Abstract
An up-to-date overview of the current state of the art of polysaccharide-based spherical particles as carriers of active/bioactive substances, with a particular emphasis on their applications in the food industry, is provided. Owing to the rapid advances in nanotechnology, much effort has been dedicated to the synthesis and potential uses of these particles. This review outlines recent research on the preparation of spherical nanoparticles, including micro-/nanoencapsulates, micelles, and liposomes, that utilise polysaccharides as carriers and stabilisers. It also discusses the potential application of these nanostructures to the field of food technology. The review aims to provide an objective assessment of the current state of research on this topic. Owing to the distinctive characteristics of spherical nanostructures and the requirement to investigate and scrutinise their potential employment in diverse aspects of the food sector, there are significant opportunities for researchers worldwide to devise innovative solutions.
1. Introduction
Although nanoscale structures have existed in nature on our planet for millennia, the science of nanotechnology has only recently emerged [1]. The field began with the vision of American physicist and Nobel Prize winner Richard Feynman, who, in the 1950s, delivered a celebrated lecture called “Plenty of Room at the Bottom” [2]. This speech is considered the first glimpse into the potential of nanotechnology for miniaturising objects [3,4]. In recent decades, there has been a dynamic development of nanotechnology and nanoscience, which can be referred to as the ‘nanoera’ [5]. Nanotechnology has developed in various industries such as food, cosmetics, and pharmaceutics. Another accomplishment is the industrial use of nanotechnology, which extends beyond the laboratory scale. Notable achievements include active packaging for monitoring food’s shelf life and prolonging the effects of drugs. The precise dosing of medicine and extending the shelf life of bioactive compounds are further milestones. Nanotechnology is an interdisciplinary field with diverse applications in various industries. It has made significant contributions to the pharmaceutical [6,7], medical [8,9,10], cosmetic [11,12], transportation [13,14], electronics [15,16], textile [17,18,19], and other industries [20,21,22,23,24]. Nanotechnology, as applied in the food industry [25], involves encapsulating and precisely delivering bioactive substances [26,27], using nanoparticles with antimicrobial properties [28,29,30], extending food freshness [31], detecting contaminants, and ameliorating food storage systems [32,33,34,35]. Furthermore, through strategic selection of suitable nanoparticles, it is feasible to enhance the sensory attributes of food, such as taste, aroma, or texture, prolonging its shelf life and consistently monitoring its handling at each stage. Spherical particles are a significant area of investigation in nanotechnology. Currently, there is a new and rapidly developing trend in research and development worldwide, which involves micro-/nanopreparations based on polysaccharides [36]. Designing and acquiring nanoscale materials using natural components has the potential to partially solve today’s global challenges. These challenges include civilisation diseases, dietary deficiencies, food waste, petroleum-based material-related environmental pollution, harmful substance release in the atmosphere, and the reduction in fossil fuel consumption [37,38,39,40,41]. The extent of the solution depends on the type of nanostructures and carriers employed. It is common knowledge that chemical synthesis processes necessitate specific reagents and apparatus, resulting in a substantial financial expenditure. Furthermore, the processes require harmful and poisonous substances, causing detrimental effects on the environment. As a result, one of the primary research directions is the implementation of eco-friendly techniques referred to as green chemistry methods. The aim of such solutions is to decrease the utilisation and creation of dangerous substances by utilising accessible organic resources including flora, algae, and microorganisms [42,43]. Polysaccharides are a group of naturally occurring compounds that have garnered significant attention in various fields in recent times. The appeal of polysaccharides is due to their impressive characteristics, including but not limited to, biocompatibility, non-toxicity, biodegradability, renewability, ease of modification, and sourcing from renewable raw materials. Moreover, they are inexpensive and readily obtainable [44]. The properties of polysaccharides previously mentioned are advantageous towards the synthesis of nanoparticles [45]. In recent years, numerous scientific papers have been published detailing the preparation and characterisation of materials derived from natural polysaccharides [46]. There is a demand for this technology, and ongoing scientific research aims to explore the potential of spherical nanoparticles and their properties. Additionally, technical terms are defined when first introduced. The current trend toward green and clean labelling, as well as a decrease in energy use, solvent use, and sales volumes, is driving the use of nanoscale materials. Ensuring the utmost quality of the food product while reducing costs, waste, and additive volumes is paramount in achieving the anticipated outcome. Accordingly, the demand for this technology’s development is consistently on the rise. This review will concentrate on the description and characterisation of spherical particles, specifically micro-/nanoencapsulates, micelles, and liposomes, which are primarily derived from natural polysaccharides. Special attention will be paid to their potential applications in the food industry.
2. Spherical Nanoparticles
The prevalent spherical nanostructures used as carriers for bioactive compounds are nanocapsules, micelles, inverted micelles, and liposomes (Figure 1).
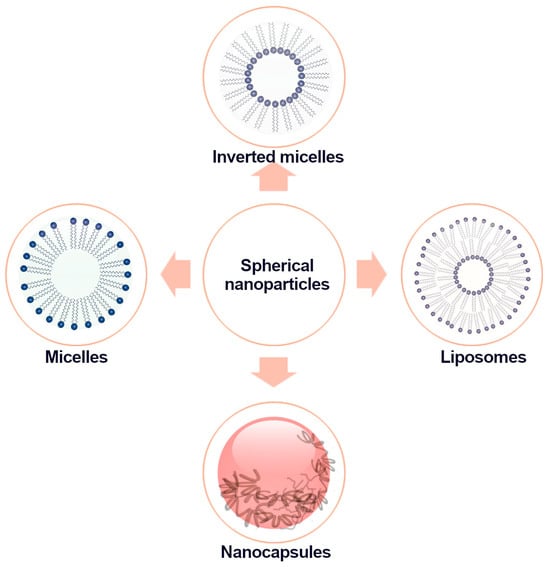
Figure 1.
Schematic diagram of the structure of spherical nanoparticles.
Polysaccharides are a promising biomaterial for developing nutrient delivery systems due to their high stability, hydrophilicity, biodegradability, non-toxicity, and biocompatibility, coupled with their versatile chemical functions. Particle size, shape, and potential have a crucial impact on nutrient uptake by spherical nanoparticles, which are obtained primarily through hydrophobic interactions, hydrogen bonds, and electrostatic interactions [47]. The shape and size of a nanoparticle have an impact on the biological processes of living organisms. The size determines the potential for intracellular transport such as phagocytosis, endocytosis, and transportation through blood vessels, which, in turn, influences permeation through cell membranes, tissues, and organs. According to scientific reports, it is advised that the nanoparticle size should not be too small. It is established that renal excretion ranges from 6 to 8 nm. Additionally, particles smaller than 6 nm may be coated with serum proteins in the bloodstream, resulting in a larger hydrodynamic diameter, which can hinder renal excretion. Nanoparticles larger than 100 nm are likewise captured by macrophages in the lung, spleen, or liver [48]. Moreover, nanoparticles’ high surface area-to-volume ratio has an impact on the quick release potential of the core substance or drug.
Recent scientific research indicates that spherical polysaccharide nanoparticles possess the potential to be utilised as functional biomaterials in the food industry, particularly for the encapsulation of hydrophobic natural phytochemicals (such as zein-Mesona quinensis) [47,49]. The capsule core wall includes polysaccharides that act as a surface decorator. This can potentially improve cellular uptake and increase the anticancer effectiveness of nanomaterials [50]. The polysaccharide used affects the potential of the particles, which further influences their applicability and usage. For instance, chitosan, currently the most widely used polysaccharide, is capable of electrostatic interaction with negatively charged mucosal surfaces, resulting in favourable mucoadhesive properties. Furthermore, the presence of a positively charged NH3+ group in chitosan allows for interaction with negatively charged proteoglycans on the cell surface, leading to improved absorption of the nanoparticle core in the gut by promoting the opening up of connections between epithelial cells [51,52]. Depending on the utilisation and characteristics of the polysaccharide, it can be determined how the active ingredient of the nanocapsule’s core will impact the consumer within the product. For example, dextran possesses multiple hydroxylated groups, making it easily conjugated to proteins [51].
2.1. Nanocapsules
2.1.1. Design, Shape, and Size
The process of encapsulation entails enclosing or coating the active substance (core) with a carrier material (such as wall material, coating, external phase, or membrane) in order to generate capsules or particles at either a macrometric or nanometric scale. The encapsulation process produces macrocapsules (measuring more than 1000 μm), micro-capsules (measuring 1–1000 μm), and nanocapsules (measuring less than 1 μm), which have a profound impact on enhancing product effectiveness [53]. Nanoencapsulation has become increasingly popular in recent times as a result of nanocapsules’ distinct characteristics, including enhanced bioavailability, high encapsulation efficiency, and loading capacity (due to their larger surface area), as well as their sustained release profile and ability to mask undesirable flavours. Additionally, nanocapsules offer improved stability, reduced particle size, and a narrower particle distribution [54]. The shape and size of the capsules are determined by the methodology and the polysaccharide properties. For instance, the nanocapsules resulting from spray drying exhibit diverse diameters and spherical forms. Moreover, they possess a concave structure due to speedy moisture loss following cooling [55].
Nanocapsule core–shell structures can be categorized into inorganic/inorganic, organic/organic, inorganic/organic, and organic/inorganic based on the type of material and coating. The properties of core–shell microparticles, including morphology, size, and structure, greatly influence their application in the food industry [56].
