Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. Determination of Total Iodine
2.3. Determination of Iodoquinolines
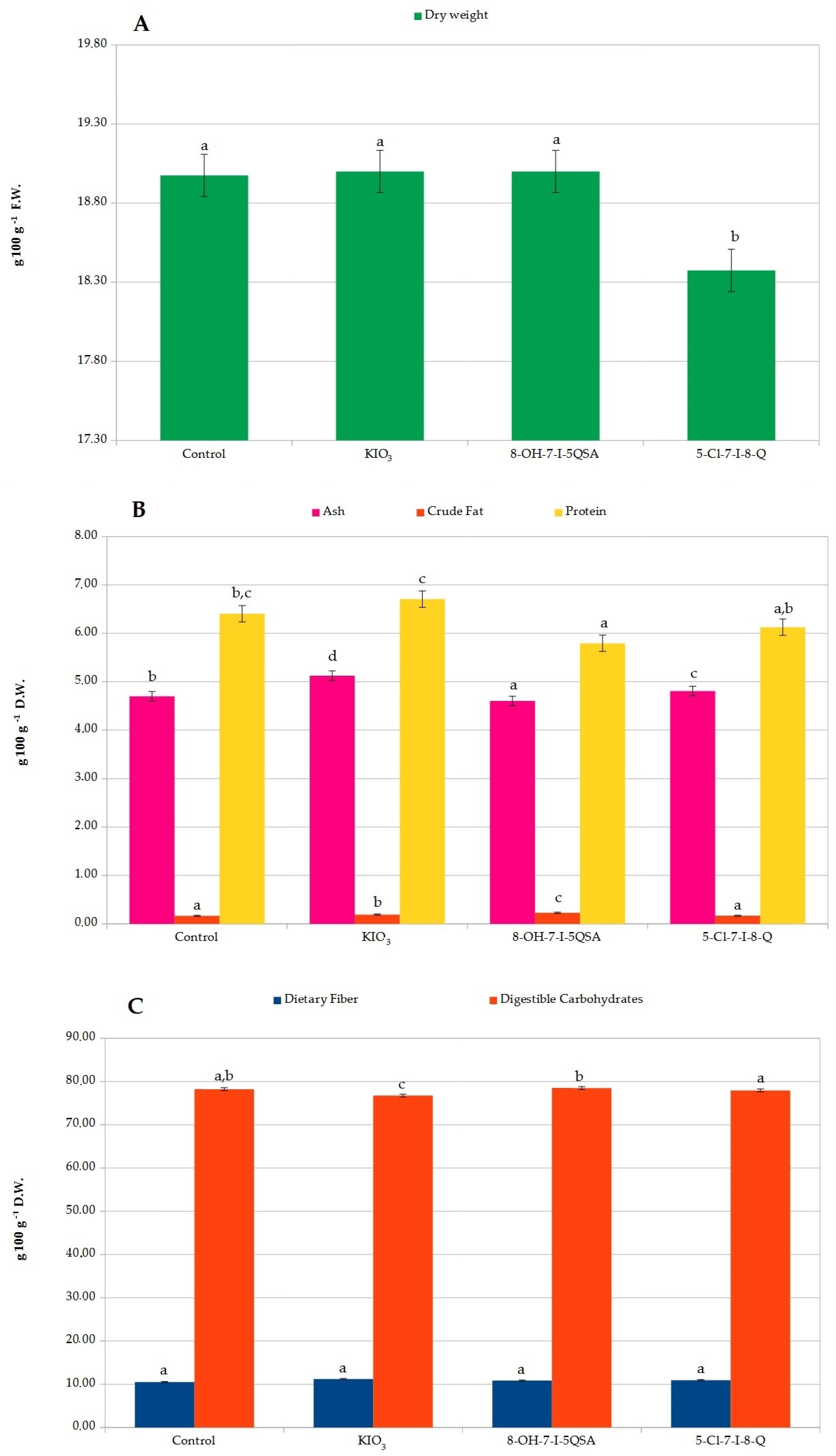
2.4. Potato Analysis after Sample Drying
2.5. Determination of Macro, Microelements and Trace Elements
2.6. Determination of Nitrogenous Ions, and Chlorides
2.7. Determination of the Antioxidant Capacity
Preparation of Ethanol Extracts
2.8. Determination of the Polyphenols Content
2.9. Determination of the Total Carotenoids Content
2.10. Determination of the Antioxidant Activity Using the ABTS Method
2.11. Determination of L-Ascorbic Acid
2.12. Basic Chemical Composition
2.13. Assessment of Iodine-Fortified Potato Tubers for Consumer Health Safety
2.14. Statistical Analyses
3. Results and Discussion
3.1. Description of Potato Tuber Yield
3.2. Determination of Total Iodine
3.3. Determination of Iodoquinolines
3.4. Determination of Macro- and Microminerals
3.5. Determination of Nitrogenous Ions, and Chlorides
3.6. Determination of the Polyphenols Content
3.7. Determination of the Total Carotenoids Content
3.8. Determination of the Antioxidant Activity Using the ABTS Method
3.9. Determination of L-Ascorbic Acid
3.10. Basic Chemical Composition
3.11. Assessment of Iodine-Fortified Potato Tubers for Consumer Health Safety
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koronowicz, A.; Kopec, A.; Master, A.; Smoleń, S.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Ledwożyw-Smoleń, I.; Skoczylas, L.; Rakoczy, R.; Leszczyńska, T.; et al. Transcriptome profiling of Caco-2 cancer cell line following treatment with extracts from iodine-biofortified lettuce (Lactuca sativa L.). PLoS ONE 2016, 11, e0147336. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Polish Population and Their Application; National Institute of Public Health—National Institute of Hygiene: Warsaw, Poland, 2020; ISBN 978-83-65870-28-5.
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Hlušek, J.; Jůzl, M.; Čepl, J.; Lošák, T. The effect of selenium supplementation on its concentration in potato tubers. Chem. Listy 2005, 99, 515–517. [Google Scholar]
