Investigation of High-Sensitivity pH Sensor Based on Au-Gated AlGaN/GaN Heterostructure
Abstract
1. Introduction
2. Materials and Methods
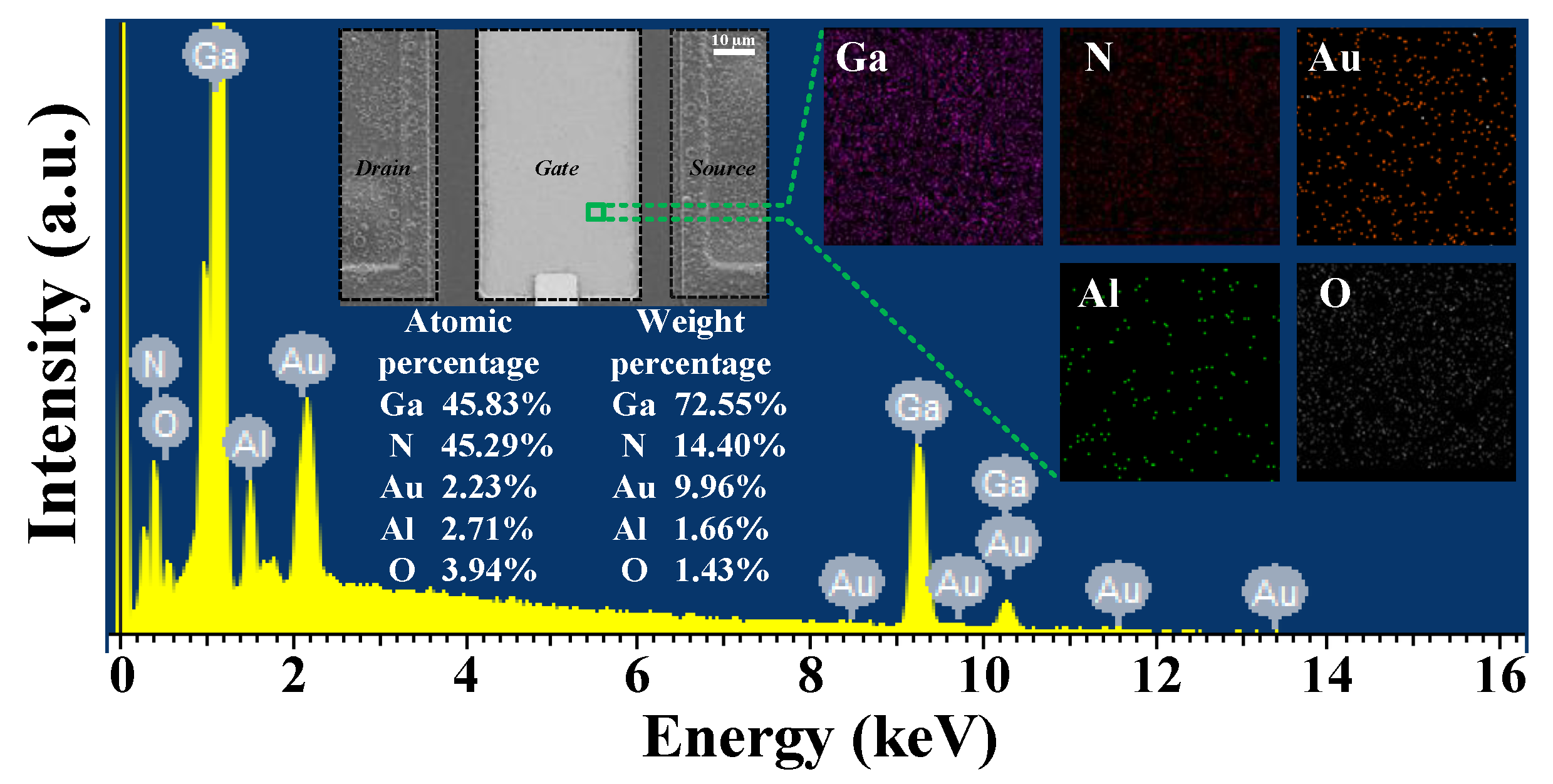
2.1. Epitaxy Growth and Device Fabrication
2.2. Measurement Setup
3. Results and Discussion
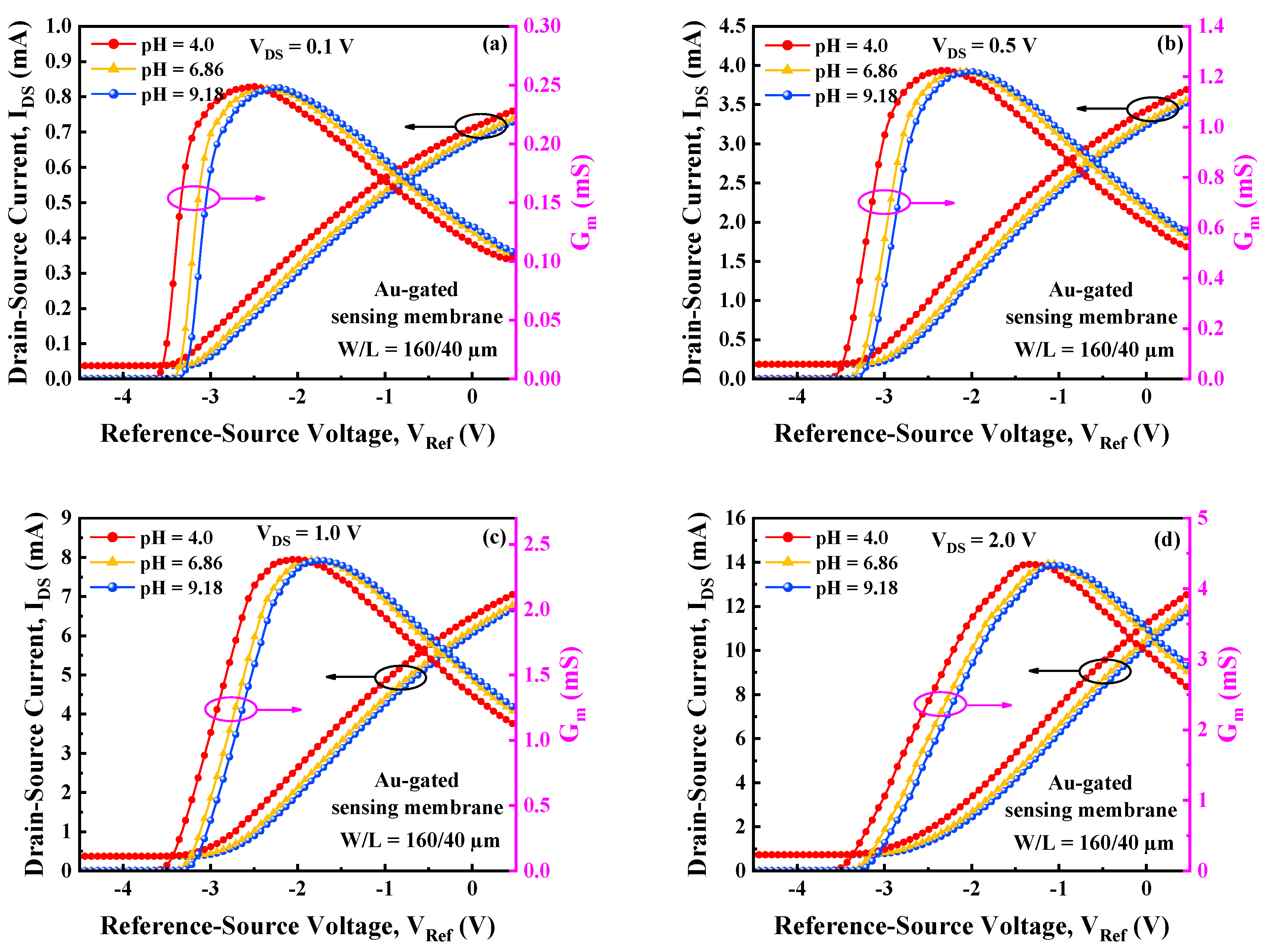
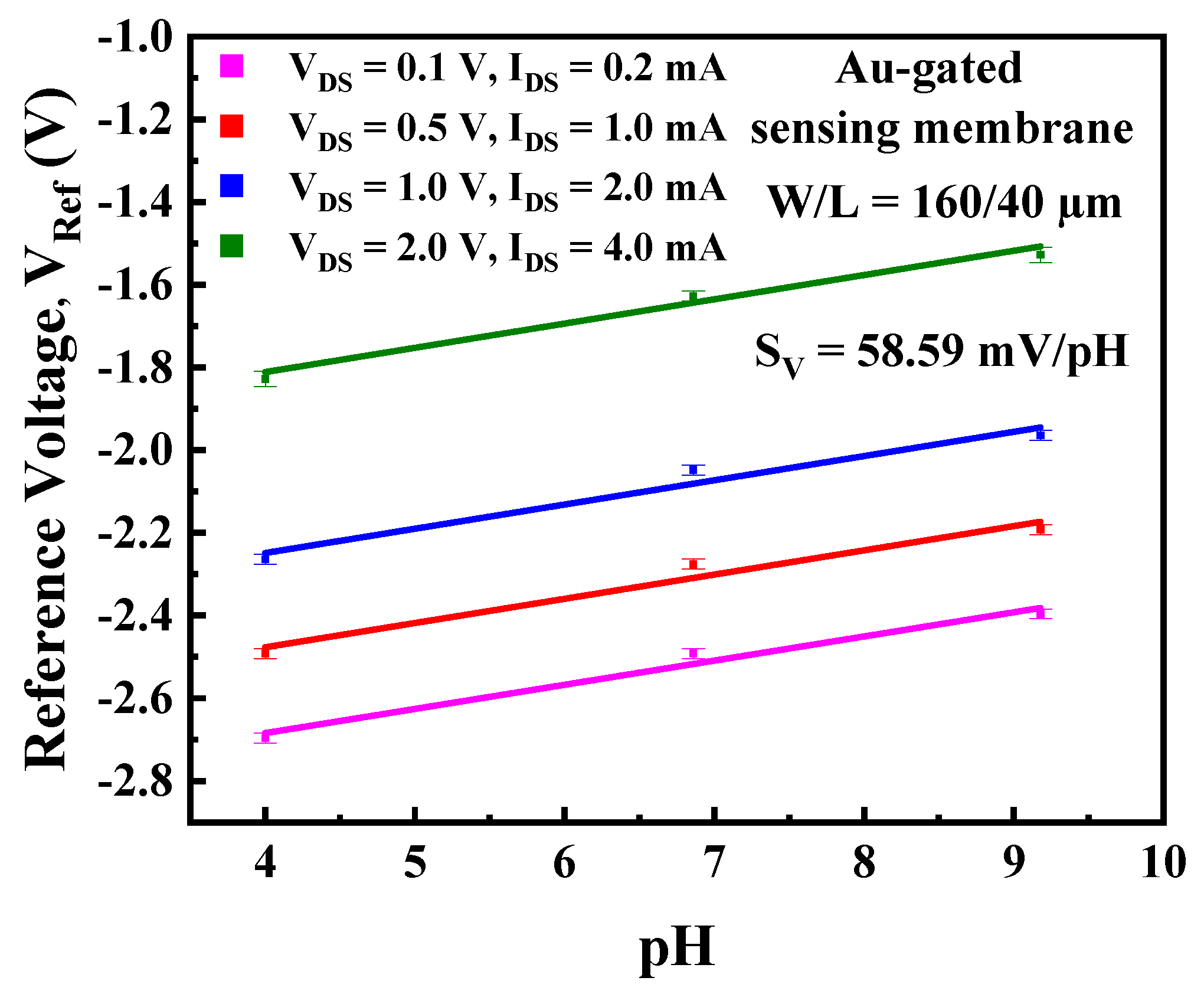
3.1. Transfer Characteristics and Potential Sensitivity
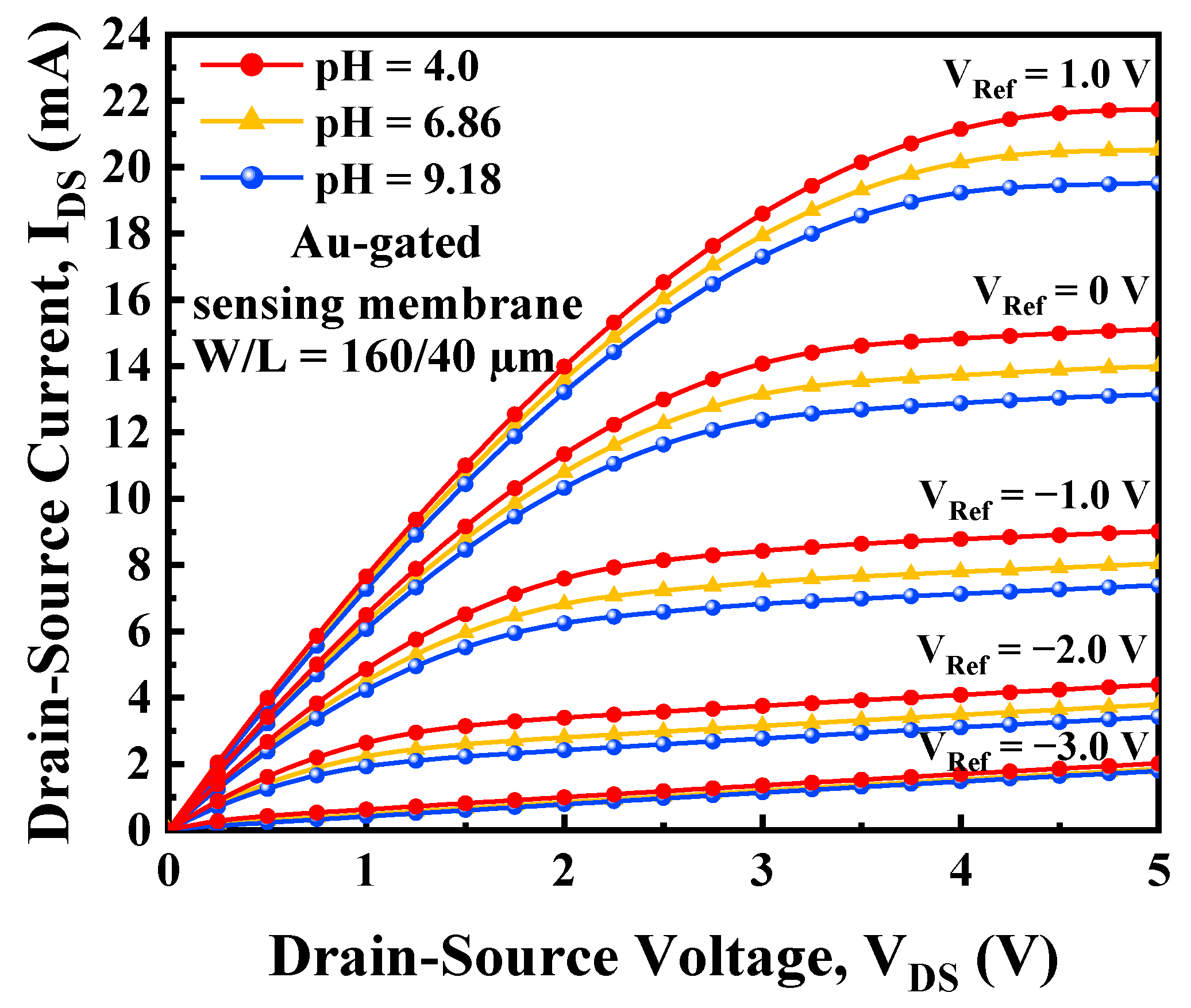
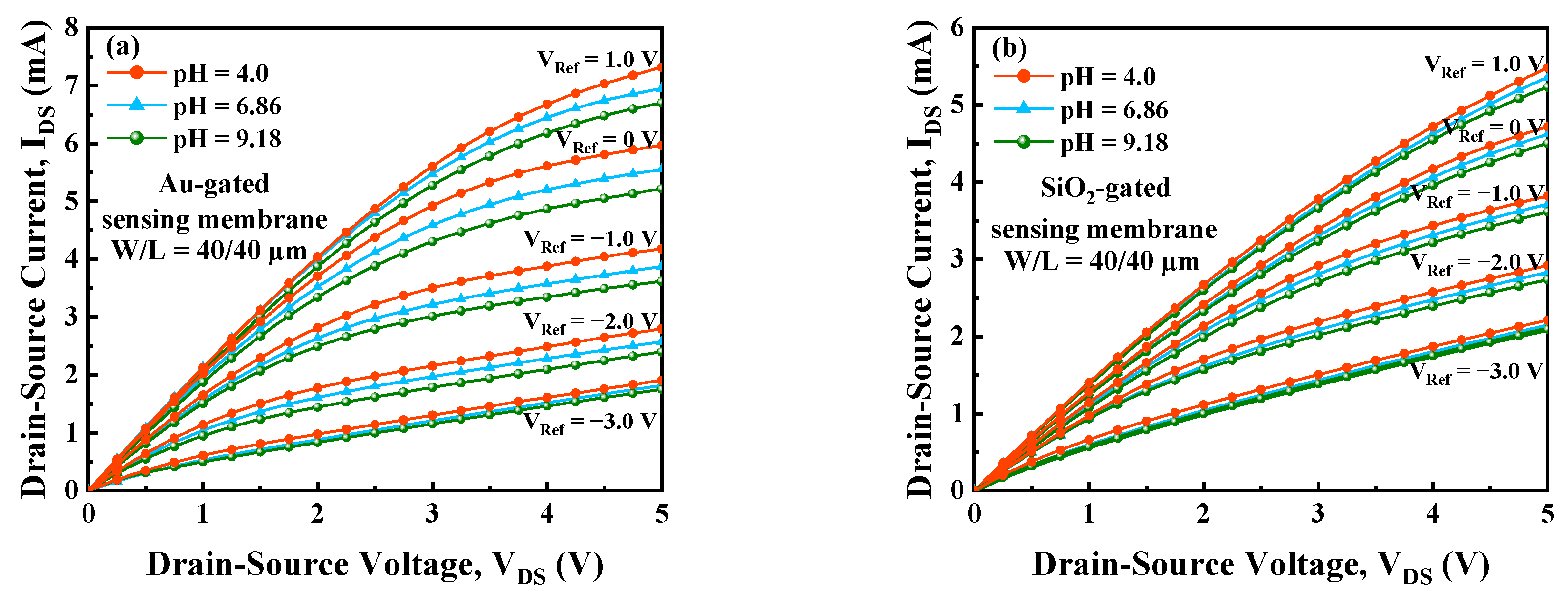
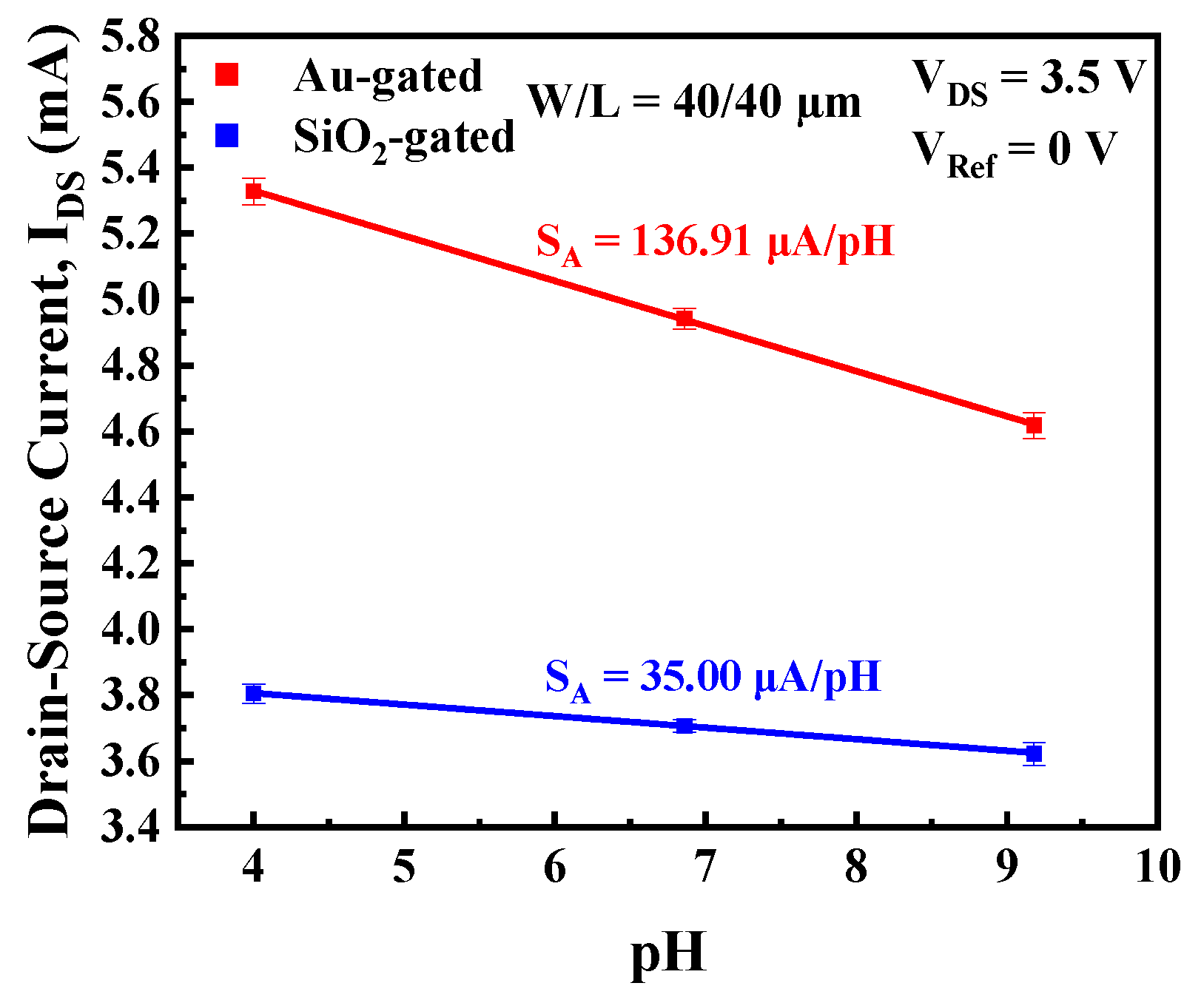
3.2. I-V Characteristics and Current Sensitivity
3.3. Au-Gated vs. SiO2-Gated Sensing Membrane
3.4. Real-Time Measurement
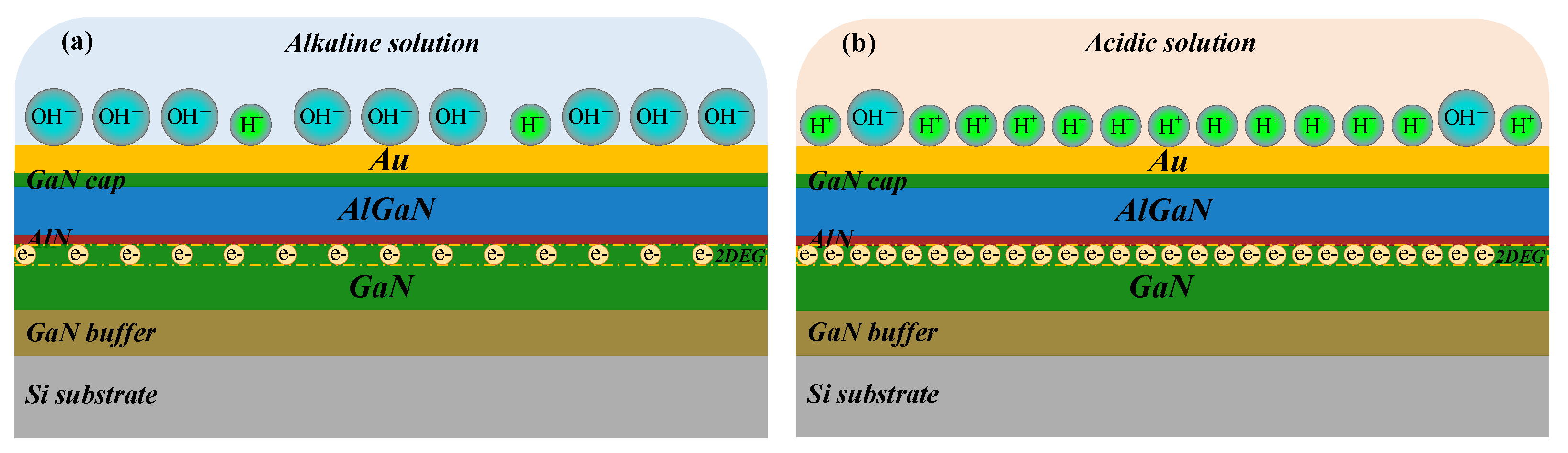
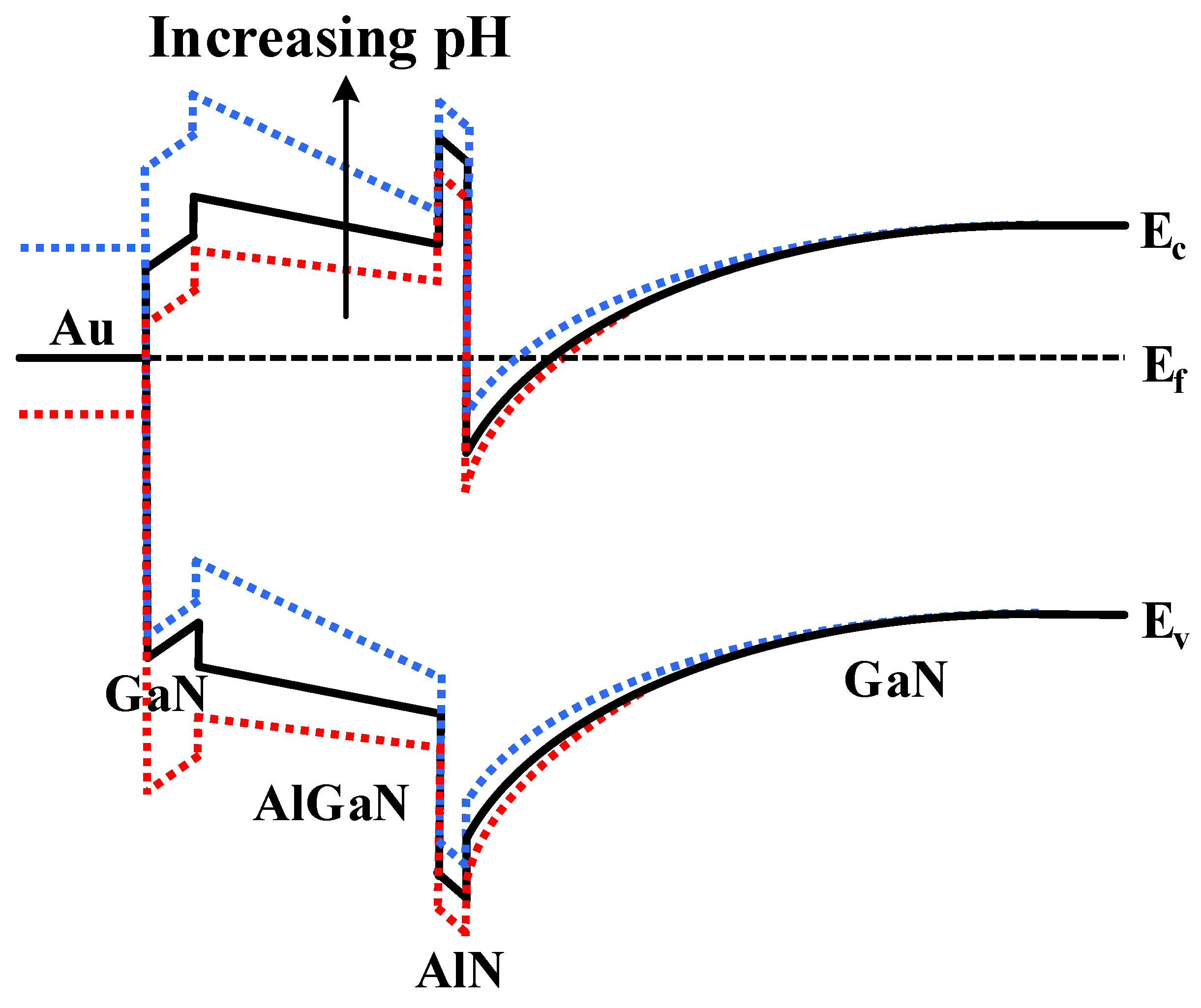
3.5. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakata, S.; Shiomi, M.; Fujita, Y.; Arie, T.; Akita, S.; Takei, K. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 2018, 1, 596–603. [Google Scholar] [CrossRef]
- Roy, S.; Zhu, D.; Parak, W.J.; Feliu, N. Lysosomal Proton Buffering of Poly(ethylenimine) Measured In Situ by Fluorescent pH-Sensor Microcapsules. ACS Nano 2020, 14, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive Seawater pH Measurement by Capacitive Readout of Potentiometric Sensors. ACS Sens. 2020, 5, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, J.M.; Hinz, W.; Rearick, T.M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J.H.; Johnson, K.; Milgrew, M.J.; Edwards, M.; et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348–352. [Google Scholar] [CrossRef]
- Jung, S.-H.; Seo, Y.-M.; Gu, T.; Jang, W.; Kang, S.-G.; Hyeon, Y.; Hyun, S.-H.; Lee, J.-H.; Whang, D. Super-Nernstian pH Sensor Based on Anomalous Charge Transfer Doping of Defect-Engineered Graphene. Nano Lett. 2021, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bu, Y.; Li, L.; Ao, J.-P. Effect of thermal oxidation treatment on pH sensitivity of AlGaN/GaN heterostructure ion-sensitive field-effect transistors. Appl. Surf. Sci. 2017, 411, 144–148. [Google Scholar] [CrossRef]
- Huang, W.; Diallo, A.K.; Dailey, J.L.; Besar, K.; Katz, H.E. Electrochemical processes and mechanistic aspects of field-effect sensors for biomolecules. J. Mater. Chem. C 2015, 3, 6445–6470. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X. Group III nitride nanomaterials for biosensing. Nanoscale 2017, 9, 7320–7341. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Zhang, T.; Liu, X.; Ao, J.-P. Enhanced pH sensitivity of AlGaN/GaN ion-sensitive field effect transistor with Al2O3 synthesized by atomic layer deposition. Appl. Surf. Sci. 2018, 427, 1199–1202. [Google Scholar] [CrossRef]
