Rapid Synthesis of Silver Nanowires in the Polyol Process with Conventional and Microwave Heating
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.3. Characterisation of Materials
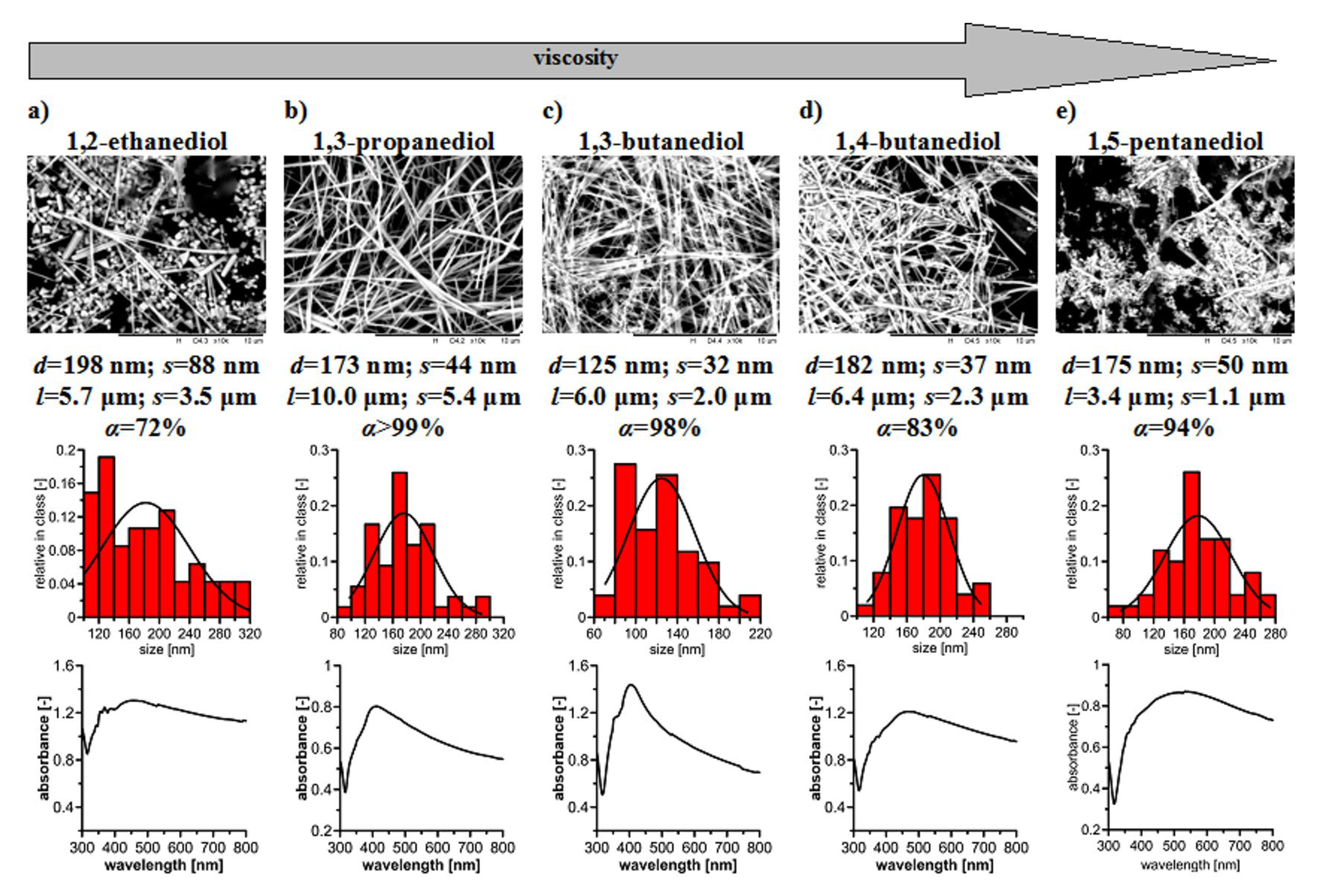
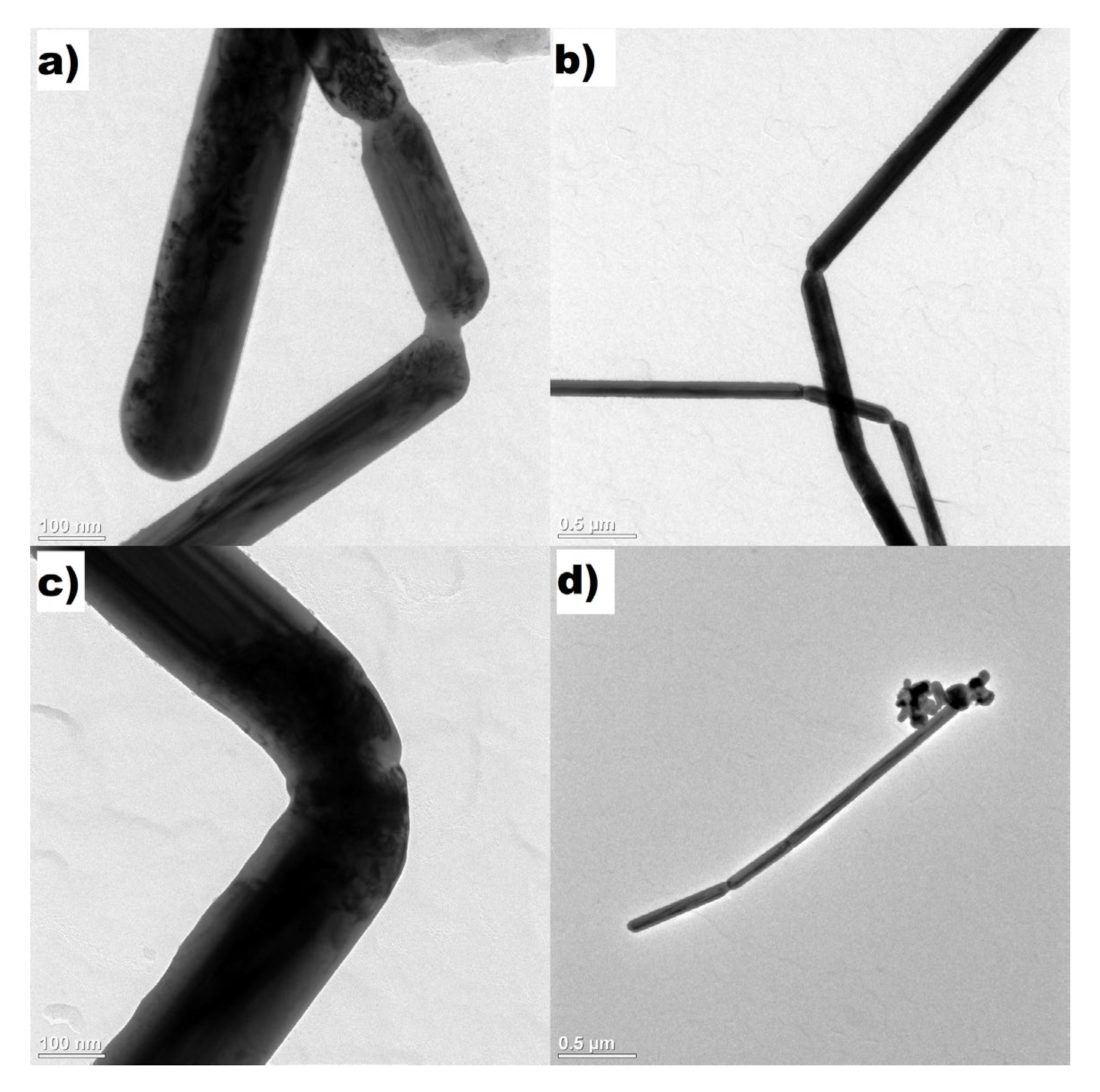
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heebo, H.; Amicucci, C.; Matteini, P.; Hwang, B. Mini review of synthesis strategies of silver nanowires and their applications. Colloid Interface Sci. Commun. 2022, 50, 100663. [Google Scholar] [CrossRef]
- Li, R.; Peng, Q.; Han, B.; Ke, Y.; Wang, X.; Lu, X.; Wu, X.; Kong, J.; Ren, Z.; Akinoglu, E.M.; et al. Plasmonic refraction-induced ultrahigh transparency of highly conducting metallic networks. Laser Photonics Rev. 2016, 10, 465–472. [Google Scholar] [CrossRef]
- Yang, X.; Bao, D.; Li, B. Light transfer from quantum-dot-doped polymer nanowires to silver nanowires. RSC Adv. 2015, 5, 60770–60774. [Google Scholar] [CrossRef]
- Valodkar, M.; Sharma, P.; Kanchan, D.K.; Thakore, S. Conducting and antimicrobial properties of silver nanowire-waxy starch nanocomposites. Int. J. Green Nanotechnol. Phys. Chem. 2010, 2, 10–19. [Google Scholar] [CrossRef]
- Li, J.; Qi, S.; Li, J.; Zhanga, M.; Wang, Z. A highly thermostable and transparent lateral heat spreader based on silver nanowire/polyimide composite. RSC Adv. 2015, 5, 59398–59402. [Google Scholar] [CrossRef]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef]