2.1.2. Methods of Obtaining, Factors Affecting Stability, and Properties
Two methods are employed in the creation of encapsulated systems: top-down and bottom-up. The top-down technique involves reducing the size of large structures by shaping them with external mechanical forces. Techniques used under this approach include the emulsification technique and solvent evaporation. The bottom-up approach involves particle association to develop large particles. Techniques such as spray drying, blanketing, and electrospinning have been used based on this approach [53].
Various techniques can be employed depending on factors such as the core composition, thermolability, coating type, and size parameters required for capsule application. These techniques include pulverisation, coacervation, lyophilisation, molecular inclusion, and the extrusion method, which involves passing bioactive compound-containing polysaccharide solutions through a syringe into a gelling solution. The molecular inclusion technique entails trapping apolar molecules within an apolar cavity through non-covalent bonds, whereas the coacervation process includes the separation of a substance into two phases by adding a third component. Polysaccharide materials can be applied through fluid bed coating, which involves suspending particles in air. Other techniques including vacuum drying, electrospinning, and emulsification are also available. Emulsification requires an emulsifier to stabilise the system [55,57,58]. The Vallejo-Castillo et al. [59] study effectively utilised the extrusion technique, employing both in situ and two-step encapsulation, to produce capsules composed of alginate and pectin (55:45 ratio) containing a core of papaya fruit extract and gallic acid (a model polyphenol). Barbosa et al. [60] adopted the coacervation technique in their study, using carboxymethylcellulose and lactoferrin as the wall material to encapsulate β-carotene in sachia inchi oil. The formation of complexes between the biopolymers occurred in two stages. In the first stage, electrostatic interactions dominated, and in the second stage, hydrophobic and hydrogen interactions took over.
Currently, the electrospinning technique is a frequently employed method due to its high encapsulation process efficiency, as well as its capacity to maintain compound stability during storage, based on Lamarra et al.’s techniques [61]. In addition, the electrospinning technique does not entail high-temperature requirements, which is crucial when working with bioactive compounds that are typically thermolabile. The electrospinning process for encapsulation is primarily influenced by the extent of chain entanglement, the chemical composition of the polysaccharide utilised, its molecular weight, and the appropriate concentration required to enable adequate molecular proximity, which allows a chain to bind to the next one [62]. Empirical research has indicated that polysaccharides like starch, recognized by their unique attributes, play a significant role in this process. Polysaccharides, such as alginate, methyl cellulose, pullulan, or dextran, exhibit low shear thinning and are, therefore, not as efficient in encapsulation as those with an anionic group in their structure. This is due to their insufficient entanglement of polymer chains and limited viscosity. As a result, when applying high voltages during electrospinning, such solutions are not able to form continuous streams for fibre formation, as evidenced by Balik et al. [63] and Ma et al. [64]. The benefits of electrospinning and electrospraying encompass several crucial features, including low energy costs, control over encapsulation morphology and physical properties, high encapsulation efficiency (increased surface area-to-area ratio), efficacy for components that are poorly soluble in water, encapsulation of hydrophilic and lipophilic bioactive compounds, compatibility with animal and plant polymers, ability to use both water and organic solvents, preservation of the functional and structural stability of encapsulated biomolecules, uniform size distribution, and reduction in aggregation [65].
In terms of the encapsulation process, chemical (polymerisation, interaction, in-situ emulsion) and physical (physical-chemical, e.g., layer-by-layer adsorption, sol–gel encapsulation; physical-mechanical, e.g., spray-drying, electro-spraying–coaxial electrohydrodynamic atomisation (CEHDA)) methods can be distinguished. The layer-by-layer technique typically leads to the formation of core–shell capsules and operates on the principle of bottom-up assembly. This technique provides control over the physical properties of the coating and enables the customisation of the size and properties of the resulting capsules [56].
In their research, Tan et al. [66] implemented a layer-by-layer approach to enclose anthocyanins in yeast capsules, which were progressively layered with oppositely charged polysaccharides. Chitosan, a positively charged polysaccharide, was complexed with chondroitin sulphate, a negatively charged polysaccharide. This complex was used to coat the yeast surface, which subsequently promoted the formation of anthocyanin-containing capsules.
Obtaining core–shell structures via convective techniques presents various challenges, such as limited control of morphology, polydispersity, and low reproducibility. State-of-the-art techniques, like electrospraying, electrospinning, or microfluidics, offer a promising solution to these difficulties. These techniques provide more precise microscale flow control, resulting in uniform capsules that are biologically and chemically compatible. These methods are applicable to an extensive variety of coating or core materials [56]. The process of encapsulation typically entails active components that are sensitive to various external factors (such as light, temperature, oxygen, pH, and enzymes), as noted by Hu et al. [67] and Maleki et al. [68]. Among the most frequently encapsulated active compounds are bioactive compounds from plants (polyphenols, anthocyanins, carotenoids), which are recognised for their therapeutic and/or preventative effects on cardiovascular diseases, metabolic diseases, degenerative diseases, urinary tract infections, gastric ulcers, and some types of cancer [53,69]. Consequently, to improve the quality of bioactive compounds whilst enhancing their quantity, it is necessary to take proper measures.
2.2. Micelles
2.2.1. Design, Shape, and Size
Micelles, which are formed through the self-organisation of amphiphilic polymers in aqueous solutions, are nanosized aggregates. Due to their unique properties, they are a suitable matrix for encapsulation, particularly in the context of polysaccharide micelles. Polysaccharides exhibit hydrophilic characteristics, and their hydroxyl, carboxyl, and amino groups can react with hydrophobic compounds to generate amphiphilic polysaccharides [70].
Micelles possess a hydrophobic core and a hydrophilic shell, making them an exceptional carrier for hydrophobic functional food ingredients. The solubility of hydrophobic ingredients increases due to the hydrophilic coating of micelles, while hydrophobic ingredients can be adsorbed or bound by the hydrophobic core of micelles. The hydrophilic coating encapsulates and stabilises the hydrophobic core of the chain (in aqueous solutions). Therefore, amphiphilic di- and tri-block copolymers exceeding 100 nm in size may still be classified as micelles according to Malekhosseini et al. [71] and Lu et al. [72].
To form a micelle through self-organisation, hydrophobic molecules attached to the polysaccharide backbone are commonly utilised. Polysaccharides, namely chitosan, dextrin, starch, and maltodextrin [73], are often used in food products. Chitosan-based micelles can be created by adjusting chitosan with hydrophobic groups such as polylactic acid, polycaprolactone, palmitic anhydride, and stearic acid. The chitosan-based polymer that has been modified can naturally produce spherical micelles in aqueous solutions with a size ranging from 20 to 500 nm [73,74,75]. To form dextrin-based micelles, the amphiphilic properties of dextrin are modified via techniques such as the Maillard reaction [76,77] and esterification [78,79]. Amphiphilic polymers are produced by hydrophobic modification of dextrans to create functional delivery systems for bioactive ingredients. The introduction of maltodextrin and spray drying, when compared to micelles acquired through acid, freeze-drying, or heat treatment, achieved a significantly higher level of stability in curcumin micelles [73]. Starch-based micelles form because of alterations that entail the binding of hydrophobic groups. Such changes attribute amphiphilic properties to the starch. Modification by OSA (octenyl succinic anhydride) esterification is currently the most commonly used method for the hydrophobic modification of starch [54,73]; in addition, acetic acid anhydride [80] and, in order to synthesise an amphiphilic conjugate (curcumin–hydrophobic component, hydroxyethyl starch–hydrophilic component), an acid-labile ester linker were used [81]. In the literature, we can also encounter polyion complex micelles (also called block ionomer complex micelles) and complex coacervate core micelles. This type of micelle is prepared from oppositely charged polymers under conditions of stoichiometric charge ratios. The presence of a neutral hydrophilic block (usually poly(ethyleneglycol)) attached to one or both components allows electrostatic interactions to be limited to the nanometric dimension of the micelle core, protected from surrounding media by a hydrophilic crown of neutral blocks [82].
To effectively use micelles in a biological setting, it is essential to consider factors such as particle size and shape, critical micelle concentration (CMC), surface potential, and loading efficiency to predict micelle behaviour in a specific environment. The size and percentage of micelle aggregation is dependent on the volume fraction of the hydrophobic block, the degree to which the CMC is exceeded, the mixing speed during micelle synthesis, and the substrate concentration [70]. The dimensions and morphology of micelles can be investigated through DLS (dynamic light scattering), TEM (transmission electron microscopy), and AFM (atomic force microscopy). The self-assembly of amphiphilic molecules leads to an array of nanostructured aggregates exhibiting distinct geometric configurations. While surfactants are inclined to form spherical micelles, they can also produce ellipsoidal and cylindrical micelles. Moreover, micelles can adopt vesicular configurations [83]. Polysaccharide chains can adopt rod, helical, or helical conformations, which have a significant impact on the movement of hydrophobic molecules within the chains and the process of self-organisation [70]. The definition of surfactants is closely linked to the micelle’s shape, but measuring the degree of packing proves arduous due to the absence of a rigid boundary. The final shape of the self-aggregates is determined by the microphase separation of the amphiphilic block copolymers, as well as the critical packing parameter (CPP). CPP is computed based on factors such as chain volume, length, and cross-sectional area per molecule at the aggregate interface. Spherical micelles are formed when CPP is less than or equal to 1/3, cylindrical micelles are formed when 1/3 < CPP ≤ 1/2, vesicular micelles are formed when 1/2 < CPP < 1, bilayers (lamellae) are formed when CPP equals 1, and inverted micelles are formed when CPP is greater than 1 [70,83,84]. Nevertheless, pure contact potentials do not always determine the interactions that drive aggregation, particularly with ionic amphiphiles where Coulomb forces mediated by electrolytes dominate intermolecular forces. In addition, electrostatic interactions have been attributed to the emergence of quasi-crystalline Franck–Kasper phases in ionic surfactants. Thus, in such cases, theoretical models are employed, which consider the free energy contribution that results in hydrophilic head-to-head repulsion and the translational entropy of surfactant monomers, counterions, and aggregates, as well as the hydrophobic contribution responsible for tail packing [73,85].
