Abstract
Tendon-related disorders are a common condition in both sports medicine and orthopedic clinical practice. Ultrasonography, power doppler, and magnetic resonance imaging (MRI) are the most widespread technologies, but the use of ultrasound elastography—including strain elastography and shear wave elastography—has been increasing in the last years. The aim of this paper is to evaluate the use of ultrasound elastography in tendon-related disorders. Research in PubMed, Scopus, and Web of Science databases is performed, and 364 papers are exported. After the study selection process, 38 papers are included in this systematic review. The risk of bias of each paper is evaluated using the RoBANS tool. Blinding, confounding variables, and measurement of exposure are the most affected items. From the included papers, tendinopathy is the most analyzed pathology, followed by tenosynovitis and rotator cuff disease. The Achilles tendon, patellar tendon, and common extensor tendon of the hand are the most analyzed tendons. Ultrasound elastography results in being the method providing good sensitivity and specificity (up to 100% and 100%, respectively, in tendinosis of the long head of the biceps tendon, in transverse plan examination) and accuracy (up to 97.8% in Achilles tendinopathy); furthermore, this technique is able to ensure real-time feedback on tissue elasticity and appears more sensitive than B-mode alone.
1. Introduction
Tendon-related disorders are common conditions in both sports medicine and orthopedic clinical practice; they are among the most important causes of suspension for sports activity and limitation in working ability [1]. Tendons can be affected by various pathological conditions; some of them could be associated with a systematic disease, and others result from local inflammation [2]. Tendinitis, tendinopathy, tenosynovitis, and peri-tendinitis are common terms, but as Maffulli and colleagues observed, all these terms were misused and confusing [3]. Ultrasonography, power doppler ultrasound, and magnetic resonance imaging (MRI) are the most common methodologies used as diagnostic imaging modalities for assessing and monitoring tendon pathologies [4].
Ultrasound (US) elastography was initially developed in the 1990s for a precise evaluation of tissue stiffness, with the aim of replicating/substituting the subjective clinician’s evaluation performed through manual palpation [5]. Two types of US elastography are available. The first is strain elastography (SE), which requires an external rhythmical pressure applied by the examiner on the target tissue, allowing the software to measure the tissue stiffness. The strain ratio obtained is the ratio between the examined tissue and the reference tissue [6]. Strain elastography provides a qualitative evaluation thanks to a color diagram [7]. The second is shear wave elastography, which is based on the use of acoustic radiation impulses to displace the tissue at several points of interest. This technique returns quantitative information about tissue stiffness (in kPa) based on the shear wave propagation velocity, quantified in meters/second (m/s) [6]. US elastography ensures the possibility of implementing several applications with respect to the body tissues, being commonly used to evaluate fascial structures, nerves, soft tissue masses, muscles, and tendons [8]. Multiple studies have been published on the use of US elastography on human tendons [9,10], most of them being experimental trials with small sample sizes [11]. To our knowledge, no systematic reviews focused on ultrasound elastography addressing clinical efficiency [12] in tendon-related disorders have been published, so far. Therefore, the aim of this SR was to evaluate the clinical efficiency of US elastography (strain elastography and/or shear wave elastography) in the aforementioned application field.
2. Materials and Methods
2.1. Information Source
An a priori study protocol was developed for this systematic review and registered in Prospero (CRD42022300598). This project was conducted following the PRISMA statement [13]. Moreover, a methodological paper focused on the SRs of observational studies [14] has been used as a main reference to perform this project.
2.2. Eligibility Criteria and Search Strategy
The research was conducted in three electronic databases, PubMed, Web of Science, and Scopus, and it was completed in January 2023.
The search strategy was developed following the PICO [15] reported below:
P: Symptomatic patients (over 18 years) affected by tendon-related disorders;
I: Tissue evaluation through US elastography (strain, shear wave elastography);
C: None;
O: Clinical efficiency of US elastography in clinical practice.
The full search strategy employed is available in Table 1. A different search strategy was developed for each database.

Table 1.
Search Strategy.
2.3. Study Selection
The inclusion criteria considered were cross-sectional studies written in English, published in a range time from 1 January 2010 to 31 December 2022, conducted on humans, and involving symptomatic subjects.
Exclusion criteria considered were studies conducted on cadavers or on animals, studies with populations affected by systematic pathologies, studies written in a language other than English, and studies published before 1 January 2010. Concerning the study design, systematic, scoping, or narrative reviews, commentary articles, letters to the editor, and conference papers were excluded.
2.4. Data Items and Collection
After the database research, data were exported and uploaded to Rayyan QCRI, a web application specifically developed to simplify and accelerate the systematic review process [16]. Two reviewers (D.M.D. and L.G.) screened the titles and abstracts following the eligibility criteria. A third reviewer (M.G.) helped in case of disagreement.
The extracted data were organized in a database that followed the Cochrane model [17]. Before starting the data extraction, the authors analyzed three papers chosen from the papers excluded with reasons [18,19,20] to familiarize themselves with the dataset.
2.5. Quality Assessment: Risk of Bias in Individual Studies
The risk of bias was evaluated using the risk of bias assessment of nonrandomized studies (RoBANS) tool [21]. Each study was analyzed independently by two reviewers (G.R. and N.F.L). In case of disagreement, a third reviewer (P.C.) was consulted.
