Food and Drug Administration (FDA), adults must consume 50 g of protein per day as part of a 2000-calorie diet. Nevertheless, an individual’s specific requirements for protein will depend on their age, sex, activity levels, and other factors. The recommended dietary allowance to prevent protein deficiency in an average sedentary adult is 0.8 g/kg of body weight [26,27]. Generally, protein quality is ascertained by measuring its digestibility and quantity of essential amino acids. FAO has recommended the DIAAS (digestible indispensable amino acid score) as a metric to measure protein quality, which is determined using the following equation [28]:
Though plant-based meat products are made from protein-rich sources such as soy, wheat gluten, and pea, they often lack a balanced composition of essential amino acids. Factually, plant-based meat products have a higher content of glutamic acid and cysteine and a lower amount of alanine, glycine, and methionine [29]. This could be because of the deficiency of these amino acids in their respective sources of plant protein. Cereals lack lysine, and legumes are lacking in sulfur-containing essential amino acids such as methionine and cysteine. Hence, plant proteins are nutritionally incomplete due to their deficiency in one or more essential amino acids. Table 2 compares the essential amino acid content between proteins derived from different plant and animal sources. The deficiency of plant proteins in essential amino acids can lead to lower muscle protein synthesis associated with the consumption of plant-based foods [30]. This is a major concern with respect to the elderly population, who are vulnerable to muscle depletion [31]. The recommendation is to consume a broad variety of plant proteins to warrant the intake of an adequate amount of all the amino acids [32].

Table 2.
Comparison of the essential amino acid content between selected sources of plant proteins and animal proteins (values expressed in g/100 g of protein).
3.2. Anti-Nutritional Factors
The presence of a high amount of anti-nutritional factors, including phytic acid, lectins, oxalates, tannins, saponins, and enzyme inhibitors (Table 3), in plant-based meat is another major nutritional challenge [36]. “Anti-nutrients” are chemicals naturally produced by plants as a defense mechanism or synthetic compounds that interfere with nutrient absorption and reduce nutrient intake, digestion, and utilization in the human body [37]. The primary health consequence of anti-nutritional factors associated with plant-based meat is reduced nutrient bioavailability [38]. In addition, anti-nutrients can also impart an unpleasant flavor, such as bitterness, when used in plant-based meat product analogues [16]. Apart from anti-nutritional factors, plant sources may also contain indigestible constituents such as non-starch polysaccharides or fibers that limit enzyme access to proteins and reduce protein digestibility [39].

Table 3.
Plant-based sources and effects of anti-nutritional factors.
Among all anti-nutritional factors, phytic acid is of prime concern for human nutrition. It is a compound present in plants, soils, legumes, cereals, seeds, and nuts (Table 4) [40]. It can be chemically described as the hexaphosphoric ester of the hexahydric cyclic alcohol meso-inositol and is the principal storage form of phosphorus in plant tissues. Its unique structure, with 12 replaceable hydrogen ions and a highly negative charge [41], helps it to strongly bind to minerals (iron, zinc, magnesium, and calcium) and amino acids [42,43,44], forming an insoluble complex called phytate, which inhibits protein and micronutrient absorption by the body [40,45,46]. Several single-meal isotope studies have shown phytate influencing absorption and bioavailability from added and inherent iron [47,48]. Phytate works in a broad pH region and has low solubility even at very low intestinal pH.

Table 4.
Examples of high-phytate plant materials, as a percentage of dry weight.