- Kang, B.S.; Wang, H.T.; Ren, F.; Gila, B.P.; Abernathy, C.R.; Pearton, S.J.; Johnson, J.W.; Rajagopal, P.; Roberts, J.C.; Piner, E.L.; et al. pH sensor using AlGaN/GaN high electron mobility transistors with Sc2O3 in the gate region. Appl. Phys. Lett. 2007, 91, 012110. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Zhou, J.Y.; Wang, T.T.; Ren, M.K.; Chen, G.Q.; Pu, T.F.; Li, X.B.; Jia, M.; Bu, Y.Y.; et al. Enhanced pH Sensitivity of AlGaN/GaN Ion-Sensitive Field-Effect Transistor by Recess Process and Ammonium Hydroxide Treatment. IEEE Trans. Electron Devices 2021, 68, 1250–1254. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, J.; Miao, B.; Liu, X.; Zhao, L.; Peng, H.; Wu, D.; Li, J. Ethanolamine Modified ZnO Nanorods-Based Disposable Gate-AlGaN/GaN High Electron Mobility Transistor for pH Sensing. IEEE Sens. J. 2021, 21, 2552–2558. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, Y.-S. Photoelectrochemical passivated ZnO-based nanorod structured glucose biosensors using gate-recessed AlGaN/GaN ion-sensitive field-effect-transistors. Sens. Actuators B Chem. 2015, 210, 756–761. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hsu, W.C.; Lee, C.S.; Chou, B.Y.; Chen, W.F. Enhanced Performances of AlGaN/GaN Ion-Sensitive Field-Effect Transistors Using H2O2-Grown Al2O3 for Sensing Membrane and Surface Passivation Applications. IEEE Sens. J. 2015, 15, 3359–3366. [Google Scholar] [CrossRef]
- Wang, L.; Bu, Y.; Ao, J.-P. Effect of oxygen plasma treatment on the performance of AlGaN/GaN ion-sensitive field-effect transistors. Diam. Relat. Mater. 2017, 73, 1–6. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, H.; ul Ahmad, A.; Liang, H.; Liu, J.; Xia, X.; Guo, W.; Huang, H.; Xu, N. Enhancing the sensitivity of the reference electrode free AlGaN/GaN HEMT based pH sensors by controlling the threshold voltage. Sens. Actuators B Chem. 2020, 306, 127609. [Google Scholar] [CrossRef]
- Xing, L.; Wang, C.; Cao, Y.; Zhang, J.; Xia, H. Macroscopical monolayer films of ordered arrays of gold nanoparticles as SERS substrates for in situ quantitative detection in aqueous solutions. Nanoscale 2021, 13, 14925–14934. [Google Scholar] [CrossRef]
- Medhat, A.; Salah, D.; Boichuk, N.; Hassan, I.; Vitusevich, S.; Kasry, A. Graphene Nanoplatele–Au Nanoparticle Hybrid as a Capacitive-Metal–Oxide–Semiconductor pH Sensor. ACS Appl. Electron. Mater. 2021, 3, 430–436. [Google Scholar] [CrossRef]
