Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Details
2.3. Microbiology Analysis
2.4. Extraction of Fatty Acids
2.5. Characterization of Milk Fatty Acids Using GC-MS Analysis
2.6. Identification of the Obtained Microorganisms by MALDI-ToF MS
2.7. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandan, R.C. Dairy ingredients for food processing: An overview. In Dairy Ingredients for Food Processing; Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 3–33. [Google Scholar] [CrossRef]
- Kailasapathy, K. Chemical composition, physical, and functional properties of milk and milk ingredients. In Dairy Processing and Quality Assurance; Blackwell Publishing: Hoboken, NJ, USA, 2015; pp. 77–105. [Google Scholar] [CrossRef]
- NIIR Board. Modern Technology of Milk Processing & Dairy Products; NIIR Project Consultancy Services: Delhi, India, 2013. [Google Scholar]
- Achaw, O.W.; Danso-Boateng, E. Milk and dairy products manufacture. In Chemical and Process Industries: With Examples of Industries in Ghana; Springer International Publishing: Cham, Switzerland, 2021; pp. 293–374. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A. The role of lactic acid bacteria in milk fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef]
- Illingworth, D.; Patil, G.R.; Tamime, A.Y. Anhydrous Milk Fat Manufacture and Fractionation. In Dairy Fats and Related Products; Blackwell Publishing: Hoboken, NJ, USA, 2009; pp. 108–166. [Google Scholar] [CrossRef]
- Gauvin, M.-P.; Pouliot, Y.; Britten, M. Characterization of buttermilk serum fractions and their effect on rennet-induced coagulation of casein micelle dispersions. Int. Dairy J. 2017, 76, 10–17. [Google Scholar] [CrossRef]
- Barłowska, J.; Litwińczuk, Z.; Król, J.; Kędzierska-Matysek, M. Fatty acid profile and mineral content in milk from cows of various breeds over spring-summer feeding period. Pol. J. Food Nutr. Sci. 2006, 15, 13–16. [Google Scholar]
- Palmquist, D.L.; Beaulieu, A.D.; Barbano, D.M. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Bazmi, A.; Relkin, P. Effects of processing conditions on structural and functional parameters of whipped dairy emulsions containing various fatty acid compositions. J. Dairy Sci. 2009, 92, 3566–3574. [Google Scholar] [CrossRef]
- Kelly, A.L.; Fox, P.F. Biochemistry of milk processing. In Food Biochemistry and Food Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 465–490. [Google Scholar] [CrossRef]