Moreover, phytate can impact carbohydrate and lipid absorption as well. Phytate–carbohydrate complexes inhibit amylase activity and decrease carbohydrate degradation, hence reducing glucose’s solubility, digestibility, and absorption. The formation of lipid–phytate complexes leads to metallic soaps in the gut lumen, resulting in lower lipid availability and metabolism. Hence, phytates must be eliminated to improve the nutritional value of plant-based meat. There are various preparation and processing techniques to reduce phytates in food [45,46,49]. In the last decade, research and development (Table 5) have focused on developing methods and solutions such as extrusion, soaking, germination, malting, cooking, fermentation, and adding isolated phytase [43,45]. All methods help to degrade phytates in food at some level. However, enzymatic methods, such as phytase addition and fermentation, have demonstrated superior benefits over physical extraction methods [50,51]. The use of added or native phytase during food processing or food consumption can result in complete dephytinization and increase the bioavailability of added and native minerals [48,52,53]. Hence, phytase can potentially enhance the effects of fortification and absorption of inherent amino acids and minerals in plant-based meat.

Table 5.
Methods used to reduce phytic acid in food material.
This next section will highlight the potential solutions and their effects on nutrition of plant-based meat.
4. Current and Potential Solutions to Improve Plant-Based Meat Nutrition
According to our analyses, the most efficient solution should address improving the protein quality and reducing phytate content in plant-based meat sustainably.
4.1. Balanced Amino Acid Composition for Better Meat Protein Quality
With respect to addressing the deficiency of one or more essential amino acids in plant proteins, arriving at a strategic combination of two or more protein sources has often been the approach to achieve a balanced amino acid composition in plant-based meat. For instance, combining protein derived from chickpea (a legume deficient in cysteine, methionine, and tyrosine) and rice (a cereal deficient in lysine) can aid in increasing the PDCAAS (protein digestibility corrected amino acid score) of the resultant protein blend. Alternatively, plant-based meat manufacturers may supplement their products with the missing essential amino acids [38].
According to the FAO/WHO, the protein digestibility–corrected amino acid score (PDCAAS) is the preferred method to measure the protein value in human nutrition. The method is based on comparing the concentration of the first limiting essential amino acid in the test protein with that of the amino acid in a reference (scoring) pattern. This scoring pattern is obtained from the essential amino acid requirements of the preschool-age child. The resultant chemical score is corrected for true fecal digestibility of the test protein (Schaafsma, 2000).
4.2. Phytase Treatment to Reduce Phytate Content in Plant-Based Meat
Phytase is the phytate-degrading enzyme found naturally in plants, animals, and microorganisms [50]. It is chemically described as myoinositol(l,2,3,4,5,6)-hexakisphosphate phosphohydrolase. Phytase sequesters orthophosphate groups from the inositol ring of phytic acid to produce free inorganic P and break the phytate complex, thereby freeing minerals and amino acids (Figure 1) [45,46]. In food processing, phytase of microbial origin is the most stable in wide-ranging pH and thermal conditions, and much work has been done with microbial-derived phytase on an industrial scale [70]. In cereal- and legume-based foods, phytase has been used to increase the bioavailability of iron, zinc, and calcium and the quality and digestibility of amino acids [45,46,71,72,73].
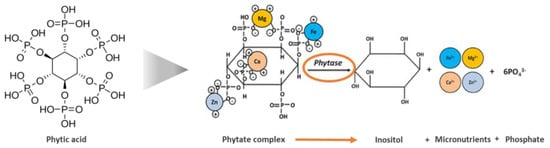
Figure 1.
Phytic acid, phytate complex, and phytase enzymatic reaction representation (adapted from [46]).
The effect of phytase on micronutrient absorption has been assessed in various studies. Table 6 summarizes eight studies that showed statistically significant improvements in iron absorption, ranging from 1.0- to 11.6-fold more absorption [74,75,76,77,78,79,80,81]. Across the iron absorption studies included in the review, three studies specifically examined the effect of phytase on iron bioavailability in humans using phytase to degrade phytate during food processing [75,76,77]. Five studies used phytase as an active enzyme ingredient [74,78,79,80,81]. Regarding the type of microorganism used, one processing study used Aspergillus niger. In four of the studies that added the active enzyme to meals, a phytase from Aspergillus niger was used, while the last used endogenous phytase. Concerning zinc absorption, six studies were analyzed, all of them showing statistically significant improvements in zinc absorption (1.4- to 2.0-fold greater absorption) [82,83,84,85,86,87]. Four used phytase derived from Aspergillus niger or cereals to support food processing, and two used phytase derived from Aspergillus niger as a food ingredient in fortified porridges.

