Abstract
Scope: Aqueous suspensions of spelt and wheat starch were irradiated with ultraviolet radiation for 5–50 h. The changes in the molecular structure of the starch induced by UV radiation were related to the photodegradation of starch chains and the subsequent recombination of the resulting polysaccharide radicals during prolonged irradiation. Methods and Results: Aqueous suspensions of spelt and wheat starch were irradiated with ultraviolet radiation for 5–50 h. The HPSEC-MALLS-RI method was used to measure the weight-average molecular weights of the starch polysaccharide chains and the distribution of the amylopectin side chains. FTIR spectroscopy of the modified samples and a study of the starch surface morphology were conducted using a scanning electron microscope. However, an increase in both the rate constant of the first stage of hydrolysis (k1) and its final level were demonstrated. The changes in the molecular structure of the starch induced by UV radiation were related to the photodegradation of starch chains and the subsequent recombination of the resulting polysaccharide radicals during prolonged irradiation. Conclusions: Changes in starch granules could influence some of their functional properties. Rearrangement in the polysaccharide chains could obtain novel starches with defined properties.
1. Introduction
Starches and their products often have to be modified to improve their essential performance characteristics for many industries. Currently, enzymatic and chemical methods are primarily used to obtain starch degradation products on an industrial scale. To date, the physical modification of starch has taken place on a much smaller scale. Physical methods are environmentally friendly, lead to the creation of unique products, and do not generate unwanted by-products. One method of physically modifying starch is exposure to ultraviolet (UV) light. UV-illumination may be recognized as a green and accessible method for the modification of starch-based films [1].
Exposure to UV radiation leads to oxidative depolymerization, especially to the cleavage of glycosidic bonds [2,3]. An absorption of UV-C leads to, in particular, the destruction of amylopectin while the amylose chains are attacked by UV-B radiation [4]. Depending on the wavelength and intensity of the radiation, the products may be dextrins, [5,6] mono- and oligosaccharides [7], or even carbon dioxide and water. If this process takes place in an oxygen atmosphere, it may result in the oxidation of the resulting products [8]. In 1981, authors of [9] suggested that irradiating starch with ultraviolet radiation led to photo-oxidation, which is associated with C2–C3 bond cleavage in glucopyranose. This creates dialdehyde starch, followed by formaldehyde, formic acid, and carbon dioxide [10], while the cross-linking observed during UV irradiation is due to the formation of highly reactive peroxides on the acetal carbon of the D-glucose unit.
It is worth knowing that ultraviolet radiation leads to the degradation of D-glucose units in water solutions. Currently, the literature proposes a mechanism by which the ultraviolet radiation energy is absorbed by the D-glucose unit (acetal carbon atom chromophore) and consequently leads to a series of photoreactions [9,10]. The presence of oxygen improves the degradation efficiency of the polysaccharide chains, possibly due to the formation of peroxides at the C-1 carbon. In [3,11], the research demonstrated that ozone and reactive oxygen species (ROS) such as radicals HO. and HO2 may be formed under the presence of water, oxygen, and UV light.
The influence of UV on the molecular structure of the starch polysaccharide chains is less known and described. However, the works [12,13] concerning the structural transformations of maize and cassava starch bear mentioning. Under aerobic conditions, UV illumination leads to structural changes induced via photodegradation reactions. Prolonged irradiation may provide crosslinking reactions [12,14].
Since the molecular structure of the polysaccharide chains of individual starches is different, the influence of UV radiation on the rearrangement of these polysaccharide chains can be varied. Therefore, the aim of this study was to determine the effect of ultraviolet light as an energy source on the molecular structure of spelt and wheat starches and on their selected functional parameters (XRD pattern, maximum absorption wavelength, and susceptibility to enzymatic hydrolysis).
2. Experimental Section
2.1. Materials
Wheat and spelt starches were isolated from common wheat flour (PZZ in Krakow, Poland) and from spelt flour (Biopont, Budapest, Hungary, ECCO) using the laboratory method developed by [15]. The weight-average molecular mass (Mw) of spelt and wheat starches were determined in our previous work [16] and indicated values of 8.2 × 106 and 23 × 106 g mol−1, respectively. The following enzymes were used in the work: porcine pancreatic α-amylase (EC.3.2.1.1; Merck, Darmstadt, Germany), isoamylase from Pseudomonas amyloderamosa (Sigma Aldrich, Poznan, Poland) and barley β-amylase (Sigma Aldrich, Poznan, Poland). Other reagents used in the experiments were analytical grade and purchased from Avantor Performance Materials Poland SA (Gliwice, Poland).
2.2. Irradiation of Starches with Ultraviolet (UV) Radiation
Aqueous starch suspensions (30% by weight) were irradiated from a distance of 30 cm using an L8 burner lamp (type L8759, 17 A, 220 V, 50 Hz) continuously emitting UV radiation with a wavelength greater than 250 nm. The suspensions were irradiated in aerobic conditions for 5, 15, 25, and 50 h at room temperature (the temperature was monitored with a thermometer). No change in the temperature of the suspensions was observed during the irradiation. After the irradiation was completed, the starches were filtered off and dried at 50 °C for 24 h. Control samples were kept in the same conditions but without access to light (non-irradiated starches).