- Santin Junior, I.A.; Silva, K.C.C.; Cucco, D.C. Milk Fatty Acids Profile and the Impact on Human Health. Dairy Vet. Sci. J. 2019, 10, 555779. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, M.H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prakash, D.; Gupta, C.; Prakash, D.; Sharma, G. Phytochemicals of nutraceutical importance: Do they defend against diseases. In Phytochemicals of Nutraceutical Importance; CABI: Oxon, UK, 2014; pp. 1–9. [Google Scholar] [CrossRef]
- Gabbi, A.M.; McManus, C.M.; Marques, L.T.; Abreu, A.S.; Machado, S.C.; Zanela, M.B.; Barbosa, R.S.; Fischer, V. Different levels of supplied energy for lactating cows affect physicochemical attributes of milk. J. Anim. Feed Sci. 2018, 27, 11–17. [Google Scholar] [CrossRef]
- Reklewska, B.; Bernatowicz, E.; Reklewski, Z.; Kuczyńska, B.; Zdziarski, K.; Sakowski, T.; Słoniewski, K. Functional Components of Milk Produced by Polish Black-And-White, Polish Red and Simmental Cows. Electron. J. Pol. Agric. Univ. 2005, 8, 25. [Google Scholar]
- Christie, W.W. Handbook of Chromatography; MH, K., Ed.; CRC Press: Boca Raton, FL, USA, 1984; Volume 1, pp. 33–46. [Google Scholar]
- Martínez, B.; Miranda, J.M.; Franco, C.M.; Cepeda, A.; Rodríguez, J.L. Development of a simple method for the quantitative determination of fatty acids in milk with special emphasis on long-chain fatty acids. J. Food 2012, 10, 27–35. [Google Scholar] [CrossRef]
- Walczak-Skierska, J.; Monedeiro, F.; Rudnicka, J.; Pomastowski, P. Optimizing Milk Quality and Shelf Life: Investigating Refrigeration Effects on Fatty Acid and Protein Profiles. ACS Food Sci. Technol. 2024, 4, 382–391. [Google Scholar] [CrossRef]
- Bruker Daltonics. MALDI Biotyper 3.1 User Manual; Bruker Daltonics: Billerica, MA, USA, 2012; Volume 1, pp. 1–212. [Google Scholar]
- Czeszewska-Rosiak, G.; Złoch, M.; Radosińska, M.; Florkiewicz, A.B.; Tretyn, A.; Pomastowski, P. The usefulness of the MALDI–TOF MS technique in the determination of dairy samples’ microbial composition: Comparison of the new EXS 2600 system with MALDI Biotyper platform. Arch. Microbiol. 2024, 206, 172. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Mercan, E. Characterisation of physicochemical, microbiological, thermal, oxidation properties and fatty acid composition of butter produced from thermosonicated cream. Int. Dairy J. 2020, 109, 104777. [Google Scholar] [CrossRef]
