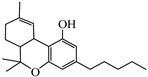
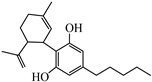
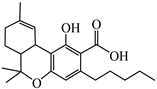
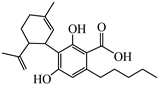
Abstract
Cannabis sativa L. has health benefits, principally due to the levels and ratios of two important cannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC:CBD ratio affects their pharmacological interaction for the treatment of different diseases as well as its modulation allows for a custom-made product that utilizes the distinguishing effects of CBD, THC, or both, for a peculiar patient or clinical effect. This study aims to investigate the total content of THC, CBD, and their ratio in 34 dried inflorescence legally sold in physical and online stores, by using a validated liquid chromatography-ultraviolet (HPLC-UV) method, after cannabinoids identification performed through MSn studies. Cannabinol (CBN) content was also monitored to evaluate hemp age or conservation status. CBN content always resulted lower than limit of quantification, thus confirming well-stored fresh hemp. All investigated samples showed a total THC amount below 0.59% w/w, thus responding to legal requirements.. The total CBD amount ranged from 2.62 to 20.27% w/w and it was not related to THC level. THC:CBD ranged among 1:3 and 1:26, thus ascertaining their suitability for different target pharmacological uses. In vitro studies using human hepatoblastoma cell line HepG2 suggested that hemp extracts with THC:CBD ratios of 1:9 exhibited higher toxicity than pure cannabinoids.
Keywords:
hemp; light cannabis; THC; CBD; THCA; CBDA; CBN; THC:CBD ratio; liquid chromatography; mass spectrometry; collision-induced dissociation; UV detection; HepG2 1. Introduction
Cannabis sativa L. is a cannabinoid-rich herbaceous plant with various pharmacological activities. During the COVID-19 pandemic, statistical studies revealed that cannabis use is increased in many countries, including Italy. This alleged increase in cannabis use is due to several factors, including stress and anxiety, social isolation, and loneliness exacerbated by the pandemic [1]. Recently, the interest in Cannabis sativa L. increased mostly due to the latest Italian legislation [2] and European regulations, which legalized the sale of light cannabis in physical stores and online. Such legislative framework classified Cannabis sativa L. into two types as a function of the content of Δ9-tetrahydrocannabinol (∆9-THC or THC). In particular, fiber-type plants of Cannabis sativa L., also called “hemp” or “light cannabis”, are characterized by a low amount of THC (<0.2% w/w), with a tolerance of up to 0.6%. Conversely, when the THC content exceeds 0.6% w/w, Cannabis sativa L is known as “medicinal” or “marijuana “ and is considered a drug-type. Generally, fiber-type plants are used for industrial purposes and less for pharmaceuticals, where drug-type plants are more commonly used [3,4,5].
As a result of the large variety and complexity of phytocannabinoids, the classification of cannabis cultivars is a fundamental requirement for the quality control of medical cannabis. Alongside THC, cannabidiol (CBD) became a crucial compound for confirming cannabis chemotypes, depending on the dry weight ratio of THC/CBD in the plant: chemotype I, including marijuana, has THC/CBD > 1, chemotype II has THC ≈ CBD, and chemotype III, including hemp, has THC/CBD << 1, with low THC content [6,7]. However, while the THC content in cannabis light must be within 0.6%, it was shown that CBD levels vary greatly, (from 2% w/w up to 40% w/w), without legal indication on the authorized percentage content [8]. THC and CBD levels are influenced also by the presence of the corresponding non-psychoactive carboxylated forms, ∆9-tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), immediately converted to THC and CBD under the influence of high temperature leading to an intensification of pharmacological effects. The level of decarboxylated substances can vary with the type of plant tissue, variety, age, growing conditions, harvest period, and conditions of storage. For this reason, also the determination of the cannabinoid acidic precursors, even if not mandatory for cannabis products, should be performed, in order to not underestimate the total THC and CBD contents, which are used for the assessing of the legal purposes, as well as the health risks/benefits [9,10]. Liquid chromatography coupled with UV and/or mass spectrometry is an analytical technique that was shown to effectively distinguish and individually measure ∆9-THC, CBD, and their acid precursors, ∆9-THCA and CBDA [11,12].
In addition to chemotype definition, THC/CBD ratio is becoming important also as a marker to categorize Cannabis sativa for medicinal purposes, and research is underway to better understand the therapeutic properties of the various formulations and dosages. Although both THC and CBD interact with the body’s endocannabinoid system, i.e., receptor types 1 (CB1) and 2 (CB2) [13], they have very different effects [14]. Several studies confirmed the efficacy of THC for treatment of glaucoma, spastic disorders, acute and chronic pain, prevention of nausea and vomiting from cancer medicines, as well as cancer treatment through cell cycle arrest, induction of apoptosis, inhibition of neovascularization, migration, adhesion, invasion, and metastasis [3,4,5,15]. In spite of the numerous positive results of THC and related cannabinoids in the study of cancer, their use as medicinal drugs is limited because of their psychotropic side effects. THC side effects might be mitigated by the presence of CBD, which is not psychoactive, thus it recently became the subject of extensive research in a number of therapeutic fields, notably cancer [16]. As a matter of fact, THC-induced inhibitory effects of cell growth and suppression of tumour growth were obtained at larger concentrations as compared with CBD effects [13].