3. Results
3.1. Study Selection
A total of 364 papers were exported from the electronic databases, and 155 papers were identified as duplicates. From the residual 209 papers, 148 were eliminated via title and abstract analysis. Subsequently, 61 papers underwent full-text analysis, and 23 papers were excluded with motivation. Supplementary Table S1 contains references and reasons for the exclusions.
Finally, 38 papers were included in this systematic review. In Figure 1, the entire selection process is represented.
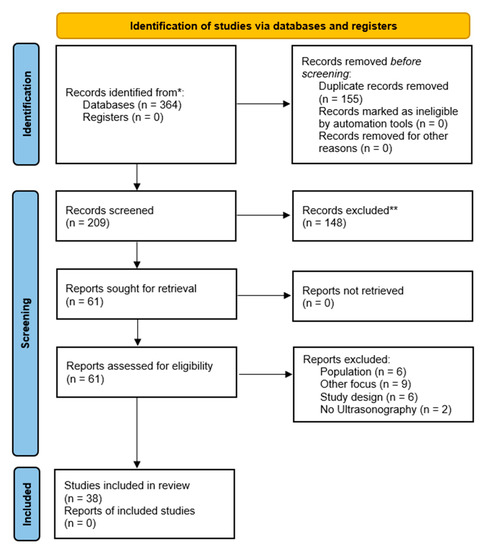
Figure 1.
PRISMA flow chart. * PubMed: 65; Scopus: 135; Web of Science: 164. ** manually excluded.
3.2. Risk of Bias
The risk of bias in each study was evaluated. The most problematic area of the evaluation was item D4: “Blinding” with 14 papers graded as high risk, and 5 papers graded as medium risk. The second important item in terms of risk is item D2, “Confounding variables”, which was graded as high risk in 2 papers, and 22 papers were graded as unclear risk. In the third position was item D3, “Measurement of exposure”, which was evaluated as high risk in 2 papers, and 18 papers resulted as unclear risk. As fourth, item D1, “Selection of Participants” was graded as unclear risk in 23 papers. To follow was item D5, “Incomplete Outcome Data”, which was graded as unclear risk in 3 papers. Finally, item D6, “Selective outcome reporting”, was graded as unclear risk in 2 studies. A visual representation of the risk of bias analysis is available in Figure 2.
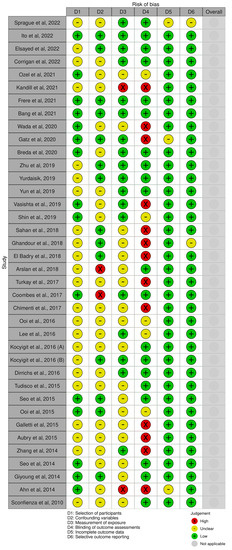
Figure 2.
Risk of bias ([22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]).
3.3. Study Characteristics
From the included studies, the most frequent evaluation technique was the longitudinal scan, used in 20 papers. Seventeen papers evaluated both longitudinal and transverse scans. Only one paper conducted only transverse scan. One paper did not declare the adopted probe’s position. The general and methodological features of the studies are resumed in Supplementary Table S2.
Sensitivity and specificity of US elastography were evaluated in 14 papers [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Accuracy value was evaluated in 6 papers [22,30,34,35,36,37]. Moreover, inter-observer reliability with MRI was evaluated in 3 papers [38,39,40].
From the included studies, the most reported outcome was the strain ratio, in 20 papers. Ten papers concerning shear wave elastography reported shear wave velocity expressed in meters/second; 11 papers instead reported shear modulus in kPa concerning tissue stiffness. One paper reported information concerning shear wave elastography in both shear wave velocity and tissue stiffness. Only 2 papers reported all three values of interest, i.e., strain ratio, shear wave velocity, and shear modulus.
Reliability indexes of included studies, concerning specificity, sensitivity, and accuracy of included papers, are hosted in Table 2.

Table 2.
Reliability indexes of included studies.
Study characteristics, concerning the type of US machine, examination position of the patient, and location of the probe, are resumed in Supplementary Table S2.
Specific values and results related to shear wave elastography are resumed in Supplementary Table S3. Supplementary Table S4 hosted values and results related to strain elastography.
3.3.1. Population
- Twenty papers involved only symptomatic subjects [22,23,24,25,34,36,37,38,39,40,41,43,44,45,48,51,52,53,54,55];
- Eighteen papers involved both symptomatic and asymptomatic subjects [26,27,28,29,30,31,32,33,35,42,46,47,49,50,56,57,58,59].
3.3.2. Ultrasound Mode
- Twenty papers described the use of B-mode + strain elastography [22,23,26,28,30,33,34,35,36,37,38,39,40,50,51,52,53,55,58,59];
- Fifteen papers described the use of B-mode + shear wave elastography [25,29,31,32,41,42,43,44,45,46,47,48,54,56,57];
- Three papers used B-mode + strain elastography + shear wave elastography [24,27,49];
- Power doppler was used in 10 papers [24,30,34,36,37,46,47,54,56,58];
- Only one paper used UTC [46];
- Only one paper used Transient [29].