- Brożek, O.; Kiełczewska, K.; Bohdziewicz, K. Characterisation of selected emulsion phase parameters in milk, cream and buttermilk. Pol. J. Food Nutr. Sci. 2022, 72, 5–15. [Google Scholar] [CrossRef]
- Bumbadiya, M.R.; Maji, S.; Sao, K.; Ranvir, S.G. Butter Oil (Ghee): Composition, Processing, and Physicochemical Changes during Storage. In The Chemistry of Milk and Milk Products; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 159–184. [Google Scholar]
- Khan, I.T.; Nadeem, M.; Imran, M.; Khalique, A. Impact of post fermentation cooling patterns on fatty acid profile, lipid oxidation and antioxidant features of cow and buffalo milk set yoghurt. Lipids Health Dis. 2020, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.; Ruegg, P.L.; Jordan, K.; O’Brien, B.; Gleeson, D. The effect of storage temperature and duration on the microbial quality of bulk tank milk. J. Dairy Sci. 2016, 99, 3367–3374. [Google Scholar] [CrossRef]
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Pasvolsky, R.; Zakin, V.; Ostrova, I.; Shemesh, M. Butyric acid released during milk lipolysis triggers biofilm formation of Bacillus species. Int. J. Food Microbiol. 2014, 181, 19–27. [Google Scholar] [CrossRef]
- Sanchez-Juanes, F.; Alonso, J.M.; Zancada, L.; Hueso, P. Glycosphingolipids from bovine milk and milk fat globule membranes: A comparative study. Adhesion to enterotoxigenic Escherichia coli strains. Biol. Chem. 2009, 390, 31–40. [Google Scholar] [CrossRef]
- Ali-Vehmas, T.E.R.H.I.; Westphalen, P.; Myllys, V.; Sandholm, M. Binding of Staphylococcus aureus to milk fat globules increases resistance to penicillin-G. J. Dairy Res. 1997, 64, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.; Keenan, T.W. The milk fat globule membrane. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1975, 415, 273–309. [Google Scholar] [CrossRef]
- Teh, K.H.; Flint, S.; Brooks, J.; Knight, G. (Eds.) Biofilms in the Dairy Industry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.A.; McSweeney, P.L.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and metabolism of fatty acids in cheese. In Cheese; Academic Press: Cambridge, MA, USA, 2017; pp. 423–444. [Google Scholar] [CrossRef]