In general, four THC:CBD ratio categories whose cannabinoid interactions are pharmacologically different could be distinguished: at a ratio ≥1:1, CBD can enhance THC effects, while for ratios ~1:2 or 1:>2 < 6, CBD can either have no effect or can attenuate THC effects. On the contrary, CBD protects against the effects of THC for ratios ≤1:6 [17]. Therefore, various health-related problems could be treated by varying their percentages. Mild to moderate pain due to inflammations can be well managed with CBD-dominant products, such as CBD:THC around 9:1 or more [18,19,20].
This study aimed to take an overview of the concentration of THC, CBD, THCA, and CBDA, in 34 cannabis light dried inflorescences commercially available in Italian local shops and online by using high-performance liquid chromatography coupled with ultraviolet detection (HPLC-UV). Although at present, no state that legalized cannabis for medical or recreational purposes considers THC/CBD ratio in the drafted regulations, in this work, a particular attention was paid to THC/CBD ratio, because it is important to identify “best practices” for treating different disease processes and their after-effects [21]. Changing the CBD:THC ratio allows for a custom-made product that utilizes the distinguishing effects of CBD, THC, or both for a peculiar patient or clinical effect. Cannabinol (CBN) content was also monitored to evaluate hemp freshness. A LC-UV-based quantitative analysis was performed after the development and validation of a suitable analytical method. Cannabinoids identification, besides quantitative analysis, was accomplished by tandem mass spectrometry (MS/MS and MSn) studies. Finally, the cytotoxicity of Cannabis sativa L. light extracts with a THC:CBD ratio of 1:9, on HepG2 cancer cell line, was assessed using a MTT assay.
2. Results and Discussion
2.1. LC-UV Method Validation
In this work, the main aim was to develop a chromatographic method able to separate the various cannabinoids. Since THC/CBD and THCA/CBDA are isomers with similar UV spectra (see panels in Figure 1), their identification is only possible depending on their retention time. The separation of the compounds under investigation was carried out on core-shell column in reverse phase mode, with good results in terms of analytes retention, peak shape, and resolution power [6]. The optimized gradient elution allowed for a good separation of cannabinoids within 16.0 min of chromatographic analysis. In detail, CBD elutes (8.9 min) after its acidic precursor CBDA (8.2 min) because of its higher lipophilicity. The same could be said about the acid precursor THCA (15.7 min) compared to THC (13.1 min). CBN elutes (11.7 min) after CBD, due to higher lipophilicity of the additional pyran ring, but before THC because of the higher polarity of the aromatic ring compared to the cyclohexane occurring in the THC molecule. THC and THCA elute following CBD and CBN due to the presence of the dihydropyran ring and the simultaneous absence of a free hydroxyl group leading to higher lipophilia. Figure 1 reports the chromatographic profile of a cannabinoid standard mix at 1 mg/mL used to evaluate the reliability of the chromatographic method.

Figure 1.
HPLC-UV chromatographic profile of a cannabinoid standard mixture at 1 mg/mL. The observed retention times were 8.2 min for CBDA, 8.9 min for CBD, 11.7 min for CBN, 13.1 min for THC, and 15.7 for THCA. The UV detector was set at 220 nm for THCA, CBDA, and CBN detection and at 210 nm for THC and CBD detection.
Method validation results are reported in Table 1. In the analyzed concentration range, the linearity was good for all the analytical standards of the cannabinoids studied, being R2 > 0.9998. The instrumental LOD and LOQ were determined by the calibration curve, based on the formulas expressed in Section 4.4, and ranged between 0.05 and 0.08 mg/L and 0.15 and 0.25 mg/L, respectively. Compared to literature [22], lower LOD and LOQ values were found for all the cannabinoids under study, confirming the sensitivity of the developed method. Repeatability and intermediate precision results (Table 1) demonstrate a very high precision of the method over the linearity range. In fact, the %RSD varied from 0.04 to 1.51% for repeatability and from 0.31 to 3.49% for intermediate precision at 1 mg/mL. Additionally, retention times precision resulted very good, with %RSD less than 1.51%. As shown in Table 1, the percentage of recovery values was higher than 87.2%, thus proving the accuracy of the method was similar to those previously reported in literature [22].

Table 1.
Validation parameters of the LC-UV method used for the quantitative analysis of cannabinoids understudy, THC (Δ9-tetrahydrocannabinol), CBD (cannabidiol), THCA (∆9-tetrahydrocannabinolic acid), CBDA (cannabidiolic acid), and CBN (cannabinol).