2.3. Molecular Weight Distribution
The weight-average molecular weights (Mw) and radii of gyration (Rg) of the polysaccharides were measured by high-performance size exclusion chromatography (HPSEC) with a refractive index detector (RI) (L-7490, Merck, Darmstadt, Germany) and a multiangle laser light scattering (MALLS) (Dawn-DSP-F, Wyatt Technology, Santa Barbara, CA, USA) detector. The measurement conditions (e.g., the flow rate of the solvent and sample injection volume) with HPSEC-MALLS-RI apparatus were similar to those in our previous publications [16,17].
For the calculations of Mw and Rg, it was assumed that dn/dc (refractive index change depending on the concentration change) = 0.146 [12]. The data obtained from the RI and MALLS detectors were used to calculate the weight-average molecular weight (Mw) and radii of gyration (Rg) using the Astra 4.73.04 program (Wyatt Technology, Santa Barbara CA, USA). A Berry plot with a third-order polynomial fit was used to calculate the Mw and Rg values [18].
The solutions to be measured were obtained by adding the appropriate starch (10 mg) to a 10 mL volumetric flask containing water (1 mL). The contents were gently mixed with a magnetic stirrer (MR Hei-Tec, Heidolph, Schwabach, Germany) (30 rpm/min) to moisten and disperse the starch granules. With continued stirring, DMSO (99.9% HPLC grade, Aldrich (Steinheim, Germany) (6 mL) was added and the temperature was gradually increased to 80 °C while continually stirring until clear solutions were obtained. After the solution had cooled to 25 °C, the flask was filled with DMSO to a final volume of 10 mL. The solutions thus obtained were filtered through 0.8 µm membrane filters (PTFE, Whatman, Maidstone, UK).
2.4. Determining the Size of the Structural Units of Amylopectin
Enzymatic hydrolysis with isoamylase was performed to determine the size of the A and B amylopectin chains for the native and illuminated starch samples. The sample preparation procedure was similar to that presented in our earlier publication [17] with slight modifications. Incubation was completed after 24 h by bringing the samples to the boil. A 100–500 µL sample was analyzed with the SEC system. The samples were filtered through cellulose acetate filters (PTFE, 0.8 µm, Whatman) before injection to the HPSEC system.
2.5. ATR-FTIR Spectroscopy
The spectral measurements were made with an ATR-FTIR spectrophotometer MATTSON 3000 FTIR (Madison, WI, USA). This spectrophotometer was equipped with a 30SPEC 30 Degree Reflectance adapter fitted with the MIRacle ATR accessory from PIKE Technologies Inc., Madison, WI, USA. The FTIR spectra were recorded in the range of 4000–700 cm−1 at a resolution of 4 cm−1. All spectra were performed at room temperature (23 ± 0.5 °C).
2.6. Morphology
Scanning electron microscopy (SEM-JEOL 7550, JEOL Ltd., Akishima, Tokyo) was used to analyze the structure, shape, and size of the starch grains. The starch samples were coated with a gold layer. The starch samples were coated with gold layer before the analysis. The samples of native wheat and spelt starches irradiated for 50 h were tested at 500× and 1000× magnifications.
2.7. X-ray Diffraction (XRD)
The crystal structure of the native and modified starch samples was determined by measuring the diffraction spectra. X-ray powder diffraction was performed according to the method described in the literature [19]. Diffraction spectra were recorded using a Philips X’pert apparatus (Eindhoven, The Netherlands) equipped with a cobalt lamp emitting radiation with a wavelength of λ = 1.78896° A, using a current of 30 mA, and a voltage of 40 kV. The measurement range covered the region of 2θ reflex angles from 5° to 60° in 0.02° intervals.
2.8. Susceptibility to α-Amylolysis
The sample preparation procedure was previously described in our earlier article [16,17]. The samples were incubated for 360 min in a water bath at 37 °C with mechanical agitation. At the designated time intervals, 2 mL of the solution was collected, and the amount of solubilized starch content was determined using the method with 3,5-dinitrosalicylic acid.
The degree of starch amylolysis was calculated as the ratio of the amount of hydrolyzed starch to the total amount of substrate. The linear relationship (1/c) as a function of time for the first stage of the enzymatic hydrolysis was defined by linear regression (R = 0.97–0.99). The slope of the lines are provided to calculate the rate constants for the first step of the reactions.
2.9. Wavelength of Maximum Absorption
The blue value and λmax of the irradiated samples were investigated according to the method described in the literature [20]. Spectra in the range of 500–800 nm were measured for all samples using a Shimadzu 2101 PC UV–VIS spectrophotometer (Shimadzu, Japan). The iodine value was defined as the absorbance at 640 nm. λmax was the maximum absorbance value for the studied wavelengths. All measurements were performed in three repetitions.
2.10. Statistical Analysis
Statistical analysis was performed using the STATISTICA 13.3 software (StatSoft, Inc., Tulsa, OK, USA). In order to determine the effect of the irradiation, analysis of variance (ANOVA) and the Tukey test were used at the p < 0.05 level.
3. Results and Discussion
3.1. Molecular Weight Distribution
The HPSEC-MALLS-RI method allowed us to determine the molecular weights of the polysaccharides included in the amylopectin fraction. Based on the shape of the chromatograms, they were divided into two regions corresponding to the amylopectin and amylose eluted fractions. Values of Mw and Rg taken from the analyzed chromatograms for cereal starches irradiated with UV radiation are presented in Table 1.

