Statistical Analysis Applied to the Production of Mirto Liqueur
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection
2.3. Design of Experiments
2.4. Extraction Procedure
2.5. Volatile Organic Compounds Determination
SPME Conditions
2.6. Anthocyanin Measurements
2.7. Determination of Total Phenols
2.8. Statistical Analysis
2.9. Multivariate Analysis
3. Results
3.1. Multivariate Analysis
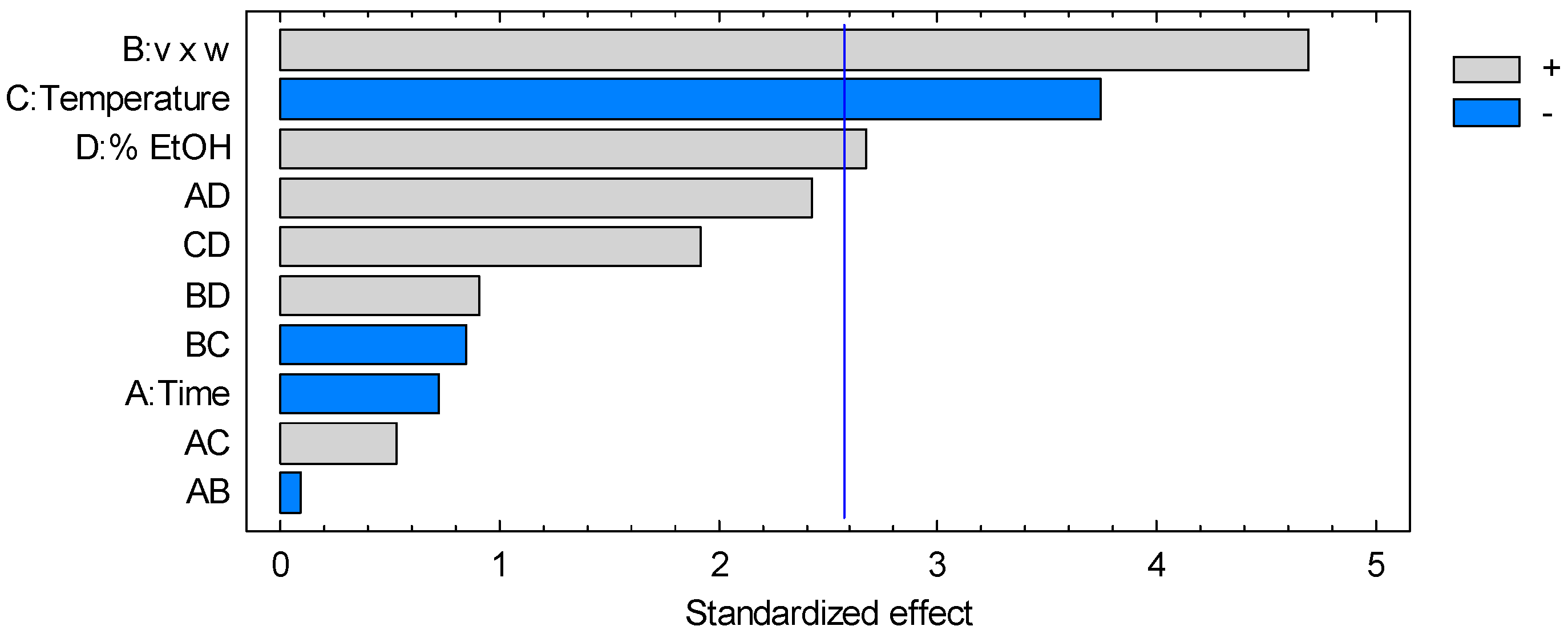
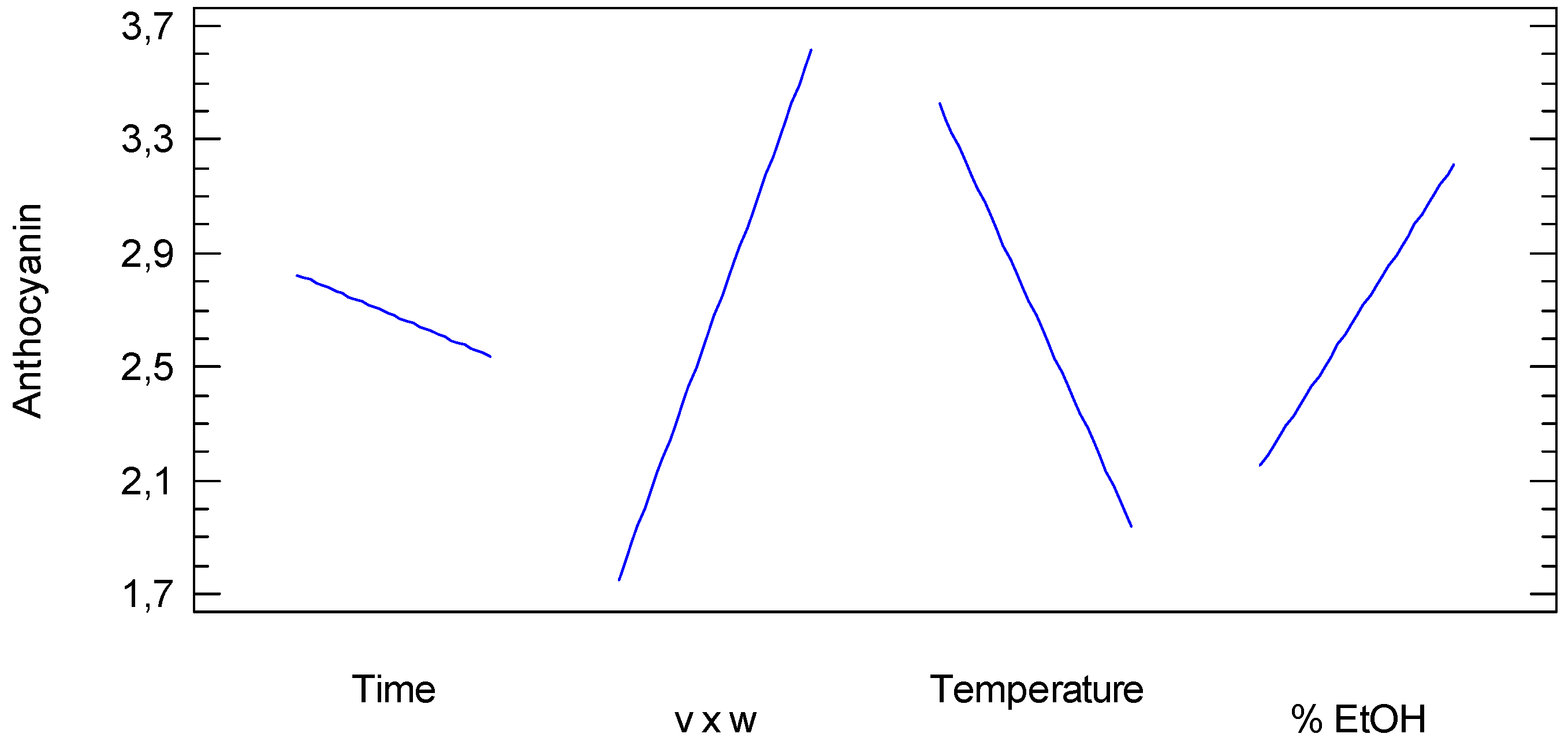
3.1.1. Anthocyanin
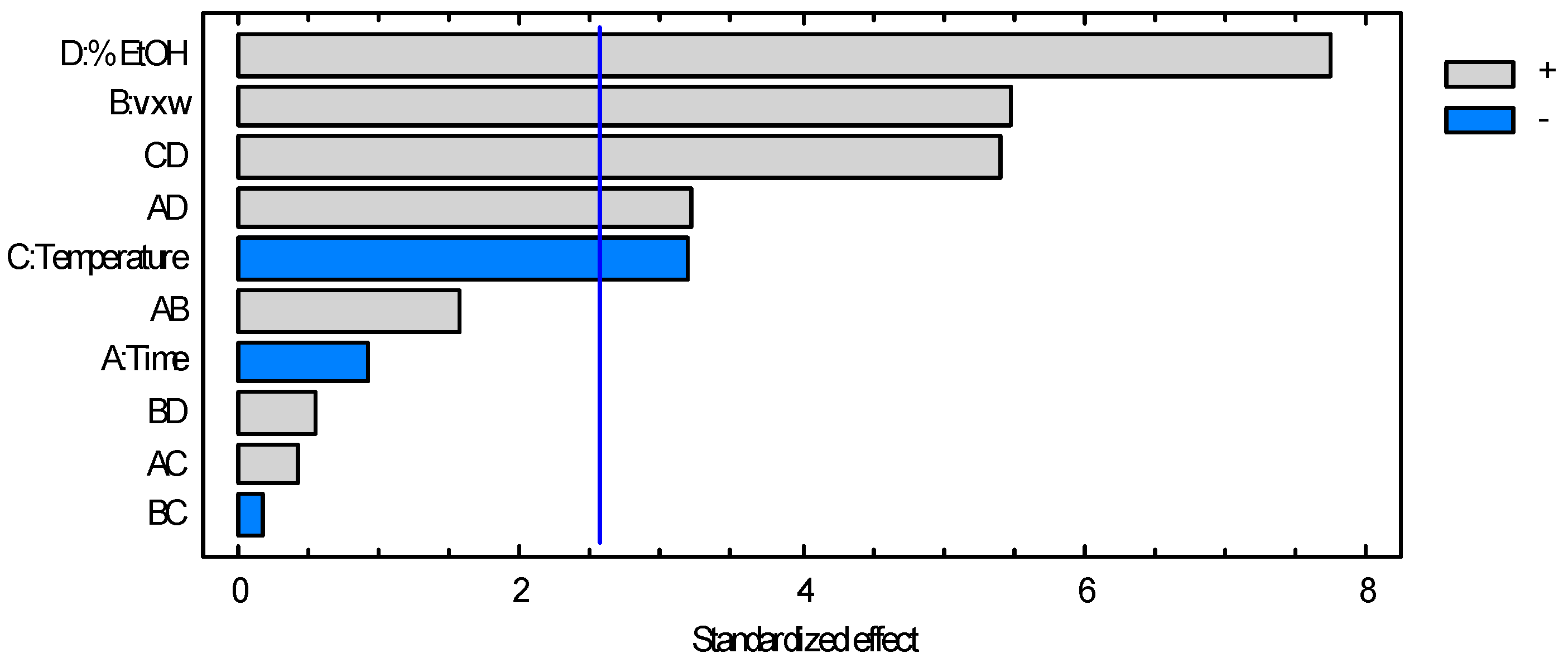
3.1.2. Dry Matter
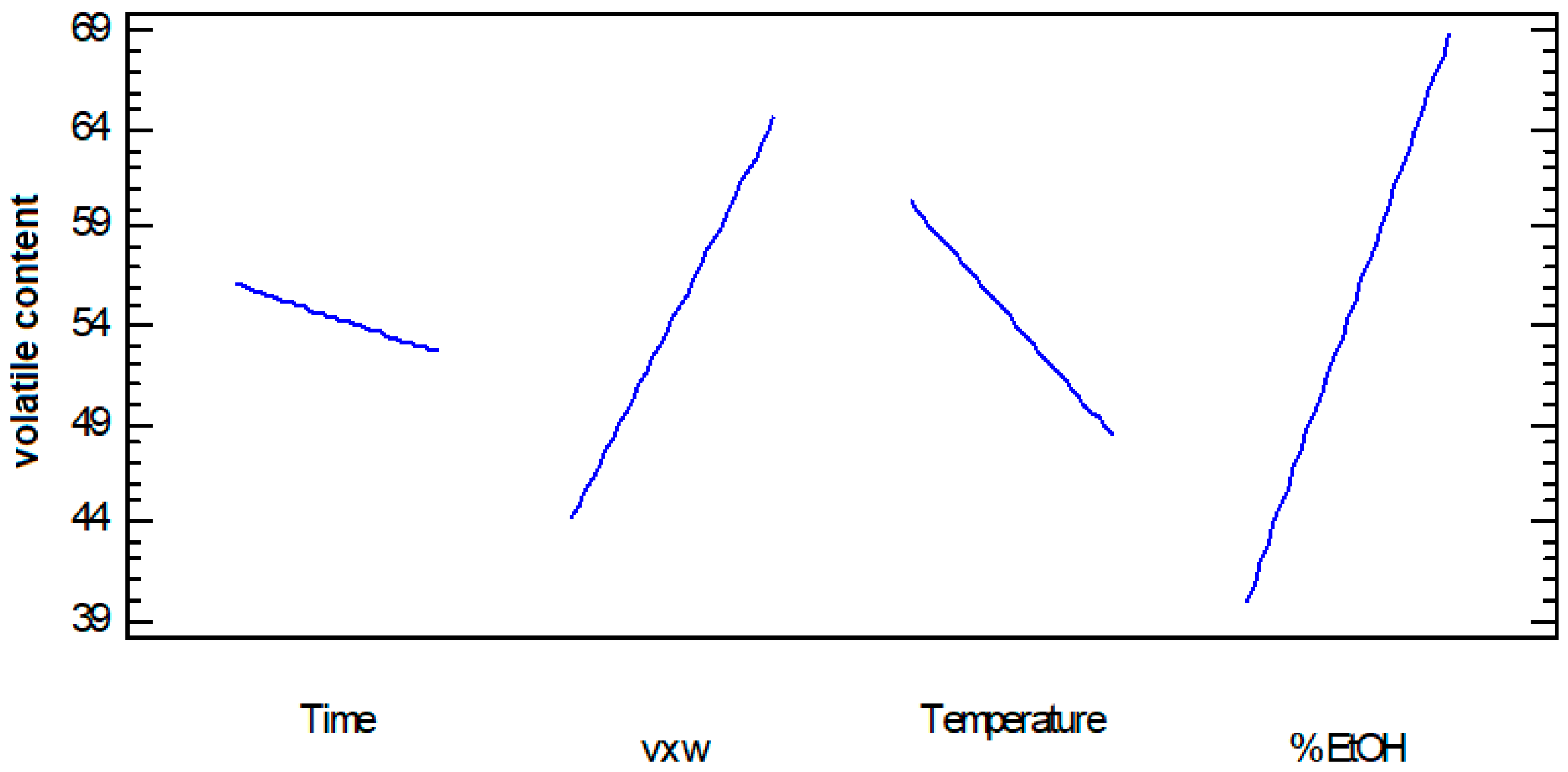
3.1.3. Volatiles Content
3.1.4. Multiple Response Optimization
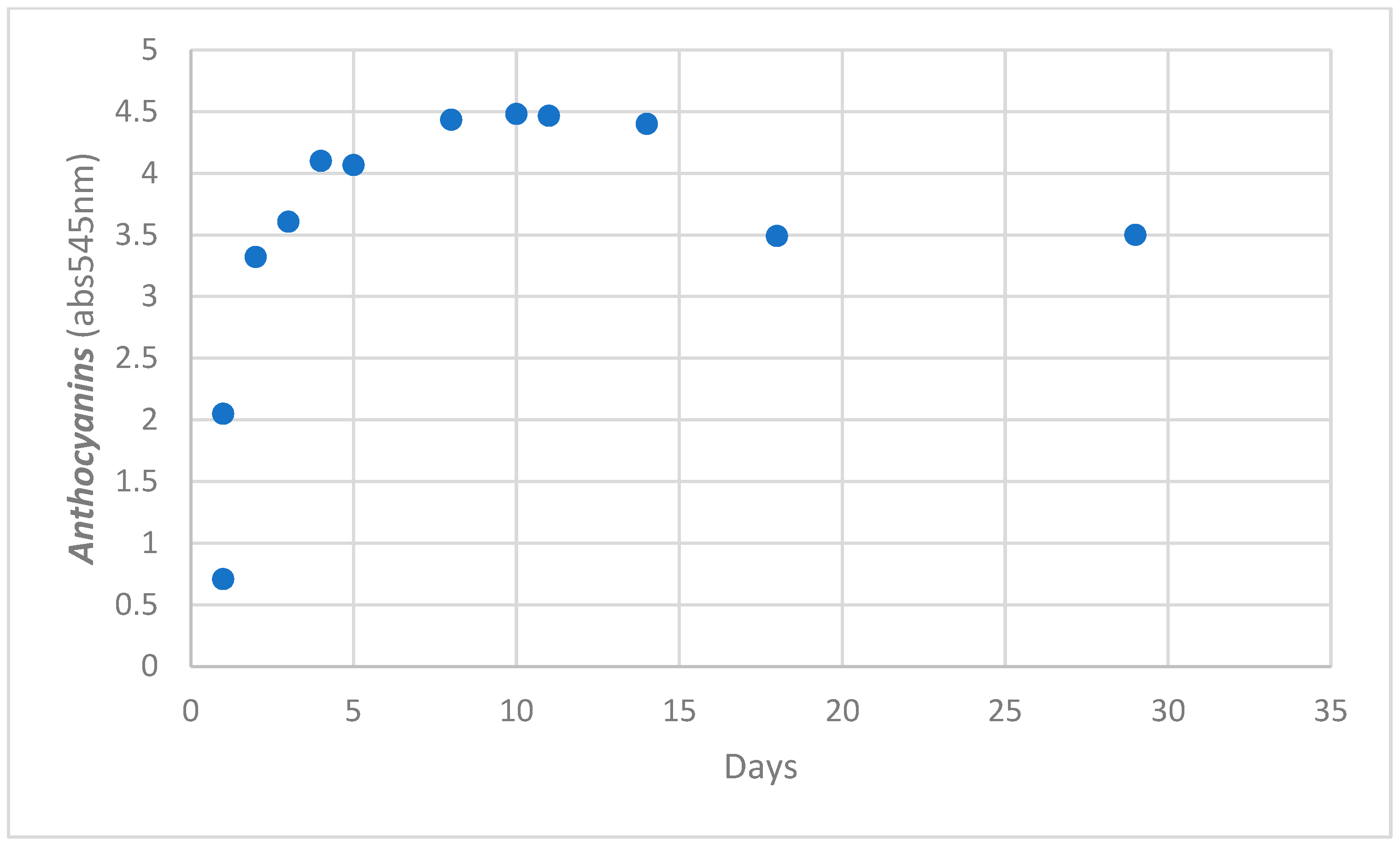
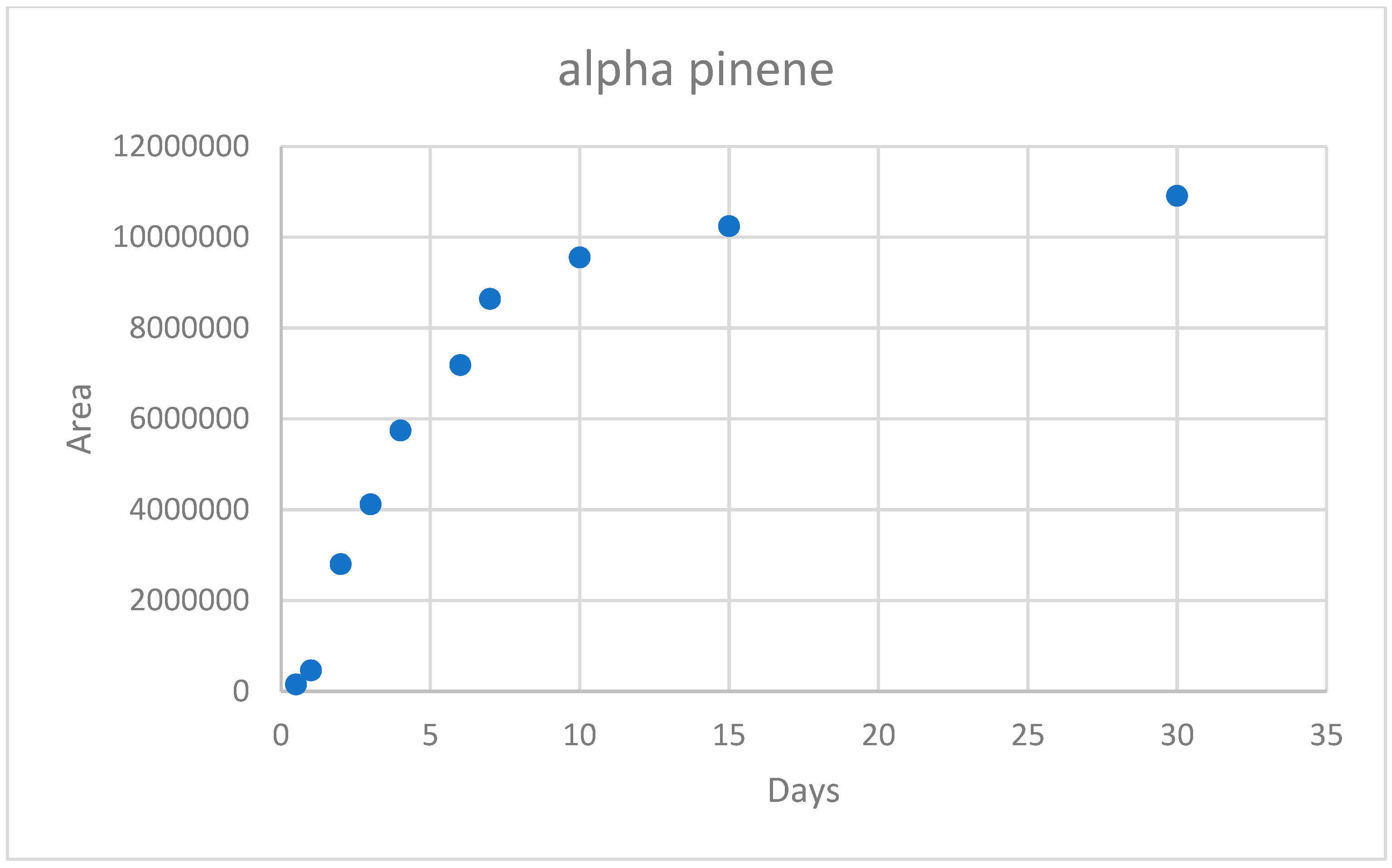
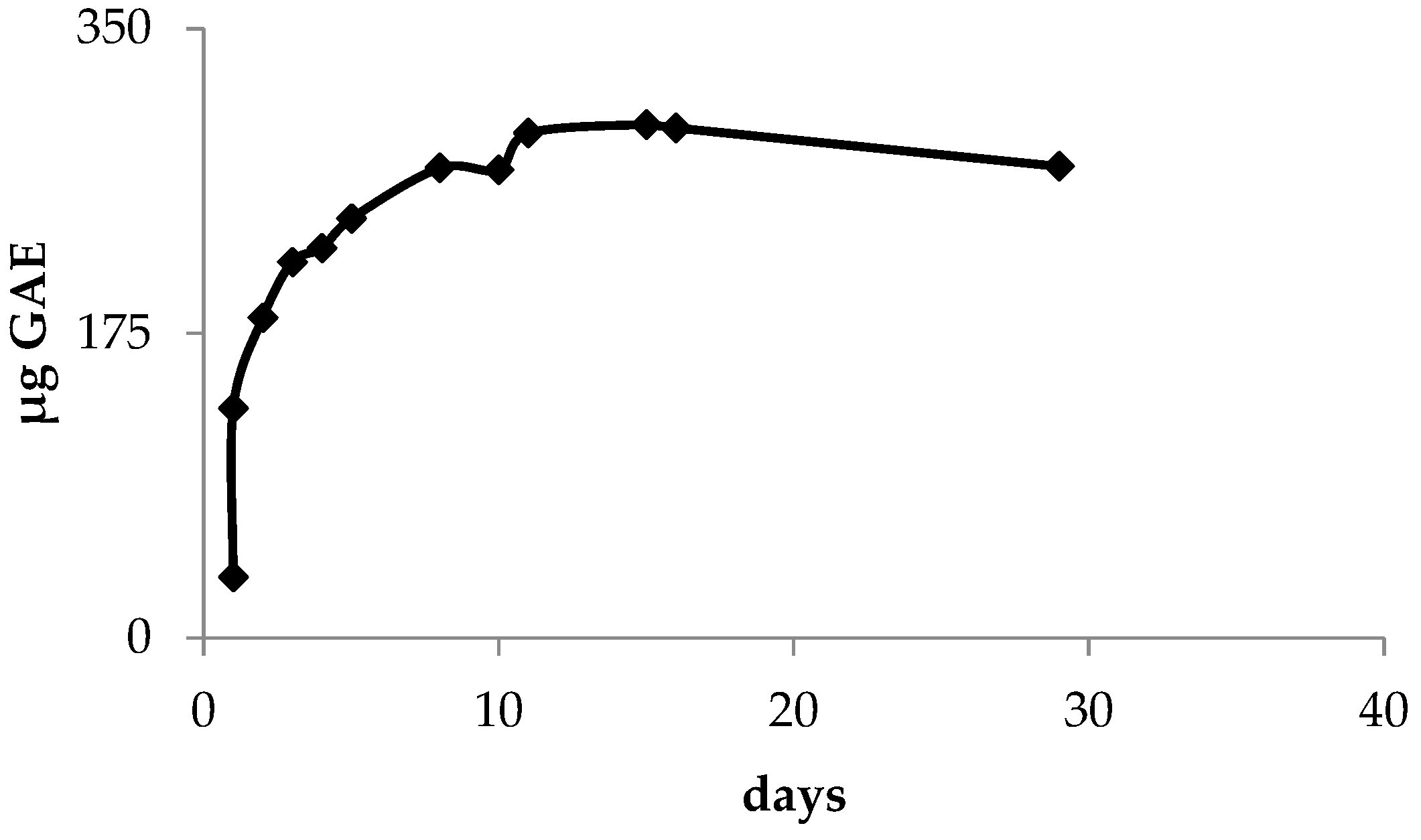
3.2. Kinetics Measurement
3.3. Anthocyanin Measurements
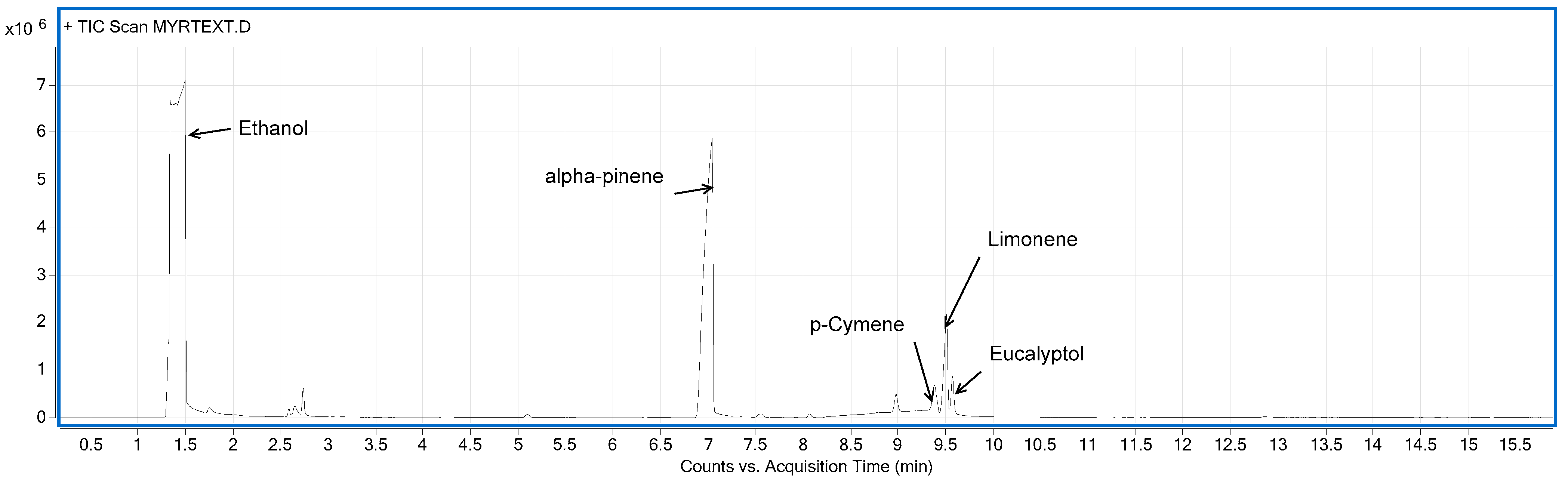
3.4. Volatile Organic Compounds
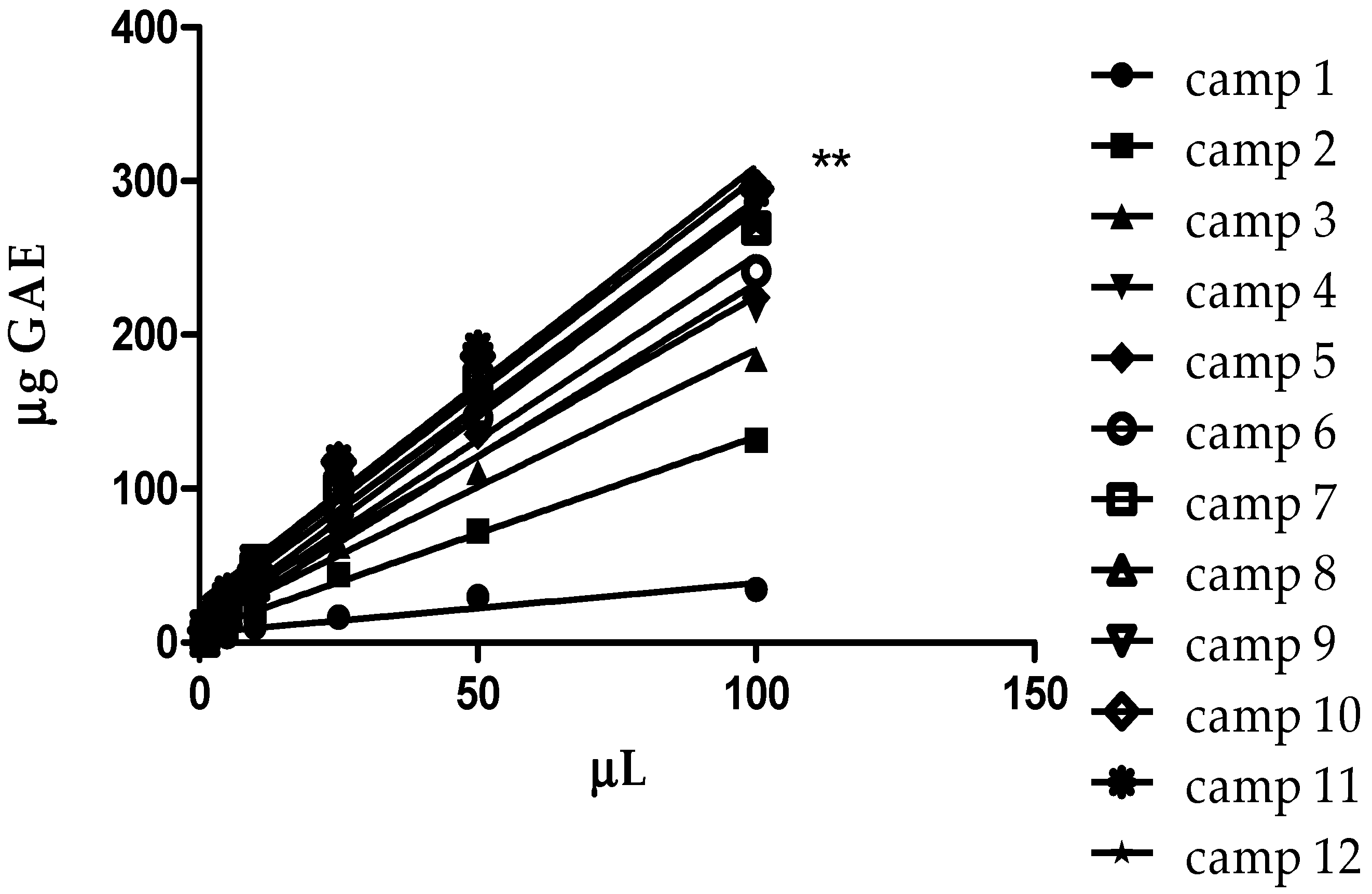
3.5. Total Polyphenols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Migliore, J.; Baumel, A.; Juin, M.; Médail, F. From Mediterranean shores to central Saharan mountains: Key phylogeographical insights from the genus Myrtus. J. Biogeogr. 2012, 39, 942–956. