Boron Carbonitride Films with Tunable Composition: LPCVD and PECVD Synthesis Using Trimethylamine Borane and Nitrogen Mixture and Characterization
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
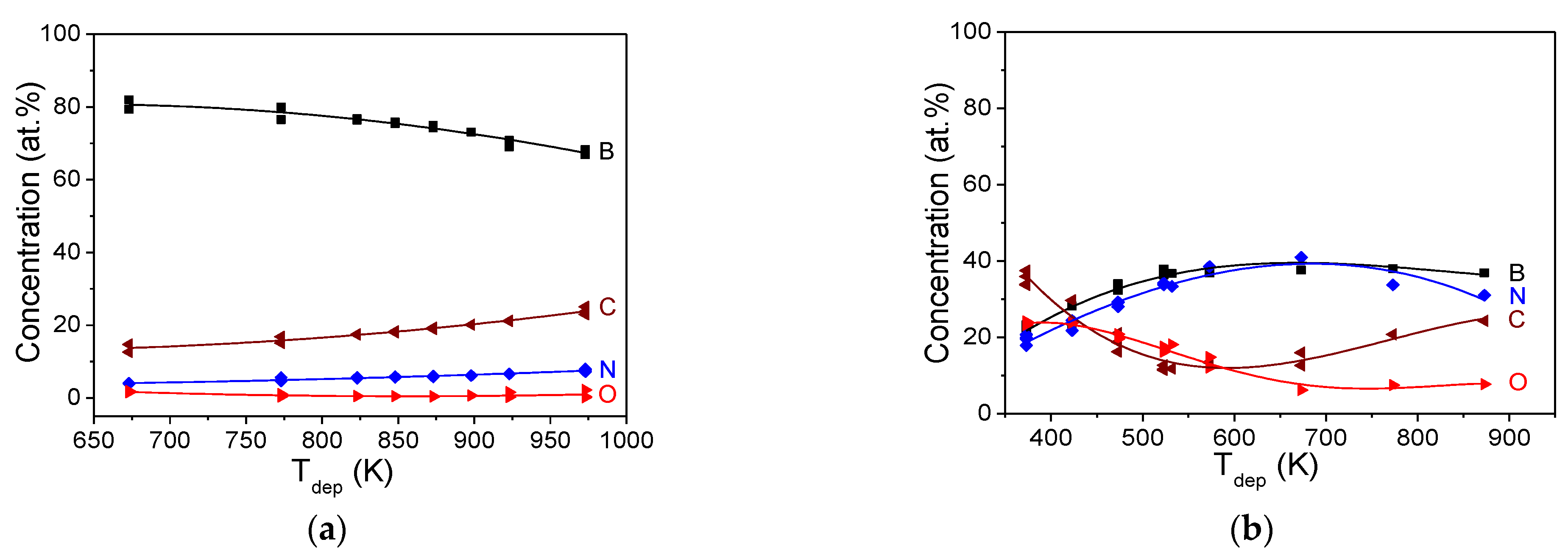
3.1. Film Deposition Rate
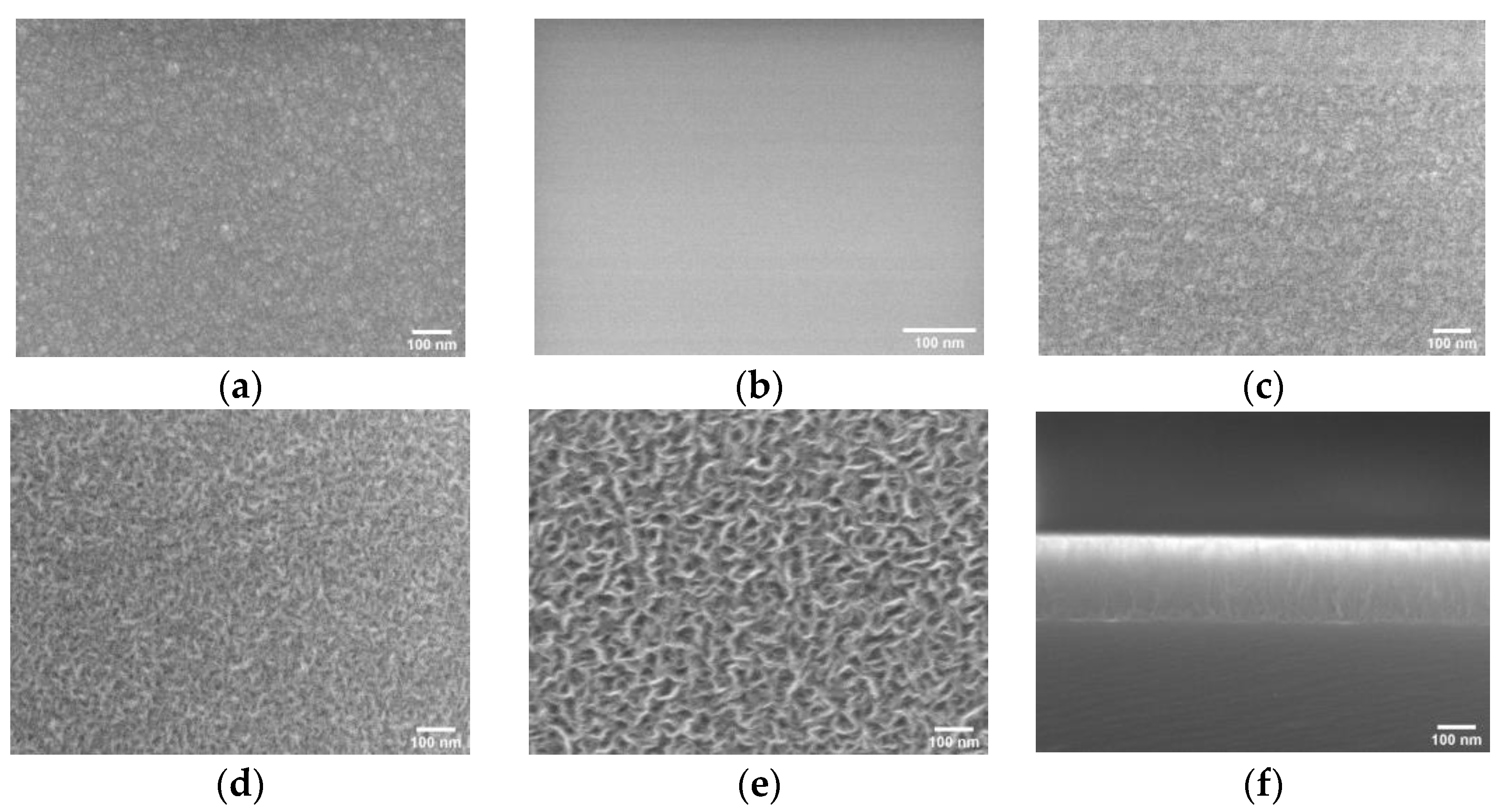
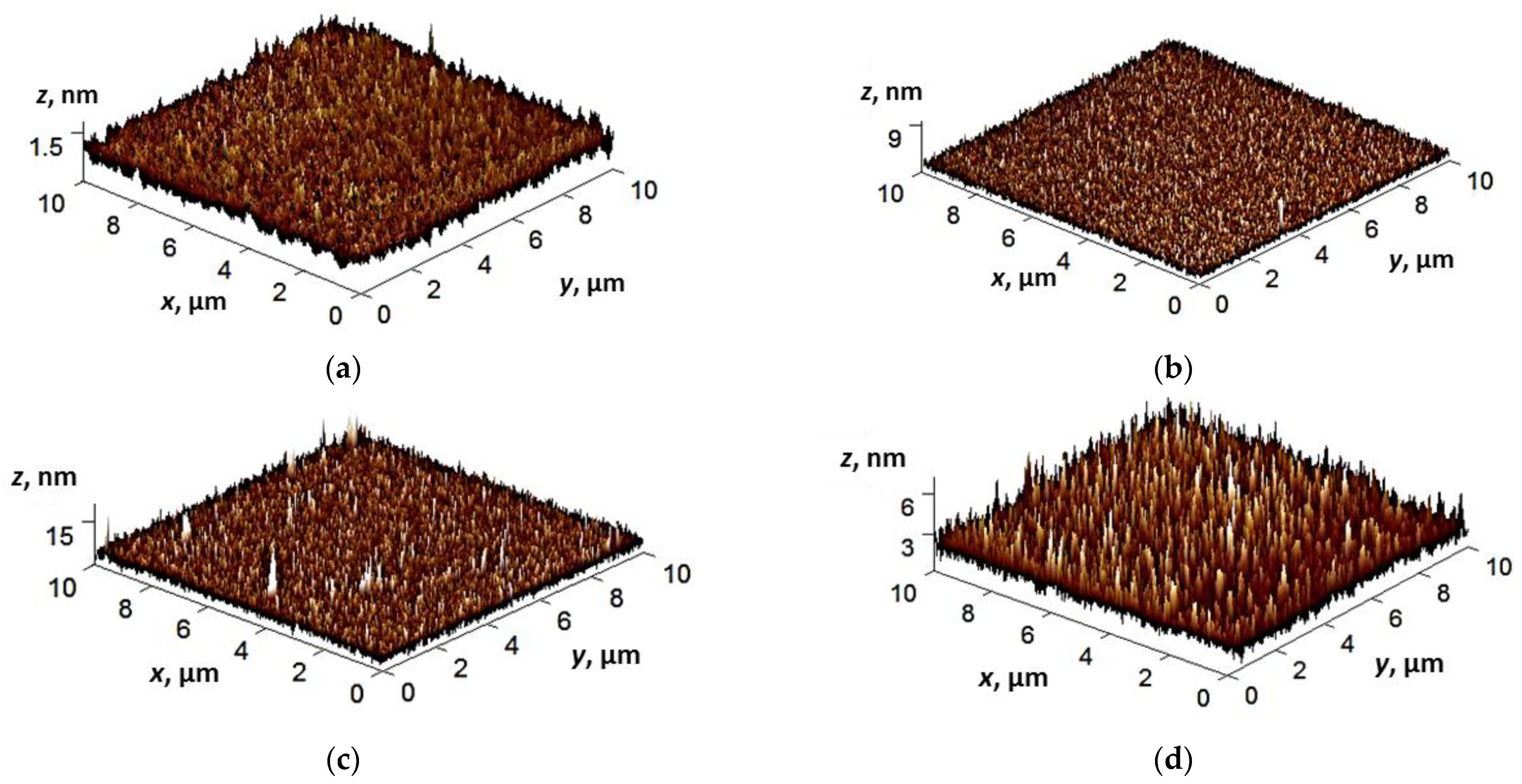
3.2. Film Surface Morphology, Topology, and Structure Study
3.3. Films’ Composition and Chemical Bonding State
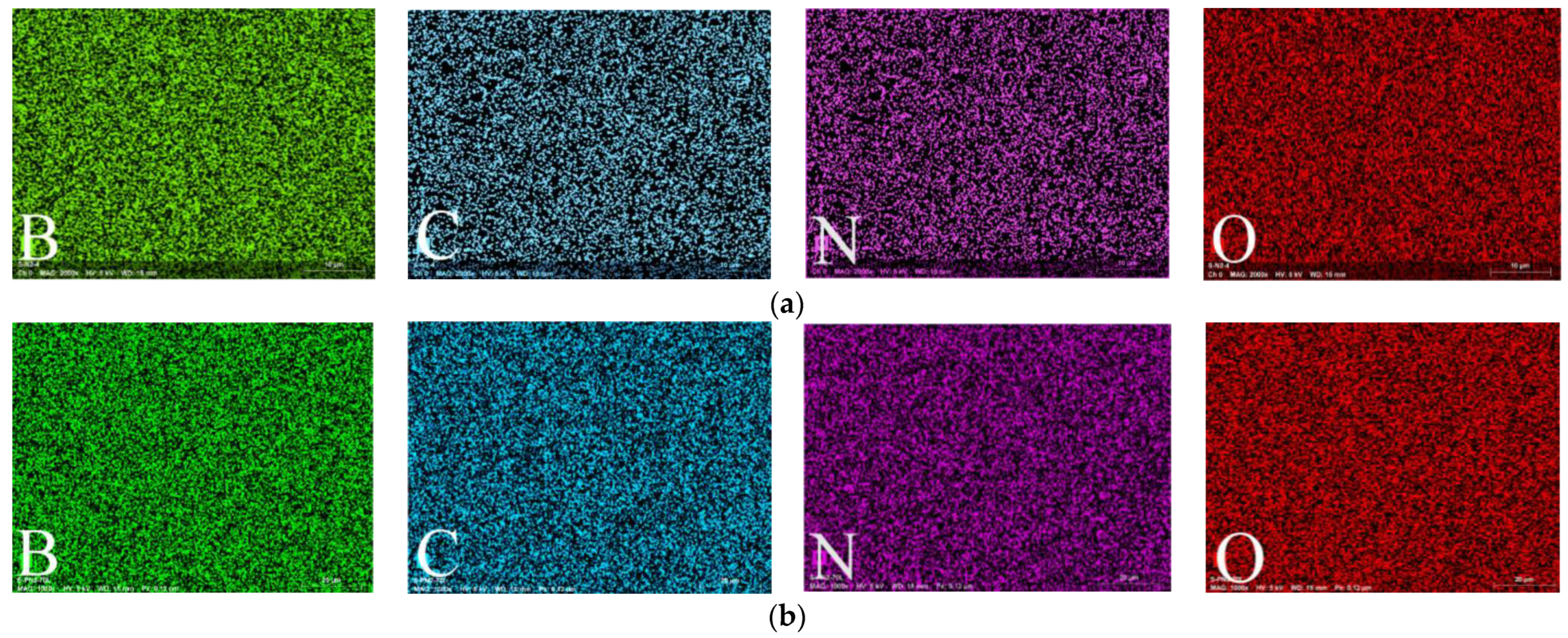
3.3.1. EDX Spectroscopy Data
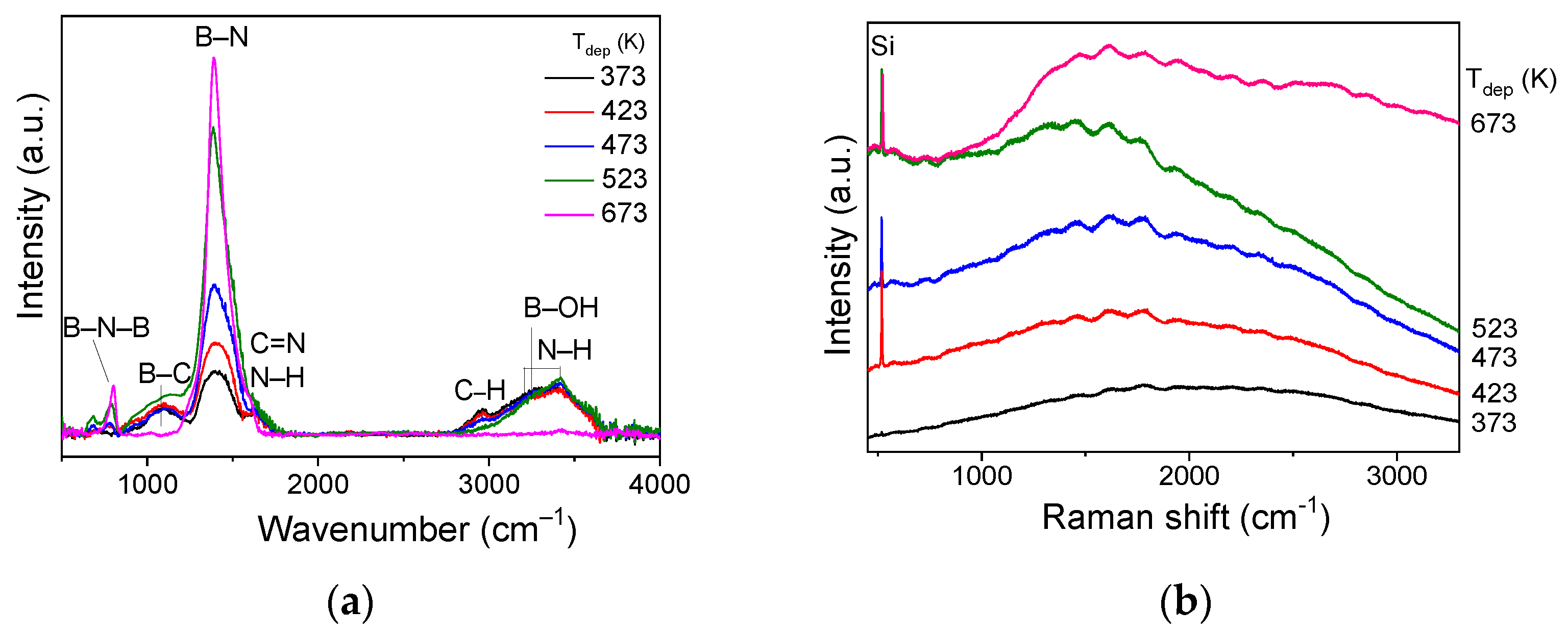
3.3.2. FTIR Spectroscopy Data
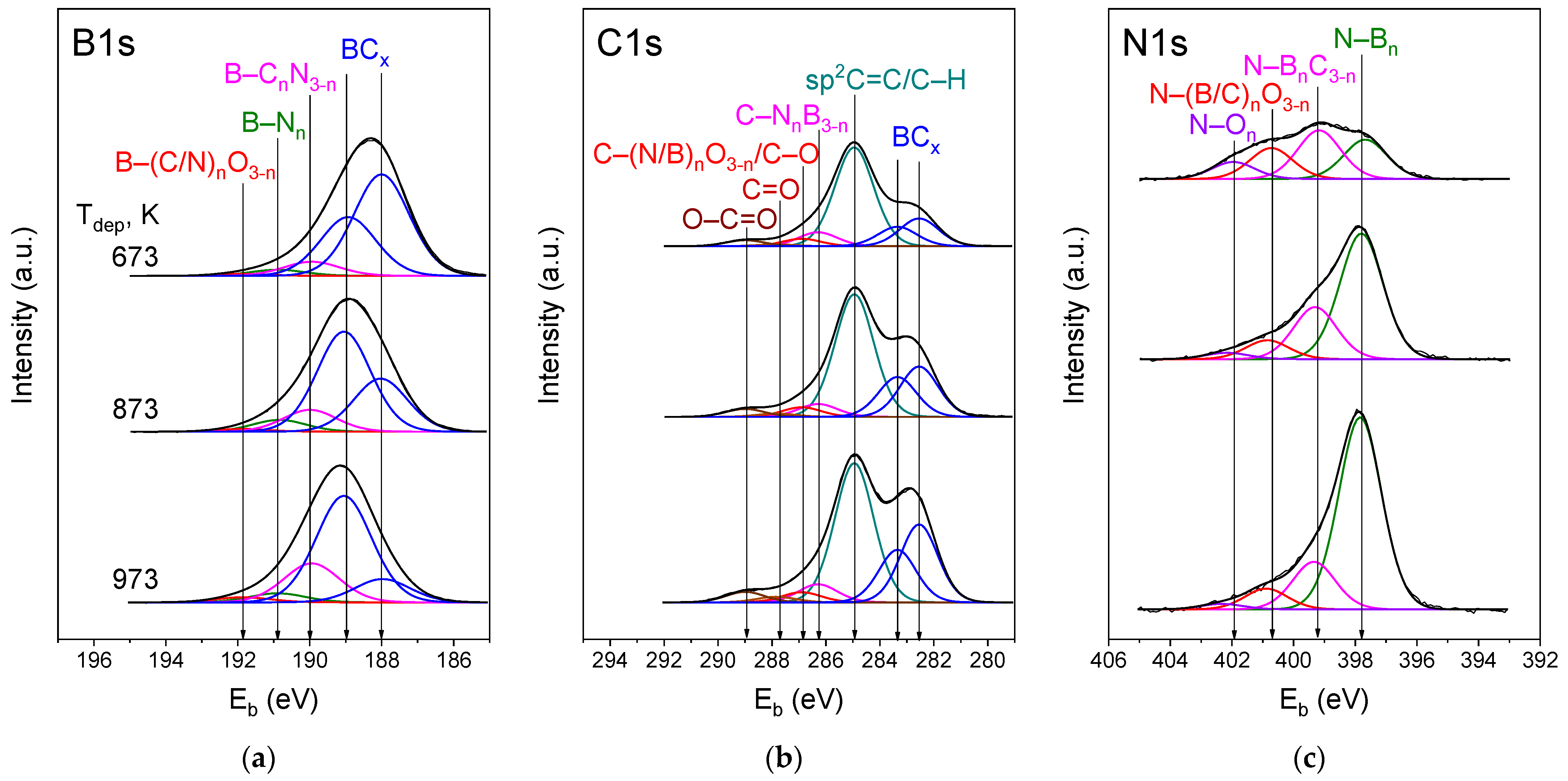
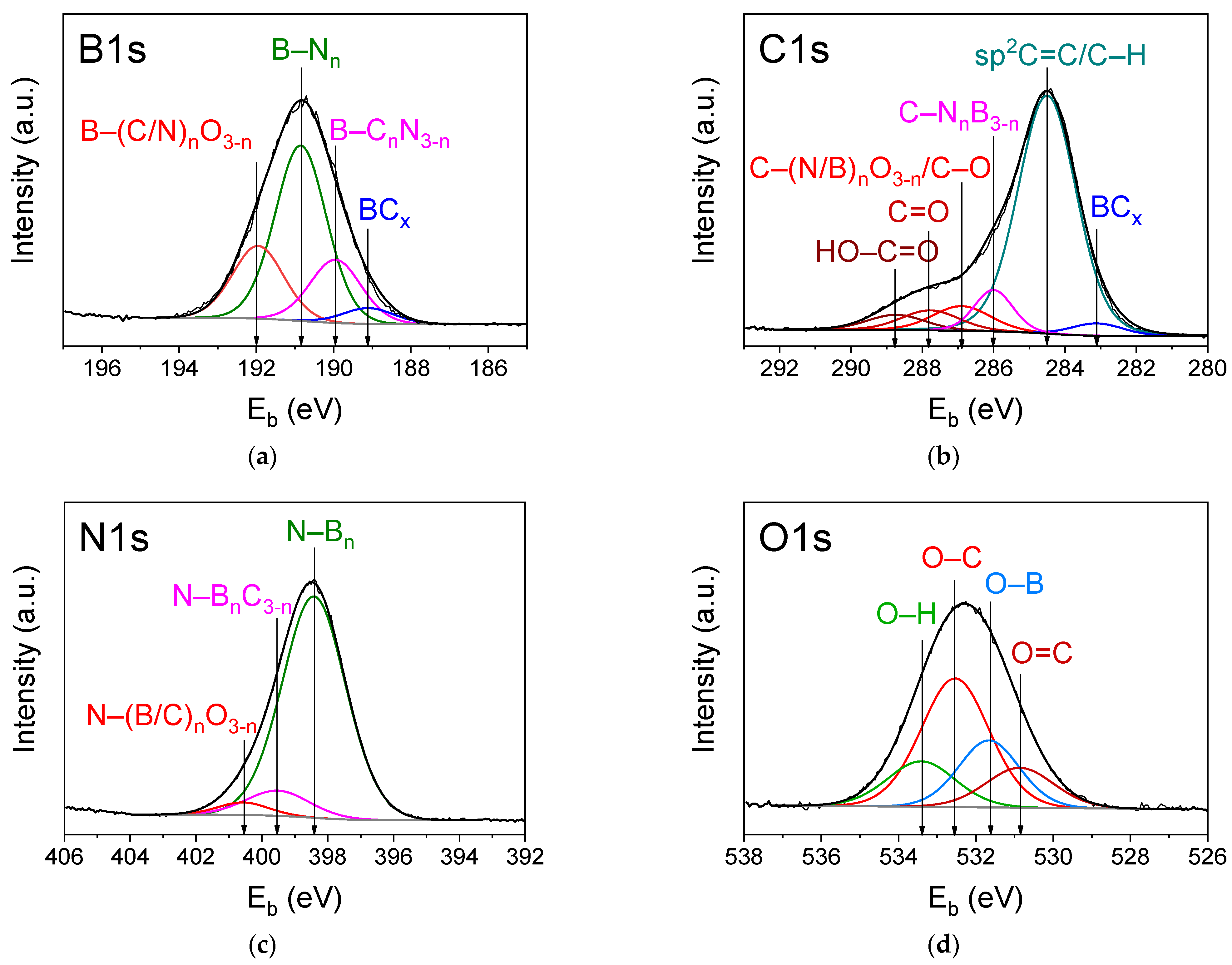
3.3.3. XPS Analysis
3.4. Functional Characteristics of BCxNy Films
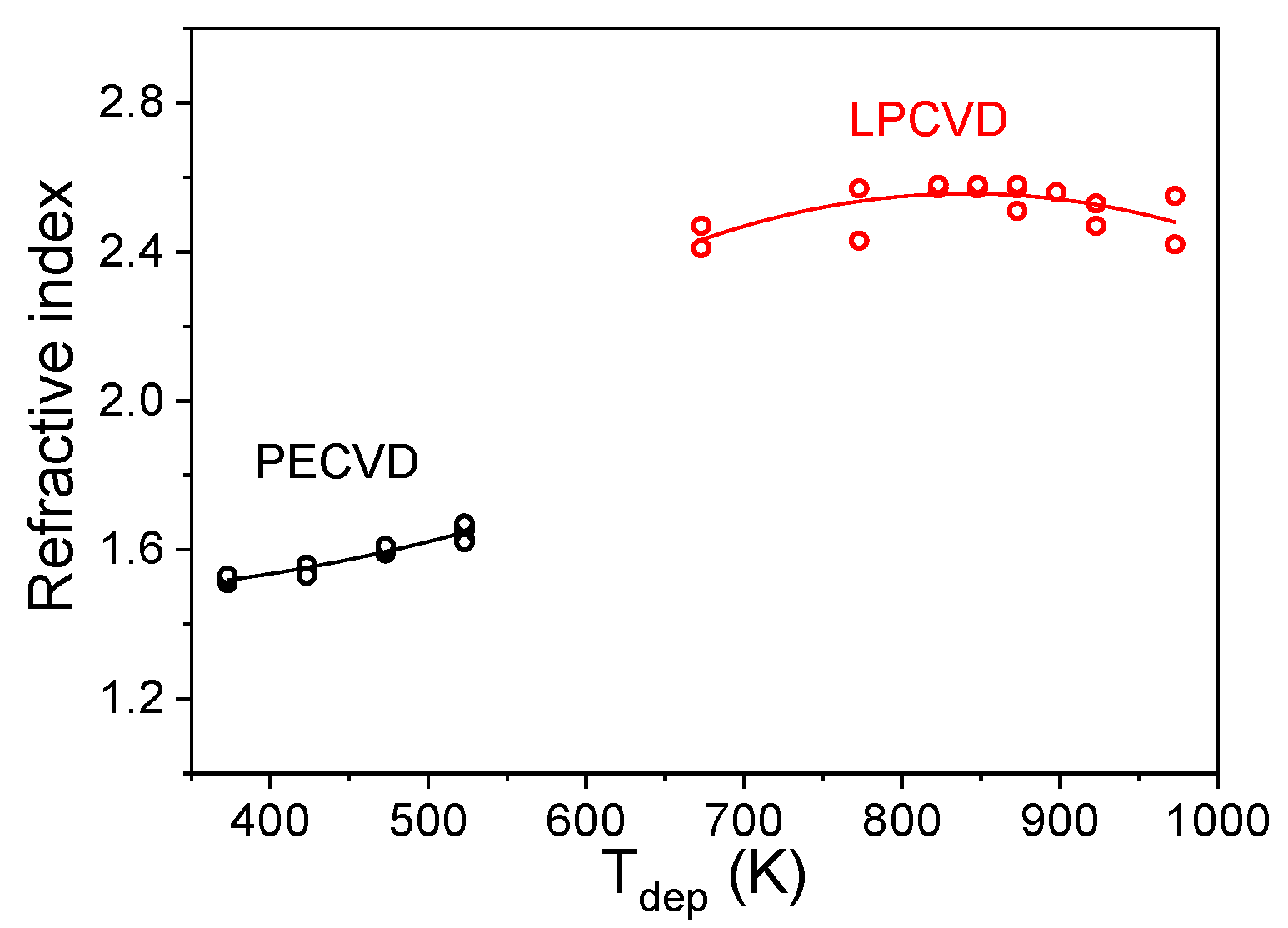
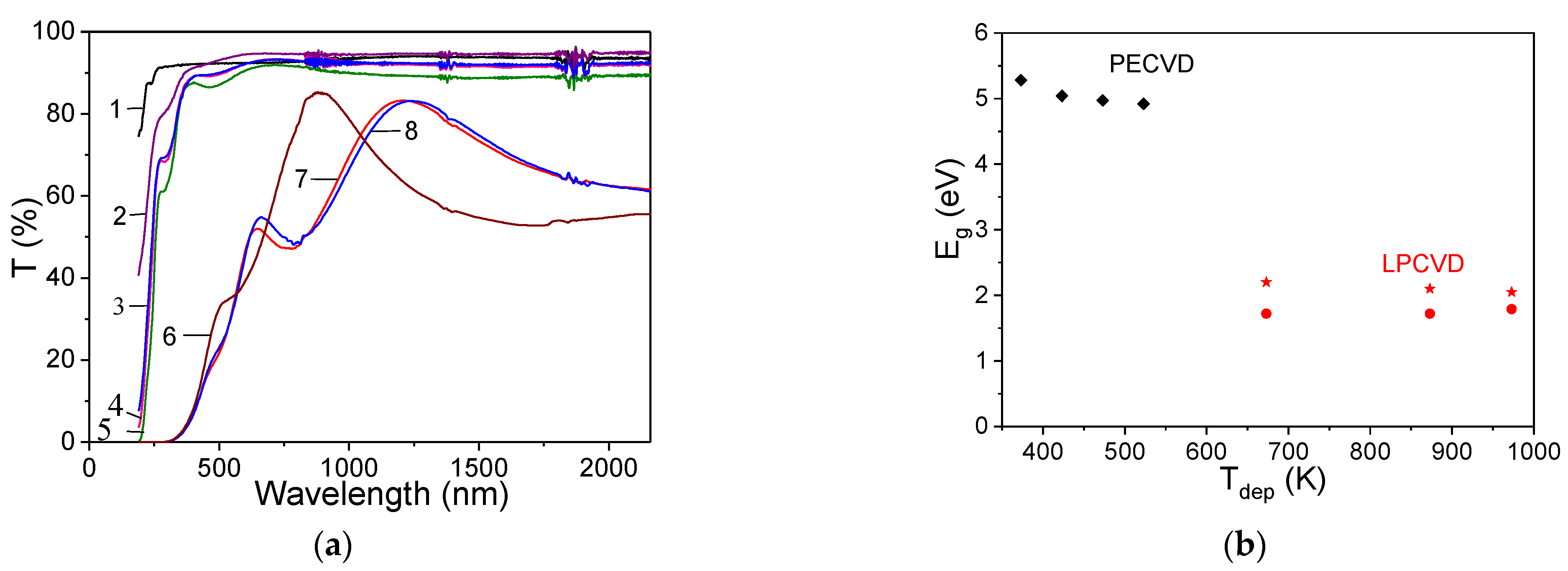
3.4.1. Optical Properties
3.4.2. Wettability
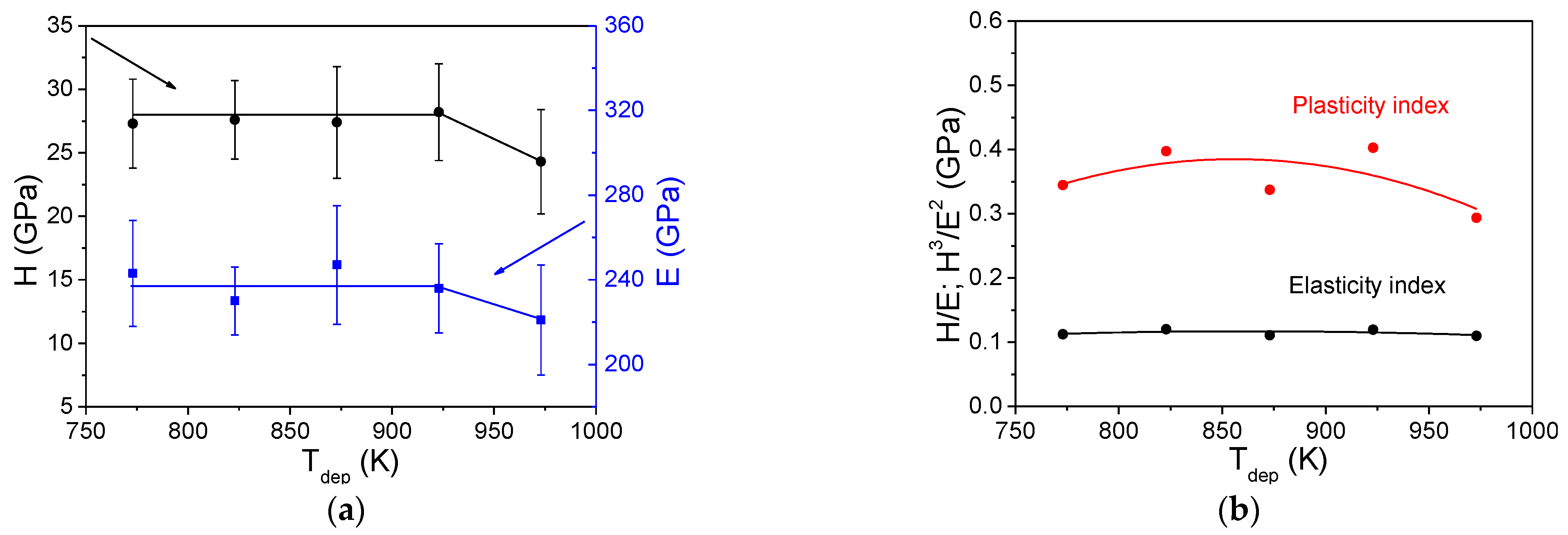
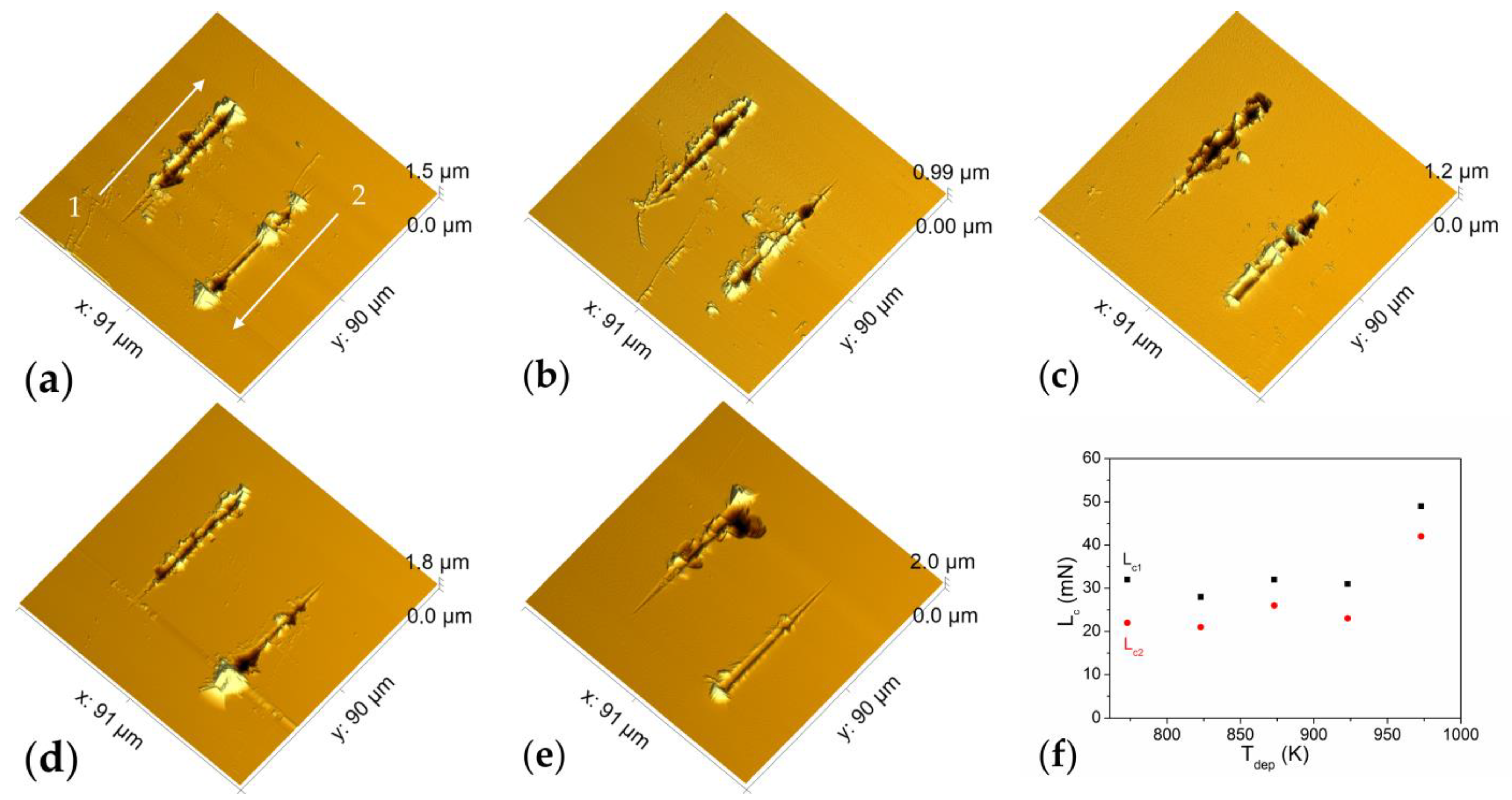
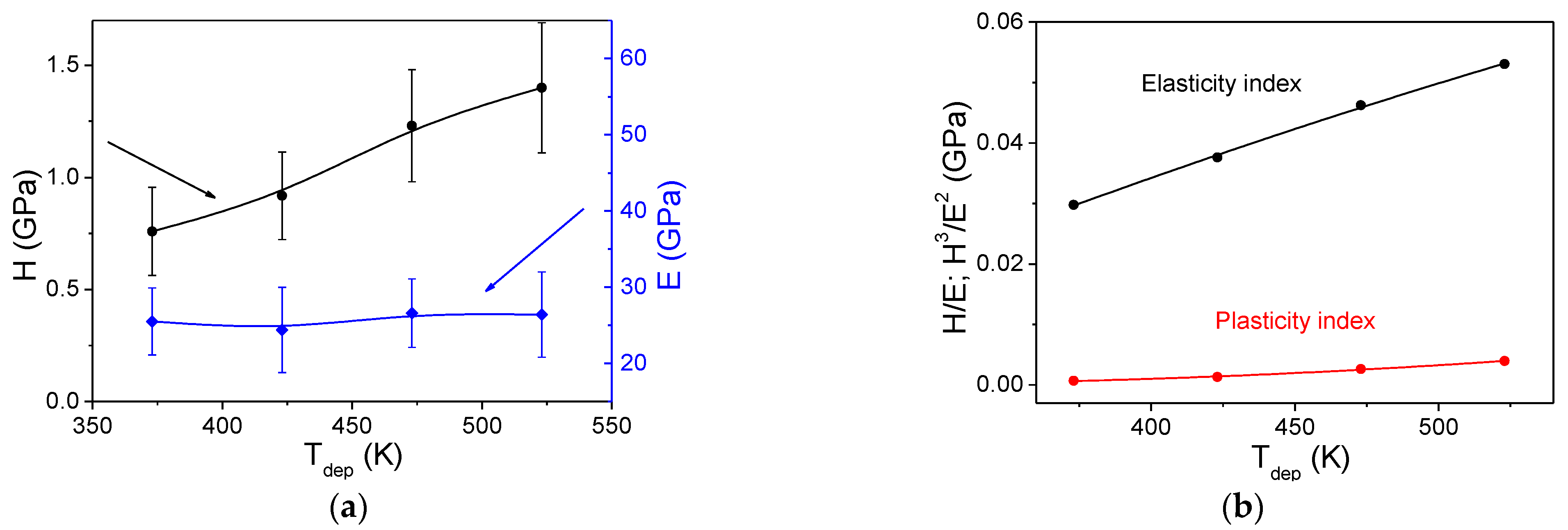
3.4.3. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skakalova, V.; Kaiser, A. (Eds.) Graphene: Properties, Preparation, Characterization and Applications, 2nd ed.; Woodhead Publishing: Philadelphia, PA, USA, 2021; p. 544. [Google Scholar]
- Hayat, A.; Sohail, M.; Hamdy, M.S.; Taha, T.A.; AlSalem, H.S.; Alenad, A.M.; Amin, M.A.; Shah, R.; Palamanit, A.; Khan, J.; et al. Fabrication, characteristics, and applications of boron nitride and their composite nanomaterials. Surf. Interfaces 2022, 29, 101725. [Google Scholar] [CrossRef]
- Nehate, S.D.; Saikumar, A.K.; Prakash, A.; Sundaram, K.B. A review of boron carbon nitride thin films and progress in nanomaterials. Mater. Today Adv. 2020, 8, 100106. [Google Scholar] [CrossRef]
- Yu, L.A.; Chebanova, G.E.; Artemkina, S.B.; Lavrov, A.N. Crystalline and nanostructured materials based on transition metal dichalcogenides: Synthesis and electronic properties. J. Struct. Chem. 2022, 63, 176–226. [Google Scholar]
- He, Z.; Zhang, S.; Zheng, L.; Liu, Z.; Zhang, G.; Wu, H.; Wang, B.; Liu, Z.; Jin, Z.; Wang, G. Si-Based NIR Tunneling Heterojunction Photodetector with Interfacial Engineering and 3D-Graphene Integration. IEEE Electron Device Lett. 2022, 43, 1818–1821. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, S.; Zhang, G.; He, Z.; Feng, X.; Liu, Z.; Wang, G.; Tao, W.; Zheng, L.; Yang, S.; et al. Dual-Enhanced Photodetectors Combining Graphene Plasmonic Nanoresonators With Germanium-on-Insulator Optical Cavities. IEEE Trans. Electron Devices 2022, 69, 3246–3250. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, W.; Ning, Z.; Wang, G.; Cheng, X.; Hu, W.; Zhou, W.; Liu, Z.; Yang, S.; Xu, K.; et al. Ambipolar Graphene–Quantum Dot Phototransistors with CMOS Compatibility. Adv. Opt. Mater. 2018, 6, 1800985. [Google Scholar] [CrossRef]
- Pristáš, G.; Werheit, H.; Gabáni, S.; Shalamberidze, S.; Flachbart, K. Low Temperature Specific Heat Anomaly with Boson Peak in Isotope-Enriched Boron Carbides B4.3C–B10C. Solid State Sci. 2020, 101, 106140. [Google Scholar] [CrossRef]
- Suri, A.K.; Subramanian, C.; Sonber, J.K.; Murthy, T.S.R.C. Synthesis and Consolidation of Boron Carbide: A Review. Int. Mater. Rev. 2010, 55, 4–40. [Google Scholar] [CrossRef]
- Beauvy, M. Stoichiometric Limits of Carbon-Rich Boron Carbide Phases. J. Less-Common Met. 1983, 90, 169–175. [Google Scholar] [CrossRef]
- Werheit, H.; Shalamberidze, S. Advanced Microstructure of Boron Carbide. J. Phys. Condens. Matter 2012, 24, 385406. [Google Scholar] [CrossRef]
- Miele, P.; Bechelany, M. (Eds.) Boron Nitride Nanostructures; MDPI: Basel, Switzerland, 2018; p. 104. [Google Scholar]
- Gonzalez-Ortiz, D.; Salameh, C.; Bechelany, M.; Miele, P. Nanostructured boron nitride-based materials: Synthesis and applications. Mater. Today Adv. 2020, 8, 100107. [Google Scholar] [CrossRef]
