Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Juice Production
2.2. Chemicals and Reagents
2.3. Physicochemical Analysis
Determination of pH, Titratable Acidity, Total Soluble Solids, and Colour
2.4. Determination of Total Phenolic Content
2.5. Determinations of Total Vitamin C (TAA), AA, and DHAA Content
2.6. DPPH Assay
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
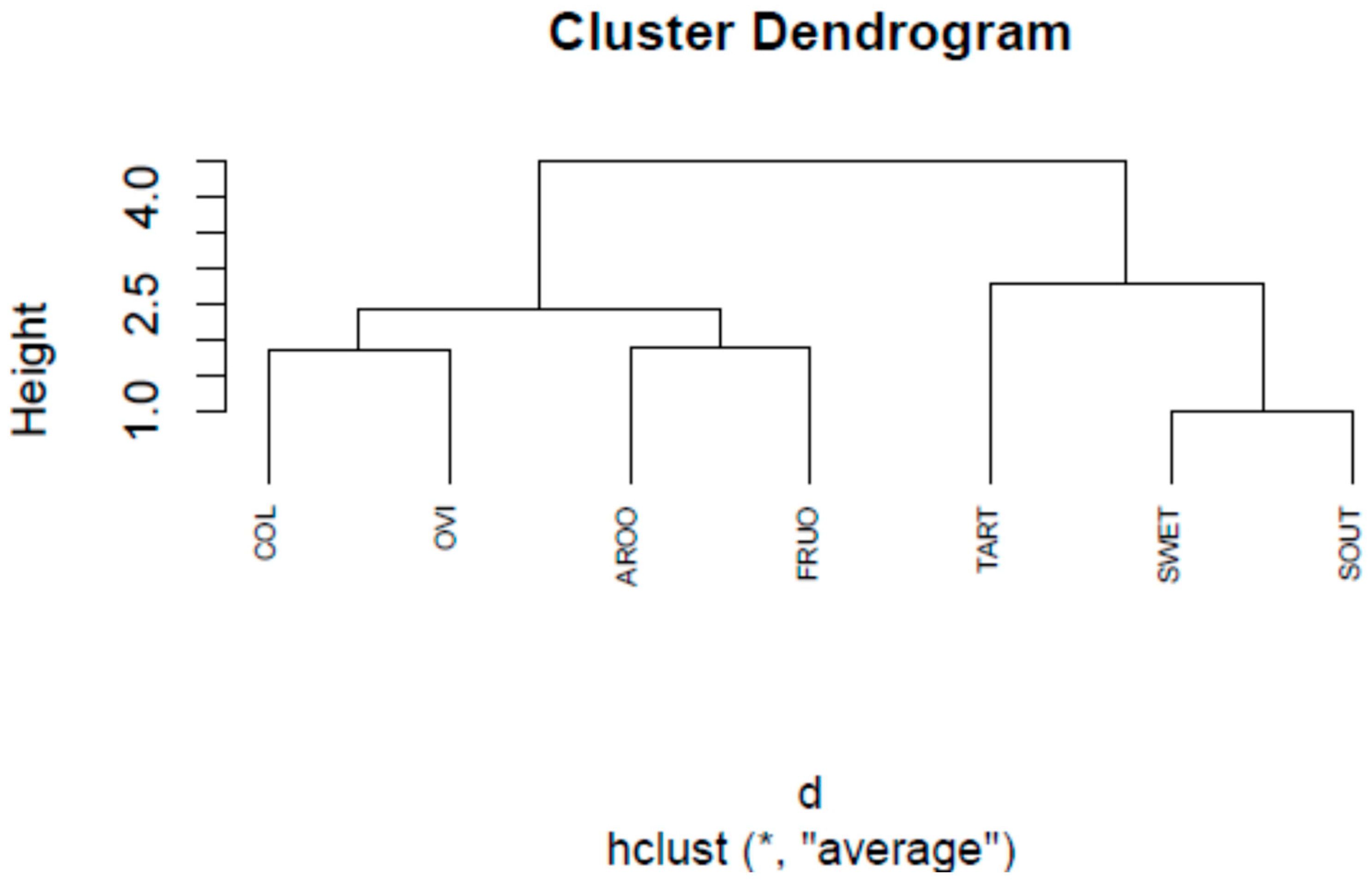
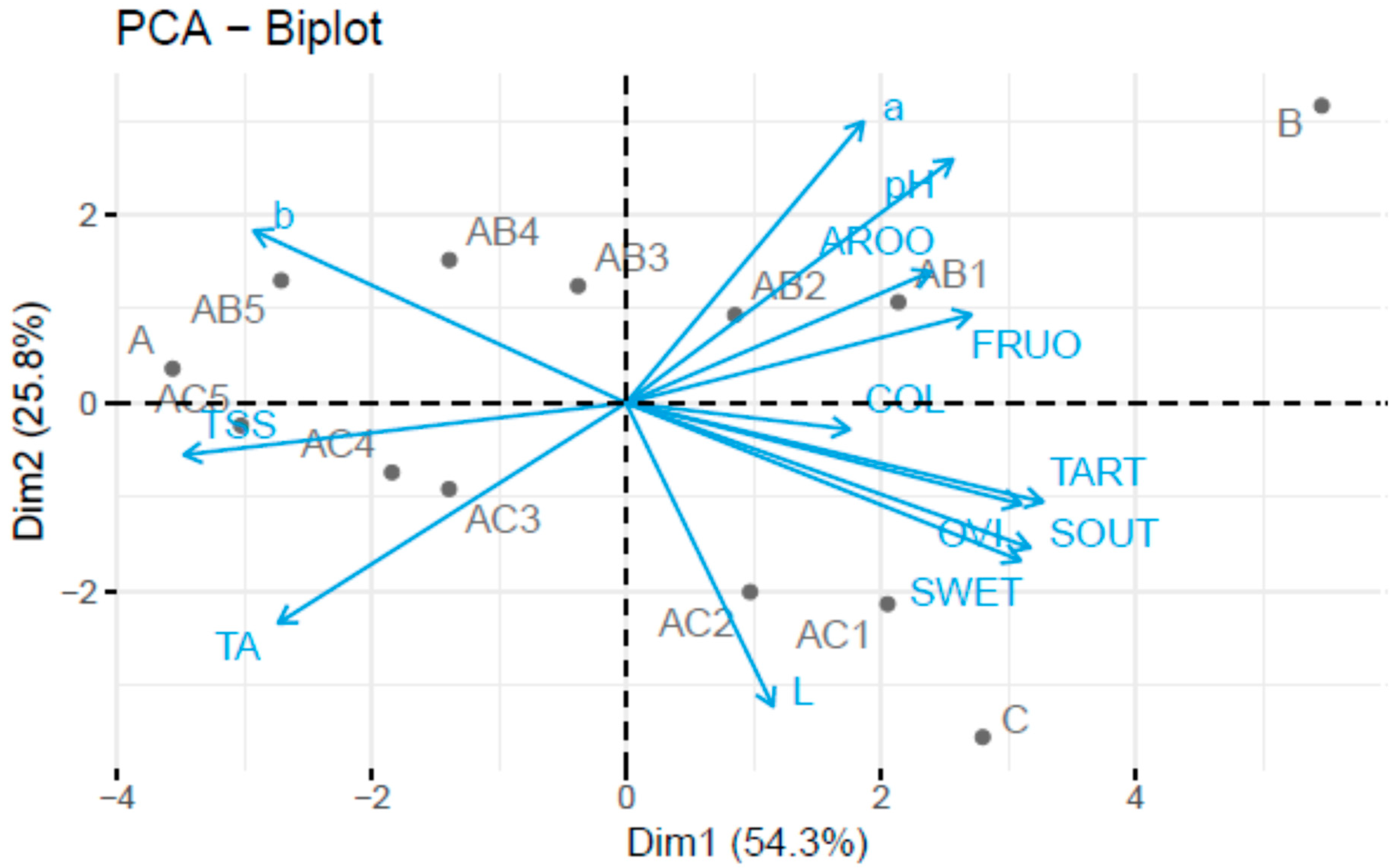
3.1. Sensory and Physicochemical Analysis of Fruit Juice Formulations
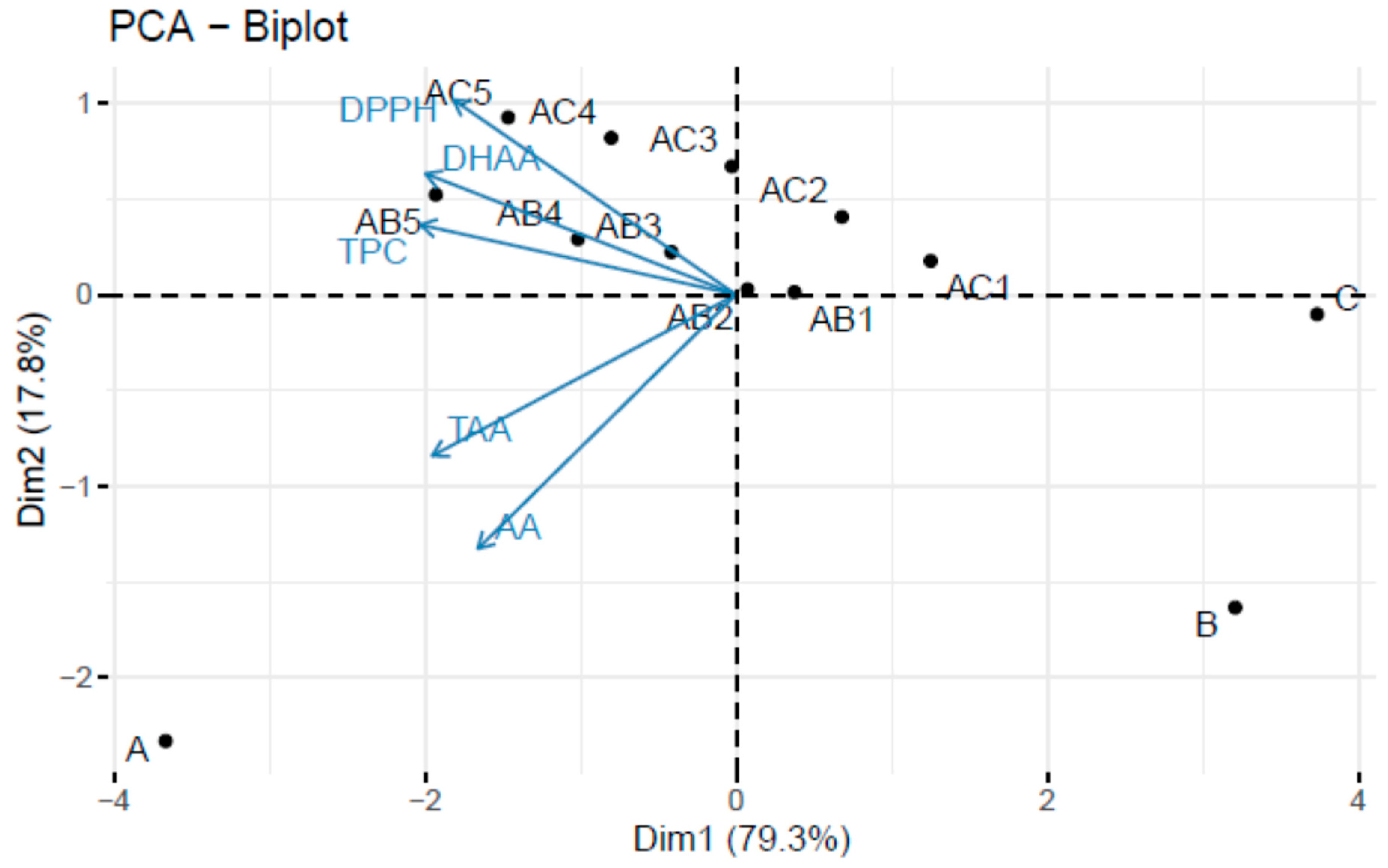
3.2. Vitamin C, Total Polyphenols, and Antioxidant Activity Analysis of Fruit Juice Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Paula, F.J.A.; Guiné, R.P.F.; Cruz-Lopes, L.; Duarte, A.C.; Fragata, A.O.S.; Reis, M.A.L. Effects of pre- and post-parvest factors on the selected elements contents in fruit juices. Czech. J. Food Sci. 2015, 33, 384–391. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Rogowska, A.; Pączkowski, C.; Szakiel, A. Distribution of Triterpenoids and Steroids in Developing Rugosa Rose (Rosa rugosa Thunb.) Accessory Fruit. Molecules 2021, 26, 5158. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Klewicka, E.; Piekarska-Radzik, L.; Milala, J.; Klewicki, R.; Sójka, M.; Rosół, N.; Otlewska, A.; Matysiak, B. Antagonistic Activity of Lactic Acid Bacteria and Rosa rugosa Thunb. Pseudo-Fruit Extracts against Staphylococcus spp. Strains. Appl. Sci. 2022, 12, 4005. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 13, 7–27. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a source of dietary phytonutrients: Bioavailability and evidence of protective effects against human cardiovascular disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef]
- Quan, W.; Tao, Y.; Lu, M.; Yuan, B.; Chen, J.; Zeng, M.; Qin, F.; Guo, F.; He, Z. Stability of the phenolic compounds and antioxidant capacity of five fruit (apple, orange, grape, pomelo and kiwi) juices during in vitro-simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2017, 53, 1131–1139. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.J.; Yahia, E.M.; Ramírez-Bustamante, N.; Pérez-Martínez, J.D.; del Pilar Escalante-Minakata, M.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Newerli-Guz, J.; Śmiechowska, M.; Drzewiecka, A.; Tylingo, R. Bioactive ingredients with health-promoting properties of strawberry fruit (Fragaria x ananassa Duchesne). Molecules 2023, 28, 2711. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Roshan, R.; Ahmed, S.; ul Hassan, M.M. Fragaria nubicola (Rosaceae): A review of medicinal uses, phytochemistry and pharmacology. J. Pharmacogn. Phytochem. 2019, 8, 3390–3393. [Google Scholar]
- Basu, A.; Morris, S.; Nguyen, A.; Betts, N.M.; Fu, D.; Lyons, T.J. Effects of dietary strawberry supplementation on antioxidant biomarkers in obese adults with above optimal serum lipids. J. Nutr. Metab. 2016, 2016, 3910630. [Google Scholar] [CrossRef]
- Apple Production by Country 2023. Available online: https://worldpopulationreview.com/country-rankings/apple-production-by-country (accessed on 10 December 2023).
- Pawlak, J.; Wróblewska, W.; Paszko, D. Production and economic efficiency in the cultivation of strawberry (Fragaria x ananassa Duch.) depending on the method of production—Case study. Agron. Sci. 2022, LXXVII. [Google Scholar] [CrossRef]
- Chiusano, L.; Cravero, M.C.; Borsa, D.; Tsolakis, C.; Zeppa, G.; Gerbi, V. Effect of the addition of fruit juices on grape must for natural beverage production. Ital. J. Food Sci. 2015, 27, 375–384. [Google Scholar] [CrossRef]