Table 1.
The Mw (g/mol) and Rg values of UV irradiated native spelt and wheat starches *.
Native spelt and wheat starches contain the polysaccharide chains with Mw of the order of 8.2 × 106 g/mol and 23 × 106 g/mol, respectively, as presented in our earlier publication [16]. For spelt starch, five hours of exposure to UV radiation caused a decrease in the molecular weight values, which indicates the degradation of the polysaccharide chains in the granule. On the other hand, prolonged irradiation leads to an increase in the molecular weight of the polysaccharide chains. The observed increase indicates the possibility of interactions between macroradicals and radicals from the polysaccharide chains formed during the breakdown of glycosidic bonds between glucose molecules. For the polysaccharide chains, longer UV exposure leads to further transformations that significantly reduce the weight-average molecular weight of the polysaccharides. Irradiation for 50 h resulted in the chains with the highest molecular weights. This may result from the recombination of the intermediates produced or the oxidation of the resulting products. When performed as such, the irradiation resulted in a twofold increase in the weight-average molecular weights of the starch polysaccharides compared to the non-irradiated starch.
The obtained results, reflecting the effects of 5 h of UV radiation exposure on wheat starch, suggest that this radiation has a significant effect on the starch granule. Taking into account the rapid decrease in Mw of the polysaccharide chains, it suggests that wheat starch grains are more sensitive to this radiation. This fact may be due to the lower lipid content of the native wheat starch compared to spelt starch. According to the authors, lipids have a protective effect on the starch granule and are the first to absorb UV radiation [21]. Similar results were obtained by the researchers who irradiated oat and barley starches with UV light for 5 or 30 min [22]. Greater degradation of the starch polysaccharide chains occurred for barley, which has a lower lipid content. Prolonged exposure caused a large increase in the mass of the chains, with a simultaneous increase in their radii of gyration. Longer irradiation led to a twofold reduction in the molecular weights of the polysaccharides while further irradiation caused a slow decrease in Mw to an average weight of about 7 × 106 g/mol.
Research conducted by [13] shows that the molecular weight values of cassava amylopectin decreased with increasing exposure time (4–16 h). Studies by the authors [13] on the effect of UV radiation on corn and cassava starch showed that the exposure time had a significant effect on the properties of the starch polysaccharides. With an increase in irradiation time, parameters such as the molecular weight of the starch polysaccharides and the viscosity of the starch pastes decreased. The mechanism proposed by the authors assumes that UV radiation disrupts the C2–C3 bond of glucopyranose units and, consequently, causes the gradual degradation of polysaccharide chains. The authors in [13] also reported that the botanical origin of starch influences its degree of UV depolymerization. Additionally, they indicate that cassava starch is more susceptible to depolymerization than corn starch. Other researchers [21] suggest that oxidative depolymerization occurs to a greater extent for cassava than for other grain starches, which may be due to the lack of lipids in cassava starch. As previously mentioned, lipids are more susceptible to oxidation and are the first to oxidize, which may provide protection for starch in the UV depolymerization process. It is noteworthy that some of the authors [4] conducted research on the influence of UV radiation on the properties of cassava starch products previously treated with lactic acid. Using HPSEC chromatography, they determined that UV-B radiation only degraded amylose chains, and only the application of higher energy radiation modified amylose and amylopectin. Prolonged exposure (15 h) to UV-C radiation reduced the susceptibility for baked good growth. According to the literature [23], the researchers illuminated four types of starch with UV light for 8 h. The data suggest a significant drop in the molecular weights of amylose and amylopectin in wheat starch, corn starch, and waxy corn starch. The authors concluded that this was evidence of main chain scission. A different type of behavior after UV-irradiation was observed with potato starch. An increase in in Mw values was assigned to photoaggregation or photocrosslinking reactions.
3.2. Determining the Size of the Structural Units of Amylopectin
Samples of native and UV-irradiated starches were subjected to isoamylolysis. The enzyme isoamylase hydrolyzes α-1,6-glycosidic bonds, breaking down the amylopectin macromolecule into straight chains.
The molar mass (Mw) distribution of the amylopectin polysaccharide chains in the debranched starches was determined using the HPSEC-MALLS-RI method. The obtained chromatograms were divided into fractions depending on the molecular weights. According to [24], polysaccharide chains with molecular weight values of Mw < 2000 are classified as A-type amylopectin side chains, while polysaccharide chains with higher molecular weights are classified as B1, B2, and B≥3. The chromatograms were divided arbitrarily into fractions belonging to the type of A-chains, B-chains as well as amylose-type chains.
For the polysaccharide chains of the individual fractions, the weight-average molecular weight and the percentage mass of a given fraction in the total weight of the sample were determined. The Mw numbers of the amylopectin structural units of the spelt and wheat starches are summarized in Table 2 and Table 3, respectively. Figure A1 and Figure A2 also represent the percentage of each amylopectin fraction (m%) in the total sample weight.

Table 2.
Weight-average molecular weight (Mw) (g/mol) of the structural units of the amylopectin of UV irradiated spelt starch *.

Table 3.
Weight-average molecular weight (Mw) (g/mol) of amylopectin structural units of UV-irradiated wheat starch *.