- Deeth, H.C. Lipoprotein lipase and lipolysis in milk. Int. Dairy J. 2006, 16, 555–562. [Google Scholar] [CrossRef]
- Klungel, G.H.; Slaghuis, B.A.; Hogeveen, H. The effect of the introduction of automatic milking systems on milk quality. J. Dairy Sci. 2000, 83, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Coolbear, T.; Daniel, R.M. Characteristics of proteinases and lipases produced by seven Bacillus sp. isolated from milk powder production lines. Int. Dairy J. 2004, 14, 495–504. [Google Scholar] [CrossRef]
- Montanhini, M.T.M.; dos Santos Bersot, L. Evaluation of psychrotrophic behavior and lipolytic and proteolytic activity of Bacillus cereus isolated from refrigerated dairy products. Acta Sci. Technol. 2013, 35, 163–167. [Google Scholar] [CrossRef]
- Parkash, M.; Rajasekar, K.; Karmegam, N. Bacterial Population of Raw Milk and Their Proteolytic and Lipolytic Activities. Res. J. Appl. Sci. 2007, 3, 848–851. [Google Scholar]
- Baur, C.; Krewinkel, M.; Kranz, B.; von Neubeck, M.; Wenning, M.; Scherer, S.; Stoeckel, M.; Hinrichs, J.; Stressler, T.; Lutz Fischer, L. Quantification of the proteolytic and lipolytic activity of microorganisms isolated from raw milk. Int. Dairy. J. 2015, 49, 23–29. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.H.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34. [Google Scholar] [CrossRef]
- Chakraborty, K.; Raj, R.P. An extra-cellular alkaline metallolipase from Bacillus licheniformis MTCC 6824: Purification and biochemical characterization. Food Chem. 2008, 109, 727–736. [Google Scholar] [CrossRef]
- Chakraborty, K.; Raj, R.P. Selective enrichment of n−3 polyunsaturated fatty acids with C18–C20 acyl chain length from sardine oil using Pseudomonas fluorescens MTCC 2421 lipase. Food Chem. 2009, 114, 142–150. [Google Scholar] [CrossRef]
- Gururaj, P.; Ramalingam, S.; Devi, G.N.; Gautam, P. Process optimization for production and purification of a thermostable, organic solvent tolerant lipase from Acinetobacter sp. AU07. Braz. J. Microbiol. 2016, 47, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.R.M.; Daniel, R.M.; Coolbear, T. Detection and impact of protease and lipase activities in milk and milk powders. Int. Dairy J. 2003, 13, 255–275. [Google Scholar] [CrossRef]