Table 6.
Summary of clinical trials on phytase effects with statistically significant improvements in iron and zinc absorption.
Phytase may enhance the digestibility and bioavailability of a denatured protein [88]. Studies have demonstrated that adding phytase to food products such as bread and defatted soymilk can increase soluble protein by 41% and 28%, respectively [89,90]. Protein digestibility of a food product increases with rising protein solubility/soluble protein. A study using simulated in vitro digestion explored how enzymatic phytase treatment and lactic acid bacteria fermentation impacted phytic acid reduction, protein quality, and digestibility of fava bean flour. Phytase treatment significantly decreased phytic acid levels by up to 89%, leading to improved protein solubility at lower pH levels. This enhancement in solubility boosted the digestibility of fava bean proteins and the release of free amino nitrogen during the initial digestion phase [91].
When used in plant-derived protein products such as soy-protein isolate, soy formula, pea formula, and oat drink, phytase increased iron absorption by one- to [75,77,82]. A recent study [58] showing application of phytase to a pea-protein blend resulted in phytate reduction by 32% improving nutritional properties of the blend. Phytase addition had statistically insignificant impacts on extrusion process and physiochemical, textural, and sensorial properties of extrudates because it did not add to the release of volatile compounds or their precursors [58,92,93]. Hence, phytase addition enhances nutritional properties of plant protein while maintaining the intended taste.
5. Conclusions and Future Directions
The information presented in this perspective article provided an overview of the nutritional advantages and current challenges associated with plant-based meat products. Analysis of published literature in this context shows that researchers have been focusing on preemptive rather than reactive approaches to mitigating these challenges. The achievements of strategic combinations of plant proteins and using phytase as a solution to alleviate the concerns associated with incomplete amino acid profile and anti-nutrients, respectively, endorse the upstream approaches followed by researchers and manufacturers. A recommendation from the present work would be to assess the techno-economic potential and study the cost-effectiveness of these approaches to transform them into commercially viable options to address the nutritional challenges of plant-based proteins and plant-based meat products. The present article encourages the study of phytase-enriched plant-based meat products to understand phytase impact on phytic acid degradation, mineral absorption, amino acid digestibility and overall human health. This could be established through in vitro digestive system trials, stable isotope studies, and human intervention trials. As plant-based meat products are gaining prominence in the global market, it is imperative to demonstrate the effectiveness of formulations and processing-based interventions in enhancing their nutritional and health benefits using scientific evidence. With increasing consumer awareness, these technical validations can aid in resolving the ambiguities within this product category and increase repeat purchases.
Author Contributions
Conceptualization, P.I.S. and P.K.; writing—original draft, P.I.S. and P.K.; writing—review and editing, P.I.S. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the philanthropic organization Children’s Investment Fund Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Da Silva, J.G. Feeding the World Sustainably. The Future We Want? XLIX. 2012. Available online: https://www.un.org/en/chronicle/article/feeding-world-sustainably (accessed on 5 March 2023).
- United Nations. Goal 2: Zero Hunger. Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 14 March 2024).
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- English, L.K.; Ard, J.D.; Bailey, R.L.; Bates, M.; Bazzano, L.A.; Boushey, C.J.; Brown, C.; Butera, G.; Callahan, E.H.; de Jesus, J.; et al. Evaluation of dietary patterns and all-cause mortality. JAMA Netw. Open 2021, 4, e2122277. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, N.; Mousavi, S.M.; Santos, H.O.; Aune, D.; Hasani-Ranjbar, S.; Larijani, B.; Esmaillzadeh, A. Legume consumption and risk of all-cause and cause-specific mortality: A systematic review and dose–response meta-analysis of prospective studies. Adv. Nutr. 2023, 14, 64–76. [Google Scholar] [CrossRef]
- The EAT-Lancet Commission Summary Report. Healthy Diets from Sustainable Food Systems: Food Planet Health. 2021. Available online: https://eatforum.org/content/uploads/2019/07/EAT-Lancet_Commission_Summary_Report.pdf (accessed on 14 March 2024).
