Microsporidiosis Causing Necrotic Changes in the Honeybee Intestine
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wittner, M.; Weiss, L.M. The Microsporidia and Microsporidiosis; ASM Press: Washington, DC, USA, 1999. [Google Scholar]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e00010-20. [Google Scholar] [CrossRef]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Münch. Bienenz. 1909, 31, 196–204. [Google Scholar]
- Fries, I.; Martín, R.; Meana, A.; García-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Chemurot, M.; Smet, L.; Brunain, M.; de Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Huang, W.F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Demetraki-Paleolog, J. Differentiation of Nosema apis and Nosema ceranae spores under Scanning Electron Microscopy (SEM). J. Apic. Res. 2014, 53, 537–544. [Google Scholar] [CrossRef]
- Tauber, J.P.; McMahon, D.; Ryabov, E.V.; Kunat, M.; Ptaszynska, A.A.; Evans, J.D. Honeybee intestines retain low yeast titers, but no bacterial mutualists, at emergence. Yeast 2021, 39, 95–107. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Latoch, P.; Hurd, P.J.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Gancarz, M.; et al. Amplicon Sequencing of Variable 16S rRNA from Bacteria and ITS2 Regions from Fungi and Plants, Reveals Honeybee Susceptibility to Diseases Results from Their Forage Availability under Anthropogenic Landscapes. Pathogens 2021, 10, 381. [Google Scholar] [CrossRef]
- Mazur, E.D.; Gajda, A.M. Nosemosis in Honeybees: A Review Guide on Biology and Diagnostic Methods. Appl. Sci. 2022, 12, 5890. [Google Scholar] [CrossRef]
