Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Storage
2.2. Shelf Life Evaluation
2.3. Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
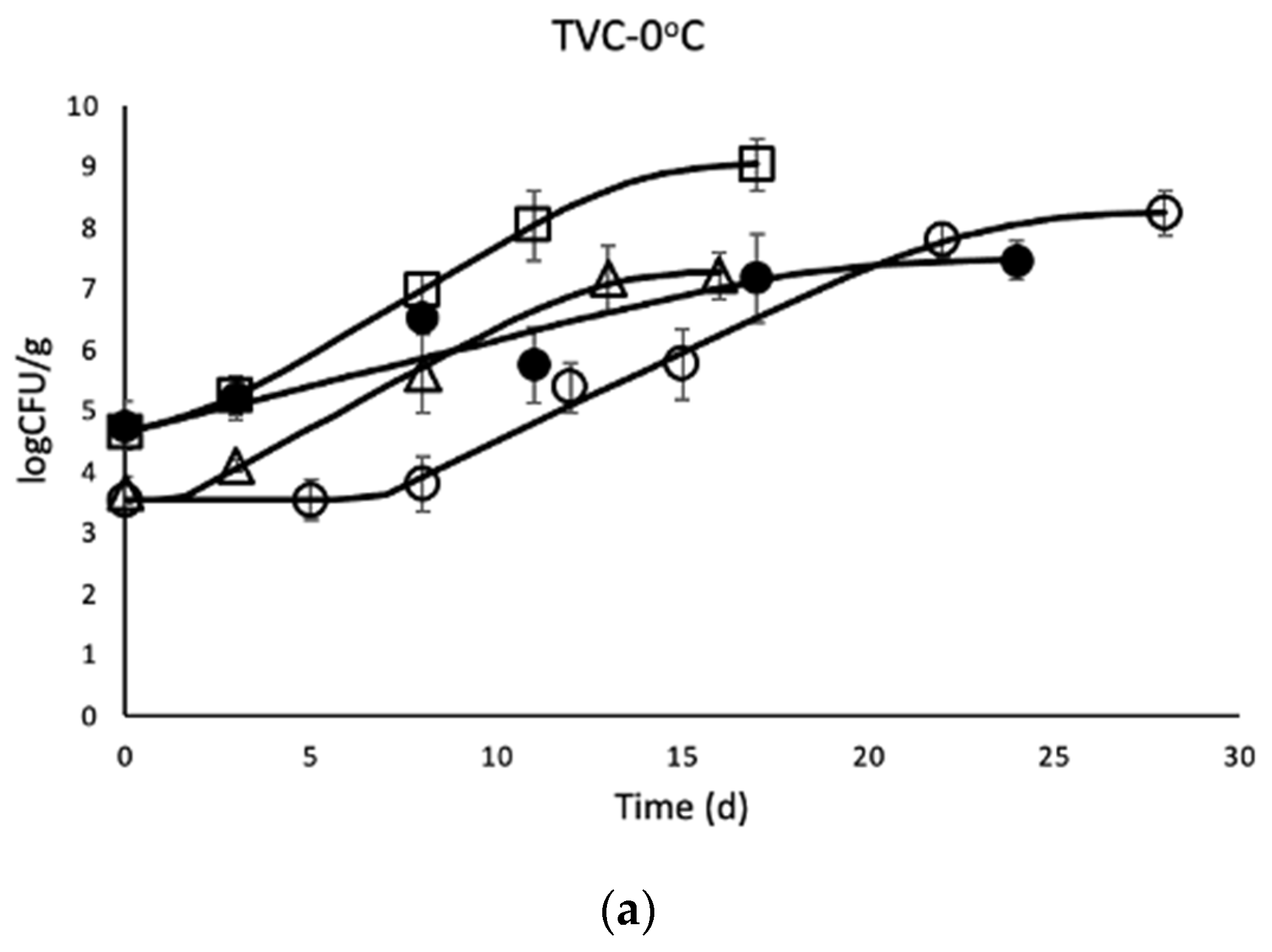
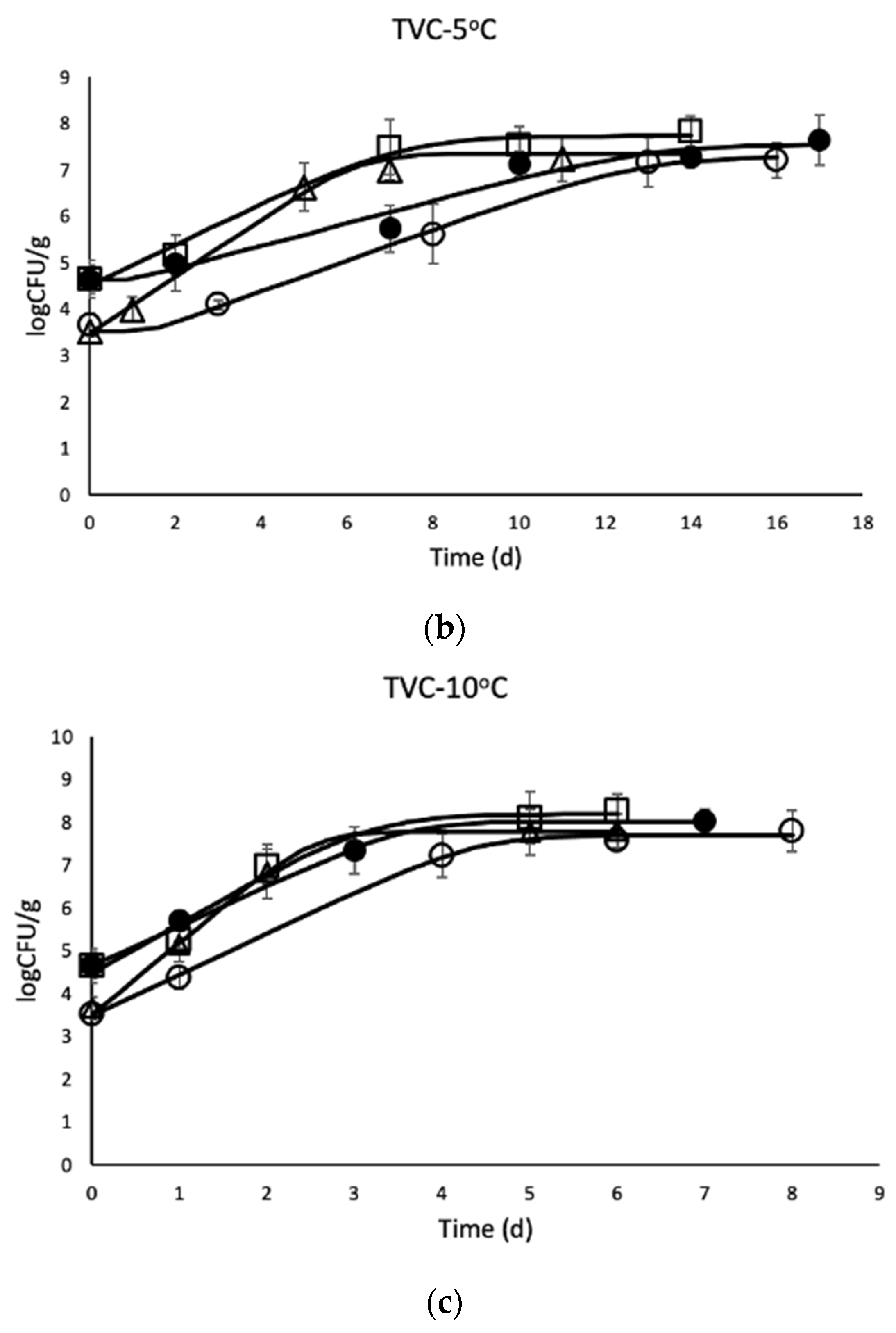
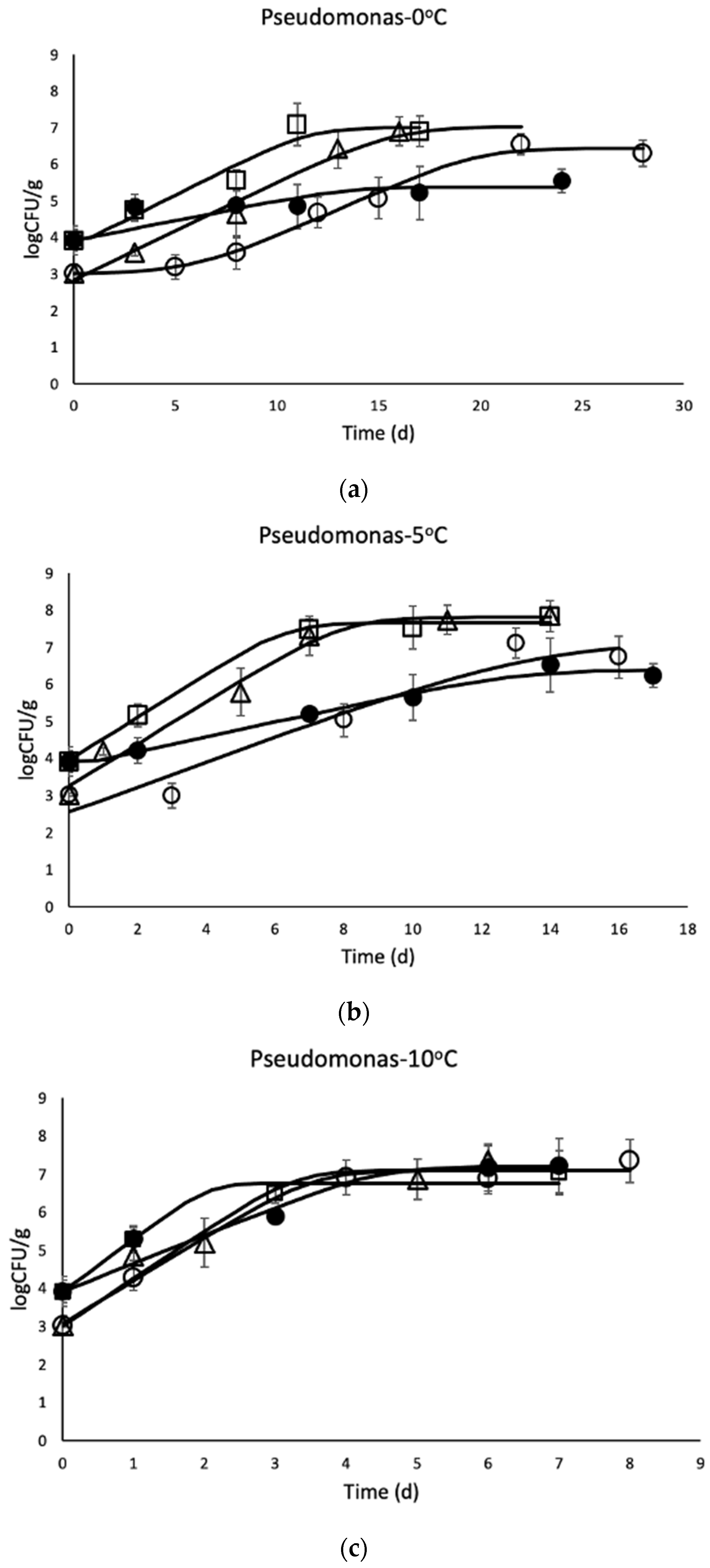
3.1. Microbial Enumeration of Chicken Thighs