- Caffagni, A.; Pecchioni, N.; Meriggi, P.; Bucci, V.; Sabatini, E.; Acciarri, N.; Ciriaci, T.; Pulcini, L.; Felicioni, N.; Beretta, M.; et al. Iodine uptake and distribution in horticultural and fruit tree species. Ital. J. Agron. 2012, 7, 32. [Google Scholar] [CrossRef]
- Ritter, E.; Angulo, B.; Riga, P.; Herran, C.; Relloso, J.; Jose, M.S. Comparison of hydroponic and aeroponic cultivation systems for the production of potato minitubers. Potato Res. 2001, 44, 127–135. [Google Scholar] [CrossRef]
- Farran, I.; Mingo-Castel, A.M. Potato minituber production using aeroponics: Effect of plant density and harvesting intervals. Am. J. Potato Res. 2006, 83, 47–53. [Google Scholar] [CrossRef]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current Strategies for Selenium and Iodine Biofortification in Crop Plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef] [PubMed]
- Aparo, N.O.; Olum, S.; Atimango, A.O.; Odongo, W.; Aloka, B.; Ongeng, D.; Gellynck, X.; De Steur, H. Farmers’ Intention to Adopt Agronomic Biofortification: The Case of Iodine Biofortified Vegetables in Uganda. Horticulturae 2023, 9, 401. [Google Scholar] [CrossRef]
- Bowley, H.E.; Mathers, A.W.; Young, S.D.; Macdonald, A.J.; Ander, E.L.; Watts, M.J.; Zhao, F.J.; Mcgrath, S.P.; Crout, N.M.J.; Bailey, E.H. Historical trends in iodine and selenium in soil and herbage at the Park Grass Experiment, Rothamsted Research, UK. Soil Use Manag. 2017, 33, 252–262. [Google Scholar] [CrossRef]
- Mageshen, V.R.; Santhy, P.; Meena, S.; Latha, M.R.; Senthil, A.; Saraswathi, T.; Janaki, P. Residual effect of biofortified iodine in soil, plant, crop yield and quality of tomato (Solanum lycopersicum L.). Res. Crops 2022, 23, 4. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Skrypnik, L.; Tallarita, A.; Caruso, G. Joint Biofortification of Plants with Selenium and Iodine: New Field of Discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef]
- Helgilibrary. Available online: https://www.helgilibrary.com/charts/which-country-eats-the-most-potatoes/ (accessed on 14 February 2023).