3.3.3. Pathologies
- Tendinopathy was analyzed in 32 [22,24,25,26,27,29,30,32,33,34,35,36,37,38,39,40,41,42,43,44,46,47,48,49,50,51,53,54,56,57,58,59,60];
- Two papers analyzed tenosynovitis [28,31];
- Rotator cuff disease was analyzed by [23]; small supraspinatus tears were studied in [55]; frozen shoulder in [45]; sub-acromial impingement syndrome was studied by [52].
3.3.4. Tendons
- Supraspinatus tendon evaluation was present in 9 papers [23,37,38,39,40,45,49,53,55];
- Subscapularis tendon evaluation was present in 2 papers [23,38];
- Infraspinatus tendon was present in 3 papers [23,37,45];
- Teres minor tendon evaluation was present in 1 paper [23];
- Biceps tendon evaluation was presented in 4 papers [27,35,37,45];
- Common flexor tendons of the Hand evaluations were presents in 4 papers [24,26,37];
- Common extensor tendons of the Hand evaluations were presented in 8 papers [25,30,36,37,43,52,54,58]
- Abductor and extensor Pollicis evaluation were presented in 2 papers [28,31]
- Patellar tendon evaluation was present in 9 papers [32,34,37,41,42,47,48,54,57]
- Achilles tendon evaluation was present in 11 papers [22,29,32,33,37,44,46,50,54,56,59].
3.3.5. Reliability Indexes of Included Studies
- Achilles tendon was evaluated in 6 papers [22,29,32,33,37,59];
- Patellar tendon was evaluated in 3 papers [32,34,47];
- Rotator cuff was evaluated in 3 papers [23,37,40,55];
- Epicondylitis was evaluated in 6 papers [24,25,26,30,36,37];
- Biceps tendon was evaluated in 2 papers [27,35];
- De Quervain tenosynovitis was evaluated in 2 papers [28,31].
4. Discussion
4.1. Discussion
To our knowledge, this is the first review focused on ultrasonography and ultrasound elastography in tendon-related disorders. We have included studies with a cross-sectional design, which is the most appropriate and frequent study design used to assess diagnostic accuracy [61].
Ultrasound elastography has been undergoing rapid growth in recent years in terms of use and in the number of publications [62].
Tendinopathy conditions are characterized by a degenerative process that occurs in the long term because of overuse and repetitive stress. Inflammation occurs in the first few weeks as a response to damage to repair and reconstitute the involved tissue. Often the reparative mechanism fails, thus leading to degenerative changes in the collagenous matrix and irregular fiber structure, hypercellularity, hypervascularity, accumulation of fluid between fibers, and a lack of inflammatory cells [63].
In tendinopathy, these pathologic changes occur concurrently with the attempts at tissue repairing (“degeneration” and attempts of “regeneration”). The presence of disorganization of collagen fibers (normally arranged in a parallel configuration and densely packed) leads to a localized decrease in tendon stiffness. Therefore, stiffness analysis can help in diagnosing tendinopathy [64].
Ultrasound elastography analyzes the elasticity of the investigated tissue through ultrasound. Both ultrasound elastography types (shear wave elastography and strain elastography) can be used to investigate tendon elastic properties, resulting in different measurements. In shear wave elastography, two outcomes can be obtained: shear modulus measured in kPa or shear wave velocity (SWV) measured in m/s. In strain elastography, the outcome is the strain, defined as the amount of displacement from the probe. Knowing the strain and identifying a reference, the strain elastography software calculates the strain ratio, which is an index of the relative elasticity of an objective region of interest (ROI) and a reference ROI with a constant elasticity [65].
Therefore, it is expected that in cases of tendinopathy, the tendon is “softer” and stiffness decreases, with a reduction in shear modulus, in shear wave velocity for the shear wave elastography, and in strain ratio for the strain elastography (higher strain for softer tissues) [66].
In some cases, US elastography analysis provides conflicting results; as shown by Breda [47], Coombes [32], and Zhang [57], a pathologic patellar tendon (both in athletes and non-athletes) is less elastic (increased stiffness) than controls. The opposite result was obtained by Ooi et al. [34] and Dirrichs et al., [54] who showed that symptomatic patellar tendons (both in athletes and nonathletes) have decreased stiffness compared to nonsymptomatic tendons. This finding is in contrast to previous studies but in agreement with what is expected. These contradicting results have already been mentioned by Breda [47], who hypothesized how different methods of image analysis, different ultrasound equipment, and different positions of the knee can be potential explanations for the discordant findings. In particular, it has been mentioned that a more flexed position of the knee leads to increased stiffness. Therefore, a standardized position is required to obtain compatible results. Finally, different US equipment can lead to different results but also different transducers and different acquisition depths [47].
Despite the different results, most of the studies that considered this type of pathology [32,34,47,54] concluded that US elastography significantly improves diagnostic accuracy when used in combination with the conventional US, providing good-to-excellent inter-operator and intra-operator reliability.