- Uddin, M.R.; Majety, S.; Li, J.; Lin, J.Y.; Jiang, H.X. Layer-structured hexagonal (BN)C semiconductor alloys with tunable optical and electrical properties. J. Appl. Phys. 2014, 115, 093509. [Google Scholar] [CrossRef]
- Mannan, M.A.; Noguchi, H.; Kida, T.; Nagano, M.; Hirao, N.; Baba, Y. Growth and characterization of stoichiometric BCN films on highly oriented pyrolytic graphite by radiofrequency plasma enhanced chemical vapor deposition. Thin Solid Film. 2010, 518, 4163–4169. [Google Scholar] [CrossRef]
- Hoffmann, P.; Fainer, N.; Kosinova, M.; Baake, O.; Ensinger, W. Chapter 21. Compilation on Synthesis, Characterization and Properties of Silicon and Boron Carbonitride Films. In Silicon Carbide—Materials, Processing and Applications in Electronic Devices; Mukherjee, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 487–546. [Google Scholar]
- Husain, E.; Narayanan, T.N.; Taha-Tijerina, J.J.; Vinod, S.; Vajtai, R.; Ajayan, P.M. Marine Corrosion Protective Coatings of Hexagonal Boron Nitride Thin Films on Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, H.; Fan, H.; Liu, Q.; Lv, C.; Zhao, X.; Yang, M.; Wu, J.; Cao, X. Corrosion resistance of modified hexagonal boron nitride (h-BN) nanosheets doped acrylic acid coating on hot-dip galvanized steel. Materials 2020, 13, 2340. [Google Scholar] [CrossRef]
- Dong, P.V.; Phan, N.H.; Patil, S.; Shirguppikar, S.; Kalel, S.; Thanh, L.T.P.; Hien, D.M. Effect of boron carbide reinforcement on properties of stainless-steel metal matrix composite for nuclear applications. J. Mech. Behav. Mater. 2022, 31, 390–397. [Google Scholar] [CrossRef]
- Kharanzhevsky, E.V.; Reshetnikov, S.M.; Efimov, A.V.; Gil’mutdinov, F.Z.; Krivilev, M.D. Application of some inhibitors for improving the corrosion resistance of ceramic coatings deposited on non-alloy steel by short-pulse laser treatment. Int. J. Corros. Scale Inhib. 2020, 9, 44–55. [Google Scholar]
- Shayapov, V.R.; Zakharchenko, K.V.; Kapustin, V.I.; Merenkov, I.S.; Khomyakov, M.N.; Maksimivskiy, E.A. Hardness and Strengthening Effect of Low-Pressure Chemical Vapor Deposition BCxNy Coatings Deposited on Ti-6Al-4V Alloy. J. Mater. Eng. Perform. 2022, 31, 3792–3798. [Google Scholar] [CrossRef]
- Duan, C.; Li, X.; Ji, Y.; He, L.; Qian, J.; Zhao, Z. In-Situ Catalytic Preparation of Two-Dimensional BCN/Graphene Composite for Anti-Corrosion Application. Catalysts 2022, 12, 1618. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Conde, O. Synthesis and properties of BxCyNz coatings. J. Mater. Res. 2001, 16, 734–743. [Google Scholar] [CrossRef]
- Yamada, M.; Nakaishi, M.; Sugishima, K. Improvements of stress controllability and radiation resistance by adding carbon to boron-nitride. J. Elecrochem. Soc. 1990, 137, 2242–2246. [Google Scholar] [CrossRef]
- Yasui, H.; Awazu, K.; Ikenaga, N.; Sakudo, N. Synthesis and characterization of BN thin films prepared by plasma MOCVD with organoboron precursors. Vacuum 2009, 83, 582–584. [Google Scholar] [CrossRef]
- Kesler, V.G.; Kosinova, M.L.; Rumyantsev, Y.M.; Sulyaeva, V.S. X-ray photoelectron and auger spectroscopic study of the chemical composition of BCxNy films. J. Struct. Chem. 2012, 53, 699–707. [Google Scholar] [CrossRef]
- Baake, O.; Hoffmann, P.S.; Kosinova, M.L.; Klein, A.; Pollakowski, B.; Beckhoff, B.; Fainer, N.I.; Trunova, V.A.; Ensinger, W. Analytical characterization of BCxNy films generated by LPCVD with triethylamine borane. Anal. Bioanal. Chem. 2010, 398, 1077–1084. [Google Scholar] [CrossRef]
- Mannan, M.A.; Baba, Y.; Hirao, N.; Kida, T.; Nagano, M.; Noguchi, H. Hexagonal Nano-Crystalline BCN Films Grown on Si (100) Substrate Studied by X-Ray Absorption Spectroscopy. Mater. Sci. Appl. 2013, 4, 11–19. [Google Scholar] [CrossRef]