- Young, S.J.; Chu, Y.J.; Chen, Y.L. Enhancing pH Sensors Performance of ZnO Nanorods with Au Nanoparticles Adsorption. IEEE Sens. J. 2021, 21, 13068–13073. [Google Scholar] [CrossRef]
- Zhan, T.; Sun, J.; Feng, T.; Zhang, Y.; Zhou, B.; Zhang, B.; Wang, J.; Sarro, P.M.; Zhang, G.; Liu, Z.; et al. Electrical characteristics and photodetection mechanism of TiO2/AlGaN/GaN heterostructure-based ultraviolet detectors with a Schottky junction. J Mater. Chem. C 2023, 11, 1704–1713. [Google Scholar] [CrossRef]
- Stock, D.; Müntze, G.M.; Figge, S.; Eickhoff, M. Ion sensitive AlGaN/GaN field-effect transistors with monolithically integrated wheatstone bridge for temperature- and drift compensation in enzymatic biosensors. Sens. Actuators B Chem. 2018, 263, 20–26. [Google Scholar] [CrossRef]
- Zhang, H.; Tu, J.; Yang, S.; Sheng, K.; Wang, P. Optimization of gate geometry towards high-sensitivity AlGaN/GaN pH sensor. Talanta 2019, 205, 120134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.; Pu, T.; He, Y.; Wang, X.; Bu, Y.; Li, L.; Ao, J.-P. Surface sensibility and stability of AlGaN/GaN ion-sensitive field-effect transistors with high Al-content AlGaN barrier layer. Appl. Surf. Sci. 2021, 570, 151190. [Google Scholar] [CrossRef]