2.2.2. Methods of Obtaining
Micelles are created through the self-organisation of amphiphilic polymers in aqueous solutions. Conversely, amphiphilic polymers exist as unimers at low concentrations. When the concentration of amphiphilic polymers is close to the critical micelle concentration (CMC), unimers aggregate to form micelles [71,72,86]. The formation of micelles and reverse micelles depends on environmental and structural factors, with their creation predominantly influenced by intermolecular bonds including hydrogen bonds, hydrophobic interactions (amphiphilic micelles), and electrostatic interactions (polyionic micelles). The reduction in interfacial free energy is the primary driver of micellization and its stabilisation. Hydrophobic interaction prevails as the primary mechanism for reducing the interfacial free energy of a block copolymer in aqueous environments. In cases of micellization, the separated core-forming molecules necessitate additional proximity forces to further stabilise the micelles [70].
There are two primary approaches for creating micelles: physical and chemical. The latter involves the formation of a reversible bond between the amphiphilic polymer and the hydrophobic functional component, which enables the encapsulation of the hydrophobic substance into the micelle’s core. Unfortunately, this technique is unfit for implementation in the food industry due to the creation of new chemical bonds. In contrast, the physical method involves the self-organisation of the amphiphilic polymer in a solution, with the hydrophobic functional component being encapsulated within the core via hydrophobic interactions and/or hydrogen bonds. Physical methods for the preparation of polymer nanoparticles include direct dissolution, solvent evaporation, dialysis, film hydration, ultrasound-assisted, nanoprecipitation, polymerisation-induced self-assembly (PISA), and oil-in-water emulsion methods [70,71,73]. The direct dissolution method is typically utilised for polymers with high water solubility. Micelles are formed when the concentration of polymer exceeds CMC. The polymer self-assembles with gentle agitation in water [87]. In the solvent evaporation method, volatile organic solvents are utilised to enhance the solubility of hydrophobic polymer chains and hydrophobic functional components [73]. The dialysis technique is employed to prepare micelles for poorly soluble polymers and/or functional components. It entails transferring the material and polymer from a solvent in which they can be jointly dissolved to a solvent selective for the hydrophilic chains of the polymer. Under the influence of the selective solvent, hydrophobic polymer chains combine gradually, leading to the emergence of micelle cores. Concurrently, the hydrophobic functional component transfers to the micelle core. Dimethylsulfoxide, ethanol, acetone, and tetrahydrofuran are among the usual solvents employed in this method [88]. The cellular structure of micelles acquired using the dialysis method is more stable and compact in comparison to the solvent method. However, this technique results in significant losses and requires an extended duration [73]. Conversely, the film hydration method utilises volatile organic solvents to dissolve hydrophobic functional components and copolymers. Evaporation eliminates these components in a subsequent step to produce a lean polymer film. Subsequently, polymer micelles are formed upon dissolving the polymer film in water, into which hydrophobic components are loaded [89]. The technique for obtaining micelles assisted by ultrasound is generally reserved for polymers with favourable water solubility, as ultrasound alone is insufficient for encapsulating hydrophobic components and hydrophilic polymers [87]. The encapsulation of water-insoluble components through the oil-in-water emulsion method is a convenient technique for preparing micelles. First, the hydrophobic components and polymer dissolve in water-insoluble organic solvents before being added to the aqueous solution with vigorous mixing (homogenisation, ultrasound), forming an oil-in-water emulsion. The resulting outer phase is a continuous aqueous phase, the inner phase is an organic phase, and the polymer self-organises to create a micelle structure [90]. The categorisation of micelles formed by standard block polymers is determined by analysing the interactions that cause core segments to aggregate from a solution of water. The categories are amphiphilic micelles (caused by hydrophobic interactions), polyionic micelles (caused by electrostatic interactions), and metal complexing micelles [70].
2.2.3. Factors Influencing Stability and Properties
The stability of the micelles is influenced by the structural parameters of the polysaccharides and environmental factors like pH, solution temperature, and salt ions. Specifically, intermolecular interactions such as electrostatic, hydrogen bonds, and hydrophobic interactions [82] play a significant role. The size and hydrophobicity of the polymer particles’ hydrophobic region directly determine the stability of the micelles. The efficiency of micelle formation is impacted by the degree of substitution in hydrophobic polymer groups and the hydrophobic chain length. Reduction in temperature results in a decrease in micelle size and may be attributed to improved hydrophobic interactions between amphiphilic polymer chains or the weakened hydrogen bonds of water molecules to polymer hydrophilic chains. Furthermore, the temperature can impact the charge and storage of functional elements within micelles, while the existence of other ions enhances micelle stability. An increase in ionic strength results in a greater aggregation of micelles, as the surface charge of the micelles correspondingly diminishes [91]. Additionally, the solubility of hydrophobic compounds is affected by ionic strength. As the concentration of salt ions increases, the solubility of the hydrophobic component in cationic surfactant micelles also increases. This phenomenon arises due to electrostatic repulsion between the polar main groups of the surfactants, which impacts the conversion of spherical micelles into elongated micelles [92]. Micelle stability impacts the encapsulation process and delivery of functional ingredients. The stability of micelles is dictated by the dendritic architecture and the molecular weight of the polysaccharides [82]. Dendritic-polysaccharide micelles can be classified based on their stiffness, and this polysaccharide-dendritic synchronisation influences the stability and compactness of micelles, as well as the kinetics of the intracellular release of biomolecules [82]. Better stability of dendritic-polysaccharide micelles is related to the intrinsic stiffness and globular shape of the dendrimers. Lower polydispersity and longer micelle stability were observed when the molecular weight of charged polysaccharides such as chitosan, alginate, and hyaluronic acid was decreased, which is due to the high rigidity of these polysaccharides. Lopez-Blanco et al. [82] conducted a study which demonstrated the possibility of preparing charged polysaccharide PIC (dendritic-polysaccharide polyion complex) micelles using PEG-dendritic block copolymers. The polysaccharides used were chitosan, alginate, and hyaluronic acid, and the resulting micelles were found to be more stable than those produced by linear copolymers [73]. Micelles have garnered significant interest owing to their distinct features, namely small size, effective solubilisation ability, high encapsulation efficacy, and precision-targeted release of hydrophobic bioactive agents. Thus, micelles serve as critical agents for enclosing functional ingredients to enhance their bioavailability and stability [93].
2.3. Inverted Micelles
In an apolar environment, amphiphiles spontaneously form inverted micelles, which are polar at the core and hydrophobic on the outer surface. While the literature uses “inverted microemulsion” and “inverted micelle” interchangeably, they represent two distinct colloidal systems that differ in thermodynamic stability. Reverse micelles exhibit an organized structure, as opposed to reverse microemulsions, because of the petite water droplets that make up the surfactant monolayer [94].
Inverted micelles are surfactant aggregates with a water molecule at the core in a non-polar solution. The size and structure of these micelles depend on various factors such as the quantity of water added, expressed as mole [water]/mol [surfactant], the type and amount of apolar solvent used, the structure of the encapsulated polar solvent, the temperature at which the process of inverted micelle formation occurs, and even the quantity of water employed [94]. Water molecules enclosed within inverted micelles are immobilised due to local water interactions with the counter ions and dipoles of the surfactant core groups. Inverted micelles possess cores that resemble the physiological environment (water), which allows them to protect encapsulated biomolecules from denaturation [94].
Due to the ability of the aqueous cores of inverted micelles to encapsulate biomolecules and then release the trapped compounds, they have become useful in the food industry. In particular, they are utilised for extracting, separating, and purifying food compounds such as enzymes, peptides, proteins, and edible oils. They are also used for protecting food enzymes and antioxidants, developing analytical and detection media, and creating multifunctional nanomaterials [94,95]. Given that most inverted micelles are made of undesirable components (e.g., iso-octane, sodium bis-[2-ethylhexyl] sulfosuccinate), the development of secure inverted micelles employing polysaccharides in non-polar media, such as ethanol, glycerol, or vegetable oils, presents a promising solution for the use of inverted micelles in food applications. Nonetheless, the utilisation of inverted micelles presents several benefits, such as the ability to retrieve surfactants and non-polar solvents, which consequently reduces cost [94]. Additionally, inverted polysaccharide micelles have practical implications for the food industry in serving as nanocarriers for the encapsulation, targeted delivery, and controlled release of hydrophilic food compounds within fat-based food products such as spreads, cocoa butter, and vegetable oils [70]. In the context of food applications, including the detection of food and the inhibition of edible oil oxidation in non-aqueous foods, the synthesising of inverted micelles with biodegradable polysaccharides is an area of great interest that necessitates further research [70].