- Leardini, F.; Jiménez-Arévalo, N.; Jimenez-Ferrer, I.; Ares, J.-R.; Molina, P.; Gomez-Navarro, C.; Manzanares, Y.; Granados, D.; Urbanos, F.J.; Garcia-Garcia, J.; et al. A fast synthesis route of Boron-Carbon-Nitrogen ultrathin layers towards highly mixed ternary B-C-N phases. 2D Mater. 2019, 6, 035015. [Google Scholar] [CrossRef]
- Nappini, S.; Bondino, F.; Píš, I.; Chelleri, R.; Greco, S.L.; Lazzarino, M.; Magnano, E. Chemical composition and interaction strength of two-dimensional boron-nitrogen-carbon heterostructures driven by polycrystalline metallic surfaces. Appl. Surf. Sci. 2019, 479, 903–913. [Google Scholar] [CrossRef]
- Kosinova, M.L.; Rumyantsev, Y.M.; Golubenko, A.N.; Fainer, N.I.; Ayupov, B.M.; Dolgovesova, I.P.; Kolesov, B.A.; Kaichev, V.V.; Kuznetsov, F.A. Chemical Composition of Boron Carbonitride Films Grown by Plasma-Enhanced Chemical Vapor Deposition from Trimethylamineborane. Inorg. Mater. 2003, 39, 366–373. [Google Scholar] [CrossRef]
- Burg, A.B.; Schlesin, H.I. Hydrides of Boron. VII. Evidence of the Transitory Existence of Borine (BH3): Borine Carbonyl and Borine Trimethylammine. J. Am. Chem. Soc. 1937, 59, 780–786. [Google Scholar] [CrossRef]
- Alton, E.R.; Brown, R.D.; Carter, J.C.; Taylor, R.C. Vapor Pressures of the Methylamine-Boranes and Ammonia-Triborane. J. Am. Chem. Soc. 1959, 81, 3550–3551. [Google Scholar] [CrossRef]
- Kosinova, M.L.; Fainer, N.I.; Sulayeva, V.S.; Rumyantsev, Y.M.; Kuznetsov, F.A.; Maximovski, E.A.; Cao, Z.X.; Terauchi, M.; Shibata, K.; Satoh, F. Synthesis, nanoindentation and AFM studies of CVD boron carbon nitride films. ECS Proc. EUROCVD-15 2005, 2005-09, 1082–1087. [Google Scholar]
- Hoffmann, P.; Baake, O.; Beckhoff, B.; Ensinger, W.; Fainer, N.; Klein, A.; Kosinova, M.; Pollakowski, B.; Trunova, V.; Ulm, G.; et al. Chemical bonding in carbonitride nanolayers. Nucl. Instrum. Methods Phys. Res. A 2007, 575, 78–84. [Google Scholar] [CrossRef]
- Baake, O.; Hoffmann, P.S.; Klein, A.; Pollakowski, B.; Beckhoff, B.; Ensinger, W.; Kosinova, M.; Fainer, N.; Sulyaeva, V.S.; Trunova, V. Chemical character of BCxNy layers grown by CVD with trimethylamine borane. X-Ray Spectrom. 2009, 38, 68–73. [Google Scholar] [CrossRef]
- Sulyaeva, V.S.; Kesler, V.G.; Kosinova, M.L. Dielectric layers BCxNy: Synthesis by the decomposition of vapors of organoboron compounds, composition and chemical structure. J. Struct. Chem. 2021, 62, 1631–1647. [Google Scholar] [CrossRef]
- Kosinova, M.L.; Rumyantsev, Y.M.; Fainer, N.I.; Maximovskii, E.A.; Kuznetsov, F.A. The structure study of boron carbonitride films obtained by use of trimethylaminoborane complex. Nucl. Instrum. Methods Phys. Res. A 2001, 470, 253–257. [Google Scholar] [CrossRef]
- Kosinova, M.L.; Fainer, N.I.; Rumyantsev, Y.M.; Maximovski, E.A.; Kuznetsov, F.A.; Terauchi, M.; Shibata, K.; Satoh, F. Growth of homogeneous and gradient BCxNy films by PECVD using trimethylamino borane complex. In Proceedings of Chemical Vapor Deposition XVI and EUROCVD 14: Proceedings of the International Symposium, Paris, France, 27 April–2 May 2003; Electrochemical Society: Pennington, NJ, USA, 2003; Volume 2003-08, pp. 708–715. [Google Scholar]
- Mannan, M.A.; Nagano, M.; Shigezumi, K.; Kida, T.; Hirao, N.; Baba, Y. Characterization of Boron Carbonitride (BCN) Thin Films Deposited by Radiofrequency and Microwave Plasma Enhanced Chemical Vapor Deposition. Am. J. Appl. Sci. 2007, 5, 736–741. [Google Scholar] [CrossRef]
- Kida, T.; Shigezumi, K.; Mannan, M.A.; Akiyama, M.; Baba, Y.; Nagano, M. Synthesis of boron carbonitride (BCN) films by plasma-enhanced chemical vapor deposition using trimethylamine borane as a molecular precursor. Vacuum 2009, 83, 1143–1146. [Google Scholar] [CrossRef]
- Smith, D.L. Mechanism of SiNxHy deposition from N2–SiH4 plasma. J. Vac. Sci. Technol. B 1990, 8, 551–557. [Google Scholar] [CrossRef]