2.2. LC-ESI—LTQ-MSn Cannabinoids Identification
Despite tandem mass spectrometry (MS) not being routinely available to most laboratories, MS is confirmed as a powerful technique for the identification of compounds in complex mixtures [9]. Since a few recent works outline the main cannabinoid fragmentation patterns by using positive and negative ion electrospray ionization [6,23], here we performed the identification of THC, CBD, THCA, CBDA, and CBN in 34 cannabis light preparations by comparing the obtained CID-MSn spectra with literature data. Positive electrospray ionization (ESI) was used for neutral cannabinoids analysis, while negative ion mode was chosen for acid derivatives, since they can be easily deprotonated in the ESI source [24]. The obtained results are shown in Table 2. We also reported as an example the HPLC-UV chromatographic profile of the three selected samples chosen for toxicity studies (See Section 2.4).

Table 2.
Cannabinoids occurring in a sample of light Cannabis sativa extract understudy, identified as intact protonated molecules, [M + H]+ for THC, CBD, and CBN, and intact deprotonated molecules, [M − H]−, for THCA and CBDA, by using LC-ESI-LTQ MSn and collision-induced dissociation (CID) as a fragmentation technique.
THC and CBD were both identified as protonated ions ([M + H]+: C21H31O2+) at m/z 315. As they showed a very similar MS/MS spectrum in positive ion mode, retention times were used to confirm their identity by comparing the retention time of the analogous commercial standards. As reported in Table 2, THC and CBD MS/MS fragmentation patterns, together with MS3, MS4, and MS5 experiments, allowed for confirmation of their identity, as already reported in literature [6]. All fragment ions were assigned as follows: ion at m/z 259 was caused by the loss of four C units of the terpenic portion; the m/z 235 ion was consistent with terpene breakage, with only four carbon units of this group left; ion at m/z 193 corresponded to olivetol moiety linked to C2 of the benzene ring; ion at m/z 181 was attributed to the olivetol moiety; ion at m/z 135 was obtained after the bond cleavage of the aromatic portion with the cyclohexenyl group, togheter with a hydrogen shift; ions at m/z 273, 245, 233, 231, and 207 were attributable to the olivetol derivatives; ion at m/z 227 was assigned to the loss of alkyl chain from dehydrated ion at m/z 297; and ions at m/z 175, 123, and 111 were assigned to structures containing the more stable aromatic group of CBD, retaining both oxygen atoms.
A similar scenario applies to the acid precursor THCA, as well as CBDA, showed a poor informative MS/MS fragmentation spectrum in negative mode (Table 2). However, Picollela et al. [24] establish the basis for thorough discrimination between THCA and CBDA, detected as deprotonated ions ([M − H]−: C22H29O4−) at m/z 357, leading to an appropriate chemical characterization guideline. Firstly, the discrimination between THCA and CBDA was based on the MS/MS fragments they share, e.g., fragment ions due to dehydration; ([M − H−H2O]− at m/z 339) and decarboxylation ([M − H−CO2]− at m/z 313): the [M − H−CO2]−/[M − H−H2O]− abundance ratio resulted < 1 in CBDA and > 1 in THCA, as already reported in literature [24]. Additionally, ions at m/z 227 and 271 are present only in the MS3 spectrum of CBDA. The first one could be attribuited to a neutral loss of of 44 Da and 18 Da alongside an isoprenic unit (−68 Da) from the precursor ion, while the second one was lately referred to as the product of a retro Diels-Alder (RDA) reaction, involving the [M − H−H2O]− ion at m/z 339 [23]. Instead, in the MS3 spectrum of THCA, the RDA occurrence produced the ion at m/z 245 (2,2-dimethyl-7-pentyl-2H-chromen-5-olate), which promptly retro-cyclized to get the ion at m/z 191 [24].
Finally, CBN was identified as a protonated ion ([M + H]+: C21H27O2+) at m/z 311. It fragmented differently (Table 2) than other cannabinoids because of the stability of the aromatic ring [6]. The base peak at m/z 293 was related to water loss. The ions at m/z 223 and 195 detected in the CID MS3 and CID MS4 spectra, respectively, suggest that the fragment ions produced are the result of consecutive leakages of the pentyl side chain and two methyl groups of [M − H−H2O]− ion at m/z 293. The benzopyran ring opening of CBN resulted in the diagnostic fragment ion at m/z 265. A fragment ion with higher signal intensity was observed at m/z 241 and it is attribuible to the cleavage of the aliphatic 5-carbon chain from the precursor ion.
2.3. Quantitative Analysis of 34 Cannabis sativa L. Samples
Dried inflorescences of 34 hemp samples were analysed by the LC-UV previously validated method in order to determine the presence and the content of five cannabinoids, i.e., THC, CBD, CBN, THCA, and CBDA, whose identities in the samples under study were previously confirmed by LC-MS/MS analysis. In Figure 2, we reported as an example the HPLC-UV chromatographic profile of the three selected samples chosen for toxicity studies (see Section 2.4).

Figure 2.
HPLC-UV chromatographic profile of an extract of cannabis inflorescences at 220 nm: (A) Mary Moonlight-Legal Weed diluted 1:10; (B) Purple Rock-Legal Weed; and (C) Alexis Haze-Legal Weed diluted 1:10.