- Good Food Institute, India. Smart Protein Economic Analysis. 2022. Available online: https://gfi-india.org/wp-content/uploads/2022/10/gfi_economic_analysis.pdf (accessed on 14 March 2024).
- Rajyalakshmi, G. Insights on Awareness, Trial, and Purchase Behavior—Plant-Based Meat and Dairy, India. 2023. Available online: https://gfi-india.org/insights-on-awareness-trial-and-purchase-behavior-plant-based-meat-and-dairy-india/ (accessed on 14 March 2024).
- Gibbs, J.; Leung, G.-K. The effect of plant-based and mycoprotein-based meat substitute consumption on cardiometabolic risk factors: A systematic review and meta-analysis of Controlled Intervention Trials. Dietetics 2023, 2, 104–122. [Google Scholar] [CrossRef]
- Crimarco, A.; Springfield, S.; Petlura, C.; Streaty, T.; Cunanan, K.; Lee, J.; Fielding-Singh, P.; Carter, M.M.; Topf, M.A.; Wastyk, H.C.; et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study with appetizing plantfood—Meat eating alternative trial (swap-meat). Am. J. Clin. Nutr. 2020, 112, 1188–1199. [Google Scholar] [CrossRef]
- Farsi, D.N.; Gallegos, J.L.; Koutsidis, G.; Nelson, A.; Finnigan, T.J.; Cheung, W.; Muñoz-Muñoz, J.L.; Commane, D.M. Substituting meat for mycoprotein reduces genotoxicity and increases the abundance of beneficial microbes in the gut: Mycomeat, a randomised crossover control trial. Eur. J. Nutr. 2023, 62, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Toribio-Mateas, M.A.; Bester, A.; Klimenko, N. Impact of plant-based meat alternatives on the gut microbiota of consumers: A real-world study. Foods 2021, 10, 2040. [Google Scholar] [CrossRef]
- Bottin, J.H.; Swann, J.R.; Cropp, E.; Chambers, E.S.; Ford, H.E.; Ghatei, M.A.; Frost, G.S. Mycoprotein reduces energy intake and postprandial insulin release without altering glucagon-like peptide-1 and peptide tyrosine-tyrosine concentrations in healthy overweight and obese adults: A randomised-controlled trial. Br. J. Nutr. 2016, 116, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Gavin-Smith, B. Plant-Based Meats. 2020. Available online: https://sightandlife.org/resource-hub/blog/alternative-proteins-the-nutritionists-perspective (accessed on 5 March 2024).
- Sánchez-Velázquez, O.A.; Ribéreau, S.; Mondor, M.; Cuevas-Rodríguez, E.O.; Arcand, Y.; Hernández-Álvarez, A.J. Impact of processing on the in vitro protein quality, bioactive compounds, and antioxidant potential of 10 selected pulses. Legume Sci. 2021, 3, e88. [Google Scholar] [CrossRef]
- Zahari, I.; Östbring, K.; Purhagen, J.K.; Rayner, M. Plant-based meat analogues from alternative protein: A systematic literature review. Foods 2022, 11, 2870. [Google Scholar] [CrossRef]
- GFI Europe. Plant-Based Meat and Health in Europe. 2023. Available online: https://gfieurope.org/wp-content/uploads/2023/11/Final_GFI-Europe_Plant-based-meat-and-Nutrition_Nov232023.pdf (accessed on 14 March 2024).