- Alexandrov, S.E.; Hitchman, M.L. Chapter12 Plasma Enhanced Chemical Vapour Deposition Processes. In Chemical Vapour Deposition: Precursors, Processes and Applications; Jones, A.C., Hitchman, M.L., Eds.; Royal Society of Chemistry: London, UK, 2009; pp. 494–509. [Google Scholar]
- Kosinova, M.L.; Fainer, N.I.; Rumyantsev, Y.M.; Golubenko, A.N.; Kuznetsov, F.A. LPCVD Boron Carbonitride Films From Trimethylamine Borane. J. Phys. 1999, 4, Pr. 8-915–Pr. 8-921. [Google Scholar]
- Sulyaeva, V.S.; Plekhanov, A.G.; Maksimovskii, E.A.; Fainer, N.I.; Rumyantsev, Y.M.; Kosinova, M.L. Characterization of Thin Boron and Silicon Carbonitride Films by Wavelength Dispersive Spectroscopy. Prot. Met. Phys. Chem. Surf. 2017, 53, 1187–1191. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical dispersion relations for amorphous semi-conductors and amorphous dielectrics. Phys. Rev. B. 1986, 34, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interf. Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurements and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Sulyaeva, V.; Khomyakov, M.; Kosinova, M. Room-Temperature Formation of Hard BCx Films by Low Power Magnetron Sputtering. Appl. Sci. 2021, 11, 9896. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; McGurk, M.R.; Bull, S.J.; Page, T.F. On the hardness of coated systems. Surf. Coat. Technol. 1998, 99, 171–183. [Google Scholar] [CrossRef]
- Hoffmann, P.S.; Baake, O.; Kosinova, M.L.; Beckhoff, B.; Klein, A.; Pollakowski, B.; Trunova, V.A.; Sulyaeva, V.S.; Kuznetsov, F.A.; Ensinger, W. Chemical bonds and elemental compositions of BCxNy layers produced by chemical vapor deposition with trimethylamine borane, triethylamine borane, or trimethylborazine. X-Ray Spectrom. 2012, 41, 240–246. [Google Scholar] [CrossRef]
- Engbrecht, E.R.; Cilino, C.J.; Junker, K.H.; Sun, Y.-M.; White, J.M.; Ekerdt, J.G. Charac-terization of Boron Carbo-Nitride Films Deposited By Low Temperature Chemical Vapor Deposition. Mat. Res. Soc. Symp. Proc. 2003, 766, E8.21.1–E8.21.6. [Google Scholar] [CrossRef]
- Bath, A.; van der Put, P.J.; Lepley, B. Study of boron nitride gate insulators grown by low temperature plasma enhanced chemical vapor deposition on InP. Appl. Surf. Sci. 1989, 39, 135–140. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls. Synthesis and Emerging Applications; Springer: Wien, Austria, 2010; p. 161. [Google Scholar]
- Sulyaeva, V.; Kosinova, M.; Rumyantsev, Y.; Golubenko, A.; Fainer, N.; Alferova, N.; Ayupov, B.M.; Gevko, P.N.; Kesler, V.G.; Kolesov, B.A.; et al. Properties of BC x N y films grown by plasma-enhanced chemical vapor deposition from N-trimethylborazine-nitrogen mixtures. Inorg. Mater. 2010, 46, 487–494. [Google Scholar] [CrossRef]
- Zaumseil, P. High-resolution characterization of the forbidden Si 200 and Si 222 reflections. J. Appl. Cryst. 2015, 48, 528–532. [Google Scholar] [CrossRef]
- Levy, R.A.; Mastromatteo, E.; Grow, J.M.; Paturi, V.; Kuo, W.P. Low pressure chemical vapor deposition of B-N-C-H films from triethylamine borane complex. J. Mater. Res. 1995, 10, 320–327. [Google Scholar] [CrossRef]
- Sulyaeva, V.S.; Rumyantsev, Y.M.; Kesler, V.G.; Kosinova, M.L. Synthesis and optical properties of BCxNy films deposited from N-triethylborazine and hydrogen mixture. Thin Solid Film. 2015, 581, 59–64. [Google Scholar] [CrossRef]
- Merenkov, I.S.; Kasatkin, I.A.; Maksimovskii, E.A.; Alferova, N.I.; Kosinova, M.L. Vertically aligned layers of hexagonal boron nitride: PECVD synthesis from triethylaminoborane and structural features. J. Struct. Chem. 2017, 58, 1018–1024. [Google Scholar] [CrossRef]
- Sulyaeva, V.S.; Kosinova, M.L.; Rumyantsev, Y.M.; Kesler, V.G.; Kuznetsov, F.A. PECVD synthesis and optical properties of BCxNy films obtained from N-triethylborazine as a single-source precursor. Surf. Coat. Technol. 2013, 230, 145–151. [Google Scholar] [CrossRef]