- Gietka-Czernel, M. Prophylaxis of iodine deficiency. Adv. Med. Sci. 2015, 25, 839–845. [Google Scholar]
- World Health Organization. News-Room Fact-Sheets Detail Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-andoverweight (accessed on 20 January 2023).
- The World Bank Group. Seeking Agriculture Related Solutions for Obesity, an Increasing Problem within Malnutrition. Available online: https://blogs.worldbank.org/health/seeking-agriculture-related-solutions-obesity-increasing-problemwithin-malnutrition (accessed on 3 February 2022).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#search/potato (accessed on 14 February 2023).
- Dereje, B.; Chibuzo, N. Nutritional Composition and Biochemical Properties of Solanum tuberosum. In Solanum tuberosum—A Promising Crop for Starvation; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients 2019, 4, 1523. [Google Scholar] [CrossRef]
- Sulli, M.; Mandolino, G.; Sturaro, M.; Onofri, C.; Diretto, G.; Parisi, B.; Giuliano, G. Molecular and biochemical characterization of a potato collection with contrasting tuber carotenoid content. PLoS ONE 2017, 12, e0184143. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations “Statistical Database”. Available online: https://www.fao.org/faostat/en/ (accessed on 13 January 2023).
- Sahair, R.A.; Sneha, S.; Raghu, N.; Ts, G.; Karthikeyan, M.; Gnanasekaran, A.; Gk, C.; Basalingappa, K.M. Solanum tuberosum L.: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Int. J. Phytomed. 2018, 10, 115–124. [Google Scholar] [CrossRef]
- Kaplan, M.; Ulger, I.; Kokten, K.; Uzun, S.; Oral, E.V.; Ozaktan, H.; Temizgul, R.; Kale, H. Nutritional composition of potato (Solanum tuberosum L.) Haulms. Prog. Nutr. 2018, 20, 90–95. [Google Scholar] [CrossRef]
- Nemś, A.; Miedzianka, J.; Pęksa, A.; Kita, A. Content of health-promoting compounds in potatoes of varied flesh colours. Bromat. Chem. Toxicol. XLVIII 2015, 3, 473–478. [Google Scholar]
- Brown, C.R. Antioxidants in potato. Am. J. Potato Res. 2005, 82, 163–172. [Google Scholar] [CrossRef]
- Tatarowska, B.; Milczarek, D.; Jakuczun, H.; Stochmal, A.; Pecio, Ł.; Flis, F. The potential for the improvement of carotenoids level inpotato—Effect of the genotype and environment. J. Food Agric. Environ. 2014, 12, 536–540. [Google Scholar]
- Włochal, M.; Grzymisławski, M.; Bogdański, P. The possibility of use functional food for the treatment of obesity. Metab. Disord. Forum 2014, 5, 51–62. [Google Scholar]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Iodine and selenium biofortification of lettuce (Lactuca sativa L.) by soil fertilization with various compounds of these elements. Acta Sci. Pol. Hortorum Cultus 2016, 15, 69–91. [Google Scholar]
- Anschutz, P.; Zhon, S.J.; Sundby, B.; Mucci, A.; Gobeil, C. Burial efficiency of phosphorus and the geochemistry of iron in continental margin sediments. Limnol. Oceanogr. 1998, 43, 53–64. [Google Scholar] [CrossRef]
- Kostka, J.E.; Luther, G.W. Partitioning and speciation of solid-phase iron in salt-marsh sediments. Geochim. Cosmochim. Acta 1994, 58, 1701–1710. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Sady, W.; Grzanka, M.; Kutman, U.B. Changes in the Chemical Composition of Six Lettuce Cultivars (Lactuca sativa L.) in Response to Biofortification with Iodine and Selenium Combined with Salicylic Acid Application. Agronomy 2019, 9, 660. [Google Scholar] [CrossRef]
- PN-EN 15111:2008; Foodstuffs—Determination of Trace Elements—Determination of Iodine Content by ICP-MS (Inductively Coupled Plasma Mass Spectrometry). Polish Committee for Standardisation: Warsaw, Poland, 2008. Available online: https://sklep.pkn.pl/pn-en-15111-2008p.html (accessed on 22 January 2023).