2.4. Liposomes
In recent years, liposomes have become increasingly popular and the subject of numerous research papers due to their distinctive properties. Liposomes have gained attention due to their potential applications in drug delivery, cosmetics, and food industries. Their unique properties make them an attractive option for targeted drug delivery due to their ability to encapsulate hydrophilic and hydrophobic drugs, improve drug solubility, and protect drugs from degradation. The term ‘liposome’ is derived from the Greek words ‘Lipos’ meaning fat and ‘Soma’ meaning body [96]. In 1965, Alec D. Bangham, a British biophysicist, discovered them [97]. The scientific literature has introduced the concept of nanoliposomes, defined as lipid bilayer vesicles which possess and maintain a nanometric size that fluctuates during storage and use, are recognised as a novel technology that offers health benefits by encapsulating bioactive ingredients with enhanced functional properties [98]. Overall, these structures display comparable characteristics; nevertheless, nanoliposomes present superior properties such as increased surface area, enhanced solubility, and precise release in contrast to liposomes [99]. Liposomes are defined as spherical vesicular structures made up of phospholipids, where a lipophilic bilayer is sandwiched between two hydrophilic layers. The efficacy and potential of liposomes as a drug delivery system for diverse active agents have been thoroughly researched and verified in the peer-reviewed scientific literature [100,101]. The purpose of liposomal encapsulation is to safeguard delicate bioactive elements and improve their effectiveness by lessening the influence of negative environmental elements and hindering the contact of enclosed ingredients with detrimental external agents [102].
The characteristics of liposomes are impacted by their ultimate organisation, and their structure and physicochemical properties are determined by elements such as lipid type, morphology, size, concentration, and charge [103]. There is a plethora of classification systems for liposomes described in the literature. These objects can be classified according to the number of layers that compose them, which include monolayer, multilayer, oligolamellar, and multilamellar vesicles [104,105]. On the other hand, membrane size and structure form the basis for another widely used classification system. An alternative system is in use, which classifies compounds based on their intracellular mechanism of action and lipid composition, as well as their mode of preparation [98]. Liposomes present a promising approach to encapsulating different types of essential oils and fatty acids [106].
3. Polysaccharides Used to Obtain and/or Support Spherical Particles
The increasing expense of crude oil and the possibility of depletion of its natural reserves necessitate a quest for substitute sources of industrial raw materials. In the food industry, carbohydrates, lipids, and proteins are commonly used as building or coating materials [107]. Biopolymers such as chitosan, alginate, starches, cellulose, and pectin are frequently employed due to their availability from renewable resources and low cost. The safety, convenience of use, and high levels of biodegradability and biocompatibility exhibited by these compounds provide a significant advantage [108,109,110]. It is noteworthy that biopolymers derived from starch, cellulose, and lactic acid have been easily commercialised as a result of the cost-effectiveness of their production and purification processes [111]. The fascination and widespread attention these polysaccharides garner stems from their distinctive and coveted chemical properties [112]. Polysaccharides are a group of organic compounds which are abundant, economical, ecologically sound, and renewable. They represent enticing raw materials, that offer the possibility of several new biodegradable substances. The enthusiasm for natural polysaccharides and their potential uses has grown enormously in recent times. Polysaccharides provide a wide range of essential parameters necessary for their practical application. These include low, medium, and high molecular weight, varied polydispersity, formation of linear and branched macrostructures, monofunctionality (compounds containing only hydroxyl groups), and polyfunctionality (compounds with hydroxyl, carboxyl, and/or amino groups). Polysaccharides also exhibit a high degree of chirality and can have low or high water solubility, as well as low, if any, toxicity and immunogenicity. Their remarkable properties make them readily applicable in nanotechnology [113]. Algae and fungi are among the alternate sources of polysaccharides. Due to economic and environmental factors, there remains significant interest in investigating polysaccharides derived from marine organisms, such as seaweed [114]. Polysaccharides, owing to their gelling properties, are commonly used as carriers of bioactive substances in the form of solutions, coating solutions, and biopolymer films used for packaging [115]. Numerous examples exist in the scientific literature demonstrating the use of polysaccharides as carriers of spherical particles possessing valuable biological properties. Mirzaei-Mohkam and Garavand [116] prepared films made from carboxymethylcellulose that were reinforced with nano-encapsulated vitamin E through a membrane casting process. Esquerdo and Monte [117] created nanoencapsulated unsaturated fatty acids from carp oil by using chitosan and its blends with gelatine. The study demonstrated that the properties of the resulting microstructures were significantly influenced by the composition of the wall material. Even a partial substitution of chitosan with gelatine resulted in smaller droplets. Additionally, chitosan was found to be an efficient barrier against fish oil degradation. On the other hand, Anand Kumar Chaudhari and colleagues conducted a study to compare the effectiveness of unencapsulated and nano-encapsulated Origanum majorana essential oil (OmEO) against microbial and metabolite contamination and lipid peroxidation. They developed and characterized an OmEO-loaded chitosan nanoemulsion, which demonstrated the potential to inhibit the growth of A. flavus and its aflatoxin production. The authors maintain that the nanoemulsion produced will be of significant value on an industrial scale as a potent antifungal agent to prolong the shelf life of food products [118]. Nowak et al. [119] have developed novel chitosan-based films that are eco-friendly and incorporate nano-/microcapsules of ozonated olive oil. The authors have demonstrated the bactericidal effect of these composites and suggest that they could effectively serve as biodegradable active packaging for food products to prolong their shelf life. Stanisławska et al. [120] have developed nano-/microcapsules of curcumin within a binary polysaccharide composite containing alginate and chitosan. They indicate that the nano-/microcapsules are compatible with polysaccharides and report that the resulting film, which contained the highest concentration of capsules, exhibited improved aqueous mechanical properties. In recent research, for instance, polymer-based fat replacements (such as konjac) have been utilised as substitutes for fat in beef patties, which were then put through a process involving storage and thermal treatment. By using the polysaccharide, the burgers were able to be enhanced with polyunsaturated fatty acids and bioactive components discovered in acai berry oil, such as polyphenolic substances or anthocyanins [27,121].
4. Food Applications
Nanotechnology is employed at every stage of food production. Firstly, during product preparation, fat substitutes exist in the form of capsules or food additives of bioactive compounds to improve stability and prolong activity, and are subjected to an encapsulation process. Secondly, products are stored in active packaging which extends their shelf life and indicates their shelf life when packaged. The main advantages of food processing and preservation lie in the enrichment of products with bioactive compounds that extend shelf life and freshness. Capsules containing antioxidants improve the nutritional value whilst shielding against microbial growth in food. Nanotechnology in food preservation extends product shelf life and affects the physicochemical properties, altering taste, colour, and aroma to meet consumers’ demands. This practice has valuable applications in the food industry.
With growing consciousness and interest in healthy living, today’s consumers are more mindful of the quality and safety of food products [122]. As a result, the food industry is actively seeking ingenious approaches to guarantee the optimal quality and safety of food, in the belief that new techniques can increase shelf life while reducing waste production [123]. Currently, considerable research is focused on the development of methods to deliver bioactive compounds in a selective, non-invasive manner while limiting undesirable side effects [46,124,125]. In the food industry, nanotechnology has the potential to be applied in two ways: through food nanosensors or nanostructured food ingredients [126]. Additionally, the use of spherical nanoparticles can improve the microbiological quality of food products, which is important considering the negative economic and public health impacts of microbiologically contaminated food. Research aimed at developing polysaccharide carriers for spherical particles that exhibit antimicrobial properties has the potential to reduce the use of preservatives, or enhance their performance, by encapsulating these compounds in a nanostructure [127].
Durable and stable capsules have the potential to be utilised not only in the manufacturing of functional foods with designed health-promoting properties but also in enhancing conventional foods, such as mousses, yoghurts, kefirs, and fruit juices, with bioactive substances [128]. The encapsulation process has a dual role: protecting the bioactive compound in the core and preserving its properties whilst also controlling the release and precision targeting of bioactive compounds. Additionally, encapsulation masks unwanted aromas, reduces evaporation or loss of volatility, prevents the formation of solid particles, and improves physical stability [53].
Polysaccharides are suitable for the encapsulation of both hydrophobic and hydrophilic bioactive compounds. Furthermore, the encapsulation techniques using polysaccharides have become significant due to their diverse physical and chemical properties, cost-effectiveness, functional attributes, ease of incorporation into food products, and prebiotic potential [65].
It is noteworthy that one in ten people suffer from food contamination annually. Therefore, the rise in food-borne illnesses necessitates highly effective and safe food preservatives that are also natural. However, the use of naturally occurring bioactive compounds, such as phenolic compounds or essential oils, can lead to rapid degradation or deactivation due to their sensitivity towards environmental factors in the matrices they are used in and the subsequent technological processes [62].
The polysaccharide coating plays a crucial role in the encapsulation process and the antioxidant properties of the core, especially in the case of plant-derived bioactive compounds with varying core types. A study conducted by Stach et al. [128] indicates that a protective coating comprised of alginate + carrageenan and alginate + pectin demonstrates superior protective properties for the phenolic compounds within the chokeberry juice core of the capsule, compared to coatings containing a blend (in varying ratios) of pectin, alginate, carrageenan, and chitosan during storage.
Capsules can provide protective benefits not only for bioactive compounds found in plants, but also for probiotics and enzymes. These findings indicate the potential advantages of utilising such capsules for their protective properties. According to a study conducted by Farahmand et al. [129], alginate (for the shell) and chitosan (for the coating layer) proved to be suitable materials for producing capsules with Bifidobacterium animalis subsp. lactis and Lactobacillus plantarum as the core.
From an industrial standpoint, the accessibility and cost-effectiveness of encapsulating agents hold significant sway in selecting encapsulating materials. Additionally, choosing appropriate encapsulating agents necessitates a deeper knowledge of the physicochemical and rheological characteristics of both encapsulating materials and the compounds to be encapsulated.