- Curi, P.N.; de Almeida, A.B.; Tavares, B.S.; Nunes, C.A.; Pio, R.; Pasqual, M.; de Souza, V.R. Optimization of tropical fruit juice based on sensory and nutritional characteristics. Food Sci. Technol. 2017, 37, 308–314. [Google Scholar] [CrossRef]
- Ogundele, O.M.A.; Awolu, O.O.; Badejo, A.A.; Nwachukwu, I.D.; Fagbemi, T.N. Development of functional beverages from blends of Hibiscus sabdariffa extract and selected fruit juices for optimal antioxidant properties. Food Sci. Nutr. 2016, 4, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Grigio, M.L.; Moura, E.A.; Carvalho, G.F.; Zanchetta, J.J.; Chagas, P.C.; Chagas, E.A.; Durigan, M.F. Nutraceutical potential, quality and sensory evaluation of camu-camu pure and mixed jelly. Food Sci. Technol. 2022, 42, e03421. [Google Scholar] [CrossRef]
- Lagrange, V.; Norback, J.P. Product optimization and the acceptor set size. J. Sens. Stud. 1987, 2, 119–136. [Google Scholar] [CrossRef]
- The Polish Standard PN-EN-1132; Fruit and Vegetable Juices. pH Determination. Polish Committee for Standardization: Warsaw, Poland, 1999.
- The Polish Standard PN-EN-12147; Fruit and Vegetable Juices-Determination of Titratable Acidity. Polish Committee for Standardization: Warsaw, Poland, 2000.