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 144, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.A.; Wennerholm, M. Kjeldahl Mineralisation Guide-Review of the Classic Method with Company Improvements FOSS TECATOR; Labconsult: Warsaw, Poland, 1999. [Google Scholar]
- Kalisz, A.; Sękara, A.; Smoleń, S.; Grabowska, A.; Gil, J.; Komorowska, M.; Kunicki, E. Survey of 17 elements, including rare earth elements, in chilled and non-chilled cauliflower cultivars. Sci. Rep. 2019, 9, 5416. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. The phenolic constituens of Prunus domestica. The quantitive analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Czernicka, M.; Halka, M.; Kęska, K.; Sady, W. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front. Plant Sci. 2016, 7, 1553. [Google Scholar] [CrossRef]
- PN-A-75101-12; Fruit and Vegetable Preserves-Preparation of Samples and Physicochemical Test Methods-Determination of Total Carotenoids and Beta-Carotene Content. Polish Committee for Standardisation: Warsaw, Poland, 1990. Available online: https://sklep.pkn.pl/pn-a-75101-03-1990p.html (accessed on 1 February 2023).
- Re, R.; Pellergini, N.; Proteggente, A.; Pannala, A.S.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Standard Operation Procedure. Ref. Ares. 2020; p. 1848056-31/03/2020. Available online: https://ec.europa.eu/research/participants/documents/downloadPublicdocumentIds=080166e5cd8d669b&appId=PPGMS (accessed on 1 February 2023).
- Total Dietary Fiber—Assay Procedure—Megazyme. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 1 February 2023).
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical composition of lettuce (Lactuca sativa L.) biofortified with iodine by KIO3, 5-Iodo-, and 3.5-diiodosalicylic acid in a hydroponic cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Knutsen, H.K.; Johansen, N.C.; Nyheim, K.A.; Erlund, I.; Meltzer, H.M.; Henjum, S. Definedby age, life stage and vegetarian dietary practice in a norwegian convenience sample. Nutrients 2018, 10, 230. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Integrated Risk Information System—Database; Environmental Protection Agency: Washington, DC, USA, 2011.