Cannabinoid contents of 34 Cannabis sativa L. samples of industrial hemp are reported in Table 3.

Table 3.
Cannabinoid contents, THC, CBD, THCA, CBDA, total THC, total CBD, and CBN, expressed as % (w/w) ± standard deviation (n = 3 replicates), for 34 Cannabis sativa L. samples of industrial hemp. Values marked by the same letter are not significantly different (p < 0.05).
As expected, cannabinoid levels were heterogeneous among the samples, because they can fluctuate in response to genetic and environmental factors. THC, CBD, and CBDA were the major cannabinoids with concentration levels in the ranges of 0.05 (±0.01)–0.54 (±0.05)% w/w, 0.32 (±0.08)–4.8 (±0.2)% w/w, and 1.74 (±0.02)–21.7 (±0.4)% w/w, respectively. THCA represented the minor component, with a content always lower than 0.16 ± 0.02% w/w, while CBN was always lower than LOQ in the samples under study.
In regard to major cannabinoids, THC:CBD ratio affects the metabolism and therapeutic effects of cannabis, due to the different interaction with cannabinoid receptors CBD 1 and 2 [13], thus classifying the corresponding plants in fiber type (or cannabis light) and drug type (or marijuana). For most of the analyzed samples, THC:CBD ratios resulted between 1:3 and 1:11. Only two samples had much higher CBD values than THC, as the THC: CBD ratios were 1:13 and 1:26 for Moon Rock-Love Canapa (Sample 33 in Table 3) and Malana-Baby J (Sample 22 in Table 3), respectively. However, all samples showed THC:CBD ratio less than 1, thus ascertaining the fiber type of the plants. Currently, no countries consider THC:CBD ratios in their own regulations for legalized medical or recreational cannabis [21].
Furthermore, decarboxylation of THCA and CBDA acid precursors yields a greater amount of THC and CBD, respectively, making it necessary to evaluate the total content of these cannabinoids occurring in the inflorescences, in order to confirm the chemotype classification. The total THC content was determined as follows: (THCA × 0.877) + THC. Similarly, the total CBD content was determined as (CBDA × 0.877) + CBD, considering 0.877 as the ratio of the molecular mass of decarboxylated form and the carboxylated form. Indeed, the neutral compound is lighter, as it has about 87.7% of the mass of the acid precursor. When THCA is converted into THC, or CBDA into CBD, the total weight of the newly formed cannabinoid is lower than the total dry weight of the herb.
As shown in Table 3, a low total content of THC was found in all the samples, as it ranged from 0.09 to 0.59% w/w. The total CBD content ranged from 2.62 to 20.27% w/w and it was not related to THC level. The data obtained corroborated that the evaluated samples were properly classified as hemp, as the amount of THC was below the legal limits. In fact, according to the current legislation concerning the cultivation of Cannabis sativa L. the total THC content must not exceed 0.2%, and in any case, 0.6%. Finally, the CBN/THC ratio determination allowed for evaluation of the preservation status of the inflorescence samples. In “old” cannabis samples, i.e., more than 6 months, or in samples exposed to light or high temperatures, the oxidation of THC leads to the formation of CBN [25] with CBN/THC ratios higher than 0.013. In all 34 samples under study, CBN contents were lower than the LOQ, thus indicating well-stored or fresh inflorescences.
As the cannabis products analysed are also sold on websites, our results can likely represent a view of cannabis light products in the Italian and international markets.
2.4. Effect of Cannabis sativa L. Extracts on Viability of Hepatoblastoma HepG2 Cells
The main anticancer effects of cannabinoids are attributed to the induction of the endocannabinoid receptors CB1 and CB2, which activate different signaling mechanisms leading to cell death by endoplasmic reticular stress and autophagy, apoptosis, as well as inhibition of cell proliferation [26]. CBD is the most promising cannabinoid for the cancer treatment, as it lacks the psychotomimetic properties of THC. The anti-proliferative and pro-apoptotic effects of CBD were shown on a variety of cancer types both in vitro and in mouse tumor models, where it was suggested to modulate the tumor microenvironment [16].
HepG2 cell line, a good in vitro model to toxicity studies also for cannabis and derivatives, was employed [27,28].
Mary Moonlight-Legal Weed (Sample 1 in Table 3), Purple Rock-Legal Weed (Sample 5 in Table 3), and Alexis Haze-Legal Weed (Sample 20 in Table 3) extracts, with THC:CBD ratios of 1:9, were chosen as representative samples of cannabis light extracts to evaluate the effect on viability of hepatoblastoma cells HepG2 after 24, 48, and 72 h treatment. The three samples reported THC:CBD ratios of 1:9, optimal for inflammation and pain treatment. According to Kovalchuk et al. [5], which suggested that cannabis extracts might show more potent anticancer activity than the pure substances due to the presence of other compounds, including flavonoids, terpenoids, sugars, and amino acids, we found that the three extracts had a comparable if not a more cytotoxic effect than CBD already after 24 h of treatment. The IC50 were 14.35 ± 3.68, 15.25 ± 1.98 and 9.86 ± 1.87 µM for Mary Moonlight-Legal Weed, Purple Rock-Legal Weed, and Alexis Haze-Legal Weed, respectively, and 16.82 ± 2.54 µM for CBD (Figure 3 and Figure 4 and Table 4 and Table 5). The comparable activity could be related to their content of CBD equal to 3.53 (±0.09)% w/w and 3.7 (±0.2)% w/w for Mary Moonlight-Legal Weed and Alexis Haze-Legal Weed, respectively. Purple Rock-Legal Weed extracts containing 1.23 (±0.05)% w/w of CBD showed more cytotoxic effect.