- The Polish Standard PN-90/A-75101; Fruit and Vegetable Products-Preparation of Samples and Methods of Physicochemical Tests-Determination of the Content of Total Extract. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Gao, X.; Ohlander, M.; Jeppson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effect and their relationship to phytonutrients in fruit of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-Ascorbic Acid, and antioxidant activity in wines from rose fruits (Rosa rugosa). Molecules 2021, 26, 2561. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Singh-Ackbarali, D.; Maharaj, R. Sensory evaluation as a tool in determining acceptability of innovative products developed by undergraduate students in Food Science and Technology at the University of Trinidad and Tobago. J. Curric. Teach. 2014, 3, 10–27. [Google Scholar] [CrossRef]
- Villa, F.; Potrich, C.; Dall’Oglio, P.A. Sensory profile and physical-chemical analysis of integral grape juice prepared through steam extraction process. Sci. Agrar. Parana. 2018, 17, 300–304. [Google Scholar]
- Igual, M.; Garcia-Herrera, P.; Cámara, R.M.; Martinez-Monzό, J.; Garcia-Segovia, P.; Cámara, M. Bioactive compounds in rosehip (Rosa canina) powder with encapsulating agents. Molecules 2022, 27, 4737. [Google Scholar] [CrossRef]
- Touyz, L.Z.G.; Nassani, L.M. Acidity and dental erosion from apple-and grape-juice (an in vitro and in vivo Report). Int. J. Oral. Dent. Health 2018, 4, 061. [Google Scholar] [CrossRef]
- Tyburcy, A.; Ścibisz, I.; Rostek, E.; Pasierbiewicz, A.; Florowski, T. Antioxidant properties of cranberry and rose juices in meat products made of defrosted meat. Food Sci. Technol. Qual. 2014, 5, 72–84. [Google Scholar] [CrossRef]
- Basak, J.K.; Madhavi, B.G.K.; Paudel, B.; Kim, N.E.; Kim, H.T. Prediction of Total Soluble Solids and pH of strawberry fruits using RGB, HSV and HSL Colour spaces and machine learning models. Foods 2022, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Rahman, M.R. Ph in food preservation. In Handbook of Food Preservation; CRS Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxford, UK, 2020; pp. 323–332. [Google Scholar]
- Porto, M.; Okina, V.; Pimentel, T.; Prudencio, S. Physicochemical stability, antioxidant activity, and acceptance of beet and orange mixed juice during refrigerated storage. Beverages 2017, 3, 36. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant Activity of Citrus Fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Kalisz, S.; Mitek, M. The influence of wild rose nectar addition into apple juices on the antioxidant activity and the bioactive components content. Food Sci. Technol. Qual. 2007, 5, 194–201. [Google Scholar]
- Kalisz, S. Effect of production method of strawberry juices on the content of anthocyanins and colour. Food Sci. Technol. Qual. 2008, 5, 149–160. [Google Scholar]
- Kalisz, S.; Wolniak, M. Changes in selected qualitative distinguishing features during storage of restored juices from concentrares. Food Sci. Technol. Qual. 2007, 5, 203–212. [Google Scholar]
- Souza, V.R.; Pereira, P.A.P.; Pinheiro, A.C.M.; Nunes, C.A.; Pio, R.; Queiroz, F. Evaluation of the jelly processing potential of raspberries adapted in Brazil. J. Food Sci. 2014, 79, 407–412. [Google Scholar] [CrossRef]