The availability and cost of encapsulating agents, along with the use of materials recognised as generally recognised as safe (GRAS), are key factors affecting the feasibility of encapsulation within the food industry [53]. The encapsulation of various substances typically involves polysaccharides, with starch, dextrins, maltodextrins, cyclodextrins, oligosaccharides, and celluloses (such as carboxymethyl cellulose, methyl cellulose, cellulose ethers, cellulose acetate, hydroxypropyl celluloses, cellulose nanocrystals, cellulose nanofibrils, pectin, chitosan, alginate, and gums) being the most commonly used.
For instance, micelles have the capability to decrease the deterioration and evaporation of volatile functional ingredients and enhance the stability of delicate substances, including carotene [130], vitamin D [131], and turmeric [78,132], throughout the different stages of production, storage, and consumption. However, when considering the release of biomolecules, it has been found through analysis of dendritic-polysaccharide micelles with shorter chains that they exhibit greater compactness and slower release. Yet, it is necessary to overcome the challenges of food processing in order to use micelles in food applications, as opposed to their widespread use in medicine where they are not subjected to heat treatment. Heat treatment, a common food processing operation such as boiling, baking, frying, and grilling, plays a significant role in the micellization process and stability of micelles. Furthermore, it is necessary to verify the impact of the environment on the release of active substances from micelles when they function as a carrier for a bioactive ingredient. Therefore, further research is required to investigate the mechanisms of food processing effects on polysaccharide micelle properties, aiming to enhance their application in food, particularly in functional foods [70,133].
Esquerdo et al. [117] created nanoencapsulated unsaturated fatty acids from carp oil by employing chitosan and its blends with gelatine. They demonstrated that the features of the resulting microstructures were highly reliant on the wall material’s composition. Additionally, even substituting chitosan with gelatine resulted in smaller droplets. Moreover, the researchers discovered that chitosan was an efficient shield against fish oil degradation. Conversely, Chaudhari et al. [118] evaluated the effectiveness of unencapsulated and nanocapsuled Origanum majorana essential oil against lipid peroxidation and mycobiological contamination. A chitosan nanoemulsion loaded with essential oil was developed and characterised, demonstrating measurable growth inhibition potential against the test fungus and inhibiting aflatoxin production by A. flavus. The resulting nanoemulsion is proposed as a useful antifungal agent on an industrial scale, extending the shelf life of food products. Šeremet et al. [134] present their findings on the development and analysis of liposomes coated with alginate and plant protein, containing common ivy extract in agar-agar candies. Additionally, a nanogel of chitosan and myristic acid was created, which encapsulated clove essential oils to extend the shelf life of refrigerated beef chops. It is noteworthy that the antimicrobial activity against Salmonella enterica was higher in the developed coating [135]. Moreover, liposomes were employed as biological carriers for meat product applications [136]. Polysaccharide-liposomal systems, which contained cinnamon oil, were employed in the evaluation of pork by modifying liposomes of the oil with sodium alginate and chitosan. The study demonstrated that this system could effectively inhibit the increase in pH, protein, amine degradation, and lipid peroxidation compared to a group containing only liposomes of the oil. However, according to the microbiological analysis, the storage period of the alginate/chitosan/liposome group extended to 12 days compared to the control group. The polysaccharide/liposome formulation developed by the researchers demonstrated a positive antiseptic effect on the preservation of chilled pork [137]. Toprakçı et al. [138] utilised olive leaf extract as an active substance for creating microcapsules coated with alginate in their research. Likewise, liposomes have been utilised in order to guarantee food safety by serving as natural antimicrobial agents. The researchers Lopes et al. [139] established a mixture of lysozyme and nisin enclosed in phosphatidylcholine liposomes coated with either pectin or polygalacturonic acid for their study. It is worth noting that liposomes containing lysozyme inhibited Listeria monocytogens, but not Salmonella enteritidis. Conversely, liposomes that contained the nisin/lysozyme combination inhibited both bacteria. Additionally, PC-pectin liposomes lowered L. monocytogenes microbial counts below the detection threshold for up to 25 days in refrigerated skimmed milk. In their study, Lopes et al. propose that utilising polysaccharide-containing liposomes could potentially serve as a useful strategy for regulating the release of lysozyme and nisin in food products in a controlled manner [139]. In contrast, Kamkar et al. [140] have developed an active nanocomposite film based on chitosan, which includes nano-liposome garlic essential oil, with a potential application as an active packaging material to extend chicken fillet shelf-life. Hanula et al. [27] created a hydrogel emulsion using konjac flour and sodium alginate, which contained encapsulated oil and was used as a functional ingredient for polyunsaturated fatty acids in beef burgers. The study found that the fat substitute extended the shelf life of the burgers, and contributed towards a healthier meat product that meets nutritional recommendations. Table 1 presents instances of how spherical nanoparticles are employed in polysaccharide carriers.

Table 1.
Examples of polysaccharides and nanostructures and their application in food technology.
5. Benefits and Risks
One contemporary issue is the prevalence of diet-related diseases of civilisation. The positive impact of good eating habits and the design of modified foods can minimize the occurrence of these maladies and improve the overall health and functioning of the body. The integration of bioactive compounds using nanotechnology enables the creation of food products with novel functional characteristics. Consequently, the inclusion of these functional components could enhance the characteristics of foods and help to decrease the occurrence of lifestyle diseases [144]. Moreover, it is crucial that the creation of novel food preservation and modification methods not only serves health benefits but is also acceptable to the end consumers. Unfortunately, anxiety may arise for various reasons among consumers towards new techniques. The scientific literature outlines a psychological attitude that significantly affects food consumption and acceptance among consumers. This phenomenon, referred to as food technology neophobia, is characterised by consumer scepticism and negative sentiments towards novel food products. The implementation of unfamiliar technology within food products, despite assurances of efficacy and safety, could result in customer rejection [145]. Accordingly, food science researchers analyse consumer sentiment towards novel food products and technology, which proves to be decisive for future investments and the commercialisation of nanofoods [146]. Consumers ultimately determine which technologies are implemented and succeed in the global agri-food industry. Therefore, it is essential to consider consumer sentiment and opinion from the early stages of product development [147]. Although bacterial biopolymers have many advantages, their potential for biomedical and biotechnological applications, in comparison to synthetic materials, is restrained due to their GRAS status. For instance, despite extensive research into the bacterial biosynthesis of alginates, these alginates currently do not qualify for GRAS status. The materials required for the creation of the outer protective layers, used in the production of nanocarriers, must belong to the generally recognised safe (GRAS) categories. Biopolymers declared as generally recognised as safe (GRAS) by the US Food and Drug Administration exhibit organic and eco-friendly properties, whilst also serving essential nutritional and structural functions in the human body [148,149,150]. In conclusion, spherical particle technologies are of significant importance within the food sector, benefiting food quality and safety. Furthermore, they have the potential to effectively combat many civilisation-related diseases by enhancing the bioavailability of functional ingredients while reducing the negative environmental impact caused by food waste. However, promoting new food modification techniques and raising public awareness are crucial for the widespread commercialisation of nanofoods [151].
6. Legal Relationship/Legal Approach
The rapid growth of interest and application opportunities for spherical particles in the food industry raises concerns primarily centred on human and environmental safety [152]. The incorporation of nanotechnology in the production of food encounters hurdles concerning safety, toxicity, and the necessity for standardised regulation. Additionally, the bioavailability of nanoparticles, their stability during storage and cooking, and production expenses represent significant concerns [152,153]. Concurrently, establishing communication with consumers and earning their trust demand transparency in the utilisation of nanotechnology. Novel product design and implementation must account for updated regulatory requirements to guarantee the safety and effectiveness of such goods [154,155].
7. Conclusions and Outlook
The quest for nanotechnology solutions has generated significant interest in developing nanoscale conveyors for transporting bioactive substances that are inadequately absorbed from meals. For the broader implementation of nanotechnology within the food industry, it is imperative to produce nanoparticles from inexpensive ingredients using mild preparation conditions. The core materials of the capsules usually consist of plant extracts or phytochemicals obtained from the source material using eco-friendly extraction methods. Polysaccharides are a relatively low-cost option and are both biodegradable and non-toxic, making them a crucial factor in protecting the environment without jeopardising it or escalating pollution levels. Additionally, these nanoparticles must maintain their structural integrity during the digestion process until absorption, and the particle’s core should not be released prematurely before reaching its intended target. To achieve the practical implementation of nanotechnological methods for delivering bioactive compounds through food fortification, the acceptability of polysaccharide spherical nanoparticles relies heavily on public perception and government regulations. Clear governmental regulation and positive public perception are essential for the successful implementation of these approaches.
In recent years, there has been a significant rise in interest in nanotechnology employing spherical particles for various everyday life applications. The utilisation of natural polysaccharides is a prominent trend in developing numerous new techniques and nano-objects. Presently, research on food industry applications of spherical particles primarily concentrates on prolonging shelf life and enhancing foods with bioactive constituents. Based on our understanding, the literature available on potential applications to food products pales in comparison to the vast amount of research focused on drug or cosmetic implementation. The lack of substantial research into nanotechnology applications in the food industry vis-à-vis the pharmaceutical and cosmetic industries is a consequence of multiple factors. Stricter rules regarding food safety prolong and complicate the integration of novel technologies. Furthermore, the absence of explicit benchmarks and protocols for food nanotechnology impedes the cultivation of consistent industry norms. Moreover, the field of food nanotechnology may have faced competition for research funding with other research areas that are considered more prioritised or of greater strategic importance, thereby restricting the pace of advancement and implementation of new technologies in the industry. It is undeniable that the integration of nanotechnology into various production processes presents vast prospects for the food industry to enhance food safety and the bioavailability of active ingredients. Nonetheless, additional investigations are necessary to examine the broader applicability of polysaccharide nanocarriers and spherical particles, and to ensure their commercial viability.