Figure 3.
Dose–response curves of Mary Moonlight-Legal Weed, Alexis Haze-Legal Weed, and Purple Rock-Legal Weed extracts. HepG2 cells were grown for 24 h (a), 48 h (b), 72 h, and (c) in the presence of increasing concentrations of the three extracts. The viability of cells was evaluated using the MTT assay and the concentration required to reduce the cell number by 50% (IC50) for each condition was calculated using GraphPad Prism. Each data point represents the mean of at least three separate experiments and the vertical bars represent the S.D.

Figure 4.
Dose–response curves of THC, CBD, and MIX THC CBD. HepG2 cells were grown for 24 h (a), 48 h (b), 72 h, and (c) in the presence of increasing concentrations of the two cannabinoids, THC and CBD, either as single agents or in dual combinations prepared at a 1:9 ratio. The cell viability was assessed with the MTT test and the concentration required to reduce the cell number by 50% (IC50) for each condition in HepG2 cells was calculated using GraphPad Prism. Each data point represents the mean of at least three separate experiments and the vertical bars represent the standard deviation.

Table 4.
IC50 of Mary Moonlight, Alexis Haze, and Purple Rock against HepG2 cells as determined by the MTT assay.

Table 5.
IC50 of THC, CBD, and MIX THC CBD against HepG2 cells as determined by the MTT assay.
Up to 48 h, THC treatment did not show any toxic effect; at 72 h, it showed a greater effect (IC50 47.94 ± 2.30 µM) than CBD (IC50 66.93 ± 6.00 µM), and when used in combination, a synergic effect was observed (IC50 36.16 ± 3.59 µM). The cell growth inhibitory activity of the three extracts did not change up to 72 h and remained higher than the pure substances.
3. Conclusions
In conclusion, LC-UV and LC-MSn methods proved to be suitable for rapidly and precisely measuring cannabinoid contents in cannabis products. All 34 cannabis light products studied, legally commercialized in Italy, but also available on the online marketplace, complied with the national law as the total THC content was lower than 0.59%. As reported in literature, the level of CBD varied and was not associated with that of THC. CBN content was lower than LOQ in all samples. The results from the MTT assay suggested that cannabis light extracts were cytotoxic and suppressed the viability of HepG2 cells more effectively than pure compounds already after 24 h of treatment. These results may be considered timely and medically relevant in view of the proposed clinical use of cannabis-based drugs to relieve cancer-related pain. Nevertheless, further studies should be carried out to test extracts of Cannabis sativa L with different THC:CBD ratios on tumor cells and on cell line derived from normal tissues.
4. Materials and Methods
4.1. Chemicals and Reagents
Methanolic standard solutions of cannabidiol (CBD, 1.0 mg mL−1), cannabinol (CBN, 1.0 mg mL−1), and Δ9-tetrahydrocannabinol (THC, 1.0 mg mL−1), were purchased from HPC Standard GmbH (Cunnersdorf, Germany). Methanolic standard solutions of cannabidiol acid (CBDA, 1.0 mg mL−1) and Δ9-tetrahydrocannabinol acid (THCA, 1.0 mg mL−1) were purchased from THC Pharm GmbH (Frankfurt am Main, Germany). All standards were stored at −20 °C.
Methanol, acetonitrile, and formic acid (99%) used for chromatographic separation had LC-MS grade and were obtained from Sigma-Aldrich (Steinheim, Germany). Ethanol (96.0%), chloromethane, dimethyl sulfoxide (DMSO), and isopropanol were purchased from Sigma-Aldrich (Steinheim, Germany). Ultrapure water was produced using a Milli-Q RG system from Millipore (Bedford, MA, USA). Pure nitrogen (99.996%) was delivered to the LC-MS system as sheath gas. The ion trap pressure was maintained with helium 99.999%, which was used for trapping and collisional activation of the trapped ions.
The HepG2 cell line was obtained from American Type Culture Collection, Manassas, VA, USA (ATCC), Dulbecco’s modified Eagle’s medium (DMEM), and MTT reagent (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) from Sigma (St. Louis, MO, USA).