Similar findings can be found in the analysis of the long-head biceps tendon (LHBT): Sahan [27] found that chronically pathological long-head biceps tendon (identified with pain and MRI examination) becomes stiffer compared to the same tendons in a control group using both shear wave elastography and strain elastography evaluations. In this case, we must consider that some artifacts may be involved [67]. In fact, it is known that during a pathological process, while tendons become softer, joint capsules and ligaments become stiffer [68]. The LHBT takes part in shoulder stability; it is included in the rotator cuff and has an extra-synovial part and an intra-synovial part that runs under the shoulder capsule. When the LHBT is analyzed, it is nearly impossible to isolate the tendon from the capsule tissue. Therefore, in the US elastography analysis of the LHBT, the stiffness of the shoulder capsule must be considered to properly understand the results [69]. However, by comparing the diagnostic utility of US, shear wave elastography, and strain elastography to MRI, it was concluded that US elastography is a useful diagnostic tool for LHBT tendinopathy when considering usability, cost-effectiveness, and patient preference compared to MRI.
Among the included studies, biceps tendinopathy is also considered by Seo [35]. They did not specify any strain elastography results data about tendon stiffness but concluded that US elastography has a positive correlation with US images.
Other frequently analyzed tendons in the review are the Achilles tendon, supraspinatus tendon, common extensor, and common flexor tendons. The Achilles tendon has been taken into consideration by Kandil [22,29,32,33,37,50,54,56,59,60]. Most of these studies [22,29,34,37,54,56,60] found that symptomatic Achilles tendon is softer than healthy tendons and asymptomatic tendons. They also reported good to excellent sensitivity and specificity in the diagnosis of Achilles tendinopathy using both strain elastography and shear wave elastography techniques. Galletti [37] did not provide SE results data but found out that it could identify tendinopathy features in 27/35 patients with suspected pathologic tendons with negative US examination [37].
Coombes et al. [32] divided the Achilles tendon into insertional and mid-parts. Even if the tendon thickness was greater in symptomatic patients than in controls, in both mid- and insertional Achilles, the shear wave elastography analysis revealed that stiffness was very similar at the midportion and slightly greater in symptomatic tendons at the insertional area.
The paper of Chimenti et al. [50] is in agreement with the previous one, supporting that in insertional Achilles tendinopathy (IAT), tendons’ compressive modulus increases compared with healthy controls, indicating an intrinsically more rigid tissue. This outcome is due to the fact that at the Achilles tendon insertion, in patients with IAT, increased cartilage matrix proteins (type 2 collagen and aggrecan) and rounder tendon cells can be found, suggesting a metaplastic phenomenon. It is worth mentioning that this paper is the only one analyzing the Achilles tendon with the patient actively moving (active dorsiflexion, standing, and partial squat) [50].
The other paper in contrast with expectations is the one from Sconfienza and colleagues [59], which found the painful Achilles tendon to be harder with respect to pain-free controls.
Findings related to the insertional part of the tendon are controversial since other papers discovered that symptomatic tendons are globally softer than healthy controls [61]. This fact can also be due to the overlapping of muscle and tendon tissues in the explored region.
Further studies in this field are needed since there is no unambiguous judgment about sensitivity and specificity and on whether maturation, aging, or chronic load underlies these findings.
We assume that the limitations of ultrasound imaging play a primary role in generating this variety of results. Despite the controversial outcomes, these studies showed that US elastography is an accurate method in the diagnosis of Achilles tendon pathologies, and it can be useful in the evaluation of Achilles tendinopathy, especially when paired with US imaging.
Medial and lateral epicondylitis have also been analyzed multiple times [24,25,26,30,36,37,52,58]. In these cases, the available findings are not controversial and all studies suggest that pathological tendons are softer than healthy structures. Additionally, both SWE and SE are valid imaging techniques for diagnosing medial and lateral epicondylitis with good to excellent sensitivity and specificity.
The study by Kocyigit [53] revealed a higher strain ratio in symptomatic tendons, unlike other papers’ assumptions. This is probably due to the fact that they selected the subcutaneous fat tissue near the common extensor tendon origin as a reference ROI for calculating the strain ratio. As fat is softer than the tendon tissue, this may represent a convincing explanation for the resulting higher strain ratio [53].
The last series of studies concerns supraspinatus tendinopathy without tears [37,38,39,40,51]. In these papers, US elastography has been compared to MRI, showing a good to excellent correlation with MRI findings. The results are in agreement with the expectations about tendon softening.
The study by Lee [51] has to be mentioned because, in the strain ratio calculation, they used subcutaneous tissue as a reference area in one measure and a gel pad in the other measure. Despite that, the conclusion did not differ from other papers, since MRI tendinosis grade is associated with stiffness assessed using US elastography [51].
Therefore, we can state that US elastography is a useful clinical tool. It allows a quantitative measurement of tissue stiffness, and, thanks to its sensibility, it is able to detect tendinous alterations that are not evident in US B-mode evaluation [37]. However, its role in revealing early subclinical alterations is still debated [65].
Nevertheless, US elastography provides real-time feedback in tendon evaluation. However, as reported in the current literature, there is a need to combine US and clinical evaluation [70].
4.2. Strengths and Limitations
Some limitations should be considered.
4.2.1. Limitations of Included Studies
As concerns the included studies, in some of them, the study population was relatively small; moreover, the subjects were not consecutively recruited, and blinding was not performed in all studies. Furthermore, in some studies, the control group was not optimally matched for age and sex with the study population.