- Liu, X.; He, L.; Zheng, J.; Guo, J.; Bi, F.; Ma, X.; Zhao, K.; Liu, Y.; Song, R.; Tang, Z. Solar-light-driven renewable butanol separation by core–shell Ag@ZIF-8 nanowires. Adv. Mater. 2015, 27, 3273–3277. [Google Scholar] [CrossRef]
- Sun, Y. Silver nanowires—Unique templates for functional nanostructures. Nanoscale 2010, 2, 1626–1642. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Large-scale synthesis of uniform silver nanowires through a soft; self-seeding polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Fivet, F.; Brayner, R. The polyol process. In Nanomaterials: A Danger or a Promise; Brayner, R., Fievet, F., Coradin, T., Eds.; Springer: London, UK, 2013; pp. 1–25. [Google Scholar]
- Tsuji, M.; Matsumoto, K.; Jiang, P.; Matsuo, R.; Tang, X.L.; Kamarudin, K.S.N. Roles of Pt seeds and chloride anions in the preparation of silver nanorods and nanowires by microwave-polyol method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 266–277. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Jiang, G.; Yang, Q.; Wang, J.; Yu, H.; Chen, T.; Wang, C.; Chen, X. The influence of seeding conditions and shielding gas atmosphere on the synthesis of silver nanowires through the polyol process. Nanotechnology 2006, 17, 466–474. [Google Scholar] [CrossRef]
- Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, I.H.; Ooi, S.W.; Singho, N.D.; Aplop, F. Synthesis and growth mechanism of silver nanowires through different mediated agents (CuCl2 and NaCl) polyol process. J. Nanomater. 2014, 2014, 105454. [Google Scholar] [CrossRef]