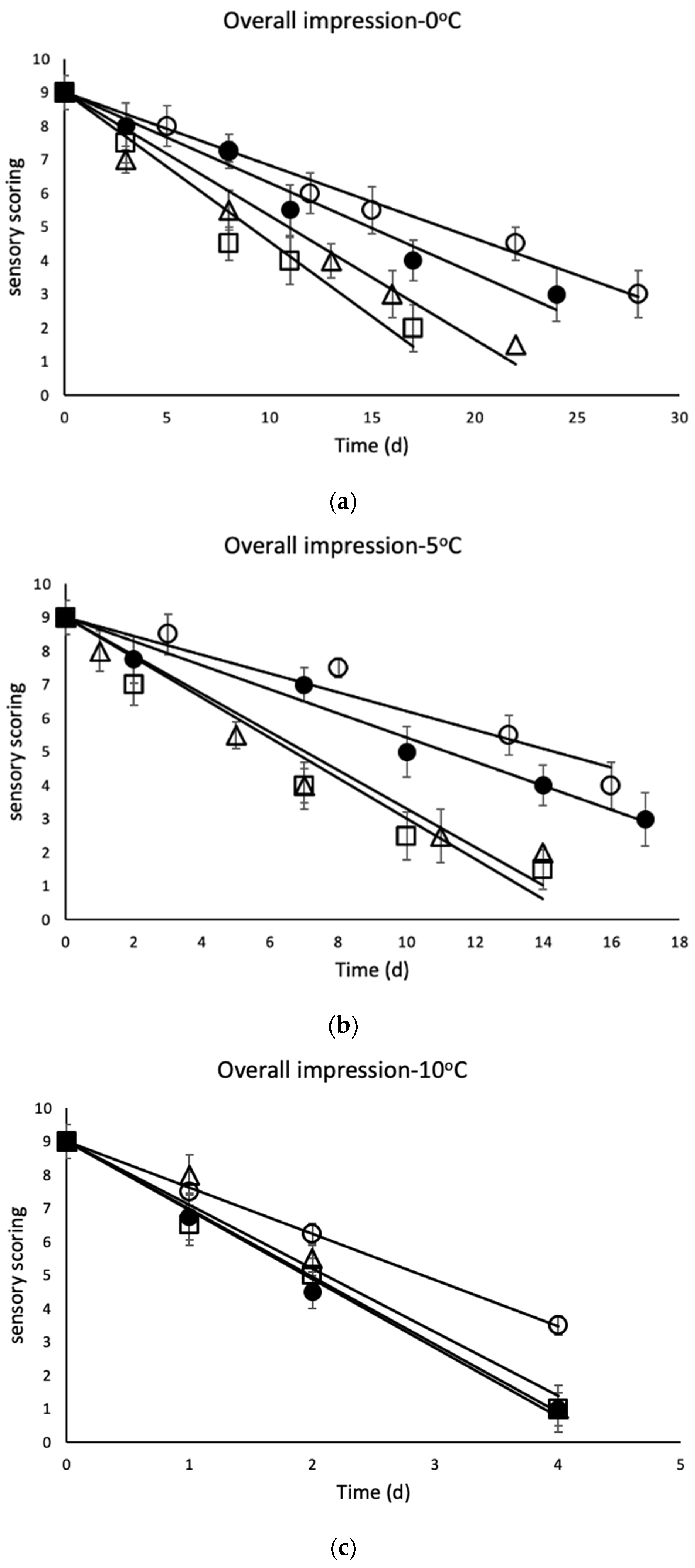
3.2. Sensory Evaluation of Chicken Thighs
3.3. Determination of Colour Parameters of Chicken Thighs
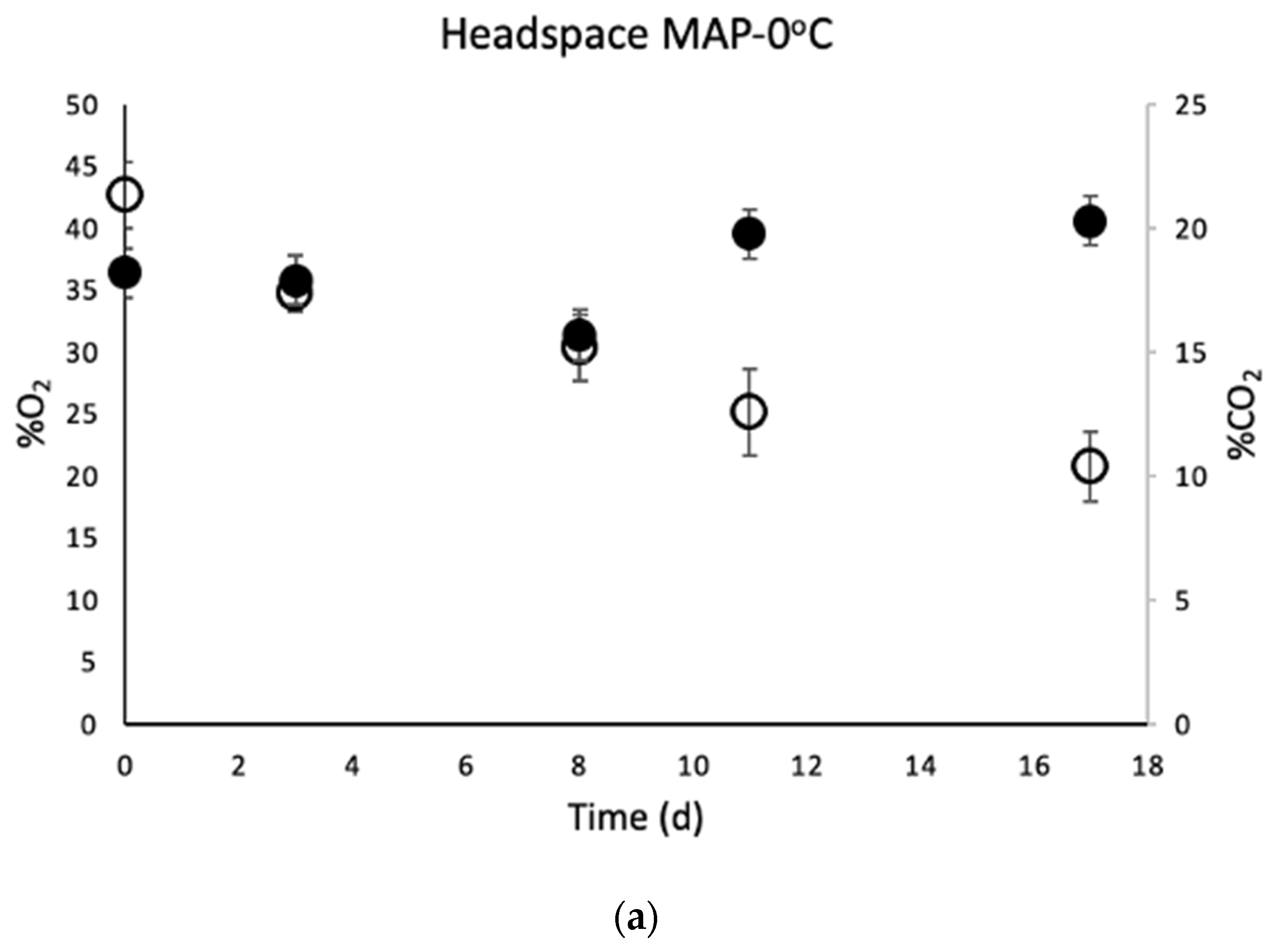
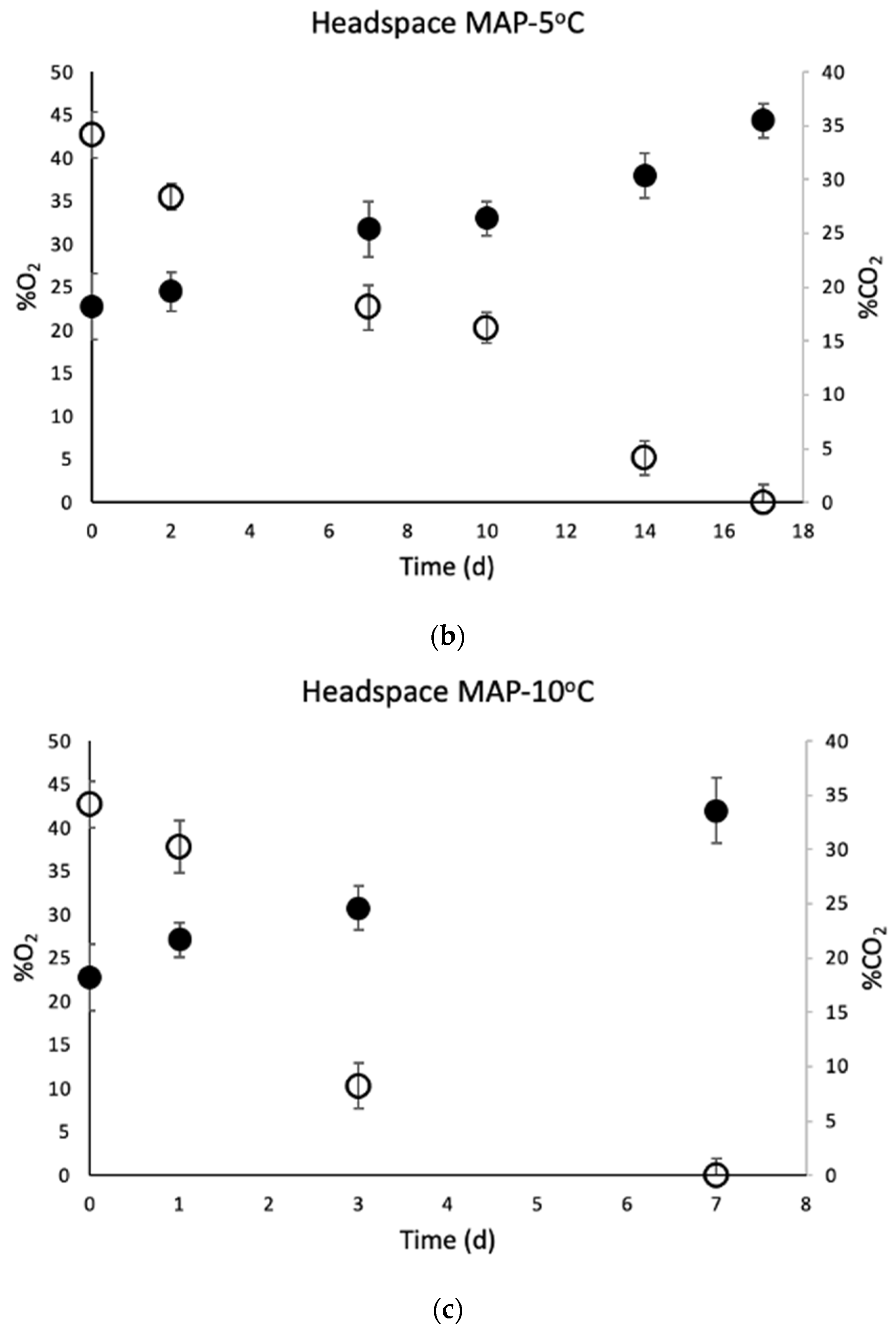
3.4. Determination of Headspace Composition of MAP Chicken Thighs