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Mulas, M. Chemical composition of essential oils of leaves and flowers from five cultivars of myrtle (Myrtus communis L.). J. Essent. Oil Res. 2015, 27, 465–476. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical composition of Myrtle (Myrtus communis L.) berries essential oils as observed in a collection of genotypes. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef] [PubMed]
- Mulas, M. The myrtle (Myrtus communis L.) case: From a wild shrub to a new fruit crop. ISHS Acta Hortic. 2012, 948, 235–242. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Orrù, C.D. Myrtle (Myrtus communis L.) berries: Composition and properties. In Berries: Properties, Consumption and Nutrition; Tuberoso, C.I.G., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 145–155. [Google Scholar]
- Tuberoso, C.I.G.; Barra, A.; Cabras, P. Effect of different technological processes on the chemical composition of myrtle (Myrtus communis L.) alcoholic extracts. Eur. Food Res. Technol. 2008, 226, 801–808. [Google Scholar] [CrossRef]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Panzeri, W.; Petretto, G.L.; Urgeghe, P.; Mele, A. Improving the recycling technology of waste cooking oils: Chemical fingerprint as tool for non-biodiesel application. Waste Manag. 2019, 96, 1–8. [Google Scholar] [CrossRef]
- Nejrotti, S.; Mannu, A.; Blangetti, M.; Baldino, S.; Fin, A.; Prandi, C. Optimization of Nazarov Cyclization of 2, 4-Dimethyl-1, 5-diphenylpenta-1, 4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules 2020, 25, 5726. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Di Pietro, M.E.; Garroni, S.; Mele, A. From deep eutectic solvents to deep band gap systems. J. Mol. Liq. 2020, 301, 112441. [Google Scholar] [CrossRef]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from Sardinia region. J. Mass Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Dao, L.T.; Takeoka, G.R.; Edwards, R.H.; Berrios, J.D.J. Improved method for the stabilization of anthocyanidins. J. Agric. Food Chem. 1998, 46, 3564–3569. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food. Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Melis, M.; Angioni, A.; Pala, M.; Cabras, P. Myrtle hydroalcoholic extracts obtained from different selections of Myrtus communis L. Food Chem. 2007, 101, 806–811. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32008R0110 (accessed on 6 July 2024).