- Zhang, L.; Langlois, E.; Williams, K.; Tejera, N.; Omieljaniuk, M.; Finglas, P.; Traka, M.H. A comparative analysis of nutritional quality, amino acid profile, and nutritional supplementations in plant-based products and their animal-based counterparts in the UK. Food Chem. 2024, 448, 139059. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, S.; Angelino, D.; Tedeschi, T.; Pellegrini, N.; Martini, D. Nutritional quality of meat analogues: Results from the food labelling of Italian Products (FLIP) project. Front. Nutr. 2022, 9, 852831. [Google Scholar] [CrossRef] [PubMed]
- Geerts, M.E.; Dekkers, B.L.; van der Padt, A.; van der Goot, A.J. Aqueous fractionation processes of soy protein for fibrous structure formation. Innov. Food Sci. Emerg. Technol. 2018, 45, 313–319. [Google Scholar] [CrossRef]
- Costa-Catala, J.; Toro-Funes, N.; Comas-Basté, O.; Hernández-Macias, S.; Sánchez-Pérez, S.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Castell-Garralda, V.; Vidal-Carou, M.C. Comparative Assessment of the Nutritional Profile of Meat Products and Their Plant-Based Analogues. Nutrients 2023, 15, 2807. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An Umbrella Review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, D.; Huang, J.; Zuo, T. The gut microbiome: Linking dietary fiber to inflammatory diseases. Med. Microecol. 2022, 14, 100070. [Google Scholar] [CrossRef]
- Coffey, A.A.; Lillywhite, R.; Oyebode, O. Meat versus meat alternatives: Which is better for the environment and health? A nutritional and environmental analysis of animal-based products compared with their plant-based alternatives. J. Hum. Nutr. Diet. 2023, 36, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Kalhoff, H.; Kersting, M. Programming Long-Term Health: Nutrition and Diet in Infants Aged 6 Months to 1 Year. In Early Nutrition and Long-Term Health Mechanisms, Consequences, and Opportunities; Saavedra, J.M., Dattilo, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 499–535. [Google Scholar] [CrossRef]
- FDA. Interactive Nutrition Facts Label. 2021. Available online: https://www.accessdata.fda.gov/scripts/InteractiveNutritionFactsLabel/assets/InteractiveNFL_Protein_October2021.pdf (accessed on 18 April 2024).
- FDA. Daily Value and Percent Daily Value on the Nutrition and Supplement Facts Labels. 2023. Available online: https://www.fda.gov/food/nutrition-facts-label/daily-value-nutrition-and-supplement-facts-labels (accessed on 18 April 2024).
- FAO. Dietary Protein Quality Evaluation in Human Nutrition—Report of an FAO Expert Consultation. 2013. Available online: https://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf (accessed on 18 April 2024).
- De Marchi, M.; Costa, A.; Pozza, M.; Goi, A.; Manuelian, C.L. Detailed characterization of plant-based burgers. Sci. Rep. 2021, 11, 2049. [Google Scholar] [CrossRef]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.A.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Table; National Institute of Nutrition: Hyderabad, India, 2017; p. 578. [Google Scholar]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Xu, Y.; Sismour, E.; Britland, J.W.; Sellers, A.; Abraha-Eyob, Z.; Yousuf, A.; Rao, Q.; Zhao, W. Physicochemical, Structural, and Functional Properties of Hemp Protein vs Several Commercially Available Plant and Animal Proteins: A Comparative Study. ACS Food Sci. Technol. 2022, 2, 1672–1680. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Inst. Food Technol. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Trommelen, J.; Snijders, T.; van Loon, L.J. The anabolic response to plant-based protein ingestion. Sports Med. 2021, 51, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.G.; Taylor JR, N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse role of phytic acid in plants and approaches to develop low-phytate grains to enhance bioavailability of micronutrients. Adv. Genet. 2021, 107, 89–120. [Google Scholar] [CrossRef]