- Pereira, A.C.S.; Wurlitzer, N.J.; Dionísio, A.P.; Soares, M.V.L.; Bastos, M.D.S.R.; Alves, R.E.; Brasil, I.M. Synergistic, additive and antagonistic effects of fruit mixtures on total antioxidant capacities and bioactive compounds in tropical fruit juices. Arch. Latinoam. De Nutr. 2015, 65, 119–127. [Google Scholar]
- Al Fata, N.; Georgè, S.; Dlalah, N.; Renard, C.M.G.C. Influence of partial pressure of oxygen on ascorbic acid degradation at canning temperature. Innov. Food Sci. Emerg. Technol. 2018, 49, 215–221. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef] [PubMed]
- Varming, C.; Petersen, M.A.; Toldam-Andersen, T.B. Ascorbic Acid Contents in Danish Apple Cultivars and Commercial Apple Juices. LWT-Food Sci. Technol. 2013, 54, 597–599. [Google Scholar] [CrossRef]
- Kalemba-Dróżdż, M.; Cierniak, A.; Kwiecień, I. Fruits, edible flowers and leaves of wild plants-polyphenolic raw materials act as effective antioxidant and genoprotective factors. In Polyphenol Raw Materials. Applications and Prospects; Oficyna Wydawnicza AFM: Kraków, Poland, 2022; Volume 3, pp. 51–98. [Google Scholar]
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, A.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic acid content in apple pulp, peel, and monovarietal cloudy juices of 64 different cultivars. Int. J. Food Prop. 2017, 20, S2626–S2634. [Google Scholar] [CrossRef]
- Schvab, M.C.; Ferreyra, M.M.; Davies, C.V.; Stefani, A.; Cayetano, M.C.; Gerard, L.M.; Gonzalez, R.F. Effects of orange winemaking variables on antioxidant activity and bioactive compounds. Food Sci. Technol Camp. 2015, 35, 407–413. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative Evaluation of UV-HPLC Methods and Reducing Agents to Determine Vitamin C in Fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: Critical review and kinetic modelling approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Michalak-Majewska, M.; Żukiewicz-Sobczak, W.; Kalbarczyk, J. Assessment of the composition and properties of fruit juices most popular with consumers. Bromatol. I Chem. Toksykol. 2009, 42, 836–841. [Google Scholar]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of pectinase treatment on extraction of antioxidant phenolsfrom pomace, for the production of puree-enriched cloudy apple juices. Food Chem. 2011, 127, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S. High-methoxyl pectins influence on the antioxidant compounds content in strawberry juices. Food Sci. Technol. Qual. 2007, 2, 145–154. [Google Scholar]
- Wolniak, M.; Kalisz, S. The influence of pectin on anthocyanins and total polyphenol content and its antiradical scavenging activity designated in strawberry juices. Food 2006, 4, 39–46. [Google Scholar] [CrossRef]
- Szajdek, A.; Dąbkowska, E.; Borowska, E.J. The influence of enzymatic treatment of berry pulp on the polyphenol content and antioxidant activity of the juice. Food Sci. Technol. Qual. 2006, 4, 59–67. [Google Scholar]
| Sample | Ingredients | ||
|---|---|---|---|
| Rose Juice (% v/v) | Strawberry Juice (% v/v) | Apple Juice (% v/v) | |
| A | 100 | 0 | 0 |
| B | 0 | 100 | 0 |
| C | 0 | 0 | 100 |
| AB1 | 50 | 50 | 0 |