We should consider some limitations caused by the intrinsic characteristics of the tool employed. Strain elastography has a high operator dependency since the application of pressure to the probe is performed manually [58]. In addition, artifacts may be found [40] and the region of interest (ROI) selected for the strain ratio measurement may not properly represent tissue properties [52]. In fact, ultrasound imaging analyzes the ROI that has a determined area. If another tissue is present in the ROI or immediately below it, it will be considered in the measurement by the software itself. This phenomenon influences the results [63].
As concerns the patellar tendon, some studies considered a very small ROI or a single ROI with flexible diameters centered in the hypoechoic region of the proximal patellar tendon, while Breda [47] evaluated average stiffness over the proximal patellar tendon assuming that pathological intra-tendinous changes are diffuse.
4.2.2. Methodological Limitation
We have some methodological limitations to cite. First: we know the importance of the agreement value between reviewers, but this value was not calculated in the selection process and in the RoB evaluation. Second: we searched only three databases: PubMed, Scopus, and Web of Science. Third: our time range from 1 January 2010 to 31 December 2022 could be a limit, but we finally included 38 studies, so we think we have provided an extensive representation of the most recent literature. Fourth: we could have used a more detailed risk of bias tool such as the ROBINS-I tool. However, a long training period is needed to accurately use this tool [71].
5. Conclusions
In conclusion, US elastography shows good to excellent reliability of the measures in both healthy and pathological Achilles tendons [42].
Shear wave elastography may offer a more objective and reproducible value of tendon elasticity [38]. It is also the most suitable imaging technology for symptom monitoring [20].
Strain elastography is sensitive to small strain alterations in early Achilles tendon alterations, before pathological changes can be seen with B-mode US and power doppler or manifest clinically [22]. In the shoulder, strain elastography could detect different rotator cuff disorders, comparable to MRI [23], but it is more observer dependent than shear wave elastography [38].
Strain elastography and shear wave elastography demonstrate no significant differences in diagnosing medial epicondylitis [24].
Some of the alterations retrieved by strain elastography are not completely understood, and further studies are needed to clarify if they are preclinical changes or false-positive results [22].
However, to ensure the appropriate use of these technological solutions, it is of paramount importance to follow methodological recommendations such as, for instance, the technical guidelines developed by the European Society of Musculoskeletal Radiology [72].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app13084920/s1: Table S1: Reason for exclusion. Paper excluded from the review and reasons for exclusion. Table S2: Study characteristics, concerning: Study population, pathology, US machine characteristics, patient position and, location of the probe. Table S3. Shear wave elastographic values, classified in: Shear wave velocity (m/s) and Shear modulus (kPa). Table S4: Strain elastographic values, classified in Strain ratio.
Author Contributions
Conceptualization and design, G.R., M.G. and N.F.L.; acquisition of the data, G.R., E.S., P.C., D.D.M. and L.G.; analysis and interpretation of data, G.R., E.S., P.C., D.D.M. and L.G.; writing—original draft preparation, G.R.; writing—review and editing, G.R., E.S., M.G. and N.F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Filardo, G.; Di Matteo, B.; Kon, E.; Merli, G.; Marcacci, M. Platelet-Rich Plasma in Tendon-Related Disorders: Results and Indications. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. The Pathogenesis of Tendinopathy. A Molecular Perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N. Overuse Tendon Conditions: Time to Change a Confusing Terminology. Arthroscopy 1998, 14, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Rath, B.; Betsch, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies: A Double-Blinded, Longitudinal Clinical Study. Acad. Radiol. 2018, 25, 265–272. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound Elastography: Principles and Techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of Ultrasound Elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Snoj, Ž.; Wu, C.H.; Taljanovic, M.S.; Dumić-Čule, I.; Drakonaki, E.E.; Klauser, A.S. Ultrasound Elastography in Musculoskeletal Radiology: Past, Present, and Future. Semin. Musculoskelet. Radiol. 2020, 24, 156–166. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Köse, K. Quantitative Assessment of Normal Soft-Tissue Elasticity Using Shear-Wave Ultrasound Elastography. AJR Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef]