- Bianchin, J.N.; Nardini, G.; Merib, J.; Neves Dias, A.; Martendal, E.; Carasek, E. Screening of volatile compounds in honey using a new sampling strategy combining multiple extraction temperatures in a single assay by HS-SPME-GC-MS. Food Chem. 2014, 145, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.A.; Anderson, N.G. Design of experiments (DoE) and process optimization. A review of recent publications. Org. Process. Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box–behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020, 10, 3595. [Google Scholar] [CrossRef]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An integrated approach to optimizing cellulose mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Garroni, S.; Taras, A.; Mele, A. Response surface analysis of density and flash point in recycled waste cooking oils. Chem. Data Collect. 2020, 25, 100329. [Google Scholar] [CrossRef]
- Alagumurthi, N.; Palaniradja, K.; Soundararajan, V. Optimization of grinding process through design of experiment (DOE)—A comparative study. Mater. Manuf. Process. 2006, 21, 19–21. [Google Scholar] [CrossRef]
- Dopar, M.; Kusic, H.; Koprivanac, N. Treatment of simulated industrial wastewater by photo-fenton process. Part I: The optimization of process parameters using design of experiments (DOE). Chem. Eng. J. 2011, 173, 267–279. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Effect of Winemaking Techniques on Phenolic Extraction. Am. J. Enol. Vitic. 2005, 56, 197. [Google Scholar] [CrossRef]
- Carew, A.L.; Kerslake, F.L.; Bindon, K.A.; Smith, P.A.; Close, D.C.; Dambergs, R.G. Viticultural and Controlled Phenolic Release Treatments Affect Phenolic Concentration and Tannin Composition in Pinot noir Wine. Am. J. Enol. Vitic. 2020, 71, 256–265. [Google Scholar] [CrossRef]
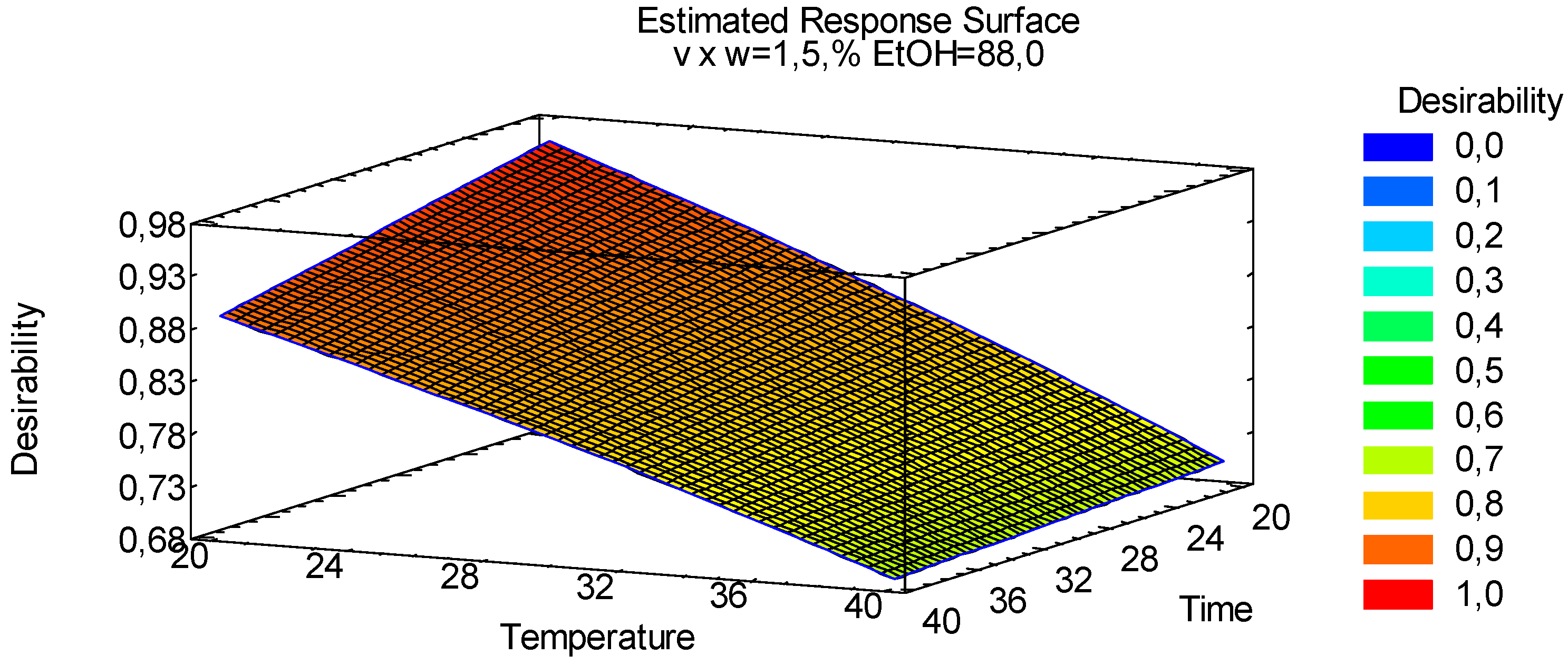
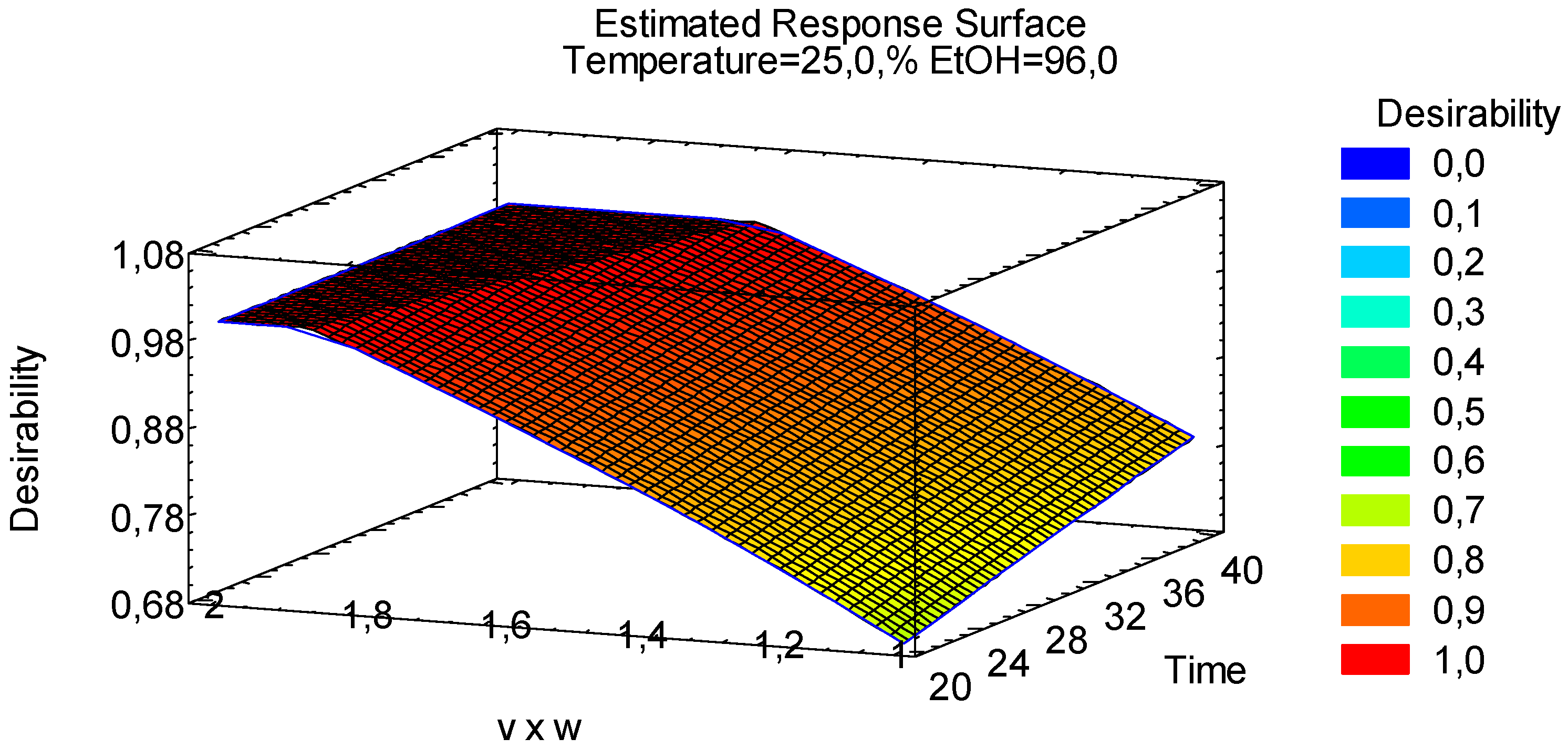
| Factors | Low | High | Units | Continuous |
|---|---|---|---|---|
| Time | 20 | 40 | days | Yes |
| v × w | 1 | 2 | l × kg | Yes |
| Temperature | 20 | 40 | °C | Yes |
| % EtOH | 80 | 96 | g/L | Yes |
| Run | Days | v × w (mL × g) | T (°C) | [EtOH] | ABS 545 nm | Fixed Residue | Volatiles |
|---|---|---|---|---|---|---|---|
| 1 | 40 | 1 | 40 | 96 | 2.1 | 0.24 | 6,019,156 |
| 2 | 40 | 1 | 20 | 96 | 3.3 | 0.25 | 5,067,420 |
| 3 | 20 | 2 | 40 | 80 | 1.9 | 0.31 | 4,078,820 |
| 4 | 40 | 2 | 40 | 96 | 4.2 | 0.26 | 9,216,100 |
| 5 | 20 | 1 | 40 | 96 | 1.3 | 0.20 | 6,499,873 |
| 6 | 20 | 1 | 20 | 80 | 3.6 | 0.20 | 5,865,395 |
| 7 | 40 | 1 | 20 | 80 | 0.7 | 0.26 | 3,545,460 |