- Thavarajah, P.; Thavarajah, D.; Vandenberg, A. Low Phytic Acid Lentils (Lens culinaris L.): A Potential Solution for Increased Micronutrient Bioavailability. J. Agric. Food Chem. 2009, 57, 9044–9049. [Google Scholar] [CrossRef]
- Feizollahi, E.; Mirmahdi, R.S.; Zoghi, A.; Zijlstra, R.T.; Roopesh, M.S.; Vasanthan, T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021, 143, 110284. [Google Scholar] [CrossRef] [PubMed]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2014, 95, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Vashishth, A.; Ram, S.; Beniwal, V. Cereal phytases and their importance in improvement of micronutrients bioavailability. 3 Biotech 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Egli, I.; Aeberli, I.; Zimmermann, M.; Hurrell, R. Strategies to Enhance Iron Absorption from Tef-injera in Young Women. Eur. J. Nutr. Food Saf. 2015, 5, 1169–1170. [Google Scholar] [CrossRef]
- Greiner, R.; Konietzny, U. Phytase for food application. Food Technol. Biotechnol. 2006, 44, 125–140. [Google Scholar]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.E.O.; Elhag, S.A. Effect of milling, soaking, malting, heat-treatment and fermentation on phytate level of four Sudanese sorghum cultivars. Food Chem. 1998, 61, 77–80. [Google Scholar] [CrossRef]
- Moretti, D.; Biebinger, R.; Bruins, M.J.; Hoeft, B.; Kraemer, K. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Ann. N. Y. Acad. Sci. 2013, 1312, 54–65. [Google Scholar] [CrossRef]
- Sandberg, A.S.; Andersson, H. Effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. J. Nutr. 1988, 118, 469–473. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Damstrup, M.L.; Thomsen, A.D.; Rasmussen, S.K.; Hansen, Å. Phytase activity and degradation of phytic acid during rye bread making. Eur. Food Res. Technol. 2007, 225, 173–181. [Google Scholar] [CrossRef]
- Akhter, S.; Saeed, A.; Irfan, M.; Malik, K.A. In vitro dephytinization and bioavailability of essential minerals in several wheat varieties. J. Cereal Sci. 2012, 56, 741–746. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Frontela, C.; Ros, G.; Martinez, C.; Monedero, V.; Haros, M. Application of bifidobacterial phytases in infant cereals: Effect on phytate contents and mineral dialyzability. J. Agric. Food Chem. 2012, 60, 11787–11792. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Wheat and Rice in Disease Prevention and Health: Benefits, Risks and Mechanisms of Whole Grains in Health Promotion; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Kaleda, A.; Talvistu, K.; Tamm, M.; Viirma, M.; Rosend, J.; Tanilas, K.; Kriisa, M.; Part, N.; Tammik, M.-L. Impact of Fermentation and Phytase Treatment of Pea-Oat Protein Blend on Physicochemical, Sensory, and Nutritional Properties of Extruded Meat Analogs. Foods 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Rebellato, A.P.; Orlando, E.A.; Thedoropoulos, V.C.T.; Greiner, R.; Pallone, J.A.L. Effect of phytase treatment of sorghum flour, an alternative for gluten free foods and bioaccessibility of essential minerals. J. Food Sci. Technol. 2020, 57, 3474–3481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egli, I.; Davidsson, L.; Juillerat, M.A.; Barclay, D.; Hurrell, R.F. The Influence of Soaking and Germination on the Phytase Activity and Phytic Acid Content of Grains and Seeds Potentially Useful for Complementary Feedin. J. Food Sci. 2006, 67, 3484–3488. [Google Scholar] [CrossRef]