- Soklič, M.; Gregorc, A. Comparison of the two microsporidia that infect honeybees—A review. Agricultura 2016, 13, 49–56. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; Mcmahon, D.P.; Martínhernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 51, 1–28. [Google Scholar] [CrossRef]
- Kurze, C.; Le Conte, Y.; Kryger, P.; Lewkowski, O.; Müller, T.; Moritz, R.F. Infection dynamics of Nosema ceranae in honey bee midgut and host cell apoptosis. J. Invertebr. Pathol. 2018, 154, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis meliffera: A 12-years post-detection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed]
- Copley, T.R.; Jabaji, S.H. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 2012, 112, 15–24. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Litkowski, A.M.; Pettis, J. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Rasekh, M.; Afkari Sayyah, A.H.; Abbaspour-Gilandeh, Y.; Karami, H.; Rasooli Sharabiani, V.; Fioravanti, A.; Gancarz, M.; Findura, P.; Kwaśniewski, D. Hyperspectral imaging coupled with multivariate analysis and artificial intelligence to the classification of maize kernels. Int. Agrophys. 2022, 36, 83–91. [Google Scholar] [CrossRef]
- Gancarz, M.; Konstankiewicz, K.; Zgórska, K. Cell orientation in potato tuber parenchyma tissue. Int. Agrophys. 2014, 28, 15–22. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Gancarz, M.; Wiącek, D.; Nawrocka, A.; Strachecka, A. Imidacloprid Pesticide Causes Unexpectedly Severe Bioelement Deficiencies and Imbalance in Honey Bees even at Sublethal Doses. Animals 2023, 13, 615. [Google Scholar] [CrossRef]