Author Contributions
Conceptualization, M.J., M.H., K.K. and G.K.; writing—original draft preparation, M.J., M.H., K.K. and G.K.; writing—review and editing, M.J., M.H., K.K. and G.K.; supervision, K.K. and G.K.; project administration, K.K. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a subsidy from the Ministry of Science and Higher Education for the University of Agriculture in Krakow for 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hochella, M.F.; Spencer, M.G.; Jones, K.L. Nanotechnology: Nature’s Gift or Scientists’ Brainchild? Environ. Sci. Nano 2015, 2, 114–119. [Google Scholar] [CrossRef]
- Feynman, R.P. Plenty of Room at the Bottom. In APS Annual Meeting; Little Brown: Boston, MA, USA, 1959; pp. 1–7. [Google Scholar]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.D.; Sahoo, A.K.; Shrivastav, O.P.; Charmode, N.; Prasad, S.R.; Kamat, R.; Kajave, N.G.; Chauhan, J.; Banga, S.; Tamboli, U.; et al. A Review on Aspects of Nanotechnology in Food Science and Animal Nutrition. ES Food Agrofor. 2022, 8, 12–46. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; AbuAlSamen, M.M.; Alzoubi, K.H. Awareness about Nanotechnology and Its Applications in Drug Industry among Pharmacy Students. Curr. Pharm. Teach. Learn. 2020, 12, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Abdussalam-Mohammed, W.; Mohammed, W.A. Review of Therapeutic Applications of Nanotechnology in Medicine Field and Its Side Effects. J. Chem. Rev. 2019, 1, 243–251. [Google Scholar] [CrossRef]
- Li, F.; Shao, H.; Zhou, G.; Wang, B.; Xu, Y.; Liang, W.; Chen, L. The Recent Applications of Nanotechnology in the Diagnosis and Treatment of Common Cardiovascular Diseases. Vascul. Pharmacol. 2023, 152, 107200. [Google Scholar] [CrossRef]
- Gera, S.; Kankuri, E.; Kogermann, K. Antimicrobial Peptides—Unleashing Their Therapeutic Potential Using Nanotechnology. Pharmacol. Ther. 2022, 232, 107990. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the Development of New Cosmetic Formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Shafique, M.; Luo, X. Nanotechnology in Transportation Vehicles: An Overview of Its Applications, Environmental, Health and Safety Concerns. Materials 2019, 12, 2493. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Joy, J.; George, S.C. Potential Applications of Nanotechnology in Transportation: A Review. J. King Saud Univ.-Sci. 2019, 31, 586–594. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Taha, T.B.; Barzinjy, A.A.; Hussain, F.H.S.; Nurtayeva, T. Nanotechnology and Computer Science: Trends and Advances. Mem.-Mater. Devices Circuits Syst. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Idumah, C.I. Influence of Nanotechnology in Polymeric Textiles, Applications, and Fight against COVID-19. J. Text. Inst. 2021, 112, 2056–2076. [Google Scholar] [CrossRef]
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of Nanotechnology in Smart Textile Industry: A Critical Review. J. Adv. Res. 2022, 38, 55–75. [Google Scholar] [CrossRef]
- Ben Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Osagie, C.; Othmani, A.; Ghosh, S.; Malloum, A.; Kashitarash Esfahani, Z.; Ahmadi, S. Dyes Adsorption from Aqueous Media through the Nanotechnology: A Review. J. Mater. Res. Technol. 2021, 14, 2195–2218. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in Applications of Nanotechnology in Global Food Industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Sharma, A.; Nagarajan, J.; Gopalakrishnan, K.; Bodana, V.; Singh, A.; Prabhakar, P.K.; Suhag, R.; Kumar, R. Nanotechnology Applications and Implications in Food Industry. Nanotechnol. Appl. Food Saf. Qual. Monit. 2023, 171–182. [Google Scholar] [CrossRef]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-Based Approaches Applied to Nutraceuticals. Drug Deliv. Transl. Res. 2021, 12, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelski, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Hydrogel Emulsion with Encapsulated Safflower Oil Enriched with Açai Extract as a Novel Fat Substitute in Beef Burgers Subjected to Storage in Cold Conditions. Molecules 2022, 27, 2397. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An Innovative Tool to Release Natural Extracts with Antimicrobial Properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Fazly Bazzaz, B.S.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The Role of Nanotechnology in Combating Biofilm-Based Antibiotic Resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Bhandari, B.; Yang, C. Shelf Life Extension of Aquatic Products by Applying Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1521–1535. [Google Scholar] [CrossRef]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current Applications and Future Scope in Food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Hanula, M.; Pogorzelska-Nowicka, E.; Pogorzelski, G.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Active Packaging of Button Mushrooms with Zeolite and Açai Extract as an Innovative Method of Extending Its Shelf Life. Agriculture 2021, 11, 653. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ. Chem. Lett. 2020, 19, 1715–1735. [Google Scholar] [CrossRef]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Yadav, N.; Mudgal, D.; Anand, R.; Jindal, S.; Mishra, V. Recent Development in Nanoencapsulation and Delivery of Natural Bioactives through Chitosan Scaffolds for Various Biological Applications. Int. J. Biol. Macromol. 2022, 220, 537–572. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Gul, I.; Basharat, A.; Qamar, S.A. Polysaccharides-Based Bio-Nanostructures and Their Potential Food Applications. Int. J. Biol. Macromol. 2021, 176, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on Mechanisms of In Vitro Antioxidant, Antibacterial and Anticancer Activities of Water-Soluble Plant Polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Shashikumar, C.; Mitra, S.; Singha, S. Microbial Polysaccharides (MPs) in Food Packaging. Biopolym. Food Packag. Innov. Technol. Appl. 2022, 225–263. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of Polysaccharides, Lipids and Proteins in Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef]
- Kumar, P.; Mahajan, P.; Kaur, R.; Gautam, S. Nanotechnology and Its Challenges in the Food Sector: A Review. Mater. Today Chem. 2020, 17, 100332. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R.; Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2021, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M. Recent Trends in Chemical Modification and Antioxidant Activities of Plants-Based Polysaccharides: A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Yang, J.; Lin, J.; Zhang, J.; Chen, X.; Wang, Y.; Shen, M.; Xie, J. Fabrication of Zein/Mesona Chinensis Polysaccharide Nanoparticles: Physical Characteristics and Delivery of Quercetin. ACS Appl. Bio Mater. 2022, 5, 1817–1828. [Google Scholar] [CrossRef]
- Fernández, E.F.; Santos-Carballal, B.; de Santi, C.; Ramsey, J.M.; MacLoughlin, R.; Cryan, S.A.; Greene, C.M. Biopolymer-Based Nanoparticles for Cystic Fibrosis Lung Gene Therapy Studies. Materials 2018, 11, 122. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of Nanoparticle Transport across Intestinal Tissue: An Oral Delivery Perspective. ACS Nano 2023, 17, 13044–13061. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface Decoration by Spirulina Polysaccharide Enhances the Cellular Uptake and Anticancer Efficacy of Selenium Nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Luisa Martínez-López, A.; Ribeiro, B.; B Pinto, R.J.; Stana Kleinschek, K.; et al. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626–647. [Google Scholar] [CrossRef]
- Noi, I.; Schlachet, I.; Kumarasamy, M.; Sosnik, A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers 2018, 10, 478. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Hemar, Y.; Yang, Z.; Singh, H. Self-Assembled Micelles Based on OSA-Modified Starches for Enhancing Solubility of β-Carotene: Effect of Starch Macromolecular Architecture. J. Agric. Food Chem. 2019, 67, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and Nano Encapsulation, Retention and Controlled Release of Flavor and Aroma Compounds: A Critical Review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Vallejo-Castillo, V.; Rodríguez-Stouvenel, A.; Martínez, R.; Bernal, C. Development of Alginate-Pectin Microcapsules by the Extrusion for Encapsulation and Controlled Release of Polyphenols from Papaya (Carica papaya L.). J. Food Biochem. 2020, 44, e13331. [Google Scholar] [CrossRef]
- El Ghazzaqui Barbosa, A.; Constantino, A.B.T.; Bastos, L.P.H.; Garcia-Rojas, E.E. Encapsulation of Sacha Inchi Oil in Complex Coacervates Formed by Carboxymethylcellulose and Lactoferrin for Controlled Release of β-Carotene. Food Hydrocoll. Health 2022, 2, 100047. [Google Scholar] [CrossRef]
- Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun Nanofibers of Poly(Vinyl Alcohol) and Chitosan-Based Emulsions Functionalized with Cabreuva Essential Oil. Int. J. Biol. Macromol. 2020, 160, 307–318. [Google Scholar] [CrossRef]