- Liang, J.; Han, B.Z.; Nout, M.J.; Hamer, R.J. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008, 110, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Duman, B. Dephytinization of wheat and rice brans by hydrothermal autoclaving process and the evaluation of consequences for dietary fiber content, antioxidant activity and phenolics. Innov. Food Sci. Emerg. Technol. 2017, 39, 209–215. [Google Scholar] [CrossRef]
- Coulibaly, A.; Kouakou, B.; Chen, J. Phytic Acid in Cereal Grains: Structure, Healthy or Harmful Ways to Reduce Phytic Acid in Cereal Grains and Their Effects on Nutritional Quality. Am. J. Plant Nutr. Fertil. Technol. 2011, 1, 1–22. [Google Scholar] [CrossRef]
- Makokha, A.; Oniang’o, R.; Njoroge, S.; Kamar, O. Effect of Traditional Fermentation and Malting on Phytic Acid and Mineral Availability from Sorghum (Sorghum Bicolor) and Finger Millet (Eleusine Coracana) Grain Varieties Grown in Kenya. Food Nutr. Bulletin 2002, 23, 241–255. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effect of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney bean. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Singh, B.; Dar, B.N. Effect of extrusion variables (temperature, moisture) on the antinutrient components of cereal brans. J. Food Sci. Technol. 2015, 52, 1670–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albarracín, M.; González, R.; Drago, S. Soaking and extrusion effects on physicochemical parameters, phytic acid, nutrient content and mineral bio-accessibility of whole rice grain. Int. J. Food Sci. Nutr. 2015, 66, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, F.; Levrat-Verny, M.A.; Chanliaud, E.; Rémésy, C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Sanz, Y.; Haros, M. Phytate reduction in bran-enriched bread by phytase-producing bifidobacteria. J. Agric. Food Chem. 2009, 57, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Pallauf, J.; Moehring, J.; Kraemer, K.; Minihane, A.M. Effect of dietary phytate and microbial phytase on mineral and trace element bioavailability—A literature review. Curr. Top. Nutraceutical Res. 2008, 6, 131–144. [Google Scholar]
- Nguyen, P.H.; Kachwaha, S.; Tran, L.M.; Sanghvi, T.; Ghosh, S.; Kulkarni, B.; Beesabathuni, K.; Menon, P.; Sethi, V. Maternal Diets in India: Gaps, Barriers, and Opportunities. Nutrients 2021, 13, 3534. [Google Scholar] [CrossRef]
- Troesch, B.; Jing, H.; Laillou, A.; Fowler, A. Absorption Studies Show that Phytase from Aspergillus niger Significantly Increases Iron and Zinc Bioavailability from Phytate-Rich Foods. Food Nutr. Bull. 2013, 34, S90–S101. [Google Scholar] [CrossRef]
- Layrisse, M.; Gracia-Casal, M.N.; Solano, L.; Baron, M.A.; Arguello, F.; LIovera, D.; Ramirez, J.; Leets, I.; Tropper, E. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L.; Dimitriou, T.; Walczyk, T.; Hurrell, R.F. Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: The effect of phytic acid and ascorbic acid. Br. J. Nutr. 2001, 85, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Onning, G.; Oste, R.; Gramatkovski, E.; Hulthén, L. Improved iron bioavailability in an oat-based beverage: The combined effect of citric acid addition, dephytinization and iron supplementation. Eur. J. Nutr. 2007, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Egli, I.; Zeder, C.; Hurrell, R.F.; de Pee, S.; Zimmermann, M.B. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am. J. Clin. Nutr. 2009, 89, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Cercamondi, C.I.; Egli, I.M.; Mitchikpe, E.; Tossou, F.; Hessou, J.; Zeder, C.; Hounhouigan, J.D.; Hurrell, R.F. Iron bioavailability from a lipid-based complementary food fortificant mixed with millet porridge can be optimized by adding phytase and ascorbic acid but not by using a mixture of ferrous sulfate and sodium iron EDTA. J. Nutr. 2013, 143, 1233–1239. [Google Scholar] [CrossRef]