- Kessler, J. Are there side effects when using supraphysiologic levels of iodine in treatment regimens? In Comprehensive Handbook of Iodine—Nutritional. Biochemical, Pathological and Therapeutic Aspects, 1st ed.; Preedy, V., Burrow, G., Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 1, pp. 107–118. [Google Scholar]
- Dobosy, P.; Endrédi, A.; Sandil, S.; Vetési, V.; Rékási, M.; Takács, T.; Gy, Z. Biofortification of Potato and Carrot with Iodine by Applying Different Soils and Irrigation with Iodine-Containing Water. Front. Plant Sci. 2020, 11, 593047. [Google Scholar] [CrossRef] [PubMed]
- Ledwożyw-Smoleń, I. Iodine Biofortification of Potato (Solanum tuberosum L.) Grown in Field. Agronomy 2020, 10, 1916. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and the antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Sánchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Leyva, R.; Romero, L.; Ruiz, J.M. A study of the interaction of iodine and minerals in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Hong, C.L.; Weng, H.X.; Yan, A.L.; Islam, E.U. The fate of exogenous iodine in pot soil cultivated with vegetables. Environ. Geochem. Health 2009, 31, 99–108. [Google Scholar] [CrossRef]
- Voogt, W.; Steenhuizen, J.; Eveleens, B. Iodine uptake and distribution in cucumber, sweet pepper, round and cherry tomato. Rep. Wagening. UR Greenh. Horic. 2014, 1329, 1–72. [Google Scholar]
- Dai, J.L.; Zhu, Y.G.; Zhang, M.; Huang, Y.Z. Selecting iodine-enriched vegetables and the residual effect of iodate application to soil. Biol. Trace Elem. Res. 2004, 101, 265–276. [Google Scholar] [CrossRef]
- Weng, H.X.; Yan, A.L.; Hong, C.L.; Xie, L.L.; Qin, Y.C.; Cheng, C.Q. Uptake of different species of iodine by water spinach and its effect to growth. Biol. Trace Elem. Res. 2008, 125, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Skoczylas, L.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Koronowicz, A.; Kapusta-Duch, J. Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front. Plant Sci. 2016, 7, 730. [Google Scholar] [CrossRef]
- Dobosy, P.; Kröpfl, K.; Óvári, M.; Sandil, S.; Németh, K.; Engloner, A.; Takács, T.; Záray, G. Biofortification of green bean (Phaseolus vulgaris L.) and lettuce (Lactuca sativa L.) with iodine in a plant-calcareous sandy soil system irrigated with water containing KI. J. Food Compos. Anal. 2020, 88, 103434. [Google Scholar] [CrossRef]
- Golob, A.; Novak, T.; Maršić, K.N.; Šircelj, H.; Stibilj, V.; Jerše, A.; Kroflič, A.; Germ, M. Biofortification with selenium and iodine changes morphological properties of (Brassica oleracea L. var. gongylodes) and increases their contents in tubers. Plant Physiol. Biochem. 2020, 150, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.L.; Weng, H.X.; Qin, Y.C.; Yan, A.L.; Xie, L.L. Transfer of iodine from soil to vegetables by applying exogenous iodine. Agron. Sustain. Dev. 2008, 28, 575–583. [Google Scholar] [CrossRef]
- Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A.; Takagi, D.; Yoshida, H.; Yoshida, S.; Sekimoto, H.; Takahashi, M. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 2013, 4, 227. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef]
- Weng, H.X.; Hong, C.L.; Yan, A.L.; Pan, L.H.; Qin, Y.C.; Bao, L.T.; Xie, L.-L. Mechanism of iodine uptake by cabbage: Effects of iodine species and where it is stored. Biol. Trace Elem. Res. 2008, 125, 59–71. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Pierdomenico, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci. 2015, 6, 450. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Grzanka, M.; Halka, M.; Sady, W. The effect of salicylic acid on biofortification with iodine and selenium and the quality of potato cultivated in the NFT system. Sci. Hortic. 2018, 240, 530–543. [Google Scholar] [CrossRef]
- Smoleń, S.; Rożek, S.; Strzetelski, P.; Ledwożyw, I. Preliminary evaluation of the influence of soil fertilization and foliar nutrition with iodine on the effectiveness of iodine biofortification and mineral composition of carrot. J. Elem. 2011, 16, 103–114. [Google Scholar] [CrossRef]
- United State Department of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168482/nutrients (accessed on 15 February 2023).