Amylose chains were assigned in accordance with the obtained chromatograms for the separated fraction (III) of spelt starch. The B2 and B≥3 chains were included in fraction (II). However, short type A and B1 chains were assigned to fraction (I). The percentage of each type of chain in the total amylopectin weight is shown in Figure A1.
The spelt sample starch contained a high proportion of type A chains (88%) [16]. As the exposure time increased (5–50 h), the percentage of this type of chain decreased. The lowest short chain content was observed in spelt starch irradiated for 50 h. At the same time, this exposure resulted in the highest number of longer chains, both type B and amylose chains.
The conducted experiments for wheat starches allowed for the isolation of a greater number of fractions containing chains characteristic of the amylopectin molecule. Fraction (V) was formed by long polysaccharide chains close to amylose units. Long type B≥3 chains were distinguished in fraction (IV), while type B1 and B2 chains were assigned to fractions (III) and (II). Type A chains constituted Fraction (I) (Table 3).
The percentage share of each fraction in the total mass of the native and UV irradiated wheat starch sample is summarized in Figure A2.
The fraction of amylose chains accounted for a small percentage of all chains; however, the largest number of such chains was observed in starch modified for 5 h. Meanwhile, for the same modified starch sample (wheat-5), the highest percentage of type A chains (Fraction I) was identified. Exposing wheat starch for 5 h resulted in polysaccharide chains with the lowest weight-average molecular weight (2.9 × 106 g/mol), hence the high percentage of the shortest chains. It is worth mentioning that the proportion of B≥3 chains was the highest for natural starch and for starch irradiated for 15 h, for which the Mw of polysaccharides exhibited the highest values. The fact that the share of A-type chains for amylopectin fraction (native spelt) was two times higher than that of wheat starch amylopectin may affect the distribution of amylopectin side chains of the irradiated starch.
3.3. ATR-FTIR Spectroscopy
The FTIR spectra for natural and modified UV tasted starches are shown in Figure A3 and Figure A4 and are typical for polysaccharides. The characteristic bands have been described in our previous work [17]. In the FTIR spectra of the UV-irradiated samples, there was a slight change compared to the spectrum of natural, examined starches, where mainly changes in the absorption intensity were recorded. The conducted research suggests that three wavenumbers (995 cm−1, 1022 cm−1, 1045 cm−1) are susceptible to changes in the crystalline structure of starch (crystalline and amorphous regions) [25].
Moreover, it has been proven that the presence of moisture influences the above wavenumbers in various ways (mainly the bands at wavenumber 995 cm−1 are sensitive) [23,26]. According to their reports, the band intensities at 1045 cm−1 and/or 995 cm−1 are related to the starch crystalline region, while intensities around 1022 cm−1 are associated with the amorphous region. The changes in water content are also a factor affecting these phases. As the 1100–900 cm−1 region is considered to be the most sensitive to changes in the starch structure [26], FTIR spectra of the natural and modified starches—in the sensitive range 1450–800 cm−1—are also presented in Figure 1.
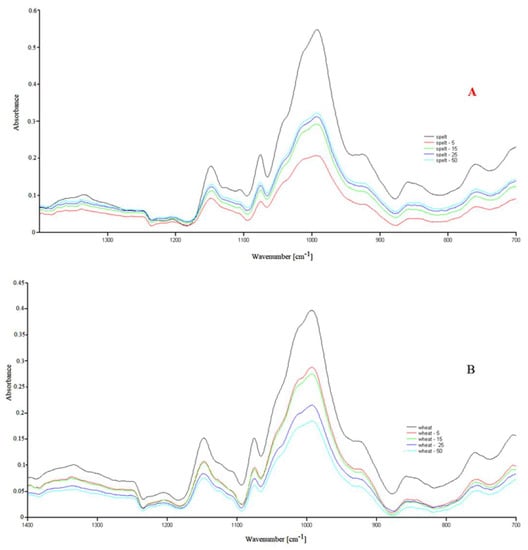
Figure 1.
FTIR spectra in the range of 1450–700 cm−1 for the native spelt (A) and wheat (B) and UV irradiated starches.
The differences in absorption intensity were the results of slight changes in the bound water content in the tested samples. The literature data showed shifts in some of the bands in the FTIR spectra of the UV-irradiated samples [1]. The authors suggest that radiation for up to 12 h could create new links between the starch chains. After illumination using UV-A and UV-C radiations for >12 h, the formed cross-links were destroyed. They concluded that the UV modification results strongly depend on the type of the biopolymers, the intensity of the light, the processing methods, and the random nature of photochemical reactions.
Different results were obtained during the modification of sago starch under UV radiation. The researchers observed no changes in the FTIR spectra compared to native starch. The authors concluded that the granular structure of the starch basically remained intact after UV illumination [27].
3.4. Morphology
SEM pictures were taken for the native starches and for starches irradiated for 50 h, which are provided in Figure 2.
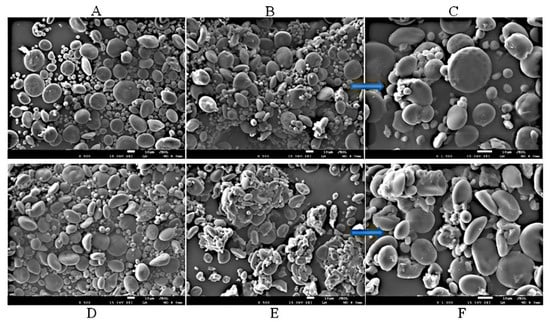
Figure 2.