| 8 | 40 | 1 | 40 | 80 | 0.4 | 0.26 | 1,170,427 |
| 9 | 40 | 2 | 20 | 80 | 4.1 | 0.28 | 5,590,749 |
| 10 | 20 | 1 | 40 | 80 | 0.9 | 0.29 | 1,731,154 |
| 11 | 20 | 2 | 20 | 96 | 4.7 | 0.26 | 6,447,419 |
| 12 | 20 | 2 | 40 | 96 | 3.8 | 0.30 | 7,448,706 |
| 13 | 40 | 2 | 40 | 80 | 0.95 | 0.32 | 2,582,121 |
| 14 | 20 | 1 | 20 | 96 | 1.7 | 0.30 | 5,446,433 |
| 15 | 20 | 2 | 20 | 80 | 4.7 | 0.28 | 7,392,442 |
| 16 | 40 | 2 | 20 | 96 | 4.6 | 0.26 | 8,919,072 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Time | 0.330625 | 1 | 0.330625 | 0.52 | 0.5015 |
| B: v × w | 13.8756 | 1 | 13.8756 | 22.00 | 0.0054 |
| C: Temperature | 8.85063 | 1 | 8.85063 | 14.03 | 0.0133 |
| D: % EtOH | 4.51563 | 1 | 4.51563 | 7.16 | 0.0440 |
| AB | 0.005625 | 1 | 0.005625 | 0.01 | 0.9284 |
| AC | 0.180625 | 1 | 0.180625 | 0.29 | 0.6155 |
| AD | 3.70563 | 1 | 3.70563 | 5.88 | 0.0598 |
| BC | 0.455625 | 1 | 0.455625 | 0.72 | 0.4341 |
| BD | 0.525625 | 1 | 0.525625 | 0.83 | 0.4031 |
| CD | 2.32563 | 1 | 2.32563 | 3.69 | 0.1129 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Time | 4.89908 × 1011 | 1 | 4.89908 × 1011 | 0.88 | 0.3907 |
| B: v × w | 1.6667 × 1013 | 1 | 1.6667 × 1013 | 30.01 | 0.0028 |
| C: Temperature | 5.67396 × 1012 | 1 | 5.67396 × 1012 | 10.22 | 0.0241 |