4.2. Cannabis Samples and Phytocannabinoids Extraction
Female dry inflorescences of cannabis light (n = 34), available online, were obtained from cannabis stores located in Potenza (Basilicata, Italy). They were ground in a mortar to reduce particulate size and then mixed carefully to assure homogeneity. Next, 200 mg of cannabis power were sonicated (BRANSON 1510 Ultrasonic Cleaner, National Institute of Standards and Technology, Gaithersburg, MD, USA) with 20 mL of methanol:chloromethane (9:1) for 30 min. Then, samples were centrifuged (Hettich Zentrifuge, MIKRO220R, Germany) at 6000 rpm for 10 min, and the clear supernatant was removed and kept, while pellets were reextracted with 10 mL of MeOH:CH3Cl (9:1). Supernatants were then collected and kept at −20 °C. Samples were filtered with a 22 μm nylon filter and injected into HPLC systems. For vitality assays, samples were extracted with Ethanol (EtOH).
4.3. LC-ESI-LTQ-MSn Qualitative Analyses
LC–MSn analyses were performed using a Surveyor HPLC system coupled to a linear ion-trap mass spectrometer (Linear Trap Quadropole [LTQ], Thermo Fisher Scientific, Bremen, Germany). HPLC separation was performed at 45 °C on a Discovery C18 column, 250 × 4.6-mm i. d., 5-µm particle size, equipped with a Discovery C18 20 × 4 mm i. d. security guard cartridge (Supelco Inc., Bellefonte, PA, USA). H2O containing 0.1% formic acid (solvent A) and ACN containing 0.1% formic acid (solvent B) were used for chromatographic separation [6]. The following elution program was adopted: 0–17 min from 35%:65% (A:B, v/v) to 5%:95%, 17–22 min from 5%:95% to 5%:95%, and 22–24 min from 5%:95% to 35%:65%. The flow rates were 1 mL/min in the column and 200 μL/min in the electrospray ionization (ESI) source (split ratio 4:1). Positive electrospray ionization, ESI(+), was chosen for the detection of CBD, THC, and CBN; instead, ESI(−) was employed for acidic form, CBDA, and THCA. The LTQ mass spectrometer was calibrated according to the manufacturer’s instructions using a solution of sodium dodecyl sulfate (m/z 265) and sodium taurocholate (m/z 514). The source voltage was set at 4.60 kV, the heated capillary temperature was set at 350 °C and the applied voltage was set at −28 V. The sheath gas (N2) flow rate was 80 arbitrary units (a.u.) and the auxiliary gas was set to zero. Full-scan MS experiments were performed in the linear ion trap in the m/z range 100–1000/MSn experiments were performed by selecting the precursor ion of interest and subjecting it to collision-induced dissociation (CID) in the linear ion trap. Helium was used as a collisional gas and the collision energy was selected according to the stability (typically 20–50 eV). Identification was based on retention time comparison and fragments match (m/z and intensity). HPLC-MS data were acquired in full scan mode and then elaborated to obtain the chromatographic profile of the ions of interest, with specific m/z values. The data acquisition was carried out with the Xcalibur package (version 2.0.7 Thermo Electron). Raw chromatographic data were imported, processed, and plotted by SigmaPlot. 12.5 (Systat Software, Inc., London, UK).
4.4. LC-UV Method Validation and Quantitative Analyses
The analytical system for cannabinoid quantitative analysis consisted of an Agilent 1200 Series Gradient HPLC System equipped with a quaternary gradient pump unit, a DAD (diode array detector, 190 nm–400 nm), and a standard autosampler (0.1–100 µL) (Agilent Technologies, Santa Clara, CA, USA). The autosampler was set to inject 20 µL. All the experiments were performed at room temperature (25 °C).
The separation was attained on a reversed-phase Luna C18, 5 μm (150 × 4.6 mm, 100 Å) analytical column, preceded by a security guard cartridge. The linear gradient was between eluent A (water containing 0.1% formic acid) and eluent B (acetonitrile). The column temperature was 25 °C and the flow rate was 1 mL/min. The elution gradient was set as below: 0–17 min (35–5% A), 17–22 min (5% A), 22–24 min (5–35% A), and 24–28 min (35% A). The wavelength value employed for UV detection was 220 nm for THCA, CBDA e CBN, and 210 nm per THC and CBD.
The LC-UV validation protocol included parameters such as linearity, precision (for both peak area and retention time), accuracy, limits of detection (LOD), and limits of quantification (LOQ). The stock solutions of CBD, THC, CBDA, THCA, and CBN were prepared by diluting concentrated standard solutions in MeOH by using 50:50 methanol/acetonitrile (v/v). All samples were analyzed in triplicate using the optimized method described above. The linearity was investigated in the range 0.1–50 mg/L for each compound, according to the regression line by the method of least squares and expressed by the coefficient of correlation (R2). Accuracy, expressed as recovery, was calculated from the spikes of 1 mg/L and 10 mg/L standard solutions to cannabis extracts. Precision was measured as percentage relative standard deviation (%RSD) for two levels (k = 2), 1 mg/L and 10 mg/L. The repeatability was calculated in the same day for six replicates (n = 6); instead, the intermediate precision was obtained within several days (p = 3) for the ten replicates (n = 10). A %RSD below 15% and 30% for repeatability and intermediate precision, respectively, were considered suitable [29]. The detection limit (LOD) is the lowest quantity or concentration of analyte in the sample that can be reliably distinguished from zero [22]. It was calculated as follows: LOD = (3.3 σ)/m, where σ is the residual standard deviation of the calibration line and m is the slope of the calibration line. The quantification limit (LOQ) is the concentration of analyte, below which it is determinable with a level of precision that is too low with inaccurate results. The LOQ can be determined by using the following formula: LOQ = (10 σ)/m, where σ is the residual standard deviation of the calibration line and m is the slope of the calibration line. All tests were performed in triplicate.