- Peng, Y.; Lee-Pullen, T.F.; Heel, K.; Millar, A.H.; Baer, B. Quantifying spore viability of the honey bee pathogen Nosema apis using flow cytometry. Cytometry 2014, 85, 454–462. [Google Scholar] [CrossRef]
- Fernández, M.; Sánchez, J. Nuclease activities and cell death processes associated with the development of surface cultures of Streptomyces antibioticus ETH 7451. Microbiology 2002, 148 Pt 2, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.; McGoverin, C.; Swift, S.; Vanholsbeeck, F. Rapid and cost-effective evaluation of bacterial viability using fluorescence spectroscopy. Anal. Bioanal. Chem. 2019, 411, 3653–3663. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae Tissue Tropism in Worker Honey Bees (Apis mellifera). Vet. Pathol. 2020, 57, 132–138. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Identification of candidate agents active against N. ceranae infection in honeybees: Establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS ONE 2015, 6, e0117200. [Google Scholar] [CrossRef]
- Kurze, C.; Le Conte, Y.; Dussaubat, C.; Erler, S.; Kryger, P.; Lewkowski, O.; Müller, T.; Widder, M.; Moritz, R.F.A. Nosema Tolerant Honeybees (Apis mellifera) Escape Parasitic Manipulation of Apoptosis. PLoS ONE 2015, 10, e0140174. [Google Scholar] [CrossRef]
- Panek, J.; Paris, L.; Roriz, D.; Mone, A.; Dubuffet, A.; Delbac, F.; Diogon, M.; El Alaoui, H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J. Invertebr. Pathol. 2018, 159, 121–128. [Google Scholar] [CrossRef]