3.5. pH Measurement in Chicken Thighs
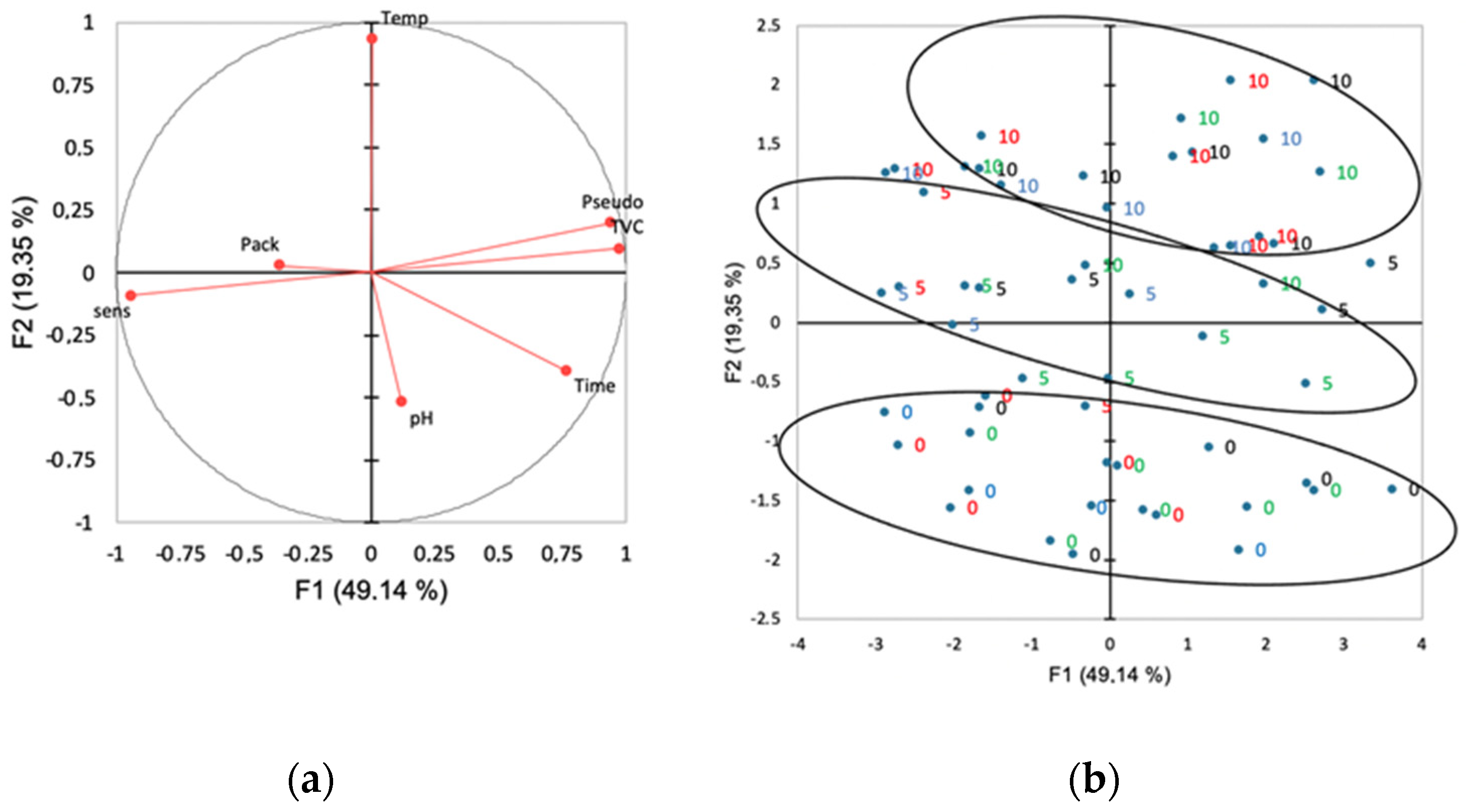
3.6. Principal Component Analysis (PCA)
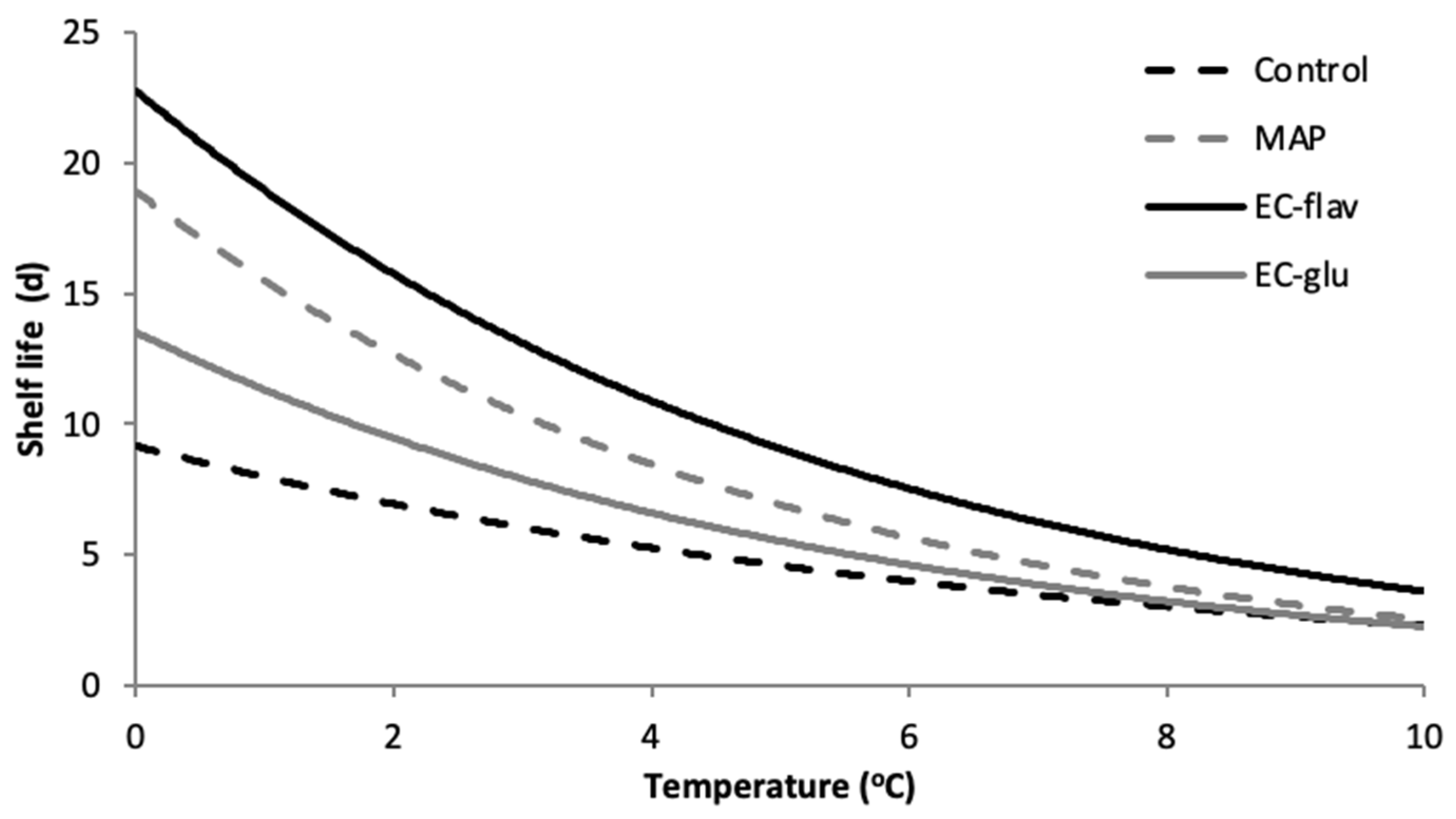
3.7. Shelf Life Evaluation of Chicken Thighs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grau, R.; Sánchez, A.J.; Girón, J.; Iborra, E.; Fuentes, A.; Barat, J.M. Nondestructive assessment of freshness in packaged sliced chicken breasts using SW-NIR spectroscopy. Food Res. Int. 2011, 44, 331–337. [Google Scholar] [CrossRef]
- Ghollasi-Mood, F.; Mohsenzadeh, M.; Hoseindokht, M.R.; Varidi, M. Quality changes of air-packaged chicken meat stored under different temperature conditions and mathematical modelling for predicting the microbial growth and shelf life. J. Food Saf. 2016, 37, e12331. [Google Scholar] [CrossRef]
- Choe, J.H.; Nam, K.C.; Jung, S.O.; Kim, B.N.; Yun, H.J.; Jo, C.R. Differences in the quality characteristics between commercial Korean native chickens and broilers. Food Sci. Anim. Resour. 2010, 30, 13–19. [Google Scholar] [CrossRef]
- FAO (The Food and Agriculture Organization). Gateway to Poultry Production and Products. 2020. Available online: http://www.fao.org/poultry-production-products/en (accessed on 19 July 2022).