Quantification of cannabinoids, and thus chemotypes definition, was carried out by usingthe external standard method. According to official methods, the peak area ratio of each standard cannabinoid was plotted versus the analyte concentration [30,31,32,33]. Values are provided as percent analyte per 100 g dry weight (%), the standard deviation (SD) being estimated for three replicates.
Data acquisition and analyses were accomplished using the HPLC 1200 offline (Agilent Technologies, Santa Clara, CA, USA). The chromatographic raw data were imported, elaborated, and plotted by SigmaPlot 11.0 (Systat Software, Inc., London, UK).
4.5. Vitality Assay
Human hepatoblastoma cells (HepG2) were kept in Dulbecco’s modified Eagle’s medium (DMEM) having 25 mM glucose, enriched with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, 2 mM L-glutamine, and 100 U/mL penicillin at 37 °C, in an atmosphere humidified with 5% of CO2.
The MTT (3-(4,5-dimethyl thiazol-2 yl)-2,5-diphenyl tetrazolium bromide) assay was used to assess cell viability as previously reported [34] with some variations. HepG2 cells were seeded into 96-well plates at a density of 1 × 104 cells per well in triplicate and incubated all night at 37 °C. After 24 h, media was changed and the cells were treated with Cannabis sativa plant extracts (Mary Moonlight-Legal Weed, Alexis Haze-Legal Weed, and Purple Rock-Legal Weed) solubilized in ethanol at various concentrations (from 2 to 100 μM) for 24, 48, and 72 h. Control cells were treated at the corresponding non toxic final percentage of ethanol. After treatment, the cells were incubated with 100 μL of (5 mg/mL) MTT reagent (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) for 4 h at 37 °C, then cells were treated with 1:1 DMSO and isopropanol with 1% of Triton X-100 to solubilize the formazan crystal. The viability of cells was estimated by light absorption at 570 nm after background subtraction at 630 nm, using a microplate reader MultiskanTM GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
The concentration of the extract required to inhibit the viability of HepG2 cells by 50% (IC50) was calculated by non-linear curve fitting. The dose–response curve was graphed by using the GraphPad Prism 6 software (GraphPad Prism Software, San Diego, CA, USA). Each test was replicated three times in triplicate. The treated cells percentage viability was calculated by using the following formula:
The viability of cells in the control group was considered 100% [35].
Author Contributions
Conceptualization, F.L., A.O. and G.B.; methodology, M.A.A., F.L., A.O. and I.M.; validation, C.T., R.P. and R.C.; formal analysis M.A.A. and C.T.; investigation, F.L., M.A.A. and A.D.C.; resources, L.S. and S.A.B.; data curation C.T., R.P., A.O. and M.A.A.; writing—original draft preparation., M.A.A., R.P. and I.M.; writing—review and editing F.L., R.C., G.B., A.D.C. and A.O.; supervision F.L. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chong, W.W.-Y.; Acar, Z.I.; West, M.L.; Wong, F. A Scoping Review on the Medical and Recreational Use of Cannabis During the COVID-19 Pandemic. Cannabis Cannabinoid Res. 2022, 7, 591–602. [Google Scholar] [CrossRef]
- Legge 2 Dicembre 2016, n.242. Disposizioni per la Promozione della Coltivazione e della Filiera Agroindustriale della Canapa (16G00258), GU Serie Generale n. 304 del 30-12-2016. GU Serie Generale n. 304 del 30-12-2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 1 December 2021).