- Sim, H.; Kim, C.; Bok, S.; Kim, M.K.; Oh, H.; Lim, G.H.; Cho, S.M.; Lim, B. Five-minute synthesis of silver nanowires and their roll-to-roll processing for large-area organic light emitting diodes. Nanoscale 2018, 10, 12087–12092. [Google Scholar] [CrossRef]
- Yang, C.; Gu, H.; Lin, W.; Yuen, M.M.; Wong, C.P.; Xiong, M.; Gao, B. Silver nanowires: From scalable synthesis to recyclable foldable electronics. Adv. Mater. 2011, 23, 3052–3056. [Google Scholar] [CrossRef]
- Nghia, N.V.; Truong, N.N.K.; Thong, N.M.; Hung, N.P. Synthesis of nanowire—Shaped silver by polyol process of sodium chloride. Int. J. Mater. Chem. 2012, 2, 75–78. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 2007, 40, 1067–1076. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Huang, R.; Pan, C.; Zhu, J. Capping modes in PVP-directed silver nanocrystal growth: Multi-twinned nanorods versus single-crystalline nano-hexapods. Cryst. Growth. Des. 2008, 8, 1916–1923. [Google Scholar] [CrossRef]
- da Silva, R.R.; Yang, M.; Choi, S.I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile synthesis of sub-20 nm silver nanowires through a bromide-mediated polyol method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J.; Jiang, R. Convenient, rapid synthesis of silver nanocubes and nanowires via a microwave-assisted polyol method. Nanotechnology 2010, 21, 025607. [Google Scholar] [CrossRef]