| D: % EtOH | 3.33726 × 1013 | 1 | 3.33726 × 1013 | 60.10 | 0.0006 |
| AB | 1.36951 × 1012 | 1 | 1.36951 × 1012 | 2.47 | 0.1771 |
| AC | 9.89477 × 1010 | 1 | 9.89477 × 1010 | 0.18 | 0.6905 |
| AD | 5.71015 × 1012 | 1 | 5.71015 × 1012 | 10.28 | 0.0238 |
| BC | 1.68895 × 1010 | 1 | 1.68895 × 1010 | 0.03 | 0.8684 |
| BD | 1.73622 × 1011 | 1 | 1.73622 × 1011 | 0.31 | 0.6002 |
| CD | 1.62712 × 1013 | 1 | 1.62712 × 1013 | 29.30 | 0.0029 |
| Total error | 2.77652 × 1012 | 5 | 5.55304 × 1011 | ||
| Total (corr.) | 8.26203 × 1013 | 15 |
| Desirability | Desirability | |||
|---|---|---|---|---|
| Response | Low | High | Goal | Weight |
| Anthocyanin | 0.0 | 4.0 | Maximize | 1.0 |
| Volatiles content | 1.0 | 9.0 | Maximize | 1.0 |
| Predicted | Observed | |||
|---|---|---|---|---|
| Row | Anthocyanin | Volatile Content | Desirability | Desirability |
| 1 | 4.2 | 9.2161 × 106 | 1.0 | 1.0 |
| 2 | 1.9 | 4.07882 × 106 | 0.732078 | 0.689202 |
| 3 | 4.1 | 5.59075 × 106 | 0.927867 | 1.0 |
| 4 | 1.7 | 5.44643 × 106 | 0.714799 | 0.65192 |
| 5 | 2.1 | 6.01916 × 106 | 0.769537 | 0.724569 |
| 6 | 4.7 | 6.44742 × 106 | 1.0 | 1.0 |
| 7 | 1.3 | 6.49987 × 106 | 0.600781 | 0.570088 |
| 8 | 3.6 | 5.86539 × 106 | 0.875892 | 0.948683 |