- Sulyaeva, V.S.; Kosinova, M.L.; Rumyantsev, Y.M.; Kuznetsov, F.A.; Kesler, V.G.; Kirienko, V.V. Optical and electrical characteristics of plasma-enhanced chemical vapor deposition boron carbonitride thin films derived from N-trimethylborazine precursor. Thin Solid Film. 2014, 558, 112–117. [Google Scholar] [CrossRef]
- Binnenbruck, H.; Werheit, H. IR-Active Phonons of Boron and Boron Carbide. J. Phys. Sci. 1979, 34, 787–798. [Google Scholar] [CrossRef]
- Werheit, H.; Manghnani, M.H.; Kuhlmann, U.; Hushur, A.; Shalamberidze, S. Mode Grüneisen parameters of boron carbide. Sol. State Sci. 2017, 72, 80–93. [Google Scholar] [CrossRef]
- Shirai, K.; Emura, S.; Gonda, S.I. Infrared study of amorphous B1-xCx, films. J. Appl. Phys. 1995, 78, 3392–3400. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.H.; Rupprecht, N.G. Modes in Hexagonal Boron Nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Demin, V.N.; Asanov, I.P.; Akkerman, Z.L. Chemical vapor deposition of pyrolytic boron nitride from borazine. J. Vac. Sci. Technol. A 2000, 18, 94–98. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Springer: Berlin, Heidelberg, Germany, 1975; p. 433. [Google Scholar]
- Weltner, W.; Warn, J.R.W. Matrix Isolation of High-Temperature Vapors: Boric Oxide. J. Chem. Phys. 1962, 37, 292–303. [Google Scholar] [CrossRef]
- Putkonen, M.; Niinistö, L. Atomic layer deposition of B2O3 thin films at room temperature. Thin Solid Film. 2006, 514, 145–149. [Google Scholar] [CrossRef]
- Parker, J.H.; Feldman, D.W.; Ashkin, M. Raman scattering by silicon and germanium. Phys. Rev. 1967, 155, 712–714. [Google Scholar] [CrossRef]
- Tallant, D.R.; Aselage, T.L.; Campbell, A.N.; Emin, D. Boron carbide structure by Raman spectroscopy. Phys. Rev. B 1989, 40, 5649–5656. [Google Scholar] [CrossRef]
- Imam, M.; Höglund, C.; Jensen, J.; Schmidt, S.; Ivanov, I.G.; Hall-Wilton, R.; Birch, J.; Pedersen, H. Trimethylboron as Single-Source Precursor for Boron-Carbon Thin Film Synthesis by Plasma Chemical Vapor Deposition. J. Phys. Chem. C 2016, 120, 21990–21997. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Samanta, S.R.; Krishnan, P.N. Raman investigation of vitreous and molten boric oxid. J. Chem. Phys. 1980, 72, 113–120. [Google Scholar] [CrossRef]
- Parsons, J.L.; Milberg, M.E. Vibrational Spectra of Vitreous B2O3·xH2O. J. Amer. Ceram. Soc. 1960, 43, 326–330. [Google Scholar] [CrossRef]
- Wang, C.B.; Xiao, J.L.; Shen, Q.; Zhang, L.M. Bonding structure and mechanical properties of B-C-N thin films synthesized by pulsed laser deposition at different laser fluences. Thin Solid Film. 2016, 603, 323–327. [Google Scholar] [CrossRef]
- Puyoo, G.; Teyssandier, F.; Pailler, R.; Labrugère, C.; Chollon, G. Boron carbonitride coatings synthesized by LPCVD, structure and properties. Carbon 2017, 122, 19–46. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B. Optical and XPS studies of BCN thin films by co-sputtering of B4C and BN targets. Appl. Surf. Sci. 2017, 396, 484–491. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. (Eds.) . Practical Surface Analysis. V.1.—Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; John Wiley & Sons: Chichester, UK, 1995. [Google Scholar]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Databas. In NIST Standard Reference Database 20, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar] [CrossRef]
- Ramana, C.V.; Vemuri, R.S.; Kaichev, V.V.; Kochubey, V.A.; Saraev, A.A.; Atuchin, V.V. X-ray Photoelectron Spectroscopy Depth Profiling of La2O3/Si Thin Films Deposited by Reactive Magnetron Sputtering. ACS Appl. Mater. Interfaces 2011, 3, 4370–4373. [Google Scholar] [CrossRef]