- Kore’issi-Dembe’le’, Y.; Fanou-Fogny, N.; Moretti, D.; Schuth, S.; Dossa, R.A.M.; Egli, I.; Zimmermann, M.B.; Brouwer, I.D. Dephytinisation with Intrinsic Wheat Phytase and Iron Fortification Significantly Increase Iron Absorption from Fonio (Digitaria exilis) Meals in West African Women. PLoS ONE 2013, 8, e70613. [Google Scholar] [CrossRef] [PubMed]
- Monnard, A.; Moretti, D.; Zeder, C.; Steingötter, A.; Zimmermann, M.B. The effect of lipids, a lipid-rich ready-to-use therapeutic food, or a phytase on iron absorption from maize-based meals fortified with micronutrient powders. Am. J. Clin. Nutr. 2017, 105, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L.; Ziegler, E.E.; Kastenmayer, P.; van Dael, P.; Barclay, D. Dephytinisation of soyabean protein isolate with low native phytic acid content has limited impact on mineral and trace element absorption in healthy infants. Br. J. Nutr. 2004, 91, 287–294. [Google Scholar] [CrossRef]
- Egli, I.; Davidsson, L.; Zeder, C.; Walczyk, T.; Hurrell, R.F. Dephytinization of a complementary food based on wheat and soy increases zinc, but not copper, apparent absorption in adults. J. Nutr. 2004, 134, 1077–1080. [Google Scholar] [CrossRef]
- Kim, J.; Paik, H.Y.; Joung, H.; Woodhouse, L.R.; Li, S.; King, J.C. Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J. Am. Coll. Nutr. 2007, 26, 1–9. [Google Scholar] [CrossRef]
- Marica, B.; Wegmüller, R.; Zeder, C.; Gabriela, S.; Hurrell, R.F. Influence of Phytase, EDTA, and Polyphenols on Zinc Absorption in Adults from Porridges Fortified with Zinc Sulfate or Zinc Oxide. J. Nutr. 2010, 144, 1467–1473. [Google Scholar] [CrossRef][Green Version]
- Brnić, M.; Hurrell, R.F.; Songré-Ouattara, L.T.; Diawara, B.; Kalmogho-Zan, A.; Tapsoba, C.; Zeder, C.; Wegmüller, R. Effect of phytase on zinc absorption from a millet-based porridge fed to young Burkinabe children. Eur. J. Clin. Nutr. 2017, 71, 137–141. [Google Scholar] [CrossRef]
- Zyba, S.J.; Wegmüller, R.; Woodhouse, L.R.; Ceesay, K.; Prentice, A.M.; Brown, K.H.; Wessells, K.R. Effect of exogenous phytase added to small-quantity lipid-based nutrient supplements (SQ-LNS) on the fractional and total absorption of zinc from a millet-based porridge consumed with SQ-LNS in young Gambian children: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1465–1475. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Satyanarayana, T. Phytase production by Sporotrichum thermophile in a cost-effective cane molasses medium in submerged fermentation and its application in bread. J. Appl. Microbiol. 2008, 105, 1858–1865. [Google Scholar] [CrossRef]
- Saito, T.; Kohno, M.; Tsumura, K.; Kugimiya, W.; Kito, M. Novel Method Using Phytase for Separating Soybean β-Conglycinin and Glycinin. Biosci. Biotechnol. Biochem. 2001, 65, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Sibakov, N.; Re, M.; Karsma, A.; Laitila, A.; Nordlund, E. Phytic Acid Reduction by Bioprocessing as a Tool to Improve the In Vitro Digestibility of Faba Bean Protein. J. Agric. Food Chem. 2018, 66, 10394–10399. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hua, Y.; Kong, X.; Zhang, C. Effects of phytase-assisted processing method on physicochemical and functional properties of soy protein isolate. J. Agric. Food Chem. 2014, 62, 10989–10997. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghian, S.; McClements, D.J.; Khalesi, M.; Garcia-Vaquero, M.; Mirzapour-Kouhdasht, A. Digestibility and bioavailability of plant-based proteins intended for use in meat analogues: A Review. Trends Food Sci. Technol. 2022, 129, 646–656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).