- Nandikonda, S.; Davis, E.W. Parameters affecting the microwave-assisted polyol synthesis of silver nanorods. ISRN Nanotechnol. 2011, 2011, 104086. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanostructures: Control of product morphology with Fe(II) or Fe(III) species. Langmuir 2005, 21, 8078–8080. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.S.; Hsua, C.Y.; Liu, B.T. Salt-mediated polyol synthesis of silver nanowires in a continuous-flow tubular reactor. RSC Adv. 2015, 5, 29872–29877. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Jiang, G.; Zhou, J.; Chen, X.; Yu, H.; Yang, Q. Study on the synthesis of silver nanowires with adjustable diameters through the polyol process. Nanotechnology 2006, 17, 3933–3938. [Google Scholar] [CrossRef]
- Liu, S.; Sun, B.; Li, J.G.; Chen, J. Silver nanowires with rounded ends: Ammonium carbonate-mediated polyol synthesis; shape evolution and growth mechanism. CrystEngComm 2014, 16, 244–251. [Google Scholar] [CrossRef]
- Amirjani, A.; Marashi, P.; Fatmehsari, D.H. Effect of AgNO3 addition rate on aspect ratio of CuCl2–mediated synthesized silver nanowires using response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 33–39. [Google Scholar] [CrossRef]