- Vithanage, N.R.; Dissanayake, M.; Bolge, G.; Palombo, E.A.; Yeager, T.R.; Datta, N. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 2016, 57, 80–90. [Google Scholar] [CrossRef]
- Salgado, C.A.; Baglinière, F.; Vanetti, M.C.D. Spoilage potential of a heat-stable lipase produced by Serratia liquefaciens isolated from cold raw milk. Lwt 2020, 126, 109289. [Google Scholar] [CrossRef]
- Hantsis-Zacharov, E. and Halpern, M. Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lipolytic traits. Appl. Environ. Microbiol. 2007, 73, 7162–7168. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, A.; Borch, K.; Barfoed, M.; Nielsen, T.B.; Gormsen, E.; Patkar, S.A. Biochemical properties of cloned lipases from the Pseudomonas family. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1259, 9–17. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J. Purification and properties of heat-stable extracellular protease from Pseudomonads fluorescens BJ-10. J. Food Sci. Technol. 2014, 51, 1185–1190. [Google Scholar] [CrossRef]
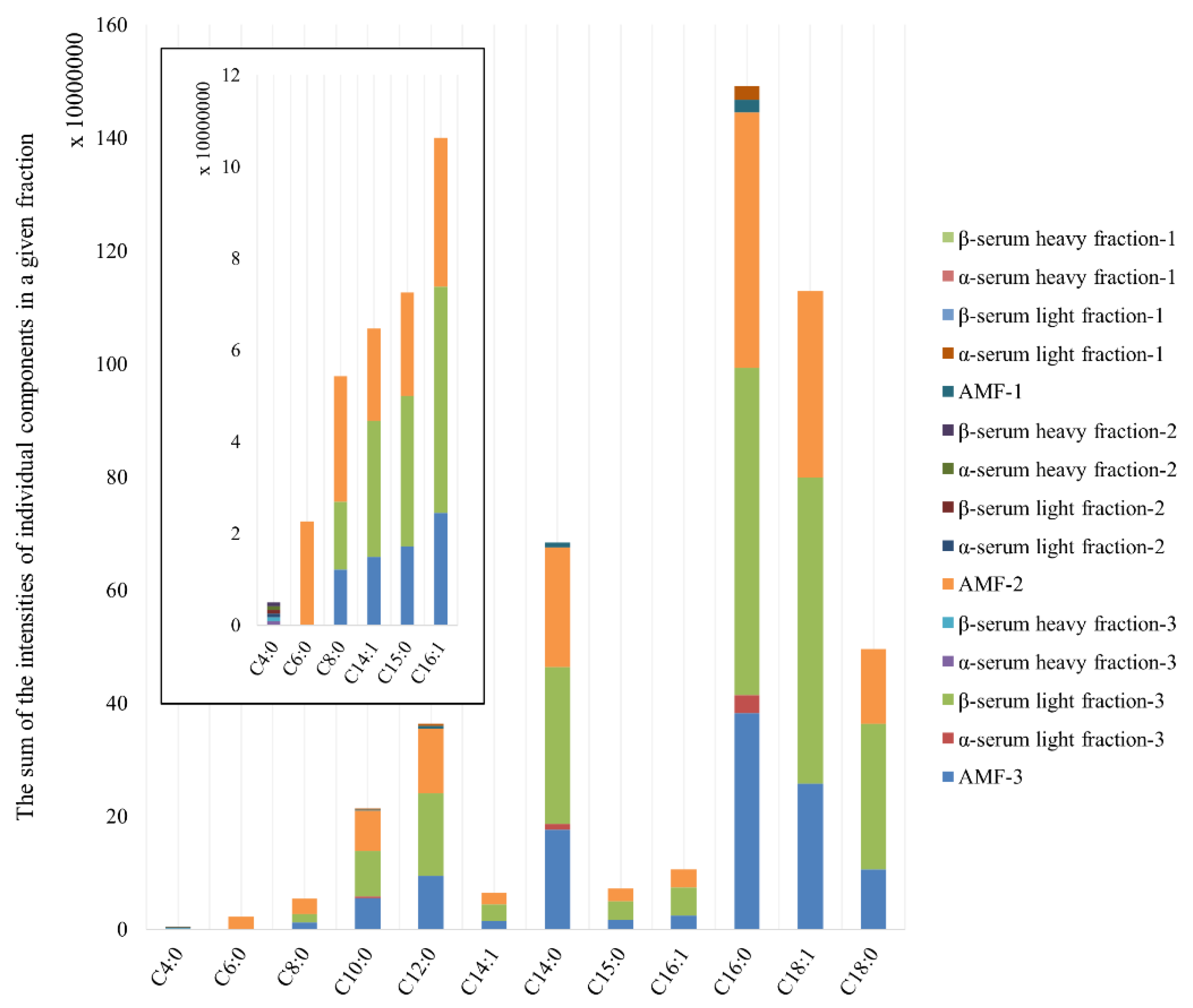
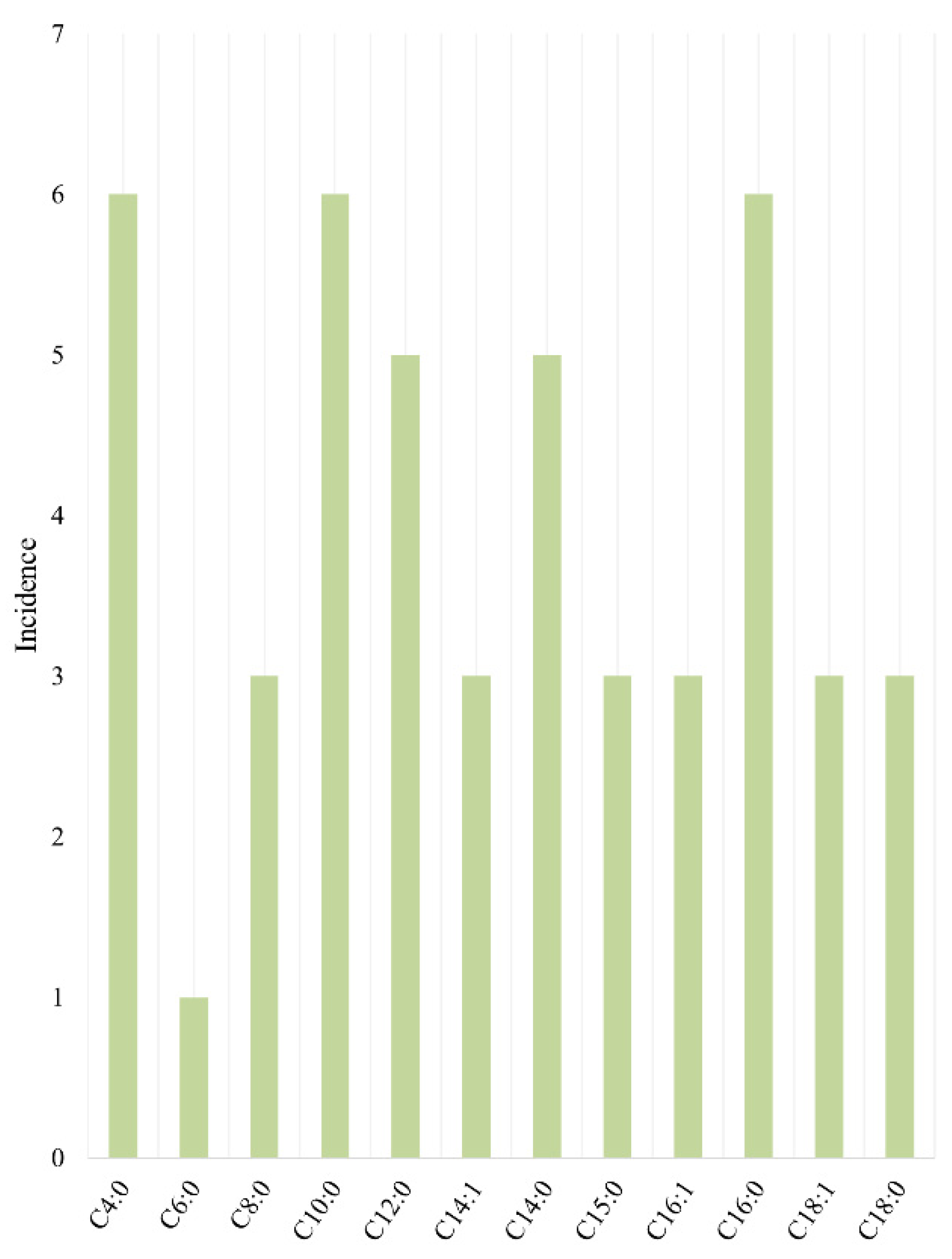
| Retention Time (min) | Volatile Organic Compound | AMF-3 | α-Serum Light Fraction-3 | β-Serum Light Fraction-3 | AMF-2 | β-Serum Heavy Fraction-2 | AMF-1 | α-Serum Light Fraction-1 |
|---|---|---|---|---|---|---|---|---|
| 6.211 | Butanoic acid (C4:0) | 0 | 0 | 0 | 0 | X | 0 | 0 |
| 9.328 | Hexanoic acid (C6:0) | 0 | 0 | 0 | X | 0 | 0 | 0 |
| 12.475 | Octanoic acid (C8:0) | X | 0 | X | X | 0 | 0 | 0 |
| 16.363 | Decanoic acid (C10:0) | X | X | X | X | 0 | X | X |
| 20.692 | Dodecanoic acid (C12:0) | X | 0 | X | X | 0 | X | X |
| 24.91 | (Z)-9-Tetradecenoic acid (C14:1) | X | 0 | X | X | 0 | 0 | 0 |
| 25.01 | Tetradecanoic acid (C14:0) | X | X | X | X | 0 | X | 0 |
| 27.086 | Pentadecanoic acid (C15:0) | X | 0 | X | X | 0 | 0 | 0 |
| 28.85 | (Z)-9-Hexadecenoic acid (C16:1) | X | 0 | X | X | 0 | 0 | 0 |
| 29.109 | Hexadecanoic acid (C16:0) | X | X | X | X | 0 | X | X |
| 33.144 | (E)-9-octadecenoic acid (C18:1) | X | 0 | X | X | 0 | 0 | 0 |
| 33.58 | Octadecanoic acid (C18:0) | X | 0 | X | X | 0 | 0 | 0 |
| Nr. | Name Sample | Microorganisms | MSP Score * |
|---|---|---|---|
| 1 | Part 1 | ||
| α–serum light fraction | Pseudomonas fluorescens Bacillus cereus Serratia marcescens Escherichia coli Micrococcus luteus Staphylococcus aureus |
2.21 2.18 2.32 2.08 2.36 2.19 | |
| α–serum heavy fraction | Micrococcus luteus | 2.03 | |