- Slane, L.C.; Martin, J.; DeWall, R.; Thelen, D.; Lee, K. Quantitative Ultrasound Mapping of Regional Variations in Shear Wave Speeds of the Aging Achilles Tendon. Eur. Radiol. 2017, 27, 474–482. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. The Biomarker Paradigm: Between Diagnostic Efficiency and Clinical Efficacy. Pol. Arch. Med. Wewn. 2015, 125, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; D’Addario, M.; Egger, M.; Cevallos, M.; Dekkers, O.; Mugglin, C.; Scott, P. Methods to Systematically Review and Meta-Analyse Observational Studies: A Systematic Scoping Review of Recommendations. BMC Med. Res. Methodol. 2018, 18, 44. [Google Scholar] [CrossRef]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enfermagem. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Higgins, J.P.D.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (Updated February 2022); John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Corrigan, P.; Cortes, D.H.; Pohlig, R.T.; Grävare Silbernagel, K. Tendon Morphology and Mechanical Properties Are Associated with the Recovery of Symptoms and Function in Patients with Achilles Tendinopathy. Orthop. J. Sports Med. 2020, 8. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, D.-B.; Wang, J.; Li, H.-Z.; Wang, Y.-C. Role of Shear Wave Elastography in the Evaluation of the Treatment and Prognosis of Supraspinatus Tendinitis. World J. Clin. Cases 2020, 8, 2977–2987. [Google Scholar] [CrossRef]
- Gatz, M.; Bode, D.; Betsch, M.; Quack, V.; Tingart, M.; Kuhl, C.; Schrading, S.; Dirrichs, T. Multimodal Ultrasound Versus MRI for the Diagnosis and Monitoring of Achilles Tendinopathy: A Prospective Longitudinal Study. Orthop. J. Sports Med. 2021, 9, 23259671211006824. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a Tool for Assessing the Risk of Bias for Nonrandomized Studies Showed Moderate Reliability and Promising Validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Kandil, N.M.; Abdelkarim, M.A.; Abdelwahab, N.M.; Hashem, A.M. In Achilles Tendon Disorders, Will Sonoelastography Add to Grey-Scale Ultrasound? Using MRI as Gold Standard. Indian J. Radiol. Imaging 2021, 31, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Frere, R.A.; Libda, I.; Tantawy, F.; Sakr, H.M.; El-Alfy, A.T. Sonoelastography, Conventional Ultrasound and Magnetic Resonance Imaging in Detection of Rotator Cuff Lesions in Patients with Chronic Shoulder Pain. Egypt. Rheumatol. 2021, 43, 17–21. [Google Scholar] [CrossRef]
- Bang, J.-Y.; Hahn, S.; Yi, J.; Lim, Y.-J.; Jung, H.K. Clinical Applicability of Shear Wave Elastography for the Evaluation of Medial Epicondylitis. Eur. Radiol. 2021, 31, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; You, Y.; Xiang, X.; Wang, L.; Qiu, L. Assessment of Common Extensor Tendon Elasticity in Patients with Lateral Epicondylitis Using Shear Wave Elastography. Quant. Imaging Med. Surg. 2020, 10, 211–219. [Google Scholar] [CrossRef]
- Shin, M.; Hahn, S.; Yi, J.; Lim, Y.-J.; Bang, J.-Y. Clinical Application of Real-Time Sonoelastography for Evaluation of Medial Epicondylitis: A Pilot Study. Ultrasound Med. Biol. 2019, 45, 246–254. [Google Scholar] [CrossRef]
- Sahan, M.H.; Inal, M.; Burulday, V.; Kultur, T. Evaluation of Tendinosis of the Long Head of the Biceps Tendon by Strain and Shear Wave Elastography. Med. Ultrason. 2018, 20, 192–198. [Google Scholar] [CrossRef]
- Ghandour, A.M.; Ghandour, T.M. Strain-Based Elastography Assessment of Patients with De Quervain Tenosynovitis: A Preliminary Study. Egypt. J. Radiol. Nucl. Med. 2018, 49, 415–418. [Google Scholar] [CrossRef]
- El Badry, A.; Ghieda, U.; El Khouly, R.M.; Elreweny, E.A. Evaluation of Sonoelastography in Achilles Tendon of Healthy Volunteers and Patients with Symptomatic Achilles Tendon. Egypt. J. Radiol. Nucl. Med. 2018, 49, 119–127. [Google Scholar] [CrossRef]
- Arslan, S.; Karahan, A.Y.; Oncu, F.; Bakdik, S.; Durmaz, M.S.; Tolu, I. Diagnostic Performance of Superb Microvascular Imaging and Other Sonographic Modalities in the Assessment of Lateral Epicondylosis. J. Ultrasound Med. 2018, 37, 585–593. [Google Scholar] [CrossRef]
- Turkay, R.; Inci, E.; Aydeniz, B.; Vural, M. Shear Wave Elastography Findings of de Quervain Tenosynovitis. Eur. J. Radiol. 2017, 95, 192–196. [Google Scholar] [CrossRef]
- Coombes, B.K.; Tucker, K.; Vicenzino, B.; Vuvan, V.; Mellor, R.; Heales, L.; Nordez, A.; Hug, F. Achilles and Patellar Tendinopathy Display Opposite Changes in Elastic Properties: A Shear Wave Elastography Study. Scand. J. Med. Sci. Sports 2018, 28, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Chadwick, M.; Connell, D.A. Diagnostic Performance of Axial-Strain Sonoelastography in Confirming Clinically Diagnosed Achilles Tendinopathy: Comparison with B-Mode Ultrasound and Color Doppler Imaging. Ultrasound Med. Biol. 2015, 41, 15–25. [Google Scholar] [CrossRef]