SEM images of the native spelt (A) and wheat (D) starches and of the spelt and wheat starches irradiated for 50 h (B,E, respectively) taken at ×500 magnification and at ×1000 (C,F, respectively).
The wheat starch consisted of small and large granules. The shape of these granules were different, and the large ones resembled a flattened spheroid, while the small granules possessed a spherical shape. As for the spelt starch, intermediate granules were additionally present, which is in line with the results presented by other authors [28]. The surface of the investigated grains was smooth, though some grooves and channels could be registered. The occurrence of pores and surface channels also varied for different types of granules and depends on the dimension of the grains [29]. Modification with UV radiation for 50 h (Figure 2B,C) did not significantly change the surface structure of the spelt starch grains. Nevertheless, a higher dose of energy caused small granules to aggregate into larger particles.
Observations from the analysis of the wheat starch images (Figure 2E,F) indicated partial degradation of the starch shell, particularly the larger granules, and that smaller granules stuck to the larger ones. The amylose level was higher in larger wheat starch granules, while smaller granules exhibited a higher lipid content, [28] which may result in the greater absorption of UV radiation by larger granules and thus their faster degradation. These observations may indicate the possibility of enzyme interference during the enzymatic hydrolysis process and a significant rising up in the hydrolysis rate for wheat starch irradiated for 50 h. Other research data showed that illumination with UV light led to insignificant changes in the morphology of the examined starch granules [22].
3.5. X-ray Diffraction (XRD)
X-ray scattering on starch granules provides information about the nature of the regularly repeating molecular structures in the starch granule.
The XRD spectra for native starches were presented and described in our previous research [16]. Spelt and wheat starches presented type A, which is characteristic of cereal starches. Native starches showed a strong intensity for the bands recorded at the 2θ reflection angles of 15° and 23° and the weakly resolved doublet at 17–18°. At the same time, the presence of a type V crystal structure was observed, reflecting the presence of amylose–lipid inclusion complexes at a 2θ reflection angle of 20°. The diffractograms for the irradiated spelt and wheat starches are shown in Figure A5 and Figure A6, respectively.
In the diffraction patterns for spelt and wheat starch irradiated with UV, no significant changes were noticed in the crystal structure of the starch granules, regardless of the exposure time. These data were in line with those reported in other studies [23]. The influence of UV radiation for starch with type A crystal structures have less effect on crystal structure reorganization than on starches with types B or C. Slight changes in the XRD patterns of natural and irradiated starch samples were observed as partially diffused peaks at 2θ = 17°, 18°. According to the literature, this small variation arises from the different arrangement of macromolecules and water content in the samples [23]. A study carried out by [13] showed no changes in the spectra compared to non-irradiated starch. The same effect was observed with acidified cassava starch subjected to UV irradiation. The authors suggest that the UV-induced photodegradation of starch is limited to the amorphous regions of the starch granule.
3.6. Susceptibility to α-Amylolysis
The size, shape, and surface porosity of the starch grains are of importance in the enzymatic hydrolysis process. Some of the authors [30] proposed that the pores may be a site of initial attack by amylases and affect the pattern of attack. The main products of the enzymatic hydrolysis of starch using α-amylase are maltose, maltotriose, and maltotetraose [31]. The dependence of the resulting concentration on the hydrolysis time of spelt and wheat starch is illustrated in Figure A7 and Figure A8, respectively.
Regardless of the irradiation time, the α-amylolysis for all starches (spelt and wheat) was carried out in two stages and the observations were similar to the values reported by others [31]. The rate of stages were different and the first (up to 40 min)—described with rate constant k1—was fast. The second step was considerably slower. Based on Figure A7 and Figure A8, the initial rate constants of the hydrolysis reaction (k1) were determined for the native and modified starches, and these values are summarized in Table 4.

Table 4.
Rate constants (k1) of the hydrolysis of the natural and irradiated cereal starches *.
Comparing the rate constants of the first stage of enzymatic hydrolysis and the final result, we may conclude that wheat starch is more responsive to the α-amylase attack. In [31,32], the authors confirmed that starch containing a high percentage of short chains together with a major branch density was less susceptible to amylases. The lower susceptibility of spelt to amylolysis was influenced by the high proportion of short chains in spelt amylopectin. Furthermore, spelt starch is built with higher number of small grains, resulting in a rich content of lipids. Lipids, however, make it difficult for the enzyme to expand inside the granule.
The UV irradiation of cereal starches (wheat and spelt) significantly increases the first step of enzymatic reaction. This fact is confirmed by the increase in the k1 values with respect to the unmodified starches. Irradiation of wheat starch granules induces a gradual growth in the k1 reaction rate along with the increase in the UV illumination time. A significant rise up susceptibility to the action of the enzyme was recorded for a sample of wheat starch exposed to UV for 50 h. This may result from the partial degradation of the starch shell during prolonged irradiation and thus the facilitation of the expansion of the enzyme inside the starch granule. Meanwhile, for spelt starch irradiated for 50 h, a reduction in the rate of enzymatic reaction was noted in relation to the samples irradiated for 25 h. The spelt starch irradiated for 50 h was characterized by the highest values of Mw polysaccharide chains, which may affect the enzymatic hydrolysis levels. Additionally, the research [31] on the hydrolysis of starch amylopectins indicates that the average chain length distribution affects the enzymatic hydrolysis rate of amylopectin after the first stage. The susceptibility to α-amylolysis is insignificant at the initial stage for different average chain length amylopectins.