- Kim, D.H.; Byon, E.; Lee, S.; Kim, J.-K.; Ruh, H. Characterization of ternary boron carbon nitride films synthesized by RF magnetron sputtering. Thin Solid Film. 2004, 447–448, 192. [Google Scholar] [CrossRef]
- Uddin, M.N.; Shimoyama, I.; Baba, Y.; Sekiguchi, T.; Nagano, M. X-ray photoelectron spectroscopic observation on B–C–N hybrids synthesized by ion beam deposition of borazine. J. Vac. Sci. Technol. A Vac. Surf. Film. 2005, 23, 497–502. [Google Scholar] [CrossRef]
- Yan, X.; Xu, T.; Chen, G.; Yang, S.; Liu, H.; Xue, Q. Preparation and characterization of electrochemically deposited carbon nitride films on silicon substrate. J. Phys. D Appl. Phys. 2004, 37, 907. [Google Scholar] [CrossRef]
- Souqui, L.; Palisaitis, J.; Högberg, H.; Pedersen, H. Plasma CVD of B-C-N thin films using triethylboron in argon-nitrogen plasma. J. Mater. Chem. C 2020, 8, 4112–4123. [Google Scholar] [CrossRef]
- Chen, C.M.; Huang, J.Q.; Zhang, Q.; Gong, W.Z.; Yang, Q.H.; Wang, M.Z.; Yang, Y.G. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 2012, 50, 659–667. [Google Scholar] [CrossRef]
- Rah, Y.; Jin, Y.; Kim, S.; Yu, K. Optical analysis of the refractive index and birefringence of hexagonal boron nitride from the visible to near-infrared. Opt. Lett. 2019, 44, 3797–3800. [Google Scholar] [CrossRef]
- Rumyantsev, S.L.; Levinshtein, M.E.; Jackson, A.D.; Mohammmad, S.N.; Harris, G.L.; Spencer, M.G.; Shur, M.S. Boron nitride (BN). In Properties of Advanced Semiconductor Materials GaN, AlN, InN, BN, SiC, SiGe; Levinshtein, M.E., Rumyantsev, S.L., Shur, M.S., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 67–92. [Google Scholar]
- Murarka, S.P.; Chang, C.C.; Wang, D.N.K.; Smit, T.E. Effect of Growth Parameters on the CVD of Boron Nitride and Phosphorus-Doped Boron Nitride. J. Elecrtochem. Soc. 1979, 126, 1951–1957. [Google Scholar] [CrossRef]
- Takahashi, T.; Itoh, H.; Kuroda, M. Structure and properties of CVD-BN thick film prepared on carbon steel substrate. J. Cryst. Growth. 1981, 53, 418–422. [Google Scholar] [CrossRef]
- Vedam, K.; Schneider, W.C. Variation of the refractive index of boric oxide glasses with hydrostatic pressure to 7 kbar. J. Appl. Phys. 1972, 43, 3623–3627. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, Y.; Luo, Y. The structure and optical properties of C doped BN thin films deposited by RF reactive magnetron sputtering. Optic. Mater. 2020, 110, 110502. [Google Scholar] [CrossRef]
- Maitz, M.F.; Gago, R.; Abendroth, B.; Camero, M.; Caretti, I.; Kreissig, U. Hemocompatibility of Low-Friction Boron–Carbon–Nitrogen Containing Coatings. J. Biomed. Mater. Res. Part B 2006, 77, 179–187. [Google Scholar] [CrossRef]
- Sulyaeva, V.S.; Gatapova, E.Y.; Kozhevnikov, A.K.; Rogilo, D.I.; Saraev, A.A.; Yushina, I.V.; Khomyakov, M.N.; Shapovalova, A.A.; Shayapov, V.R.; Bhaskar, N.; et al. Chemical Structural and Functional Properties of Amorphous Boron Carbonitride Films. J. Struct. Chem. 2021, 62, 1309–1324. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron Carbide: Structure, Properties, and Stability under Stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Stockel, S.; Weise, K.; Deietrich, D.; Thamm, T.; Braun, M.; Cremer, R.; Neuschutz, D.; Marx, G. Influence of composition and structure on the mechanical properties of BCN coatings deposited by thermal CVD. Thin Solid Film. 2002, 420-421, 465–471. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Tsui, T.Y.; Pharr, G.M.; Oliver, W.C.; Bhatia, C.S.; White, R.L.; Anders, S.; Anders, A.; Brown, I.G. Nanoindentation and nanoscratching of hard carbon coatings for magnetic disks. Mat. Res. Soc. Symp. Proc. 1995, 383, 447–452. [Google Scholar] [CrossRef]
- Lvova, N.A.; Kravchuk, K.S.; Shirokov, I.A. Algorithms for Processing Scratch Images in the Sclerometry Method. Phys. Solid State 2013, 55, 1681–1689. [Google Scholar] [CrossRef]