- Ooi, C.C.; Richards, P.J.; Maffulli, N.; Ede, D.; Schneider, M.E.; Connell, D.; Morrissey, D.; Malliaras, P. A Soft Patellar Tendon on Ultrasound Elastography Is Associated with Pain and Functional Deficit in Volleyball Players. J. Sci. Med. Sport 2016, 19, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-B.; Yoo, J.-S.; Ryu, J.-W. Sonoelastography Findings of Biceps Tendinitis and Tendinosis. J. Ultrasound 2014, 17, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Giyoung, P.; Dongrak, K.; Junghyun, P. Diagnostic Confidence of Sonoelastography as Adjunct to Greyscale Ultrasonography in Lateral Elbow Tendinopathy. Chin. Med. J. 2014, 127, 3110–3115. [Google Scholar]
- Galletti, S.; Oliva, F.; Masiero, S.; Frizziero, A.; Galletti, R.; Schiavone, C.; Salini, V.; Abate, M. Sonoelastography in the Diagnosis of Tendinopathies: An Added Value. Muscles Ligaments Tendons J. 2015, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Özel, D.; Demir, Y.; Özel, B.D.; Adaş, M. A Novel Measurement to Evaluate Supraspinatus Tendinopathies with Strain Elastography. Acta Radiol. 2021, 62, 1365–1373. [Google Scholar] [CrossRef]
- Vasishta, A.; Kelkar, A.; Joshi, P.; Hapse, R. The Value of Sonoelastography in the Diagnosis of Supraspinatus Tendinopathy-a Comparison Study. Br. J. Radiol. 2019, 92, 20180951. [Google Scholar] [CrossRef]
- Seo, J.-B.; Yoo, J.-S.; Ryu, J.-W. Sonoelastography Findings of Supraspinatus Tendon in Rotator Cuff Tendinopathy without Tear: Comparison with Magnetic Resonance Images and Conventional Ultrasonography. J. Ultrasound 2015, 18, 143–149. [Google Scholar] [CrossRef]
- Sprague, A.L.; Couppé, C.; Pohlig, R.T.; Cortes, D.C.; Silbernagel, K.G. Relationships between Tendon Structure and Clinical Impairments in Patients with Patellar Tendinopathy. J. Orthop. Res. 2022, 40, 2320–2329. [Google Scholar] [CrossRef]
- Ito, N.; Sigurðsson, H.B.; Pohlig, R.T.; Cortes, D.H.; Grävare Silbernagel, K.; Sprague, A.L. Reliability of Continuous Shear Wave Elastography in the Pathological Patellar Tendon. J. Ultrasound Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Hafez, M.R.M.; Ibrahim, M.A.H. Ultrasound with Shear Wave Elastography in Diagnosis and Follow-up of Common Extensor Tendinopathy in Cases with Lateral Epicondylitis: A Cross-Sectional Analytic Study. Egypt. J. Radiol. Nucl. Med. 2022, 53, 236. [Google Scholar] [CrossRef]
- Corrigan, P.; Hornsby, S.; Pohlig, R.T.; Willy, R.W.; Cortes, D.H.; Silbernagel, K.G. Tendon Loading in Runners with Achilles Tendinopathy: Relations to Pain, Structure, and Function during Return-to-Sport. Scand. J. Med. Sci. Sports 2022, 32, 1201–1212. [Google Scholar] [CrossRef]
- Wada, T.; Itoigawa, Y.; Yoshida, K.; Kawasaki, T.; Maruyama, Y.; Kaneko, K. Increased Stiffness of Rotator Cuff Tendons in Frozen Shoulder on Shear Wave Elastography. J. Ultrasound Med. 2020, 39, 89–97. [Google Scholar] [CrossRef]
- Gatz, M.; Betsch, M.; Bode, D.; Schweda, S.; Dirrichs, T.; Migliorini, F.; Tingart, M.; Quack, V. Intra Individual Comparison of Unilateral Achilles Tendinopathy Using B-Mode, Power Doppler, Ultrasound Tissue Characterization and Shear Wave Elastography. J. Sport. Med. Phys. Fit. 2020, 60, 1462–1469. [Google Scholar] [CrossRef]
- Breda, S.J.; van der Vlist, A.; de Vos, R.-J.; Krestin, G.P.; Oei, E.H.G. The Association between Patellar Tendon Stiffness Measured with Shear-Wave Elastography and Patellar Tendinopathy-a Case-Control Study. Eur. Radiol. 2020, 30, 5942–5951. [Google Scholar] [CrossRef]
- Yurdaışık, I. Comparison of Two-Dimensional Shear Wave Elastography and Point Shear Wave Elastography Techniques with Magnetic Resonance Findings in Detection of Patellar Tendinopathy. Eklem Hastalik Cerrahisi 2019, 30, 275–281. [Google Scholar] [CrossRef]
- Yun, S.J.; Jin, W.; Cho, N.S.; Ryu, K.N.; Yoon, Y.C.; Cha, J.G.; Park, J.S.; Park, S.Y.; Choi, N.Y. Shear-Wave and Strain Ultrasound Elastography of the Supraspinatus and Infraspinatus Tendons in Patients with Idiopathic Adhesive Capsulitis of the Shoulder: A Prospective Case-Control Study. Korean J. Radiol. 2019, 20, 1176–1185. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Bucklin, M.; Kelly, M.; Ketz, J.; Flemister, A.S.; Richards, M.S.; Buckley, M.R. Insertional Achilles Tendinopathy Associated with Altered Transverse Compressive and Axial Tensile Strain during Ankle Dorsiflexion. J. Orthop. Res. 2017, 35, 910–915. [Google Scholar] [CrossRef]
- Lee, S.-U.; Joo, S.Y.; Kim, S.K.; Lee, S.-H.; Park, S.-R.; Jeong, C. Real-Time Sonoelastography in the Diagnosis of Rotator Cuff Tendinopathy. J. Shoulder Elbow Surg. 2016, 25, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, F.; Kuyucu, E.; Kocyigit, A.; Herek, D.T.; Savkin, R.; Aslan, U.B. Investigation of Biomechanical Characteristics of Intact Supraspinatus Tendons in Subacromial Impingement Syndrome: A Cross-Sectional Study with Real-Time Sonoelastography. Am. J. Phys. Med. Rehabil. 2016, 95, 588–596, (A). [Google Scholar] [CrossRef]