3.7. Wavelength of Maximum Absorption
Visible radiation, being absorbed in the range of 525–640 nm by starch–iodine complexes, is related to the degree to which the starch chains form branches. The λmax value for starch complexes with KI5 depends on the length of the glucan chains forming the helices, which are part of the inclusion complexes with the I5− ion.
The capacity for iodine binding through the starch is determined by the E640/E525 ratio. This relationship expresses the correlation between the amylose chains (without side branches) and amylopectin chains (with short branches). The ratio of amylose/amylopectin chains in the molecule significantly affects the functional properties of the starch. An E640/E525 value greater than 1.5 indicates the occurrence of amylose-type chains [33]. The indices of the iodine number (BV), the maximum absorption for the iodine complexes (λmax) as well as the E640/E525 ratio obtained for the tested samples are presented in Table A1.
These subtle differences in the values of starch–iodine complexes resulted from the changes in the structure of the starch granules. The results thus obtained do not support an unequivocal statement that irradiation with UV light causes the differences in the arrangement of the polysaccharide chains.
4. Concluding Remarks
Illumination with UV light of the examined cereal starches caused structural changes in the starch polysaccharide chains and changes in the distribution of amylopectin side chains. In the first step, these transformations led to UV-induced photodegradation. Structural changes in the starch granule did not affect the starch crystal structure and had a significant effect on the increase in the rate of enzymatic hydrolysis, which is widely used in the food industry.
The functional properties of starch depend on the Mw of amylose and amylopectin chains as well as on their molecular structure and the starch organization. Highly branched glucans may have great applications in the beverage industry for their solubility in water. Technological processes where starch is enzymatically hydrolyzed for conversion to glucose, and finally to bioethanol by fermentation are used in food technology at a large scale. The molecular changes in the starch may be responsible for the starches’ baking expansion ability. The amylopectin with longer chain length distribution exhibited an amylose-like behavior in relation to the formation of thermoreversible starch gels with a high thermal stability. This starch gels with properties similar to gelatin, and have been applied in food technology. The presence of a high degree of short type chains in amylopectin favor the interactions and rearrangement of the chains, which enables the molding of starch films with a compact, opaque, and strong structure.
The radiation modification of spelt starch significantly widens its range of applications. Its demonstrably different properties compared to wheat starch are leading to its innovative use not only in the food industry, where it is mainly used due to its status as a health food.
Author Contributions
Conceptualization, E.N. and A.W.-Ś.; Methodology, E.N. and A.W.-Ś.; Investigation, E.N. and A.K.; Writing—original draft preparation, E.N.; Writing—review and editing, T.L.; Visualization, A.W.-Ś.; Supervision, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions (e.g., privacy or ethical). The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
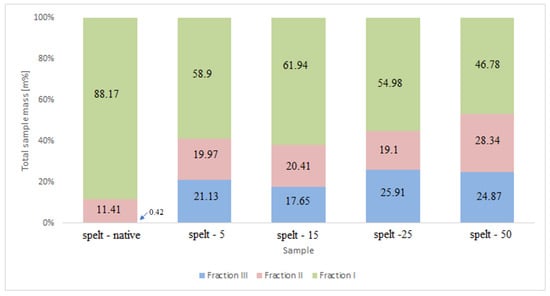
Figure A1.
Percentage share of each fraction in the total mass of the native spelt and UV irradiated spelt starch samples.
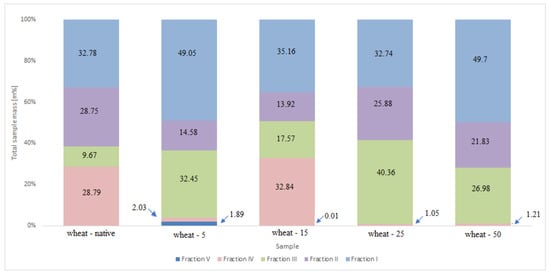
Figure A2.
Percentage share of each fraction in the total mass of the native wheat and UV irradiated wheat starch samples.
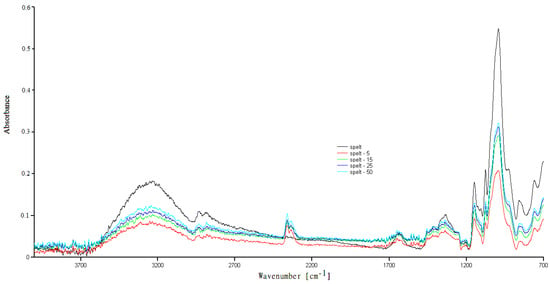
Figure A3.
FTIR spectra of the native spelt starch and UV irradiated spelt starch.
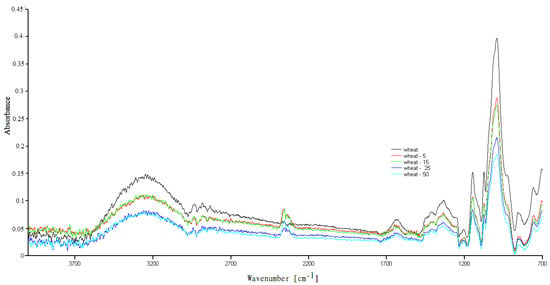
Figure A4.