- Xu, S.; Wang, L.; Ma, X.; Tang, G.; Sun, M. Effect of Target Power on Nano-Scratch Behavior of BCN Films by D.C. Reactive Magnetron Sputtering. Nanosci. Nanotechnol. Lett. 2011, 3, 276–279. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Smolyar, A.S.; Ovsiannikova, L.I.; Pokropivny, A.V.; Kuts, V.A.; Lyashenko, V.I.; Nesterenko, Y.V. Fluid synthesis and structure of a new polymorphic modification of boron nitride. Phys. Solid State 2013, 55, 878–884. [Google Scholar] [CrossRef]
- Jacobsohn, L.G.; Nastasi, M. Sputter-deposited boron carbide films: Structural and mechanical characterization. Surf. Coat. Technol. 2005, 200, 1472–1475. [Google Scholar] [CrossRef]
- Hirata, Y.; Takeuchi, R.; Taniguchi, H.; Kawagoe, M.; Iwamoto, Y.; Yoshizato, M.; Akasaka, H.; Ohtake, N. Structural and Mechanical Properties of a-BCN Films Prepared by an Arc-Sputtering Hybrid Process. Materials 2021, 14, 719. [Google Scholar] [CrossRef]



| Deposition Conditions | Elemental Composition, at.% | Functional Properties | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Mechanical | Optical | Electrical | Wettability | |||||
| Initial Gas Mixture | Tdep, K | Plasma Frequency, Power | H, GPa, E, GPA, R, % | n, T, % Eg, eV | k, ρ, Ω·cm | CA, ° SFE, mN/m | ||
| LPCVD | ||||||||
| TMAB + He | 723 773 | – | B0.72C0.23N0.04O0.01 B0.68C0.27N0.04O0.01 | 34 226 – | 1.9–2.8 | – | – | [34] |
| TMAB + NH3 | 673 | – | B0.54C0.14N0.29O0.03 | 10–15 115 – | 1.9–2.8 | – | – | [34] |
| TMAB TMAB + NH3 | 973 | – | B0.33C0.45N0.21O0.01 B0.28C0.17N0.54O0.01 | – | – | – | – | [35] |
| TMAB TMAB + He TMAB + N2 TMAB + NH3 | 973 | – | B0.33C0.57N0.08 B0.44C0.49N0.07 B0.37C0.56N0.08 B0.35–0.42C0.34–0.40N0.32–0.18 | – | – | – | – | [36] |
| TMAB + He | 723 773 | – | B0.72C0.23N0.04O0.01 B0.68C0.27N0.04O0.01 | – | 2.59, 2.72 | – 3.7 × 106; 2.4 × 106 | – | [37] |
| TMAB + N2 | 673–973 | – | B0.67–0.80C0.14–0.24N0.04–0.08O0.01-0.02 | 24–28 220–247 70–74 | 2.43–2.56 83–85 2.05–2.20 | – | 53–63 42–48 | This study |
| RF PECVD | ||||||||
| TMAB TMAB + NH3 TMAB + H2 TMAB + He | 573–973 | 40.68 MHz | B0.40C0.39N0.12O0.10 B0.41C0.21N0.32O0.06 | – | 1.56–2.27 70–90 – | – | – | [31,38,39] |
| TMAB + He | 473–723 | 40.68 MHz 70 W | B0.36–0.33C0.28–0.46N0.30–0.21O0.09–0.01 | – | 1.55–2.06 – – | 4.6–5.6 – | – | [18,37] |
| TMAB + NH3 | 473–723 | 40.68 MHz 70 W | B0.35–0.42C0.12–0.30N0.30–0.42O0.12–0.02 | – | 1.68–2.04 – – | 4.2–6.3 1013–1015 | – | [18,37] |
| TMAB | 573, 873 | 13.56 MHz 400 W | B0.50C0.23N0.12O0.15 B0.31C0.26N0.28O0.13 | 6.7, 1.9 – – | – | – | – | [40] |
| TMAB + N2 | 373–873 | 13.56 MHz 50 W | B0.22–0.39C0.12–0.36N0.19–0.40O0.07–0.24 | 0.8–1.4 25–26 19–28 | 1.51–1.67 92–95 4.92–5.28 | – | 48–55 49–51 | This study |
| MW PECVD | ||||||||
| TMAB | 1113, 1112 | 2.45 GHz 300, 400 W | B0.16C0.63N0.09O0.12 B0.33C0.22N0.33O0.12 | 2.3, 5.2 | – | – | – | [40] |
| TMAB + N2 TMAB + CH4 + H2 | 1083–1213 973 | 2.45 GHz 200, 300, 400 W | B0.36–0.52C0.06–0.11N0.07-0.32O0.10-0.46 B0.30C0.15N0.04O0.51 | – | – | – | – | [41] |
| Tdep, K | [C]/[B] | [N]/[B] | [O]/[B] | Elemental Composition, at.% | |||
|---|---|---|---|---|---|---|---|
| B | C | N | O | ||||
| 673 | 0.44 | 0.07 | 0.20 | 59 | 26 | 4 | 12 |
| 873 | 0.61 | 0.10 | 0.21 | 52 | 32 | 5 | 11 |
| 973 | 0.77 | 0.13 | 0.25 | 46 | 36 | 6 | 12 |
| Bonding Structures | Position, eV | Area, % | ||
|---|---|---|---|---|
| Tdep = 673 K | Tdep = 873 K | Tdep = 973 K | ||
| B–B/B–C in B4C | 188.0 | 56 | 28 | 13 |
| B–C in BCx | 189.0 | 32 | 53 | 58 |
| B–CnN3–n | 189.9 | 8 | 12 | 21 |
| B–N in h-BN | 190.9 | 3 | 6 | 5 |
| B–(C/N)nO3–n | 191.9 | 1 | 1 | 3 |
| Carbon State | Eb, eV | Area, % | ||
|---|---|---|---|---|
| Tdep = 673 K | Tdep = 873 K | Tdep = 973 K | ||
| C–B in B4C | 282.5 | 16 | 20 | 24 |
| C–B in BCx | 283.3 | 11 | 16 | 16 |
| sp2 C=C/C–H | 284.8 | 57 | 50 | 45 |