- Kocyigit, F.; Kuyucu, E.; Kocyigit, A.; Herek, D.T.; Savkin, R.; Aslan, U.B.; Karabulut, N. Association of Real-Time Sonoelastography Findings with Clinical Parameters in Lateral Epicondylitis. Rheumatol. Int. 2016, 36, 91–100, (B). [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef]
- Tudisco, C.; Bisicchia, S.; Stefanini, M.; Antonicoli, M.; Masala, S.; Simonetti, G. Tendon Quality in Small Unilateral Supraspinatus Tendon Tears. Real-Time Sonoelastography Correlates with Clinical Findings. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 393–398. [Google Scholar] [CrossRef]
- Aubry, S.; Nueffer, J.-P.; Tanter, M.; Becce, F.; Vidal, C.; Michel, F. Viscoelasticity in Achilles Tendonopathy: Quantitative Assessment by Using Real-Time Shear-Wave Elastography. Radiology 2015, 274, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ng, G.Y.; Lee, W.C.; Fu, S.N. Changes in Morphological and Elastic Properties of Patellar Tendon in Athletes with Unilateral Patellar Tendinopathy and Their Relationships with Pain and Functional Disability. PLoS ONE 2014, 9, e108337. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Kang, C.H.; Hong, S.-J.; Jeong, W.-K. Ultrasound Elastography of Lateral Epicondylosis: Clinical Feasibility of Quantitative Elastographic Measurements. AJR Am. J. Roentgenol. 2014, 202, 1094–1099. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Silvestri, E.; Cimmino, M.A. Sonoelastography in the Evaluation of Painful Achilles Tendon in Amateur Athletes. Clin. Exp. Rheumatol. 2010, 28, 373–378. [Google Scholar] [PubMed]
- Gatz, M.; Betsch, M.; Dirrichs, T.; Schrading, S.; Tingart, M.; Michalik, R.; Quack, V. Eccentric and Isometric Exercises in Achilles Tendinopathy Evaluated by the VISA-A Score and Shear Wave Elastography. Sports Health 2020, 12, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Thiese, M.S. Observational and Interventional Study Design Types; an Overview. Biochem. Med. 2014, 24, 199–210. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; OSullivan, C.; Hides, J.; Fernandez-Carnero, S.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with Ultrasound in Physical Therapy: What Is the PT’s Scope of Practice? A Competency-Based Educational Model and Training Recommendations. Br. J. Sports Med. 2019, 53, 1447–1453. [Google Scholar] [CrossRef]
- Fu, S.-C.; Rolf, C.; Cheuk, Y.-C.; Lui, P.P.; Chan, K.-M. Deciphering the Pathogenesis of Tendinopathy: A Three-Stages Process. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 30. [Google Scholar] [CrossRef]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon Basic Science: Development, Repair, Regeneration, and Healing. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef]
- Fusini, F.; Langella, F.; Busilacchi, A.; Tudisco, C.; Gigante, A.; Massé, A.; Bisicchia, S. Real-Time Sonoelastography: Principles and Clinical Applications in Tendon Disorders. A Systematic Review. Muscles Ligaments Tendons J. 2017, 7, 467–477. [Google Scholar] [CrossRef]
- De Zordo, T.; Fink, C.; Feuchtner, G.M.; Smekal, V.; Reindl, M.; Klauser, A.S. Real-Time Sonoelastography Findings in Healthy Achilles Tendons. AJR Am. J. Roentgenol. 2009, 193, W134–W138. [Google Scholar] [CrossRef] [PubMed]
- Dighe, M.; Hippe, D.S.; Thiel, J. Artifacts in Shear Wave Elastography Images of Thyroid Nodules. Ultrasound Med. Biol. 2018, 44, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, Y.-G.; Park, H.-S.; Cho, R.-K.; Lee, H.-J. Comparison of Gene Expression of Inflammation- and Fibrosis-Related Factors between the Anterior and Posterior Capsule in Patients with Rotator Cuff Tear and Shoulder Stiffness. Orthop. J. Sports Med. 2021, 9, 23259671211032544. [Google Scholar] [CrossRef]
- Redondo-Alonso, L.; Chamorro-Moriana, G.; Jiménez-Rejano, J.J.; López-Tarrida, P.; Ridao-Fernández, C. Relationship between Chronic Pathologies of the Supraspinatus Tendon and the Long Head of the Biceps Tendon: Systematic Review. BMC Musculoskelet. Disord. 2014, 15, 377. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Teyhen, D.S.; Elliott, J.M.; Cook, K.; Langevin, H.M.; Dahl, H.H.; Stokes, M. Rehabilitative Ultrasound Imaging: Understanding the Technology and Its Applications. J. Orthop. Sports Phys. Ther. 2007, 37, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Igelström, E.; Campbell, M.; Craig, P.; Katikireddi, S.V. Cochrane’s Risk of Bias Tool for Non-Randomized Studies (ROBINS-I) Is Frequently Misapplied: A Methodological Systematic Review. J. Clin. Epidemiol. 2021, 140, 22–32. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical Indications for Musculoskeletal Ultrasound Updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) Consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).