- Jiu, J.; Araki, T.; Wang, J.; Nogi, M.; Sugahara, T.; Naga, S.; Koga, H.; Suganum, K.; Nakazawa, E.; Hara, M.; et al. Facile synthesis of very-long silver nanowires for transparent electrodes. J. Mater. Chem. A 2014, 2, 6326–6330. [Google Scholar] [CrossRef]
- Dzido, G.; Markowski, P.; Małachowska-Jutsz, A.; Prusik, K.; Jarzębski, A.B. Rapid continuous microwave-assisted synthesis of silver nanoparticles to achieve very high productivity and full yield: From mechanistic study to optimal fabrication strategy. J. Nanoparticle Res. 2015, 17, 27. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Park, K.H.; Im, S.H.; Park, O.O. The size control of silver nanocrystals with different polyols and its application to low-reflection coating materials. Nanotechnology 2011, 22, 045602. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, S.S.; Cao, H.T.; Xie, L.H.; Hong, C.S.; Jin, L.Z.; Yu, M.N.; Zhang, H.; Zhang, Z.Y.; Huang, L.H.; et al. An eco-friendly water-assisted polyol method to enhance the aspect ratio of silver nanowires. RSC Adv. 2019, 9, 1933–1938. [Google Scholar] [CrossRef]
- Yang, T.; Han, Y.; Li, J. Manipulating silver dendritic structures via diffusion and reaction. Chem. Eng. Sci. 2015, 138, 457–464. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ji, X.H.; Zhang, Y.H.; Lu, B.H. Effect of solvent on the microstructures of nanostructured Bi2Te3 prepared by solvothermal synthesis. J. Alloys Compd. 2004, 368, 349–352. [Google Scholar] [CrossRef]