| β-serum light fraction | Pseudomonas fluorescens Lactococcus raffinolactis Serratia marcescens Micrococcus luteus Escherichia coli |
2.14 2.23 2.45 2.07 2.06 | |
| β-serum heavy fraction | Micrococcus luteus Staphylococcus aureus |
2.29 2.32 | |
| AMF ** | no identified microorganisms | ||
| Part 2 | |||
| 2 | α–serum light fraction | Micrococcus luteus Serratia marcescens |
2.20 2.37 |
| α–serum heavy fraction | Micrococcus luteus Staphylococcus aureus Lactococcus raffinolactis Escherichia coli |
2.12 2.21 2.35 2.41 | |
| β-serum light fraction | Serratia marcescens Micrococcus luteus |
2.40 2.27 | |
| β-serum heavy fraction | Pseudomonas fluorescens Staphylococcus aureus |
2.26 2.19 | |
| AMF ** | no identified microorganisms | ||
| Part 3 | |||
| 3 | α–serum light fraction | Micrococcus luteus Escherichia coli |
2.22 2.36 |
| α–serum heavy fraction | Pseudomonas fluorescens Lactococcus raffinolactis |
2.20 2.16 | |
| β-serum light fraction | Pseudomonas fluorescens Lactococcus raffinolactis |
2.36 2.14 | |
| β-serum heavy fraction | Lactococcus raffinolactis Micrococcus luteus |
2.02 2.26 | |
| AMF ** | no identified microorganisms | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gużewska, G.; Monedeiro-Milanowski, M.; Florkiewicz, A.B.; Arendowska, I.; Walczak-Skierska, J.; Białczak, D.; Pomastowski, P.P. Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile. Appl. Sci. 2024, 14, 6117. https://doi.org/10.3390/app14146117
Gużewska G, Monedeiro-Milanowski M, Florkiewicz AB, Arendowska I, Walczak-Skierska J, Białczak D, Pomastowski PP. Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile. Applied Sciences. 2024; 14(14):6117. https://doi.org/10.3390/app14146117
Chicago/Turabian StyleGużewska, Gaja, Maciej Monedeiro-Milanowski, Aleksandra Bogumiła Florkiewicz, Izabela Arendowska, Justyna Walczak-Skierska, Dorota Białczak, and Paweł Piotr Pomastowski. 2024. "Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile" Applied Sciences 14, no. 14: 6117. https://doi.org/10.3390/app14146117
APA StyleGużewska, G., Monedeiro-Milanowski, M., Florkiewicz, A. B., Arendowska, I., Walczak-Skierska, J., Białczak, D., & Pomastowski, P. P. (2024). Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile. Applied Sciences, 14(14), 6117. https://doi.org/10.3390/app14146117