- Jansen, M. The chemistry of gold as an anion. Chem. Soc. Rev. 2008, 37, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Hsu, Y.-K.; Popescu, R.; Gerthsen, D.; Lin, Y.-G.; Feldmann, C. Au@Nb@HxK1-xNbO3 nanopeapods with near-infrared active plasmonic hot-electron injection for water splitting. Nat. Commun. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ao, C.; Shen, X.; Wang, L.; Wang, S.; Cao, L.; Zhang, W.; Dong, J.; Bao, J.; Ding, T.; et al. Dynamic Surface Reconstruction of Single-Atom Bimetallic Alloy under Operando Electrochemical Conditions. Nano Lett. 2020, 20, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.-S.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, D.; Xing, M.; Zhang, Y.; Song, C. Low proton adsorption energy barrier of S-scheme p-CNQDs/VO-ZnO for thermodynamics and kinetics favorable hydrogen evolution. Chem. Eng. J. 2022, 437, 135321. [Google Scholar] [CrossRef]
- Shafa, M.; Aravindh, S.A.; Hedhili, M.N.; Mahmoud, S.T.; Pan, Y.; Ng, T.K.; Ooi, B.S.; Najar, A. Improved H2 detection performance of GaN sensor with Pt/Sulfide treatment of porous active layer prepared by metal electroless etching. Int. J. Hydrogen Energy 2021, 46, 4614–4625. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Mei, D.; Liu, C.-J.; Ge, Q. Hydrogen Adsorption on Ga2O3 Surface: A Combined Experimental and Computational Study. J. Phys. Chem. C 2011, 115, 10140–10146. [Google Scholar] [CrossRef]