- Qingqing, W.; Gang, X.; Gaorong, H. Solvothermal synthesis and characterization of uniform CdS nanowires in high yield. J. Solid State Chem. 2005, 178, 2680–2685. [Google Scholar] [CrossRef]
- Wei, X.; Xu, G.; Ren, Z.; Wang, Y.; Shen, G.; Han, G. Synthesis of highly dispersed barium titanate nanoparticles by a novel solvothermal method. In Progress in Nanotechnology Processing; Wiley: Hoboken, NJ, USA, 2010; pp. 33–36. [Google Scholar]
- Blosi, M.; Albonetti, S.; Dondi, M.; Martelli, C.; Baldi, G. Microwave-assisted polyol synthesis of Cu nanoparticles. J. Nanoparticle Res. 2011, 13, 127–138. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Sci. Rev. 1998, 27, 213–223. [Google Scholar] [CrossRef]
- Sudo, S.; Shinyashiki, N.; Kitsuki, Y.; Yagihara, S. Dielectric relaxation time and relaxation time distribution of alcohol-water mixtures. J. Phys. Chem. A 2002, 106, 458–464. [Google Scholar] [CrossRef]
- Wohlfarth, C. Permittivity (dielectric constant) of liquids. In Handbook of Chemistry and Physics, 93rd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 6, pp. 135–175. [Google Scholar]
- Gomez-Acosta, A.; Manzano-Ramirez, A.; Lopez-Naranjo, E.J.; Apatiga, L.M.; Herrera-Basurto, R.; Rivera-Munoz, E.M. Silver nanostructure dependence on the stirring-time in a high-yield polyol synthesis using a short-chain PVP. Mater. Lett. 2015, 138, 167–170. [Google Scholar] [CrossRef]
- Sundeep, D.; Kumar, D.V.; Subba Rao, P.S.; Ravikumar, R.V.S.S.N.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef]