- Katiyo, W.; de Kock, H.L.; Coorey, R.; Buys, E.M. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT Food Sci. Technol. 2020, 128, 109468. [Google Scholar] [CrossRef]
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. Worlds Poult. Sci. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Gao, J.; Srichamnong, W.; Chathiran, W.; Matthews, K.R. Influences of photosensitizer curcumin on microbial survival and physicochemical properties of chicken during storage. Poultry Sci. 2023, 102, 102417. [Google Scholar] [CrossRef]
- FAO (The Food and Agriculture Organization). FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#home (accessed on 3 June 2022).
- OECD/FAO 2021. OECD-FAO Agricultural Outlook. 2021–2030. Available online: https://www.fao.org/3/cb5332en/Meat.pdf (accessed on 23 April 2022).
- USDA (United States Department of Agriculture); Buzby, J.C.; Wells, H.F.; Axtman, B.; Mickey, J. Supermarket Loss Estimates for Fresh Fruit, Vegetables, Meat, Poultry, and Seafood and Their Use in the ERS Loss-Adjusted Food Availability Data. A Report from the Economic Research Service. 2009. Available online: https://ageconsearch.umn.edu/record/58313 (accessed on 1 September 2022).
- Aymerich, T.; Picouet, P.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef]
- Patsias, A.; Badeka, A.V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of freeze chilling and MAP on quality parameters of raw chicken fillets. Food Microbiol. 2008, 25, 575–581. [Google Scholar] [CrossRef]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.C. Shelf-life and spoilage of poultry meat. In Poultry Meat Processing and Quality; Mead, G.C., Ed.; CRC Press: Cambridge, UK, 2004; pp. 283–303. [Google Scholar]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Samelis, J. Managing Microbial Spoilage in the Meat Industry. In Food Spoilage Microorganisms; Blackburn, W., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sivarajan, M.; Lalithapriya, U.; Mariajenita, P.; AafrinVajiha, B.; Harini, K.; Madhushalini, D.; Sukumar, M. Synergistic effect of spice extracts and modified atmospheric packaging towards nonthermal preservation of chicken meat under refrigerated storage. Poult. Sci. 2017, 96, 2839–2844. [Google Scholar] [CrossRef]
- Yusof, N.L.; Mutalib, N.-A.A.; Nazatul, U.; Nadrah, A.; Aziman, N.; Fouad, H.; Jawaid, M.; Ali, A.; Kian, L.K.; Sain, M. Efficacy of Biopolymer/Starch Based Antimicrobial Packaging for Chicken Breast Fillets. Foods 2021, 10, 2379. [Google Scholar] [CrossRef] [PubMed]
- Guillard, V.; Couvert, O.; Stahl, V.; Hanin, A.; Denis, C.; Huchet, V.; Chaix, E.; Loriot, C.; Vincelot, T.; Thuault, D. Validation of a predictive model coupling gas transfer and microbial growth in fresh food packed under modified atmosphere. Food Microbiol. 2016, 58, 43–55. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Patsias, A.; Kontominas, M.G.; Savvaidis, I.N. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chem. 2007, 104, 622–1628. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2017, 109, 1095–1107. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Biopr. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Pack. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P. 20—Chitosan Polysaccharide in Food Packaging Applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagarón, J.-M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 571–593. [Google Scholar]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun antibacterial chitosan-based fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Characterization of Pectin Films Integrated with Cocoa Butter by Continuous Casting: Physical, Thermal and Barrier Properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.; Rastegar, H.; Taherian, M.; Samadi, M.; Rostami, H. The effect of nano-silver packaging in increasing the shelf life of nuts: An in vitro model. Italian. J. Food Saf. 2017, 6, 6874. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Antimicrobial Packaging Systems. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 80–107. [Google Scholar]
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-based edible coating for shelf-life extension of Ataulfo mango. J. Food Proc. Engin. 2012, 35, 572–600. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 2010, 82, 585–593. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Effect of chitosan and thyme oil on a ready to cook chicken product. Food Microbiol. 2010, 27, 132–136. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef]
- Fu, B.; Labuza, T.P. Shelf Life Testing: Procedures and Prediction Methods. In Quality in Frozen Food; Erickson, M.C., Hung, Y.C., Eds.; Chapman & Hall: New York, NY, USA, 1997; p. 377. [Google Scholar]