- de Souza, E.J.D.; Kringel, D.H.; Dias, A.R.G.; da Rosa Zavareze, E. Polysaccharides as Wall Material for the Encapsulation of Essential Oils by Electrospun Technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef] [PubMed]
- Balik, B.A.; Argin, S.; Lagaron, J.M.; Torres-Giner, S. Preparation and Characterization of Electrospun Pectin-Based Films and Their Application in Sustainable Aroma Barrier Multilayer Packaging. Appl. Sci. 2019, 9, 5136. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-Encapsulation of Probiotics in Gum Arabic-Pullulan Blend Nanofibres Using Electrospinning Technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ahmadzadeh, S.; Kandhola, G.; Kim, J.W. Polysaccharide-Based Porous Biopolymers for Enhanced Bioaccessibility and Bioavailability of Bioactive Food Compounds: Challenges, Advances, and Opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4610–4639. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, J.; Sun, B. Polysaccharide Dual Coating of Yeast Capsules for Stabilization of Anthocyanins. Food Chem. 2021, 357, 129652. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, Y.; Xie, J.; Sun, C.; Zheng, X.; Chen, W. Systematic Evaluation of Phenolic Compounds and Protective Capacity of a New Mulberry Cultivar J33 against Palmitic Acid-Induced Lipotoxicity Using a Simulated Digestion Method. Food Chem. 2018, 258, 43–50. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of Chitosan-Based Carrier as an Encapsulating Agent in Food Industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Croft, K.D.; Yamashita, Y.; O’Donoghue, H.; Shirasaya, D.; Ward, N.C.; Ashida, H. Screening Plant Derived Dietary Phenolic Compounds for Bioactivity Related to Cardiovascular Disease. Fitoterapia 2018, 126, 22–28. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhao, X.; Ye, F.; Zhao, G. Hydrophobically Modified Polysaccharides and Their Self-Assembled Systems: A Review on Structures and Food Applications. Carbohydr. Polym. 2022, 284, 119182. [Google Scholar] [CrossRef]
- Malekhosseini, S.; Rezaie, A.; Khaledian, S.; Abdoli, M.; Zangeneh, M.M.; Hosseini, A.; Behbood, L. Fabrication and Characterization of Hydrocortisone Loaded Dextran-Poly Lactic-Co-Glycolic Acid Micelle. Heliyon 2020, 6, e03975. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to Improve Micelle Stability for Drug Delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Ji, N.; Dai, L.; Dong, X.; Chen, M.; Xiong, L.; Sun, Q. Self-Assembled Micelles Based on Amphiphilic Biopolymers for Delivery of Functional Ingredients. Trends Food Sci. Technol. 2021, 114, 386–398. [Google Scholar] [CrossRef]
- Yang, T.; Feng, J.; Zhang, Q.; Wu, W.; Mo, H.; Huang, L.; Zhang, W. l-Carnitine Conjugated Chitosan-Stearic Acid Polymeric Micelles for Improving the Oral Bioavailability of Paclitaxel. Drug Deliv. 2020, 27, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S.; Sarmento, B. Novel Amphiphilic Chitosan Micelles as Carriers for Hydrophobic Anticancer Drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Zhang, Y.; Xia, S.; Cai, J.; Zhang, X.; Su, J. Improved Stability and Controlled Release of Lutein-Loaded Micelles Based on Glycosylated Casein via Maillard Reaction. J. Funct. Foods 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Assembly of Protein-Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef]
- Stanciu, M.C.; Nichifor, M.; Mocanu, G.; Tuchilus, C.; Ailiesei, G.L. Block Copolymers Containing Dextran and Deoxycholic Acid Polyesters. Synthesis, Self-Assembly and Hydrophobic Drug Encapsulation. Carbohydr. Polym. 2019, 223, 115118. [Google Scholar] [CrossRef]
- Raveendran, R.; Bhuvaneshwar, G.S.; Sharma, C.P. Hemocompatible Curcumin–Dextran Micelles as PH Sensitive pro-Drugs for Enhanced Therapeutic Efficacy in Cancer Cells. Carbohydr. Polym. 2016, 137, 497–507. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Acetylated Debranched Starch Micelles as a Promising Nanocarrier for Curcumin. Food Hydrocoll. 2021, 111, 106253. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Weng, Z.; et al. Nano-Micelles Based on Hydroxyethyl Starch-Curcumin Conjugates for Improved Stability, Antioxidant and Anticancer Activity of Curcumin. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef]
- Lopez-Blanco, R.; Fernandez-Villamarin, M.; Jatunov, S.; Novoa-Carballal, R.; Fernandez-Megia, E. Polysaccharides Meet Dendrimers to Fine-Tune the Stability and Release Properties of Polyion Complex Micelles. Polym. Chem. 2019, 10, 4709–4717. [Google Scholar] [CrossRef]
- Schäfer, K.; Kolli, H.B.; Christensen, M.K.; Bore, S.L.; Diezemann, G.; Gauss, J.; Milano, G.; Lund, R.; Cascella, M. Supramolecular Packing Drives Morphological Transitions of Charged Surfactant Micelles. Angew. Chem. 2020, 132, 18750–18757. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing Amphiphilic Polymeric Micelles for Diagnostic and Therapeutic Applications: Breakthroughs and Bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jeong, K.J.; Yethiraj, A.; Mahanthappa, M.K. Low-Symmetry Sphere Packings of Simple Surfactant Micelles Induced by Ionic Sphericity. Proc. Natl. Acad. Sci. USA 2017, 114, 4072–4077. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Afruzi, F.H.; Hajizadeh, Z.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Sehat, S.S.; Hamblin, M.R.; et al. Nanoscale Bioconjugates: A Review of the Structural Attributes of Drug-Loaded Nanocarrier Conjugates for Selective Cancer Therapy. Heliyon 2022, 8, e09577. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Fu, Y.; Li, J.; Yu, X.; Li, Y.; Wang, Y.; Wu, X.; Zhang, K.; Kong, M.; Feng, C.; et al. Multifunctional Quercetin Conjugated Chitosan Nano-Micelles with P-Gp Inhibition and Permeation Enhancement of Anticancer Drug. Carbohydr. Polym. 2019, 203, 10–18. [Google Scholar] [CrossRef]
- Abdollahi, A.R.; Firouzian, F.; Haddadi, R.; Nourian, A. Indomethacin Loaded Dextran Stearate Polymeric Micelles Improve Adjuvant-Induced Arthritis in Rats: Design and In Vivo Evaluation. Inflammopharmacology 2021, 29, 107–121. [Google Scholar] [CrossRef]
- Huerta-Ángeles, G.; Brandejsová, M.; Novotný, J.; Kopecká, K.; Šógorková, J.; Šmejkalová, D.; Velebný, V. Grafting of Steroids to Hyaluronan towards the Design of Delivery Systems for Antioxidants: The Role of Hydrophobic Core. Carbohydr. Polym. 2018, 193, 383–392. [Google Scholar] [CrossRef]
- Jinsui, Y.; Bing, S.; Muhua, L.; Yue, L.; Jianyi, L.; Meng, D.; Kuan, C.; Chaopin, Y.; Hui, Z.; Zhiyi, C. Carboxymethyl-Hexanoyl Chitosan Nanodroplets for Ultrasonic Imaging and Drug Delivery to Tumor. Curr. Pharm. Des. 2018, 24, 1682–1688. [Google Scholar] [CrossRef]
- Li, N.; Zhong, Q. Effects of Polysaccharide Charge Density on the Structure and Stability of Carboxymethylcellulose-Casein Nanocomplexes at PH 4.5 Prepared with and without a PH-Cycle. Food Hydrocoll. 2021, 117, 106718. [Google Scholar] [CrossRef]
- Wang, S.; Ye, F.; Wei, F.; Zhao, G. Spray-Drying of Curcumin-Loaded Octenylsuccinated Corn Dextrin Micelles Stabilized with Maltodextrin. Powder Technol. 2017, 307, 56–62. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Improving the Bioavailability of Oil-Soluble Vitamins by Optimizing Food Matrix Effects: A Review. Food Chem. 2021, 348, 129148. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bandara, N. Applications of Reverse Micelles Technique in Food Science: A Comprehensive Review. Trends Food Sci. Technol. 2019, 91, 106–115. [Google Scholar] [CrossRef]
- Delfanian, M.; Sahari, M.A. Improving Functionality, Bioavailability, Nutraceutical and Sensory Attributes of Fortified Foods Using Phenolics-Loaded Nanocarriers as Natural Ingredients. Food Res. Int. 2020, 137, 109555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Agrawal, M.K. A Historical Perspective of Liposomes-a Bio Nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, Physico-Chemical and Structural Characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Mahoonak, A.S.; Ghorbani, M. Liposomal/Nanoliposomal Encapsulation of Food-Relevant Enzymes and Their Application in the Food Industry. Food Bioprocess Technol. 2021, 14, 23–38. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Reddy, H.; Jyothi, G.; Maheshwari; Prasad, D.; Sudhakar, M. Nanoliposomes—A Review. World J. Pharm. Sci. 2022, 10, 299–307. [Google Scholar] [CrossRef]
- Maiti, T.K.; Parvate, S.; Dixit, P.; Singh, J.; Reddy, V.J.; Bhuvanesh, E.; Chattopadhyay, S. Liposome for Encapsulation of Essential Oil and Fatty Acids. Liposomal Encapsulation Food Sci. Technol. 2023, 113–124. [Google Scholar] [CrossRef]
- Nechita, P.; Iana-Roman, M.R. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Sharma, S.; Mulrey, L.; Byrne, M.; Jaiswal, A.K.; Jaiswal, S. Encapsulation of Essential Oils in Nanocarriers for Active Food Packaging. Foods 2022, 11, 2337. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A Comprehensive Review on the Nanocomposites Loaded with Chitosan Nanoparticles for Food Packaging. Crit. Rev. Food Sci. Nutr. 2020, 62, 1383–1416. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Altaf, A.; Usmani, Z.; Dar, A.H.; Dash, K.K. A Comprehensive Review of Polysaccharide-Based Bionanocomposites for Food Packaging Applications. Discov. Food 2022, 2, 10. [Google Scholar] [CrossRef]