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S. Evaluation of Selected Heavy Metals Contaminants in the Fruits and Leaves of Organic, Conventional and Wild Raspberry (Rubus idaeus L.). Appl. Sci. 2022, 12, 7610. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwozyw-Smoleń, I.; Rakoczy, R.; Liszka-Skoczylas, M.; Kopeć, A.; Piatkowska, E.; Biezanowska-Kopeć, R.; Koronowicz, A.; Kapusta-Duch, J.; et al. The quality of carrot (Daucus carota L.) cultivated in the field depending on iodine and selenium fertilization. Folia Hortic. 2016, 28, 151–164. [Google Scholar] [CrossRef]
- Hamouz, K.; Lachman, J.; Čepl, J.; Dvořák, P.; Pivec, V.; Prášilová, M. Site conditions and genotype influence polyphenol content in potatoes. Hortic. Sci. 2007, 34, 132–137. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of se-lected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Mahapatra, S.; Mohanta, Y.; Panda, S. Methods To Study Antioxidant Properties with Special Reference to Medicinal Plants. Int. J. Pharm. 2013, 3, 91–97. [Google Scholar]
- Haynes, K.G.; Clevidence, B.A.; Rao, D.; Vinyard, B.T. Inheritance of carotenoid content in tetraploid × diploid potatocrosses. J. Am. Soc. Hortic. Sci. 2011, 136, 265–272. [Google Scholar] [CrossRef]
- Tatarowska, B.; Milczarek, D.; Wszelaczyńska, E.; Pobereżny, J.; Keutgen, N.; Keutgen, A.J. Carotenoids Variability of Potato Tubers in Relation to Genotype, Growing Location and Year. Am. J. Potato Res. 2019, 96, 493–504. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 2, 694. [Google Scholar] [CrossRef]
- Central Statistical Office. Domestic Market Supply and Per Capita Consumption of Certain Consumer Goods in 2020. Available online: https://stat.gov.pl/download/gfx/portalinformacyjny/en/defaultaktualnosci/3285/9/11/1/domestic_deliveries_and_consumption_of_selected_consumer_goods_per_capita_in_2020.pdf (accessed on 14 January 2023).
- Iodine—Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222323/ (accessed on 15 February 2023).

| Physicochemical Soil Characteristic | Peat Substrate—Experiment 2 |
|---|---|
| pH(H2O) | 6.00 |
| EC (mS∙cm−1) | 580.5 |
| Eh(mV) | 260.6 |
| Macroelements: | |
| N-NH4 (mg∙dm−3) | 14,6 |
| N-NO3 (mg∙dm−3) | 10,7 |
| N-NH4+ N-NO3 (mg∙dm−3) | 25,3 |
| P (mg∙dm−3) | 10.9 |
| K (mg∙dm−3) | 43.2 |
| Mg (mg∙dm−3) | 158.5 |
| Ca (mg∙dm−3) | 4 363.5 |
| S (mg∙dm−3) | 73.9 |
| Na (mg∙dm−3) | 17,8 |
| Iodine (mg I∙kg−1) | 1.2 |
| Al-hydroxides (mg∙kg−1) | 1251.0 |
| Fe-hydroxides (mg∙kg−1) | 2345.6 |
| Mn-hydroxides (mg∙kg−1) | 235.4 |
| Soil organic matter (%) | 2.49 |
| Soil texture (according to the ISSS classification). | Loam soil 35 sand 28% silt 37% clay |