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Kumar, R.N.; Chambers, W.A.; Pertwee, R.G. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 2001, 56, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.C.; Crawford, W.J.; Alexander, P.C.; Anduze, A.L.; Gelbart, S.S. Effect of Marihuana on Intraocular and Blood Pressure in Glaucoma. Ophthalmology 1980, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lelario, F.; Pascale, R.; Bianco, G.; Scrano, L.; Bufo, S.A. Hemp chemotype definition by cannabinoids characterization using lc-esi(+)-ltq-fticr ms and infrared multiphoton dissociation. Separations 2021, 8, 245. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of phenotypic characteristics in three chemotype categories in the genus cannabis. HortScience 2021, 56, 481–490. [Google Scholar] [CrossRef]
- Marchei, E.; Tittarelli, R.; Pellegrini, M.; Rotolo, M.C.; Pacifici, R.; Pichini, S. Is “light cannabis” really light? Determination of cannabinoids content in commercial products. Clin. Chem. Lab. Med. 2020, 58, E175–E177. [Google Scholar] [CrossRef]
- Hädener, M.; König, S.; Weinmann, W. Quantitative determination of CBD and THC and their acid precursors in confiscated cannabis samples by HPLC-DAD. Forensic Sci. Int. 2019, 299, 142–150. [Google Scholar] [CrossRef]
- Fabresse, N.; Faltot, M.; Roux, P.; Becam, J.; Doudon, E.; Lacarelle, B.; Solas, C.; Pelissier-Alicot, A.L. Determination of cannabinoids content in light cannabis inflorescences sold in France. Drug Test. Anal. 2023, 1–6. [Google Scholar] [CrossRef]
- Nahar, L.; Onder, A.; Sarker, S.D. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019). Phytochem. Anal. 2020, 31, 413–457. [Google Scholar] [CrossRef]
- Milan, S.; Lelario, F.; Scrano, L.; Ottati, C.; Bufo, S.A.; Alpendurada, M.d.F. Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS. Water 2022, 14, 588. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Kovalchuk, I. Cannabinoids as anticancer therapeutic agents. Cell Cycle 2020, 19, 961–989. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2020, 25, 1–3. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Cannabinoids as Anticancer Drugs, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 80. [Google Scholar]
- Seltzer, E.S.; Watters, A.K.; Mackenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, S.D.; Cunnane, K.; Cash, M.C.; Romero-Sandoval, E.A. Potency and Therapeutic THC and CBD Ratios: U.S. Cannabis Markets Overshoot. Front. Pharmacol. 2022, 13, 921493. [Google Scholar] [CrossRef]
- Van De Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; Van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Tung, C.W.; Tsai, C.C.; Wu, Y.T.; Hsu, M.C. Determination of cannabinoids in hemp nut products in Taiwan by HPLC-MS/MS coupled with chemometric analysis: Quality evaluation and a pilot human study. Drug Test. Anal. 2017, 9, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Kim, E.; Kim, Y.-u.; Han, E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2020, 306, 110064. [Google Scholar] [CrossRef]
- Zeyl, V.; Sawyer, K.; Wightman, R.S. What Do You Know About Maryjane? A Systematic Review of the Current Data on the THC:CBD Ratio. Subst. Use Misuse 2020, 55, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, M.; Tura, M.; Scotti, S.; Toschi, T.G. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Molecules 2019, 24, 2113. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Piccolella, S.; Formato, M.; Pecoraro, M.T.; Crescente, G.; Pacifico, S. Discrimination of CBD-, THC- and CBC-type acid cannabinoids through diagnostic ions by UHPLC-HR-MS/MS in negative ion mode. J. Pharm. Biomed. Anal. 2021, 201, 114125. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Jiang, H.E.; Li, X.; Sutton, A.; Carboni, A.; Del Bianco, F.; Mandolino, G.; Potter, D.J.; Zhao, Y.X.; Bera, S.; et al. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J. Exp. Bot. 2008, 59, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Coppini, D.A.; Polito, L.; Ciriello, U.; Paladino, G.; Hyeraci, M.; Di Paolo, M.L.; Nordio, G.; Dalla Via, L.; Passarella, D. Cannabidiol as Self-Assembly Inducer for Anticancer Drug-Based Nanoparticles. Molecules 2023, 28, 112. [Google Scholar] [CrossRef] [PubMed]
- Janatová, A.; Doskočil, I.; Božik, M.; Fraňková, A.; Tlustoš, P.; Klouček, P. The chemical composition of ethanolic extracts from six genotypes of medical cannabis (Cannabis sativa L.) and their selective cytotoxic activity. Chem. Biol. Interact. 2022, 353, 109800. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Gembloux, Belgium, 2014; ISBN 978-91-87461-59-0. Available online: www.eurachem.org (accessed on 8 March 2023).
- CE n. 796/2004 Regolamento CE n. 796/2004 Recante Modalità di Applicazione Della Condizionalità, Della Modulazione e del Sistema Integrato di Gestione e di Controllo di cui al Reg. (CE) n. 1782/2003; Publications Office of the EU: Luxembourg, 2004.
- CE n. 1164/89 Regolamento CE n. 1164/89 della Commissione del 28 Aprile 1989 Relativo Alle Modalità D’applicazione Concernenti L’aiuto per il Lino Tessile e la Canapa; Publications Office of the EU: Luxembourg, 1989.
- ST/NAR/40: Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; UNODC: New York, NY, USA, 2009; ISBN 978-92-1-148242-3.
- ST/NAR/48: Recommended Methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials; Rev. 1.; UNODC: New York, NY, USA, 2013.
- Martinelli, F.; Cuviello, F.; Pace, M.C.; Armentano, M.F.; Miglionico, R.; Ostuni, A.; Bisaccia, F. Extracellular ATP regulates CD73 and ABCC6 expression in HepG2 cells. Front. Mol. Biosci. 2018, 5, 75. [Google Scholar] [CrossRef]
- Giglio, F.; Morelli, M.A.C.; Matera, I.; Sinisgalli, C.; Rossano, R.; Ostuni, A. Muscari comosum L. Bulb extracts modulate oxidative stress and redox signaling in hepg2 cells. Molecules 2021, 26, 416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).