- Biju, V.; Sugathan, N.; Vrinda, V.; Salini, L. Estimation of lattice strain in nanocrystalline silver from X-ray diffraction line broadening. J. Mater. Sci. 2008, 43, 1175–1179. [Google Scholar] [CrossRef]
- Jiang, P.; Li, S.Y.; Xie, S.S.; Gao, Y.; Song, L. Machinable long PVP-stabilized silver nanowires. Chem. Eur. J. 2004, 10, 4817–4821. [Google Scholar] [CrossRef] [PubMed]
- Andrés, L.J.; Menéndez, M.F.; Gómez, D.; Martínez, A.L.; Bristow, N.; Kettle, J.P.; Menéndez, A.; Ruiz, B. Rapid synthesis of ultra-long silver nanowires for tailor-made transparent conductive electrodes: Proof of concept in organic solar cells. Nanotechnology 2015, 26, 265201. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Zhang, L.; Li, Q.; Kwong, K.W.; Xu, A.W.; Lin, J. Sonochemical preparation of nanoporous composites of titanium oxide and size-tunable strontium titanate crystals. Langmuir 2003, 19, 7673–7675. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, P.; Gao, Q.; Zhang, Y.; Tang, Y. High-concentration preparation of silver nanowires: Restraining in situ nitric acidic etching by steel-assisted polyol method. Chem. Mater. 2008, 20, 1699–1704. [Google Scholar] [CrossRef]
- Zhu, J.J.; Kan, C.X.; Wan, J.G.; Han, M.; Wang, G.H. High-yield synthesis of uniform Ag nanowires with high aspect ratios by introducing the long-chain PVP in an improved polyol process. J. Nanomater. 2011, 2011, 982547. [Google Scholar] [CrossRef]