| C–NnB3–n | 286.3 | 8 | 5 | 6 |
| C–(N/B)nO3–n/C–O | 286.9 | 4 | 4 | 3 |
| C=O | 287.9 | 1 | 2 | 2 |
| HO–C=O | 289.0 | 3 | 3 | 4 |
| Nitrogen State | Eb, eV | Area, % | ||
|---|---|---|---|---|
| Tdep = 673 K | Tdep = 873 K | Tdep = 973 K | ||
| N–B in h-BN | 397.8 | 29 | 62 | 73 |
| N–BnC3–n | 399.4 | 36 | 26 | 18 |
| N–(B/C)nO3–n | 400.9 | 22 | 9 | 8 |
| N–On | 402.3 | 13 | 3 | 1 |
| Tdep, K | CA(H2O), ° | CA(DEG), ° | State Equation | Owens-Wendt Method | ||||
|---|---|---|---|---|---|---|---|---|
| Ellipse-Fitting Method | The Young-Laplace Algorithm | Ellipse-Fitting Method | The Young-Laplace Algorithm | Es, mN/m | Es, mN/m | Esd, mN/m | Esp, mN/m | |
| LPCVD | ||||||||
| 773 | 59 ± 3 | 59 ± 3 | 12 ± 2 | 12 ± 2 | 46 ± 8 | 47 ± 8 | 27 ± 5 | 19 ± 3 |
| 823 | 59 ± 5 | 60 ± 4 | 10 ± 2 | 10 ± 2 | 46 ± 10 | 47 ± 10 | 28 ± 6 | 19 ± 4 |
| 848 | 63.0 ± 0.6 | 63 ± 4 | 17 ± 3 | 17 ± 3 | 45 ± 8 | 44 ± 8 | 31 ± 5 | 13 ± 2 |
| 873 | 63 ± 2 | 63 ± 1 | 16 ± 3 | 16 ± 5 | 45 ± 8 | 45 ± 9 | 29 ± 5 | 16 ± 3 |
| 898 | 63 ± 2 | 63 ± 2 | 23 ± 5 | 23 ± 4 | 44 ± 10 | 42 ± 9 | 29 ± 6 | 14 ± 3 |
| 923 | 55 ± 3 | 55 ± 2 | 12 ± 3 | 11 ± 2 | 48 ± 12 | 48 ± 12 | 25 ± 6 | 23 ± 6 |
| 973 | 54 ± 2 | 53 ± 2 | 12 ± 2 | 11 ± 2 | 48 ± 8 | 49 ± 8 | 25 ± 4 | 24 ± 4 |
| PECVD | ||||||||
| 473 | 48 ± 2 | 48 ± 2 | 7 ± 1 | 7 ± 1 | 51 ± 8 | 51 ± 8 | 23 ± 3 | 28 ± 4 |
| 523 | 54 ± 3 | 55 ± 4 | 7 ± 1 | 5.5 ± 0.7 | 49 ± 7 | 48 ± 7 | 27 ± 4 | 21 ± 3 |
| Tdep, K | Film Composition | H, GPa | E, GPa | R, % |
|---|---|---|---|---|
| 773 | B0.80C0.15N0.05O0.00 | 27.3 ± 3.5 | 243 ± 25 | 71 |
| 823 | B0.77C0.17N0.05O0.01 | 27.6 ± 3.1 | 230 ± 16 | 70 |
| 873 | B0.75C0.19N0.06O0.01 | 27.4 ± 4.4 | 247 ± 28 | 71 |
| 923 | B0.70C0.21N0.07O0.02 | 28.2 ± 3.8 | 236 ± 21 | 70 |
| 973 | B0.67C0.25N0.08O0.00 | 24.3 ± 4.1 | 221 ± 26 | 74 |
| Tdep, K | Film Composition | H, GPa | E, GPa | R, % |
|---|---|---|---|---|
| 373 | B0.23C0.37N0.18O0.22 | 0.8 ± 0.2 | 26 ± 4 | 18.6 |
| 423 | B0.22C0.30N0.22O0.26 | 0.9 ± 0.2 | 24 ± 6 | 20.1 |
| 473 | B0.32C0.21N0.29O0.18 | 1.2 ± 0.3 | 27 ± 5 | 26.5 |
| 523 | B0.38C0.12N0.34O0.16 | 1.4 ± 0.3 | 26 ± 4 | 28.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belaya, S.V.; Maksimovsky, E.A.; Shayapov, V.R.; Shapovalova, A.A.; Kolodin, A.N.; Saraev, A.A.; Asanov, I.P.; Khomyakov, M.N.; Yushina, I.V.; Plekhanov, A.G.; et al. Boron Carbonitride Films with Tunable Composition: LPCVD and PECVD Synthesis Using Trimethylamine Borane and Nitrogen Mixture and Characterization. Appl. Sci. 2023, 13, 4959. https://doi.org/10.3390/app13084959
Belaya SV, Maksimovsky EA, Shayapov VR, Shapovalova AA, Kolodin AN, Saraev AA, Asanov IP, Khomyakov MN, Yushina IV, Plekhanov AG, et al. Boron Carbonitride Films with Tunable Composition: LPCVD and PECVD Synthesis Using Trimethylamine Borane and Nitrogen Mixture and Characterization. Applied Sciences. 2023; 13(8):4959. https://doi.org/10.3390/app13084959
Chicago/Turabian StyleBelaya, Svetlana V., Eugene A. Maksimovsky, Vladimir R. Shayapov, Aleksandra A. Shapovalova, Alexey N. Kolodin, Andrey A. Saraev, Igor P. Asanov, Maxim N. Khomyakov, Irina V. Yushina, Aleksandr G. Plekhanov, and et al. 2023. "Boron Carbonitride Films with Tunable Composition: LPCVD and PECVD Synthesis Using Trimethylamine Borane and Nitrogen Mixture and Characterization" Applied Sciences 13, no. 8: 4959. https://doi.org/10.3390/app13084959
APA StyleBelaya, S. V., Maksimovsky, E. A., Shayapov, V. R., Shapovalova, A. A., Kolodin, A. N., Saraev, A. A., Asanov, I. P., Khomyakov, M. N., Yushina, I. V., Plekhanov, A. G., Sulyaeva, V. S., & Kosinova, M. L. (2023). Boron Carbonitride Films with Tunable Composition: LPCVD and PECVD Synthesis Using Trimethylamine Borane and Nitrogen Mixture and Characterization. Applied Sciences, 13(8), 4959. https://doi.org/10.3390/app13084959