| Treatment | Tuber Yield/Plant (g) | Total Number of Tubers/Plant (Pcs.) |
|---|---|---|
| Control | 239.17 a ± 18.60 | 8.17 a ± 0.92 |
| KIO3 | 244.38 a ± 18.46 | 8.23 a ± 0.44 |
| 8-OH-7-I-5QSA | 250.63 a ± 32.30 | 8.50 a ± 0.57 |
| 5-Cl-7-I-8-Q | 243.54 a ± 13.17 | 8.60 a ± 1.07 |
| Type | Chemical Element | Treatment | |||
|---|---|---|---|---|---|
| Control | KIO3 | 8-OH-7-I-5QSA | 5-Cl-7-I-8-Q | ||
| Macrominerals (% d.w.) | Nitrogen (N) | 1.01 a ± 0.06 | 1.04 a ± 0.15 | 0.93 a ± 0.12 | 1.09 a ± 0.05 |
| Phosphorus (P) | 0.26 b ± 0.02 | 0.25 a,b ± 0.01 | 0.24 a ± 0.00 | 0.25 a,b ± 0.00 | |
| Potassium (K) | 2.12 a ± 0.08 | 2.09 a ± 0.08 | 2.03 a ± 0.02 | 2.07 a ± 0.01 | |
| Magnesium (Mg) | 0.13 b ± 0.01 | 0.12 a,b ± 0.01 | 0.12 a ± 0.00 | 0.12 a,b ± 0.00 | |
| Calcium (Ca) | 0.06 a ± 0.00 | 0.06 a ± 0.00 | 0.06 a ± 0.00 | 0.06 a ± 0.00 | |
| Sulphur (S) | 0.17 a ± 0.01 | 0.23 a ± 0.11 | 0.16 a ± 0.01 | 0.16 a ± 0.00 | |
| Sodium (Na) | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | |
| Trace elements (mg kg−1 d.w.) | Aluminium (Al) | 42.83 b ± 9.47 | 32.58 a ± 6.33 | 30.42 a ± 3.52 | 23.47 a ± 4.27 |
| Boron (B) | 2.72 a,b ± 0.05 | 2.80 a,b ± 0.53 | 2.42 a ± 0.33 | 3.35 b ± 0.88 | |
| Copper (Cu) | 8.00 a ± 0.61 | 9.38 b ± 0.59 | 8.61 a ± 0.31 | 8.55 a ± 0.42 | |
| Iron (Fe) | 62.35 b ± 22.95 | 49.25 a,b ± 11.30 | 41.27 a,b ± 1.88 | 32.81 a ± 10.53 | |
| Manganese (Mn) | 8.16 a ± 0.74 | 9.48 b ± 0.51 | 8.66 a ± 0.29 | 8.44 a ± 0.33 | |
| Zinc (Zn) | 19.88 a ± 1.08 | 25.01 b ± 1.76 | 21.92 a ± 1.98 | 21.70 a ± 1.72 | |
| Molybdenum (Mo) | 0.63 b ± 0.11 | 0.49 a ± 0.04 | 0.51 a ± 0.02 | 0.51 a ± 0.03 | |
| Trace elements (µg kg−1 d.w.) | Nickel (Ni) | 182.11 b ± 43.61 | 129.23 a,b ± 67.69 | 77.12 a ± 5.15 | 69.99 a ± 4.05 |
| Silver (Ag) | 5.61 b ± 1.54 | 4.21 a,b ± 1.43 | 3.75 a ± 0.68 | 2.85 a ± 0.41 | |
| Arsenic (As) | 31.56 a ± 2.67 | 29.60 a ± 1.47 | 26.79 a ± 3.48 | 22.39 a ± 10.72 | |
| Cadmium (Cd) | 433.20 a ± 16.39 | 426.44 a ± 34.73 | 402.21 a ± 35.08 | 411.03 a ± 27.80 | |
| Lead (Pb) | 217.02 c ± 19.12 | 144.25 b ± 9.21 | 132.11 a,b ± 14.11 | 113.18 a,b ± 22.38 | |
| Antimony (Sb) | 8.80 b ± 0.35 | 7.20 a,b ± 1.93 | 5.87 a ± 0.84 | 5.27 a ± 1.53 | |
| Thallium (Tl) | 3.99 b ± 0.26 | 1.96 a ± 0.20 | 1.93 a ± 0.36 | 1.76 a ± 0.41 | |
| Treatment | NH4+ (mg∙kg−1 f.w.) | Nitrate (V) NO3− (mg∙kg−1 f.w.) | Nitrate (III) NO2− (mg∙kg−1 f.w.) | Cl− (mg∙kg−1 f.w.) |
|---|---|---|---|---|
| Control | 19.16 a ± 1.41 | 4.77 a,b ± 1.42 | 0.30 a ± 0.19 | 423.59 b ± 76.48 |
| KIO3 | 20.04 a ± 2.03 | 3.81 b ± 0.96 | 0.32 a ± 0.14 | 333.12 a,b ± 86.80 |