- Kan, C.X.; Zhu, J.J.; Zhu, X.G. Silver nanostructures with well-controlled shapes: Synthesis; characterization and growth mechanisms. J. Phys. D Appl. Phys. 2008, 41, 155304. [Google Scholar] [CrossRef]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chem. Int. Ed. 2006, 45, 4597–4601. [Google Scholar] [CrossRef]

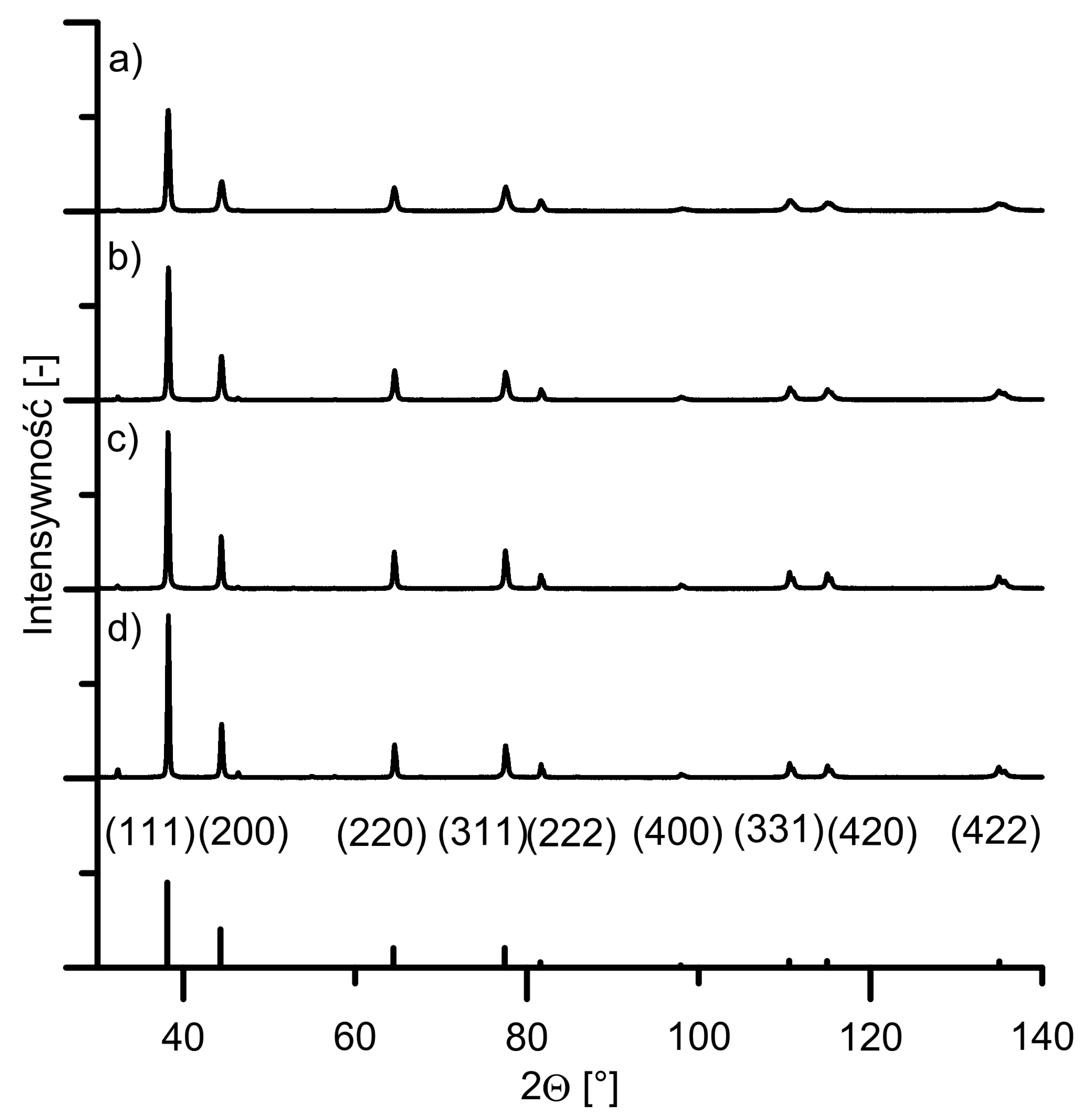
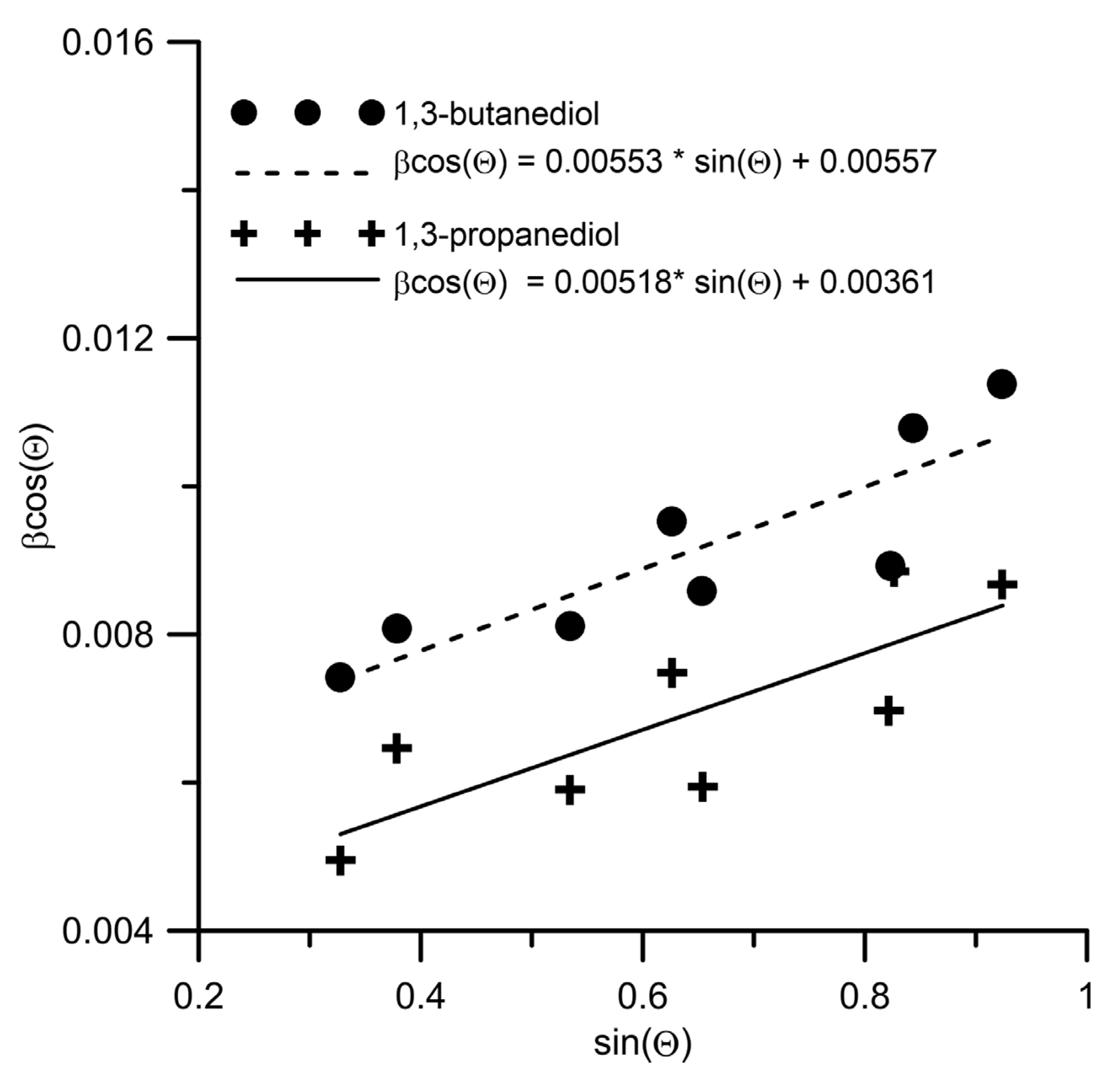
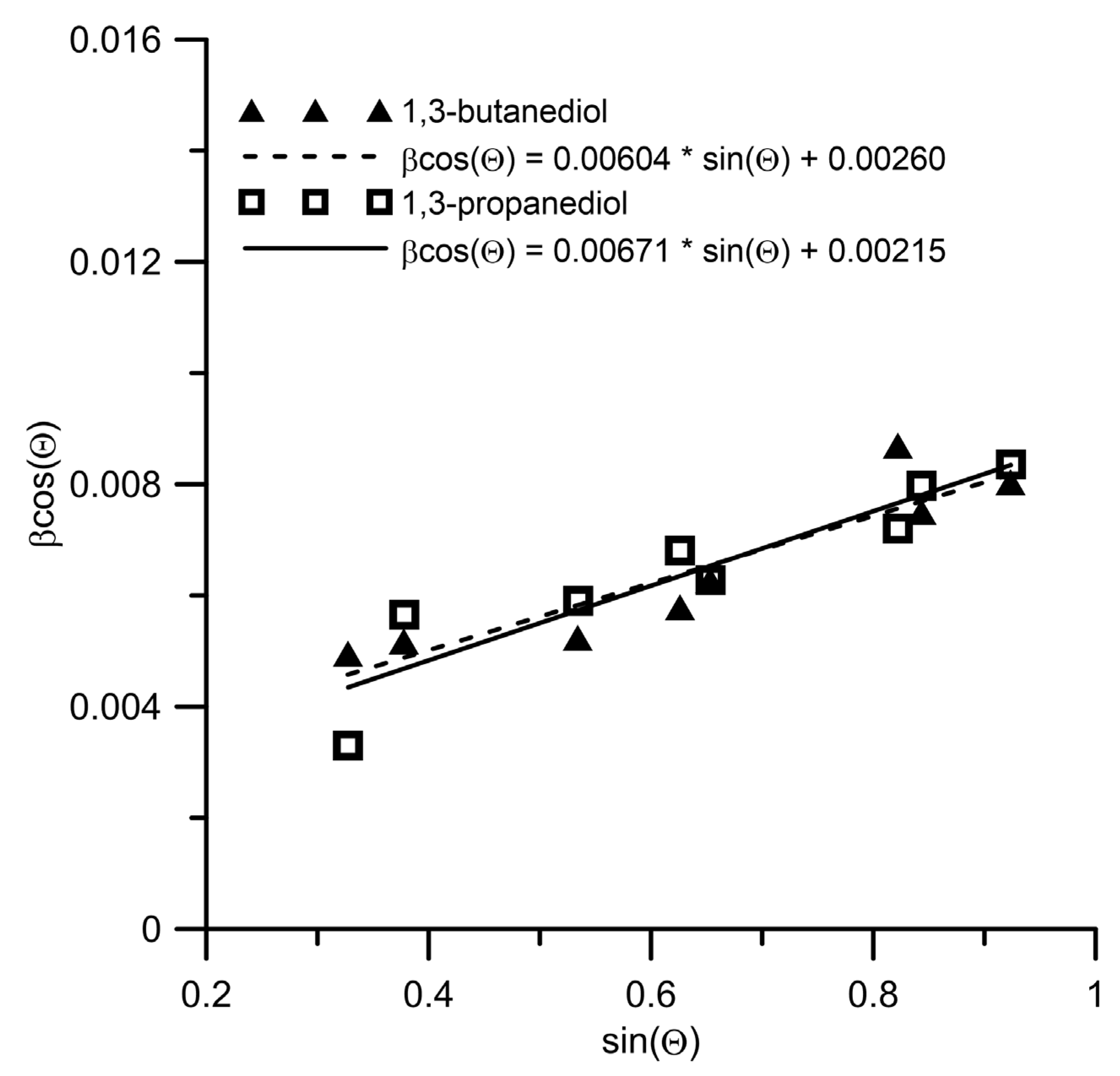
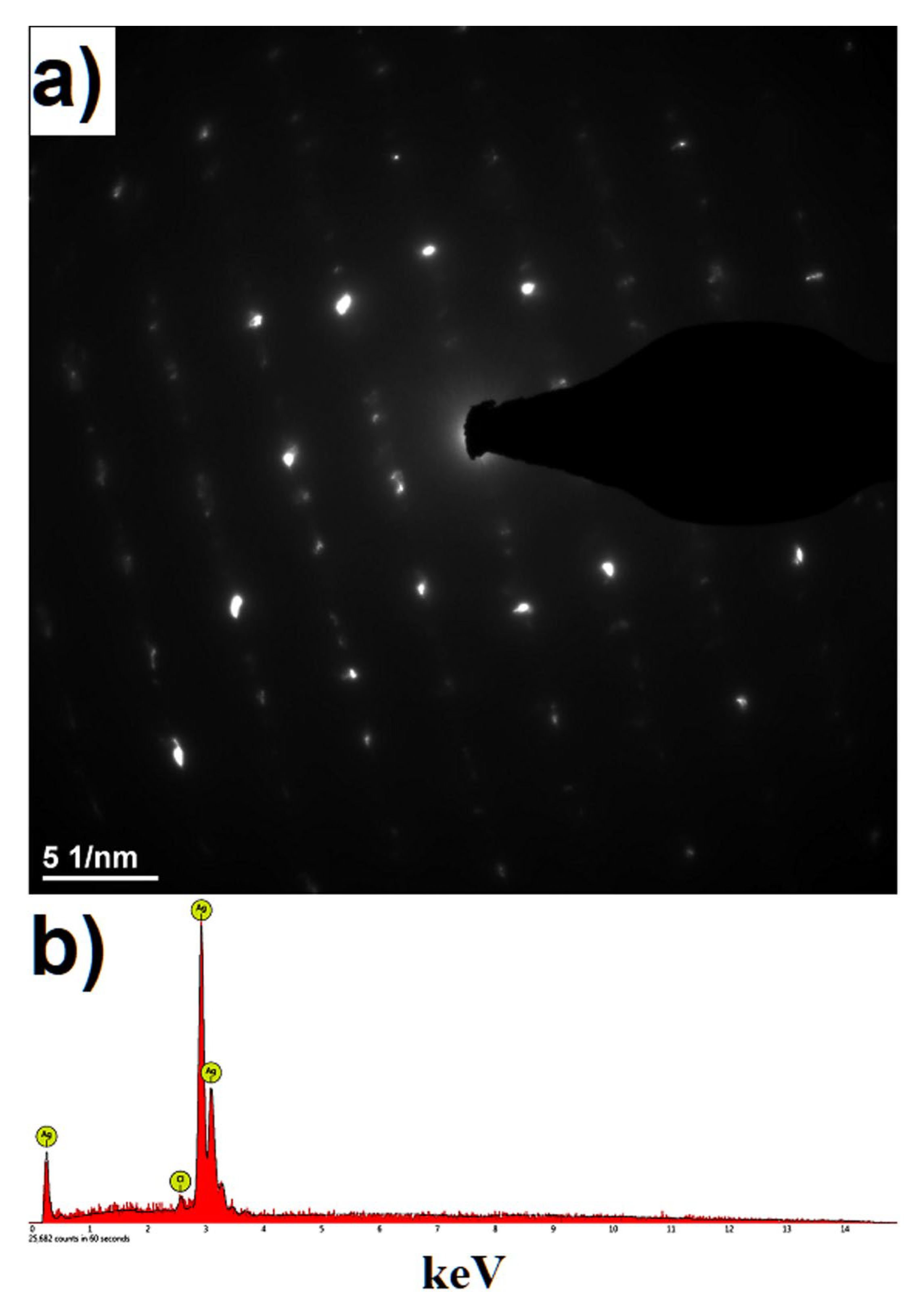

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzido, G.; Smolska, A.; Farooq, M.O. Rapid Synthesis of Silver Nanowires in the Polyol Process with Conventional and Microwave Heating. Appl. Sci. 2023, 13, 4963. https://doi.org/10.3390/app13084963
Dzido G, Smolska A, Farooq MO. Rapid Synthesis of Silver Nanowires in the Polyol Process with Conventional and Microwave Heating. Applied Sciences. 2023; 13(8):4963. https://doi.org/10.3390/app13084963
Chicago/Turabian StyleDzido, Grzegorz, Aleksandra Smolska, and Muhammad Omer Farooq. 2023. "Rapid Synthesis of Silver Nanowires in the Polyol Process with Conventional and Microwave Heating" Applied Sciences 13, no. 8: 4963. https://doi.org/10.3390/app13084963
APA StyleDzido, G., Smolska, A., & Farooq, M. O. (2023). Rapid Synthesis of Silver Nanowires in the Polyol Process with Conventional and Microwave Heating. Applied Sciences, 13(8), 4963. https://doi.org/10.3390/app13084963