| AB2 | 60 | 40 | 0 |
| AB3 | 70 | 30 | 0 |
| AB4 | 80 | 20 | 0 |
| AB5 | 90 | 10 | 0 |
| AC1 | 50 | 0 | 50 |
| AC2 | 60 | 0 | 40 |
| AC3 | 70 | 0 | 30 |
| AC4 | 80 | 0 | 20 |
| AC5 | 90 | 0 | 10 |
| Sensory Attributes | |||||||
|---|---|---|---|---|---|---|---|
| Sample | COL | AROO | FRUO | SWET | SOUT | TART | OVI |
| A | 4.96 ± 0.20 d | 4.26 ± 1.21 d | 2.56 ± 1.55 a | 1.43 ± 0.97 a | 2.15 ± 0.65 a | 1.12 ± 0.87 a | 3.20 ± 0.27 a |
| B | 4.8 ± 0.00 cd | 4.77 ± 1.12 f | 4.5 ± 1.22 e | 4.15 ± 1.11 d | 4.19 ± 0.98 e | 4.89 ± 0.99 f | 4.90 ± 0.41 e |
| C | 4.00 ± 0.23 b | 3.93 ± 1.23 b | 3.89 ± 0.92 bc | 4.83 ± 1.25 f | 4.62 ± 1.11 g | 4.96 ± 1.54 f | 4.50 ± 0.29 d |
| AB1 | 4.70 ± 0.62 c | 4.60 ± 0.72 e | 4.87 ± 0.51 f | 3.37 ± 1.22 c | 3.53 ± 1.25 d | 2.93 ± 1.48 d | 4.60 ± 0.22 d |
| AB2 | 4.45 ± 0.75 c | 4.30 ± 0.98 d | 4.37 ± 0.99 d | 3.26 ± 1.60 c | 3.63 ± 1.25 d | 2.27 ± 0.98 c | 4.20 ± 0.37 c |
| AB3 | 4.00 ± 0.49 b | 4.07 ± 1.70 c | 4.23 ± 0.97 d | 2.87 ± 1.49 bc | 3.03 ± 1.52 c | 2.10 ± 0.84 bc | 4.00 ± 0.41 c |
| AB4 | 3.7 ± 0.88 a | 3.97 ± 1.73 c | 4.13 ± 1.57 cd | 2.71 ± 0.79 b | 2.90 ± 1.45 c | 1.97 ± 0.81 b | 3.75 ± 0.17 b |
| AB5 | 3.4 ± 0.35 a | 3.70 ± 1.71 b | 3.93 ± 1.23 c | 2.62 ± 0.73 b | 2.70 ± 1.32 b | 1.86 ± 0.82 b | 3.35 ± 0.00 ab |
| AC1 | 4.97 ± 0.18 d | 4.23 ± 0.18 d | 4.40 ± 0.93 de | 4.43 ± 0.94 e | 4.73 ± 0.52 h | 3.5 ± 1.53 e | 4.93 ± 0.25 e |
| AC2 | 4.75 ± 0.55 c | 3.73 ± 1.36 b | 3.90 ± 1.40 c | 4.16 ± 1.05 d | 4.43 ± 1.22 f | 3.32 ± 1.16 e | 4.86 ± 0.44 e |
| AC3 | 4.10 ± 0.35 b | 3.5 ± 1.53 b | 3.56 ± 1.23 b | 3.10 ± 1.37 c | 2.90 ± 1.18 c | 2.56 ± 0.90 c | 4.10 ± 0.75 c |
| AC4 | 4.05 ± 0.52 b | 3.2 ± 1.35 b | 3.47 ± 1.28 b | 3.00 ± 1.32 c | 2.83 ± 1.44 c | 2.30 ± 1.21 c | 4.20 ± 0.33 c |
| AC5 | 3.85 ± 0.16 a | 2.66 ± 1.83 a | 3.17 ± 1.51 b | 2.53 ± 1.07 b | 2.50 ± 1.17 b | 2.13 ± 0.90 bc | 4.00 ± 0.49 c |
| Sample | pH | TA | TSS |
|---|---|---|---|
| A | 3.45 ± 0.03 a | 1.40 ± 0.02 i | 21.6 ± 0.3 c |
| B | 4.34 ± 0.02 f | 0.48 ± 0.01 a | 9.1 ± 0.0 a |
| C | 3.60 ± 0.03 abc | 1.28 ± 0.03 ef | 13.2 ± 0.2 b |
| AB1 | 3.96 ± 0.01 e | 0.94 ± 0.02 e | 15.8 ± 0.0 b |
| AB2 | 3.86 ± 0.06 de | 0.98 ± 0.01 e | 16.9 ± 0.1 b |
| AB3 | 3.83 ± 0.02 cde | 1.04 ± 0.03 e | 18.1 ± 0.4 ab |
| AB4 | 3.75 ± 0.02 bcde | 1.12 ± 0.01 e | 19.5 ± 0.2 b |
| AB5 | 3.70 ± 0.01 abcd | 1.24 ± 0.01 e | 20.9 ± 0.1 bc |
| AC1 | 3.58 ± 0.03 abc | 1.28 ± 0.02 ef | 17.7 ± 0.3 b |
| AC2 | 3.54 ± 0.03 ab | 1.30 ± 0.01 fg | 18.7 ± 0.1 ab |
| AC3 | 3.50 ± 0.03 ab | 1.32 ± 0.01 fgh | 19.7 ± 0.1 b |
| AC4 | 3.49 ± 0.02 a | 1.34 ± 0.01 gh | 20.2 ± 0.3 b |
| AC5 | 3.47 ± 0.02 a | 1.36 ± 0.01 hi | 21.1 ± 0.3 c |
| Sample | L* | a* | b* |
|---|---|---|---|
| A | 82.70 ± 0.05 de | 5.51 ± 0.04 f | 47.16 ± 0.06 k |
| B | 77.16 ± 0.01 a | 36.92 ± 0.01 m | 23.95 ± 0.01 b |
| C | 93.71 ± 0.01 h | 0.40 ± 0.02 a | 7.12 ± 0.01 a |
| AB1 | 83.29 ± 0.04 e | 13.90 ± 0.03 l | 34.52 ± 0.04 e |
| AB2 | 82.22 ± 0.03 cde | 11.59 ± 0.05 k | 38.25 ± 0.03 g |
| AB3 | 81.09 ± 0.07 c | 10.75 ± 0.02 j | 43.53 ± 0.03 i |
| AB4 | 78.38 ± 0.02 ab | 10.38 ± 0.07 i | 49.59 ± 0.01 l |
| AB5 | 77.46 ± 0.06 a | 9.79 ± 0.06 h | 54.39 ± 0.02 m |
| AC1 | 87.38 ± 0.04 g | 1.90 ± 0.02 b | 30.18 ± 0.04 c |