- Morteo-Flores, F.; Roldan, A. The Effect of Pristine and Hydroxylated Oxide Surfaces on the Guaiacol HDO Process: A DFT Study. ChemPhysChem 2022, 23, e202100583. [Google Scholar] [CrossRef]
- Pessoa, A.M.; Fajín, J.L.C.; Gomes, J.R.B.; Cordeiro, M.N.D.S. Ionic and radical adsorption on the Au(hkl) surfaces: A DFT study. Surf. Sci. 2012, 606, 69–77. [Google Scholar] [CrossRef]





| SV (mV/pH) | pH Range | Sensing Membrane | Ref. |
|---|---|---|---|
| 58.59 | 4–9.18 | Au | This work |
| 57.7 | 4–9 | Al2O3 | [6] |
| 57.8 | 4–9 | Al2O3 | [9] |
| 55.81 | 4–9 | AlGaN | [11] |
| 57.66 | 4–12 | ZnO | [13] |
| 55.2 | 2–12 | Al2O3 | [14] |
| 55.7 | 4–9 | AlGaN | [15] |
| 53.3 | 4–10 | GaN | [16] |
| 50 | 4–8 | GaN | [21] |
| 49 | 2–11 | AlGaN | [22] |
| 55.5 | 4–9 | AlGaN | [23] |
| Current Sensitivity, SA (μA/pH) | pH Range | Sensing Membrane | Gate Width, W (μm) | Gate Length, L (μm) | VDS (V) | VRef (V) | Ref. |
|---|---|---|---|---|---|---|---|
| 372.37 | 4–9.18 | Au | 160 | 40 | 3.5 | 0 | This work |
| 136.91 | 4–9.18 | Au | 40 | 40 | 3.5 | 0 | This work |
| 35.00 | 4–9.18 | SiO2 | 40 | 40 | 3.5 | 0 | This work |
| 37 | 3–10 | Sc2O3 | 150 | 2 | 0.25 | --- | [10] |
| 84.39 | 4–9 | AlGaN | 800 | 800 | 10 | 0 | [11] |