FTIR spectra of the native wheat starch and UV irradiated wheat starch.
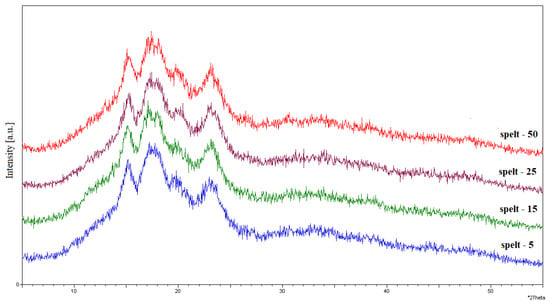
Figure A5.
Diffractograms of the spelt starch exposed to UV radiation for 5, 15, 25, and 50 h.
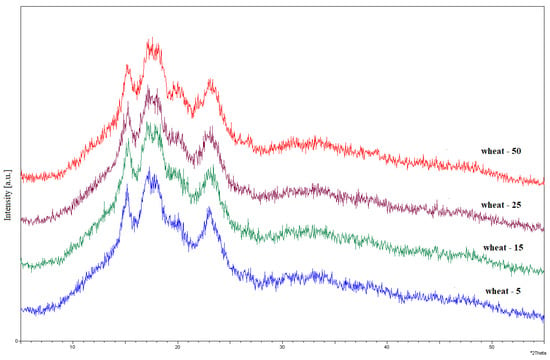
Figure A6.
Diffractograms of wheat starch exposed to UV radiation for 5, 15, 25, and 50 h.

Figure A7.
Hydrolysis of native spelt starch and UV irradiated spelt starch using α-amylase.
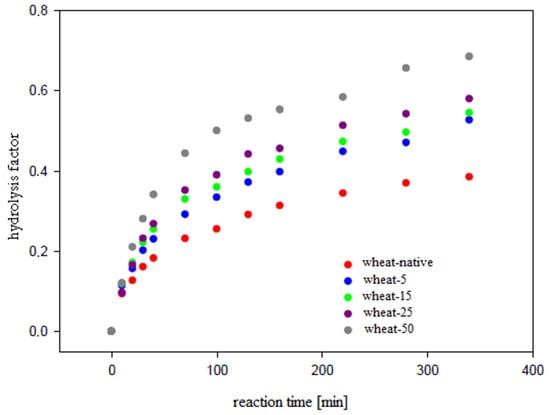
Figure A8.
Hydrolysis of native wheat starch and UV irradiated wheat starch using α-amylase.

Table A1.
Characteristics of the starch–iodine complexes for spelt and wheat starch exposed to UV radiation *.
Table A1.
Characteristics of the starch–iodine complexes for spelt and wheat starch exposed to UV radiation *.
| Starch ** | λmax [nm] | BV | E640/E525 |
|---|---|---|---|
| Spelt | 604.0 ± 0.5 | 0.363 ± 0.004 | 1.40 |
| Spelt-5 | 604.0 ± 0.5 | 0.396 ± 0.005 | 1.49 |
| Spelt-15 | 604.0 ± 0.4 | 0.379 ± 0.005 | 1.36 |
| Spelt-25 | 604.0 ± 0.5 | 0.381 ± 0.010 | 1.37 |
| Spelt-50 | 603.0 ± 0.6 | 0.396 ± 0.005 | 1.43 |
| Wheat | 605.0 ± 0.5 | 0.348 ± 0.005 | 1.47 |
| Wheat-5 | 604.0 ± 0.5 | 0.327 ± 0.004 | 1.43 |
| Wheat-15 | 604.0 ± 0.6 | 0.369 ± 0.005 | 1.46 |
| Wheat-25 | 606.0 ± 0.5 | 0.371 ± 0.006 | 1.45 |
| Wheat-50 | 606.0 ± 0.5 | 0.363 ± 0.005 | 1.45 |
* Mean of three independent experiments, ** Values indicate the exposure time [h].