| AC2 | 85.52 ± 0.02 f | 2.98 ± 0.01 c | 33.32 ± 0.02 d |
| AC3 | 83.63 ± 0.07 e | 3.41 ± 0.06 d | 36.86 ± 0.06 f |
| AC4 | 81.51 ± 0.05 cd | 3.95 ± 0.03 e | 39.18 ± 0.05 h |
| AC5 | 79.54 ± 0.07 b | 7.20 ± 0.06 g | 46.72 ± 0.09 j |
| Parameter | Factor | Degree of Freedom | Sum of Square | Mean Square | F Value | p Value |
|---|---|---|---|---|---|---|
| L* | The addition of apple or strawberry juice | 1 | 430.486 | 430.486 | 60.90 | <0.0001 |
| rose juice | 6 | 492.819 | 82.1366 | 11.62 | <0.0001 | |
| Error | 76 | 537.183 | 7.06819 | |||
| Sum | 83 | 1460.49 | ||||
| a* | The addition of apple or strawberry juice | 1 | 2314.31 | 2314.31 | 66.45 | <0.0001 |
| rose juice | 6 | 1402.41 | 233.734 | 6.71 | <0.0001 | |
| Error | 76 | 2646.85 | 34.8269 | |||
| Sum | 83 | 6363.56 | ||||
| b* | The addition of apple or strawberry juice | 1 | 1107.3 | 1107.3 | 166.36 | <0.0001 |
| rose juice | 6 | 9943.92 | 1657.32 | 248.99 | <0.0001 | |
| Error | 76 | 505.86 | 6.65606 | |||
| Sum | 83 | 11,557.1 |
| Sample | AA | DHAA | TAA | TPC | Antioxidant Capacity—DPPH |
|---|---|---|---|---|---|
| A | 198.30 ± 3.13 j | 55.30 ± 1.28 i | 253.60 ± 1.28 l | 272.93 ± 2.12 j | 36.06 ± 0.10 fg |
| B | 47.19 ± 0.50 e | 7.37 ± 0.79 b | 54.56 ± 0.28 b | 44.64 ± 0.91 a | 13.13 ± 0.05 a |
| C | 0.53 ± 0.14 a | 0.61 ± 0.15 a | 1.14 ± 0.11 a | 42.69 ± 1.11 a | 21.28 ± 0.16 b |
| AB1 | 53.29 ± 0.10 f | 36.50 ± 0.05 e | 89,79 ± 0.05 f | 164.40 ± 2.04 d | 30.38 ± 0.20 c |
| AB2 | 57.35 ± 0.17 g | 39.45 ± 0.11 f | 96.80 ± 0.29 g | 182.22 ± 3.03 e | 30.86 ± 0.53 c |
| AB3 | 60.23 ± 0.03 g | 42.79 ± 0.33 g | 103.02 ± 0.36 h | 215.89 ± 3.07 g | 32.96 ± 0.39 d |
| AB4 | 67.78 ± 0.93 h | 48.21 ± 1.73 h | 115.99 ± 0.79 i | 250.48 ± 1.44 i | 34,38 ± 0,49 e |
| AB5 | 75.65 ± 1.32 i | 56.56 ± 1.78 i | 132.21 ± 0.46 k | 306.17 ± 2.14 k | 37.16 ± 0.34 h |
| AC1 | 36.29 ± 0.58 b | 27.89 ± 0.76 c | 64.18 ± 1.34 c | 116.21 ± 2.65 b | 30.15 ± 0.35 c |
| AC2 | 39.41 ± 0.32 c | 32.11 ± 1.09 d | 71.52 ± 0.77 d | 155.42 ± 3.02 c | 32.51 ± 0.40 d |
| AC3 | 43.11 ± 0.40 d | 42.27 ± 0.64 g | 85.38 ± 0.24 e | 179.22 ± 3.65 e | 35.22 ± 0.15 ef |
| AC4 | 49.28 ± 0.54 e | 56.11 ± 0.57 i | 105.39 ± 0.03 h | 199.43 ± 3.21 f | 36.68 ± 0.48 gh |
| AC5 | 58.20 ± 0.69 g | 61.23 ± 0.15 j | 119.43 ± 0.55 j | 230.87 ± 2.98 h | 39.23 ± 0.06 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrowski, A.; Przybył, J.L.; Studnicki, M. Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry. Appl. Sci. 2024, 14, 113. https://doi.org/10.3390/app14010113
Cendrowski A, Przybył JL, Studnicki M. Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry. Applied Sciences. 2024; 14(1):113. https://doi.org/10.3390/app14010113
Chicago/Turabian StyleCendrowski, Andrzej, Jarosław L. Przybył, and Marcin Studnicki. 2024. "Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry" Applied Sciences 14, no. 1: 113. https://doi.org/10.3390/app14010113
APA StyleCendrowski, A., Przybył, J. L., & Studnicki, M. (2024). Physicochemical Characteristics, Vitamin C, Total Polyphenols, Antioxidant Capacity, and Sensory Preference of Mixed Juices Prepared with Rose Fruits (Rosa rugosa) and Apple or Strawberry. Applied Sciences, 14(1), 113. https://doi.org/10.3390/app14010113