- Naveen, C.; Shastri, N.R. Polysaccharide Nanomicelles as Drug Carriers. Polysacch. Carriers Drug Deliv. 2019, 339–363. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds Polysaccharides in Active Food Packaging: A Review of Recent Progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Physical, Mechanical, Thermal and Structural Characteristics of Nanoencapsulated Vitamin E Loaded Carboxymethyl Cellulose Films. Prog. Org. Coatings 2020, 138, 105383. [Google Scholar] [CrossRef]
- Esquerdo, V.M.; Monte, M.L.; de Almeida Pinto, L.A. Microstructures Containing Nanocapsules of Unsaturated Fatty Acids with Biopolymers: Characterization and Thermodynamic Properties. J. Food Eng. 2019, 248, 28–35. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Deepika; Prasad, J.; Dwivedy, A.K.; Dubey, N.K. Improvement of In Vitro and In Situ Antifungal, AFB1 Inhibitory and Antioxidant Activity of Origanum Majorana L. Essential Oil through Nanoemulsion and Recommending as Novel Food Preservative. Food Chem. Toxicol. 2020, 143, 111536. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Konieczna-Molenda, A.; Krzan, M.; Khachatryan, K. Preparation of Nano/Microcapsules of Ozonated Olive Oil in Chitosan Matrix and Analysis of Physicochemical and Microbiological Properties of the Obtained Films. Innov. Food Sci. Emerg. Technol. 2022, 82, 103181. [Google Scholar] [CrossRef]
- Stanisławska, N.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Makarewicz, M.; Krzan, M. Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules. Polymers 2023, 15, 919. [Google Scholar] [CrossRef]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Quality of Beef Burgers Formulated with Fat Substitute in a Form of Freeze-Dried Hydrogel Enriched with Açai Oil. Molecules 2022, 27, 3700. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zannou, O.; Karim, I.; Kasmiati; Awad, N.M.H.; Gołaszewski, J.; Heinz, V.; Smetana, S. Avoiding Food Neophobia and Increasing Consumer Acceptance of New Food Trends—A Decade of Research. Sustainability 2022, 14, 10391. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M. Chitosan-Olive Oil Microparticles for Phenylethyl Isothiocyanate Delivery: Optimal Formulation. PLoS ONE 2021, 16, e0248257. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B.; Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, M.; Liao, Y.; Kang, M.; Qi, B.; Li, Y. Pickering Emulsions Stabilized by Reassembled Oleosome Protein Nanoparticles for Co-Encapsulating Hydrophobic Nutrients. Food Hydrocoll. 2023, 138, 108445. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Tucker, N. Millifluidic-Assisted Ionic Gelation Technique for Encapsulation of Probiotics in Double-Layered Polysaccharide Structure. Food Res. Int. 2022, 160, 111699. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Native Casein Micelles as Nanocarriers for β-Carotene: PH-and Temperature-Induced Opening of the Micellar Structure. Int. J. Food Sci. Technol. 2017, 52, 1122–1130. [Google Scholar] [CrossRef]
- Cohen, Y.; Ish-Shalom, S.; Segal, E.; Nudelman, O.; Shpigelman, A.; Livney, Y.D. The Bioavailability of Vitamin D3, a Model Hydrophobic Nutraceutical, in Casein Micelles, as Model Protein Nanoparticles: Human Clinical Trial Results. J. Funct. Foods 2017, 30, 321–325. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual Targeting Curcumin Loaded Alendronate-Hyaluronan- Octadecanoic Acid Micelles for Improving Osteosarcoma Therapy. Int. J. Nanomed. 2019, 14, 6425–6437. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Xiong, L.; Li, M.; Chen, H.; Xiao, J.; Wang, S.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Preparation of Octenyl Succinic Anhydride-Modified Debranched Starch Vesicles for Loading of Hydrophilic Functional Ingredients. Food Hydrocoll. 2019, 94, 546–552. [Google Scholar] [CrossRef]
- Šeremet, D.; Štefančić, M.; Petrović, P.; Kuzmić, S.; Doroci, S.; Jarić, A.M.; Cebin, A.V.; Pjanović, R.; Komes, D. Development, Characterization and Incorporation of Alginate-Plant Protein Covered Liposomes Containing Ground Ivy (Glechoma hederacea L.) Extract into Candies. Foods 2022, 11, 1816. [Google Scholar] [CrossRef]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A Coating Based on Clove Essential Oils Encapsulated by Chitosan-Myristic Acid Nanogel Efficiently Enhanced the Shelf-Life of Beef Cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods 2022, 11, 3017. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Li, S.; Zeng, Z.; Liu, Y.; Wang, C.; Chen, S.; Hu, B.; Li, C. Cinnamon Essential Oil Liposomes Modified by Sodium Alginate-Chitosan: Application in Chilled Pork Preservation. Int. J. Food Sci. Technol. 2023, 58, 939–953. [Google Scholar] [CrossRef]
- Toprakçı, İ.; Şahin, S. Encapsulation of Olive Leaf Antioxidants in Microbeads: Application of Alginate and Chitosan as Wall Materials. Sustain. Chem. Pharm. 2022, 27, 100707. [Google Scholar] [CrossRef]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Antimicrobial Activity of Lysozyme-Nisin Co-Encapsulated in Liposomes Coated with Polysaccharides. Food Hydrocoll. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-aghaee, E.; Khanjari, A.; Akhondzadeh-basti, A.; Noudoost, B.; Shariatifar, N.; Alizadeh Sani, M.; Soleimani, M. Nanocomposite Active Packaging Based on Chitosan Biopolymer Loaded with Nano-Liposomal Essential Oil: Its Characterizations and Effects on Microbial, and Chemical Properties of Refrigerated Chicken Breast Fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef]
- Woszczak, L.; Khachatryan, K.; Krystyjan, M.; Witczak, T.; Witczak, M.; Gałkowska, D.; Makarewicz, M.; Khachatryan, G. Physicochemical and Functional Properties and Storage Stability of Chitosan–Starch Films Containing Micellar Nano/Microstructures with Turmeric and Hibiscus Extracts. Int. J. Mol. Sci. 2023, 24, 12218. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Białecka, A.; Krupka, M.; Krzan, M.; Blaszyńska, K.; Hanula, M.; et al. Synthesis and Investigation of Physicochemical and Biological Properties of Films Containing Encapsulated Propolis in Hyaluronic Matrix. Polymers 2023, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.; Khachatryan, K.; Khachatryan, G.; Krystyjan, M.; Żarska, S.; Ciesielski, W. Preparation and Characterisation of Acid–Base-Change-Sensitive Binary Biopolymer Films with Olive Oil and Ozonated Olive Oil Nano/Microcapsules and Added Hibiscus Extract. Int. J. Mol. Sci. 2023, 24, 11502. [Google Scholar] [CrossRef]
- da Costa Vieira, M.; Bakof, K.K.; Schuch, N.J.; Skupien, J.A.; Boeck, C.R. The Benefits of Omega-3 Fatty Acid Nanocapsulation for the Enrichment of Food Products: A Review. Rev. Nutr. 2020, 33, e190165. [Google Scholar] [CrossRef]
- Just, D.R.; Goddard, J.M. Behavioral Framing and Consumer Acceptance of New Food Technologies: Factors Influencing Consumer Demand for Active Packaging. Agribusiness 2023, 39, 3–27. [Google Scholar] [CrossRef]
- Yang, Y.; Hobbs, J.E. Food Values and Heterogeneous Consumer Responses to Nanotechnology. Can. J. Agric. Econ. Can. d’agroeconomie 2020, 68, 289–313. [Google Scholar] [CrossRef]
- Siegrist, M.; Hartmann, C. Consumer Acceptance of Novel Food Technologies. Nat. Food 2020, 1, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Rehm, B.H.A. Bacterial Biopolymers: From Pathogenesis to Advanced Materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of Active Food Packaging via Incorporation of Biopolymeric Nanocarriers Containing Essential Oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Subramanian, P. Liposomes in Food Industries: Challenges and Future Scope. Liposomal Encapsulation Food Sci. Technol. 2023, 269–285. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Panghal, A.; Kumar, S.; Adedeji, A.A.; Garg, M.K.; Chhikara, N. Nanocapsules as Potential Antimicrobial Agents in Food. In Nanotechnological Approaches in Food Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 331–352. [Google Scholar]
- Kumari, R.; Suman, K.; Karmakar, S.; Mishra, V.; Lakra, S.G.; Saurav, G.K.; Mahto, B.K. Regulation and Safety Measures for Nanotechnology-Based Agri-Products. Front. Genome Ed. 2023, 5, 1200987. [Google Scholar] [CrossRef]
- Fajardo, C.; Martinez-Rodriguez, G.; Blasco, J.; Mancera, J.M.; Thomas, B.; De Donato, M. Nanotechnology in Aquaculture: Applications, Perspectives and Regulatory Challenges. Aquac. Fish. 2022, 7, 185–200. [Google Scholar] [CrossRef]
- Kah, M.; Sabliov, C.; Wang, Y.; White, J.C. Nanotechnology as a Foundational Tool to Combat Global Food Insecurity. One Earth 2023, 6, 772–775. [Google Scholar] [CrossRef]
- Turek, K.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M. An Innovative Method for the Production of Yoghurt Fortified with Walnut Oil Nanocapsules and Characteristics of Functional Properties in Relation to Conventional Yoghurts. Foods 2023, 12, 3842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).