References
- Shahabi-Ghahfarrokhi, I.; Goudarzi, V.; Babaei-Ghazvini, A. Production of starch based biopolymer by green photochemical reaction at different UV region as a food packaging material: Physicochemical characterization. Int. J. Biol. Macromol. 2019, 122, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, A.C.; Mestres, C.; Colonna, P.; Raffi, J. Free radical formation in UV- and gamma-irradiated cassava starch. Carbohydr. Polym. 2001, 44, 269–271. [Google Scholar] [CrossRef]
- Kurdziel, M.; Łabanowska, M.; Pietrzyk, S.; Pająk, P.; Krolikowska, K.; Szwengiel, A. The effect of UV-B irradiation on structural and functional properties of corn and potato starches and their components. Carbohydr. Polym. 2022, 289, 119439. [Google Scholar] [CrossRef]
- Vatanasuchart, N.; Naivikul, O.; Charoenrein, S.; Sriroth, K. Molecular properties of cassava starch modified with different UV irradiations to enhance baking expansion. Carbohydr. Polym. 2005, 61, 80–87. [Google Scholar] [CrossRef]
- Takahashi, T.; Kihara, Y. Effects of far-ultraviolet radiation on potato starch. Nippon Nogei Kagaku Kaishi. 1965, 34, 88. [Google Scholar] [CrossRef]
- Gholap, A.V.; Marondeze, L.H.; Tomasik, P. Dextrinization of starch with nitrogen laser. Starch/Stärke 1993, 45, 430–432. [Google Scholar] [CrossRef]
- Azuma, J.; Hosobushi, T.; Katada, T. A method and apparatus for decomposition of polysaccharides by carbon dioxide laser beam. Jpn. Kokai Tokkyo Koho 1990, 224, 836. [Google Scholar]
- Zucca, C. Mucinolytic action of ultraviolet rays on some polysaccharides. 1st Botan. Univ. Lab. Crittoge. Pavia Atti. 1953, 10, 85. [Google Scholar]
- Merlin, A.; Fouassier, J.P. Etude de radic aux libres formés par irradiation ultaviolette de l’amidon: Application aux reactions de photodegradation et de photogreffage. Macromol. Chem. Macromol. Symp. 1981, 182, 3053–3068. [Google Scholar] [CrossRef]
- Phillips, G.O.; Rickards, T. Photodegradation of carbohydrates. Part IV. Direct photolysis of D-glucose in aqueous solution. J. Chem. Soc. B Phys. Org. 1969, 4, 445–461. [Google Scholar] [CrossRef]
- Kurdziel, M.; Filek, M.; Łabanowska, M. The impact of short-term UV irradiation on grains of sensitive and tolerant cereal genotypes studied by EPR. J. Sci. Food Agric. 2018, 98, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, M.; Tomasik, P.; You, S.; Lim, S.T. Molecular Distribution and Pasting Properties of UV-Irradiated Corn Starches. Starch/Stärke 1999, 51, 126–131. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Mestres, C.; Raffi, J.; Buleon, A.; Lerner, D.; Colonna, P. Photodegradation of cassava and corn starches. J. Agric. Food Chem. 2001, 49, 675–682. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Impact of radiation processing on starch. Compr. Rev. Food Sci. Food Saf. 2009, 8, 45–48. [Google Scholar] [CrossRef]
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewählte Methoden der Stärkechemie. Leipz. VEB Fachb. 1968, 254. [Google Scholar] [CrossRef]
- Nowak, E.; Krzeminska-Fiedorowicz, L.; Khachatryan, G.; Fiedorowicz, M. Comparison of molecular structure and selected physicochemical properties of spelt wheat and common wheat starches. J. Food Nutr. Res. 2014, 53, 31–38. [Google Scholar]
- Nowak, E.; Khachatryan, G.; Wisła-Świder, A. Structural changes of different starches illuminated with linearly polarised visible light. Food Chem. 2021, 344, 128693. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Paredes-Lopez, O.; Roger, P.; Colonna, P. Molecular characterization of some amylopectins. Cereal Chem. 1996, 73, 12–17. [Google Scholar]
- Gerard, C.; Colonna, P.; Buleon, A.; Planchot, V. Amylolysis of maize mutant starches. J. Sci. Food Agric. 2001, 81, 1281–1287. [Google Scholar] [CrossRef]
- Morrison, W.R.; Lainglet, B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Sriburi, P.; Hill, S.E.; Barclay, F. Depolymerisation of cassava starch. Carbohydr. Polym. 1999, 38, 2111–2118. [Google Scholar] [CrossRef]
- Kurdziel, M.; Łabanowska, M.; Pietrzyk, S.; Sobolewska-Zielińska, J.; Michalec, M. Changes in the physicochemical properties of barley and oat starches upon the use of environmentally friendly oxidation methods. Carbohydr. Polym. 2019, 210, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Bajer, D.; Kaczmarek, H.; Bajer, K. The structure and properties of different types of starch exposed to UV radiation: A comparative study. Carbohydr. Polym. 2013, 98, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hizukuri, S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr. Res. 1986, 147, 342–347. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydr. Polym. 2007, 68, 249–259. [Google Scholar] [CrossRef]
- Lee, J.S.; Kumar, R.N.; Rozman, H.D.; Azemi, B.M.N. Pasting, swelling and solubility properties of UV initiated starch-graft-poly(AA). Food Chem. 2005, 91, 203–211. [Google Scholar] [CrossRef]
- Wilson, J.D.; Bechtel, D.B.; Wilson, G.W.T.; Seib, P.A. Bread Quality of Spelt Wheat and Its Starch. Cereal Chem. 2008, 85, 629–638. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Fannon, J.E.; Hauber, R.J.; BeMiller, J.N. Surface Pores of Starch Granules. Cereal Chem. 1992, 69, 284. [Google Scholar]
- Guo, L.; Cui, B. The Role of Chain Structures on Enzymatic Hydrolysis of Potato and Sweet Potato Amylopectins. Starch/Stärke 2018, 70, 1800003. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Yu, B.; Tan, C.; Fang, Y.; Cui, B. Porous starches modified with double enzymes: Structure and adsorption properties. Int. J. Biol. Macromol. 2020, 164, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Pfanneműller, B.; Mayerhofer, B.H.; Schulz, R.G. Conformation of amylose in aqueous solution: Optical ratatory dispersion and circular dichroism of amylose-iodine complexes and dependence on chain length of retrogradation of amylose. Biopolymers 1971, 10, 243–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).