- Maiolino, P.; Iafigliola, L.; Rinaldi, L.; de Leva, G.; Restucci, B.; Martano, M. Histopathological findings of the midgut in European honey bee (Apis mellifera L.) naturally infected by Nosema spp. Vet. Med. Anim. Sci. 2014, 2, 4. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Anusiewicz, M.; Mułenko, W. Location of Nosema spp. spores within body of honey bee. Med. Weter. 2012, 68, 618–621. [Google Scholar]
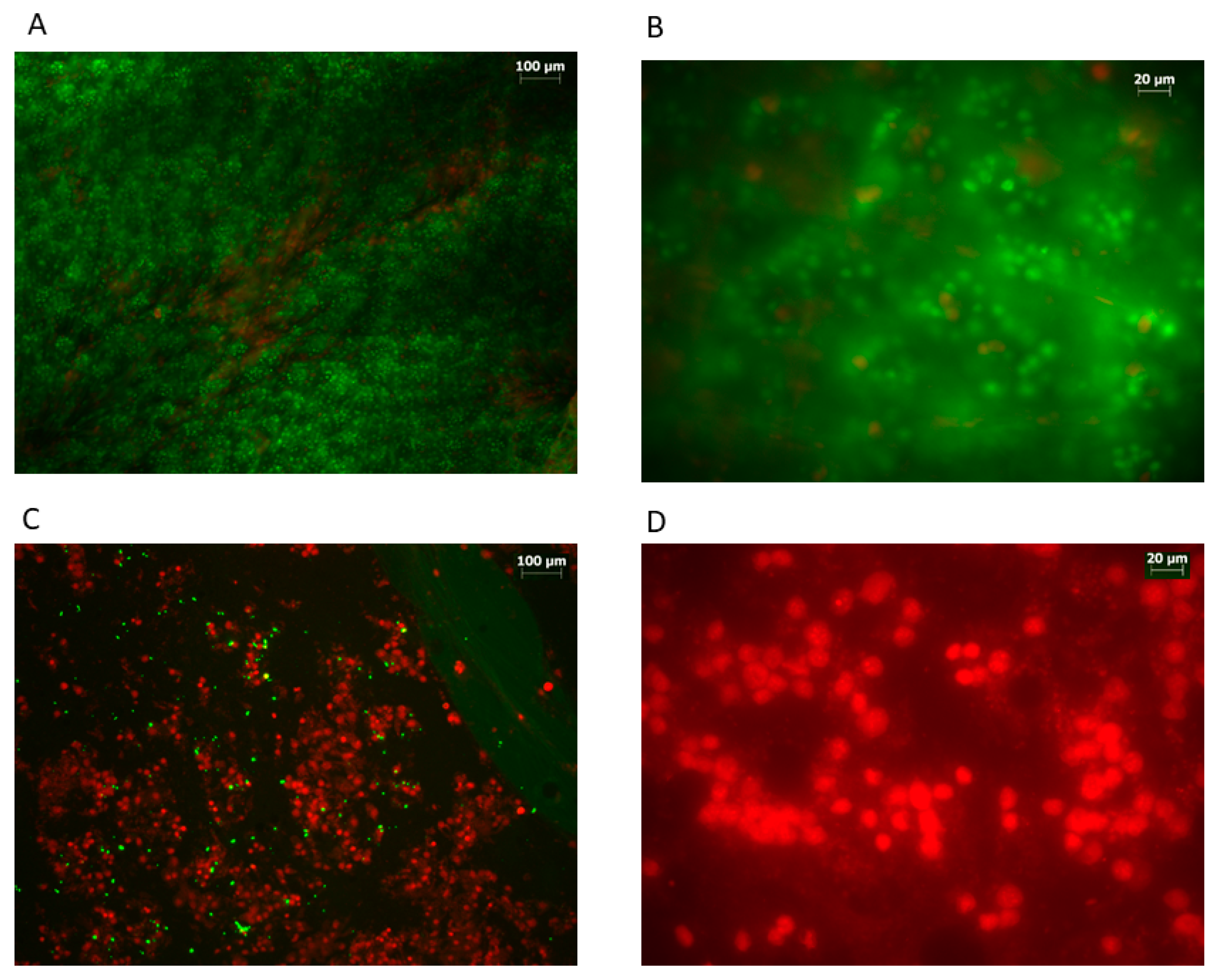
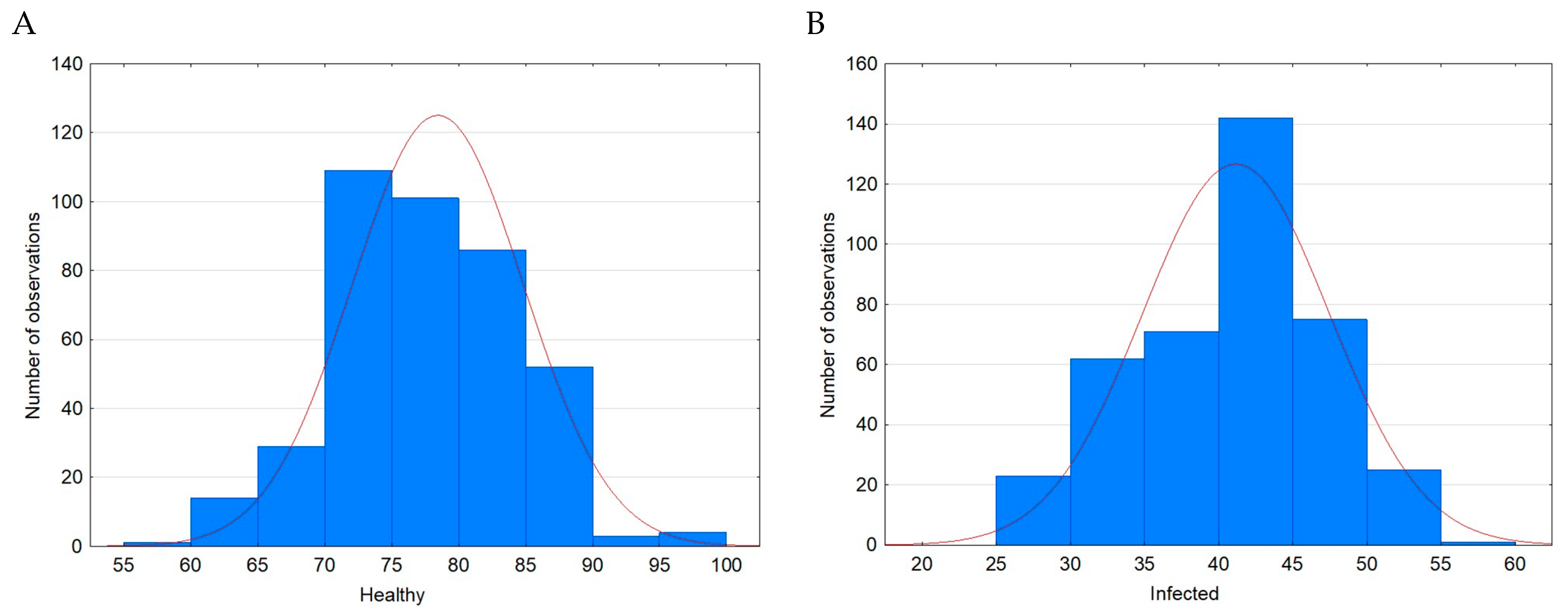
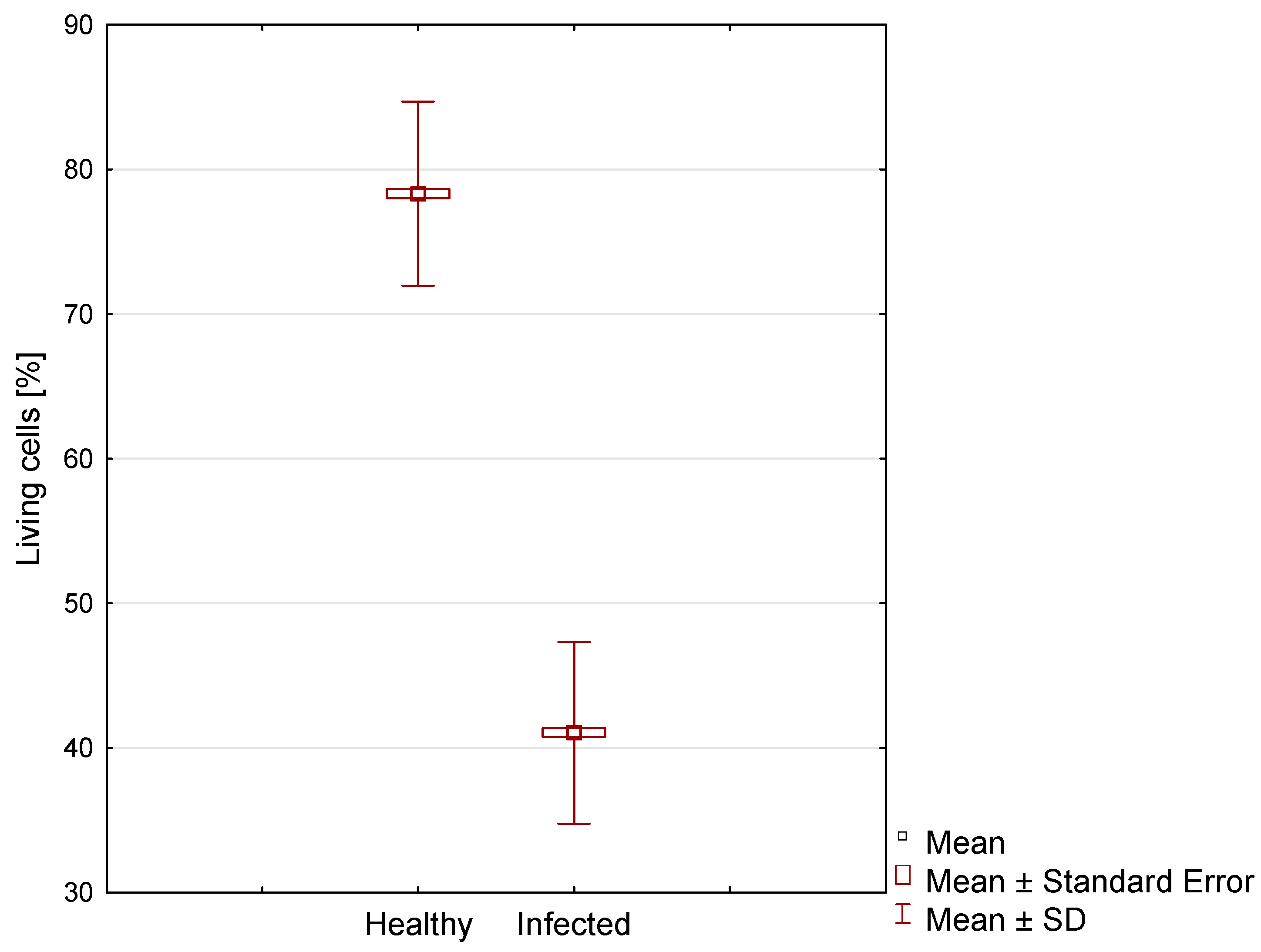
| Case | Minimum Value [%] | Maximum Value [%] | Mean [%] | SD [%] | Variance | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|
| Healthy | 59.0 | 96.0 | 78.32 a | 6.37 | 40.52 | 0.10 | −0.11 |
| Infected | 26.0 | 56.0 | 41.05 b | 6.28 | 39.49 | −0.16 | −0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszyńska, A.A.; Gancarz, M. Microsporidiosis Causing Necrotic Changes in the Honeybee Intestine. Appl. Sci. 2023, 13, 4957. https://doi.org/10.3390/app13084957
Ptaszyńska AA, Gancarz M. Microsporidiosis Causing Necrotic Changes in the Honeybee Intestine. Applied Sciences. 2023; 13(8):4957. https://doi.org/10.3390/app13084957
Chicago/Turabian StylePtaszyńska, Aneta A., and Marek Gancarz. 2023. "Microsporidiosis Causing Necrotic Changes in the Honeybee Intestine" Applied Sciences 13, no. 8: 4957. https://doi.org/10.3390/app13084957
APA StylePtaszyńska, A. A., & Gancarz, M. (2023). Microsporidiosis Causing Necrotic Changes in the Honeybee Intestine. Applied Sciences, 13(8), 4957. https://doi.org/10.3390/app13084957