| 8-OH-7-I-5QSA | 14.96 b ± 0.88 | 6.21 a ± 1.23 | 0.32 a ± 0.05 | 264.92 a ± 13.26 |
| 5-Cl-7-I-8-Q | 13.52 b ± 2.00 | 6.43 a ± 0.99 | 0.20 a ± 0.08 | 265.31 a ± 31.25 |
| Treatment | Total Phenolics (mg GAE∙100 g−1 f.w.) | ABTS (µmol Trolox∙g−1 f.w.) | Ascorbic acid (AA) (mg∙100 g−1 f.w.) | Dehydroascorbic acid (DHA) (mg∙100 g−1 f.w.) | Total Carotenoids (mg∙100 g−1 d.w.) |
|---|---|---|---|---|---|
| Control | 197.31 a ± 7.25 | 19.07 a ± 0.13 | 25.04 a ± 1.69 | 1.99 a ± 0.58 | 3.46 d ± 0.07 |
| KIO3 | 213.44 a,b ± 8.96 | 15.00 a ± 3.43 | 24.37 a ± 1.74 | 3.65 b,c ± 0.85 | 2.96 c ± 0.13 |
| 8-OH-7-I-5QSA | 233.33 b ± 9.97 | 15.87 a ± 2.67 | 24.76 a ± 1.47 | 4.81 c ± 1.55 | 2.45 b ± 0.02 |
| 5-Cl-7-I-8-Q | 197.31 a,b ± 8.77 | 14.10 a ± 2.52 | 23.98 a ± 1.98 | 3.00 b ± 0.52 | 1.47 a ± 0.06 |
| Treatment | % RDA I (in 100 g Portion of Potato) | % RDA I (in 260 g Portion of Potato) | Daily Intake of I with 100 g of Potato (mg I∙day−1) | Daily Intake of I with 260 g of Potato (mg I∙day−1) | HQ for 100 g Portion of Potato | HQ for 260 g Portion of Potato |
|---|---|---|---|---|---|---|
| Control | 0.31 b ± 0.05 | 0.82 b ± 0.12 | 0.001 b ± 0.00 | 0.001 b ± 0.00 | 0.001 b ± 0.00 | 0.003 b ± 0.00 |
| KIO3 | 17.70 c ± 0.67 | 46.02 c ± 1.75 | 0.027 c ± 0.00 | 0.069 c ± 0.00 | 0.065 c ± 0.00 | 0.170 c ± 0.01 |
| 8-OH-7-I-5QSA | 7.26 a ± 1.86 | 18.88 a ± 4.83 | 0.011 a ± 0.00 | 0.028 a ± 0.01 | 0.027 a ± 0.01 | 0.070 a ± 0.02 |
| 5-Cl-7-I-8-Q | 6.16 a ± 0.56 | 16.02 a ± 1.45 | 0.009 a ± 0.00 | 0.024 a ± 0.00 | 0.022 a ± 0.00 | 0.057 a ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzemińska, J.; Smoleń, S.; Kowalska, I.; Pitala, J.; Sularz, O.; Koronowicz, A. Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment. Appl. Sci. 2023, 13, 4659. https://doi.org/10.3390/app13084659
Krzemińska J, Smoleń S, Kowalska I, Pitala J, Sularz O, Koronowicz A. Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment. Applied Sciences. 2023; 13(8):4659. https://doi.org/10.3390/app13084659
Chicago/Turabian StyleKrzemińska, Joanna, Sylwester Smoleń, Iwona Kowalska, Joanna Pitala, Olga Sularz, and Aneta Koronowicz. 2023. "Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment" Applied Sciences 13, no. 8: 4659. https://doi.org/10.3390/app13084659
APA StyleKrzemińska, J., Smoleń, S., Kowalska, I., Pitala, J., Sularz, O., & Koronowicz, A. (2023). Effect of Biofortification with Iodine by 8-Hydroxy-7-iodo-5-quinolinesulfonic Acid and 5-Chloro-7-iodo-8-quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment. Applied Sciences, 13(8), 4659. https://doi.org/10.3390/app13084659