| 22.231 | 2–10 | ZnO | --- | --- | 0.7 | --- | [12] |
| 55.8 | 2–12 | Al2O3 | 200 | 50 | 2 | −2 | [14] |
| 14 | 4–10 | GaN | 400 | 40 | 0.5 | −1.15 | [16] |
| 6.62 | 4–8 | GaN | 500 | 1200 | 0.25 | --- | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Sun, J.; Zhan, T.; Sokolovskij, R.; Zhang, Y.; Wei, J.; Chen, S.; Liu, Z. Investigation of High-Sensitivity pH Sensor Based on Au-Gated AlGaN/GaN Heterostructure. Appl. Sci. 2024, 14, 6131. https://doi.org/10.3390/app14146131
Ye M, Sun J, Zhan T, Sokolovskij R, Zhang Y, Wei J, Chen S, Liu Z. Investigation of High-Sensitivity pH Sensor Based on Au-Gated AlGaN/GaN Heterostructure. Applied Sciences. 2024; 14(14):6131. https://doi.org/10.3390/app14146131
Chicago/Turabian StyleYe, Minjie, Jianwen Sun, Teng Zhan, Robert Sokolovskij, Yulong Zhang, Jiangtao Wei, Shaomin Chen, and Zewen Liu. 2024. "Investigation of High-Sensitivity pH Sensor Based on Au-Gated AlGaN/GaN Heterostructure" Applied Sciences 14, no. 14: 6131. https://doi.org/10.3390/app14146131
APA StyleYe, M., Sun, J., Zhan, T., Sokolovskij, R., Zhang, Y., Wei, J., Chen, S., & Liu, Z. (2024). Investigation of High-Sensitivity pH Sensor Based on Au-Gated AlGaN/GaN Heterostructure. Applied Sciences, 14(14), 6131. https://doi.org/10.3390/app14146131