- Yehia, H.M.; Elkhadragy, M.F.; Al-Megrin, W.A.; Al-Masoud, A.H. Citrox Improves the Quality and Shelf Life of Chicken Fillets Packed under Vacuum and Protects against Some Foodborne Pathogens. Animals 2019, 9, 1062. [Google Scholar] [CrossRef]
- Ntzimani, A.G.; Giatrakou, V.I.; Savvaidis, I.N. Combined natural antimicrobial treatments on a ready-to-eat poultry product stored at 4 and 8 °C. Poult. Sci. 2011, 90, 880–888. [Google Scholar] [CrossRef]
- Bruckner, S.; Albrecht, A.; Petersen, B.; Kreyenschmidt, J. Influence of cold chain interruptions on the shelf life of fresh pork and poultry. Int. J. Food Sci. Technol. 2012, 47, 1639–1646. [Google Scholar] [CrossRef]
- Yimenu, S.M.; Koo, J.; Kim, B.S.; Kim, J.H.; Kim, J.Y. Freshness-based real-time shelf-life estimation of packaged chicken meat under dynamic storage conditions. Poult. Sci. 2019, 94, 6921–6930. [Google Scholar] [CrossRef] [PubMed]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Shelf life extension of ground chicken meat using an oxygen absorber and a citrus extract. LWT Food Sci. Technol. 2012, 49, 21–27. [Google Scholar] [CrossRef]
- Herbert, U.; Albrecht, A.; Kreyenschmidt, J. Definition of predictor variables for MAP poultry filets stored under different temperature conditions. Poult. Sci. 2015, 94, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.; Stratakos, A. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Biop. Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Jay, J.M. Modern Food Microbiology, 7th ed.; Chapman & Hall, Food Science Texts: Boca Raton, FL, USA, 2005. [Google Scholar]
- Fraqueza, M.J.; Alfaia, C.M.; Barreto, A.S. Biogenic amine formation in turkey meat under modified atmosphere packaging with extended shelf life: Index of freshness. Poult. Sci. 2012, 91, 1465–1472. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Paleologos, E.K.; Kontominas, M.; Savvaidis, I.N. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4 °C: Possible role of biogenic amines as spoilage indicators. Antonie Leeuwenhoek 2006, 89, 9–17. [Google Scholar] [CrossRef]
- Jiang, Z.; Neetoo, H.; Chen, H. Efficacy of freezing, frozen storage and edible antimicrobial coatings used in combination for control of Listeria monocytogenes on roasted turkey stored at chiller temperatures. Food Microbiol. 2011, 28, 1394–1401. [Google Scholar] [CrossRef]
| Control (Total 150 Samples) | ||
|---|---|---|
| 0 °C | 5 °C | 10 °C |
|
|
|
| MAP (Total 150 Samples) | ||
|
|
|
| EC-flav (Total 150 Samples) | ||
|
|
|
| EC-glu (Total 150 Samples) | ||
|
|
|
| 0 °C | 5 °C | 10 °C | Ea (kJ/mol) | |
|---|---|---|---|---|
| Control | ||||
| kTVC | 0.3655 ± 0.0045 a3 | 0.8835 ± 0.1001 b3 | 1.7347 ± 0.0561 c2 | 100.2 (R2 = 0.9956) |
| kPseudomonas | 0.2796 ± 0.0906 a2 | 0.5811 ± 0.0828 b3 | 1.3686 ± 0.2232 c2 | 102.1 (R2 = 0.9969) |
| kB.thermosphacta | 0.3224 ± 0.0878 a2 | 0.6615 ± 0.0368 b4 | 0.8485 ± 0.0089 c3 | 62.4 (R2 = 0.9325) |
| MAP | ||||
| kTVC | 0.1511 ± 0.0607 a1 | 0.2424 ± 0.0537 a1 | 0.9304 ± 0.0974 b1 | 116.5 (R2 = 0.9223) |
| kPseudomonas | 0.1080 ± 0.0722 a1 | 0.1996 ± 0.0295 a1 | 0.7378 ± 0.3447 b1 | 123.3 (R2 = 0.9542) |
| kB.thermosphacta | 0.2024 ± 0.0385 a1 | 0.2176 ± 0.0532 a1 | 0.7135 ± 0.0239 b2 | 80.6 (R2 = 0.9245) |
| EC-flav | ||||
| kTVC | 0.2911 ± 0.0244 a2 | 0.6183 ± 0.0715 b2 | 0.9642 ± 0.0531 c1 | 77.1 (R2 = 0.9812) |
| kPseudomonas | 0.2435 ± 0.0591 a2 | 0.3825 ± 0.0169 b2 | 0.6317 ± 0.2499 c1 | 98.5 (R2 = 0.9417) |
| kB.thermosphacta | 0.2065 ± 0.0511 a1 | 0.3694 ± 0.0623 b2 | 0.5551 ± 0.0096 c1 | 63.7 (R2 = 0.9917) |
| EC-glu | ||||
| kTVC | 0.2833 ± 0.0256 a2 | 0.6605 ± 0.0236 b2 | 1.6833 ± 0.0342 c2 | 114.6 (R2 = 0.9985) |
| kPseudomonas | 0.2667 ± 0.0266 a2 | 0.5154 ± 0.1154 b3 | 1.2414 ± 0.7356 c2 | 98.8 (R2 = 0.9914) |
| kB.thermosphacta | 0.2052 ± 0.0168 a1 | 0.4791 ± 0.0119 b3 | 0.7274 ± 0.0273 c2 | 81.5 (R2 = 0.9667) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntzimani, A.; Kalamaras, A.; Tsironi, T.; Taoukis, P. Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings. Appl. Sci. 2023, 13, 4025. https://doi.org/10.3390/app13064025
Ntzimani A, Kalamaras A, Tsironi T, Taoukis P. Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings. Applied Sciences. 2023; 13(6):4025. https://doi.org/10.3390/app13064025
Chicago/Turabian StyleNtzimani, Athina, Antonios Kalamaras, Theofania Tsironi, and Petros Taoukis. 2023. "Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings" Applied Sciences 13, no. 6: 4025. https://doi.org/10.3390/app13064025
APA StyleNtzimani, A., Kalamaras, A., Tsironi, T., & Taoukis, P. (2023). Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings. Applied Sciences, 13(6), 4025. https://doi.org/10.3390/app13064025