| 9 | 0.9 | 1.73115 × 106 | 0.485734 | 0.474342 |
| 10 | 4.7 | 7.39244 × 106 | 1.0 | 1.0 |
| 11 | 3.8 | 7.44871 × 106 | 0.917708 | 0.974679 |
| 12 | 0.7 | 3.54546 × 106 | 0.641044 | 0.41833 |
| 13 | 0.4 | 1.17043 × 106 | 0.0 | 0.316228 |
| 14 | 4.6 | 8.91907 × 106 | 1.0 | 1.0 |
| 15 | 3.3 | 5.06742 × 106 | 0.797457 | 0.908295 |
| 16 | 0.9 | 2.58212 × 106 | 0.516902 | 0.474342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addis, R.; Mannu, A.; Pintore, G.; Petretto, G.L. Statistical Analysis Applied to the Production of Mirto Liqueur. Appl. Sci. 2024, 14, 5973. https://doi.org/10.3390/app14145973
Addis R, Mannu A, Pintore G, Petretto GL. Statistical Analysis Applied to the Production of Mirto Liqueur. Applied Sciences. 2024; 14(14):5973. https://doi.org/10.3390/app14145973
Chicago/Turabian StyleAddis, Roberta, Alberto Mannu, Giorgio Pintore, and Giacomo Luigi Petretto. 2024. "Statistical Analysis Applied to the Production of Mirto Liqueur" Applied Sciences 14, no. 14: 5973. https://doi.org/10.3390/app14145973
APA StyleAddis, R., Mannu, A., Pintore, G., & Petretto, G. L. (2024). Statistical Analysis Applied to the Production of Mirto Liqueur. Applied Sciences, 14(14), 5973. https://doi.org/10.3390/app14145973









