Solution Combustion Synthesis of Ni/Al2O3 Catalyst for Methane Decomposition: Effect of Fuel
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banu, A.; Bicer, Y. Review on COx-free hydrogen from methane cracking: Catalysts, solar energy integration and applications. Energy Convers. Manag. X 2021, 12, 100117. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G.; Plyasova, L.M. New Nickel Catalysts for the Formation of Filamentous Carbon in the Reaction of Methane Decomposition. J. Catal. 1999, 187, 77–84. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, D.; Huang, Y.; Long, Z.; Chen, G. COx-free hydrogen production via ammonia decomposition over mesoporous Co/Al2O3 catalysts with highly dispersed Co species synthesized by a facile method. Dalt. Trans. 2021, 50, 1443–1452. [Google Scholar] [CrossRef]
- Rategarpanah, A.; Meshkani, F.; Wang, Y.; Arandiyan, H.; Rezaei, M. Thermocatalytic conversion of methane to highly pure hydrogen over Ni–Cu/MgO·Al2O3 catalysts: Influence of noble metals (Pt and Pd) on the catalytic activity and stability. Energy Convers. Manag. 2018, 166, 268–280. [Google Scholar] [CrossRef]
- Ibrahimov, H.; Malikli, S.; Ibrahimova, Z.; Babali, R.; Aleskerova, S. Ni-γ-Al2O3 catalysts for obtaining nanocarbon by decomposition of natural gas. Appl. Petrochem. Res. 2021, 11, 123–128. [Google Scholar] [CrossRef]
- Panchan, N.; Donphai, W.; Junsomboon, J.; Niamnuy, C.; Chareonpanich, M. Influence of the Calcination Technique of Silica on the Properties and Performance of Ni/SiO2 Catalysts for Synthesis of Hydrogen via Methane Cracking Reaction. ACS Omega 2019, 4, 18076–18086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-P.; Ohta, K.; Kuroda, K.; Lei, N.; Matsuishi, K.; Gao, L.; Matsumoto, T.; Nakamura, J. Catalytic Functions of Mo/Ni/MgO in the Synthesis of Thin Carbon Nanotubes. J. Phys. Chem. B 2005, 109, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Calafat, Á.; Sánchez, N. Production of carbon nanotubes through combination of catalyst reduction and methane decomposition over Fe–Ni/ZrO2 catalysts prepared by the citrate method. Appl. Catal. A Gen. 2016, 528, 14–23. [Google Scholar] [CrossRef]
- Bannov, A.G.; Uvarov, N.F.; Ukhina, A.V.; Chukanov, I.S.; Dyukova, K.D.D.; Kuvshinov, G.G. Structural changes in carbon nanofibers induced by ball milling. Carbon 2012, 50, 1090–1098. [Google Scholar] [CrossRef]
- Bannov, A.G.; Lapekin, N.I.; Kurmashov, P.B.; Ukhina, A.V.; Manakhov, A. Room-Temperature NO2 Gas Sensors Based on Granulated Carbon Nanofiber Material. Chemosensors 2022, 10, 525. [Google Scholar] [CrossRef]
- Kuvshinov, G.G.; Popov, M.V.; Tonkodubov, S.E.; Kuvshinov, D.G. Effect of pressure on the efficiency of nickel and nickel-copper catalysts in decomposition of methane. Russ. J. Appl. Chem. 2016, 89, 1777–1785. [Google Scholar] [CrossRef]
- Kurmashov, P.B.; Bannov, A.G.; Popov, M.V.; Kazakova, A.A.; Ukhina, A.V.; Kuvshinov, G.G. Effect of Process Features and Parameters of Preparation of a Nickel Catalyst by Reduction of Nickel Nitrate with Hexamethylenetetramine on the Catalyst Performance in Synthesis of Nanofibrous Carbon. Russ. J. Appl. Chem. 2018, 91, 1874–1881. [Google Scholar] [CrossRef]
- Kuvshinov, G.G.; Chukanov, I.S.; Krutsky, Y.L.; Ochkov, V.V.; Zaikovskii, V.I.; Kuvshinov, D.G. Changes in the properties of fibrous nanocarbons during high temperature heat treatment. Carbon 2009, 47, 215–225. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Chuvilin, A.L.; Kuvshinov, G.G. Decomposition of methane over iron catalysts at the range of moderate temperatures: The influence of structure of the catalytic systems and the reaction conditions on the yield of carbon and morphology of carbon filaments. J. Catal. 2001, 201, 183–197. [Google Scholar] [CrossRef]
- Kurmashov, P.B.; Bannov, A.G.; Popov, M.V.; Brester, A.E.; Ukhina, A.V.; Ishchenko, A.V.; Maksimovskii, E.A.; Tolstobrova, L.I.; Chulkov, A.O.; Kuvshinov, G.G. COx-free catalytic decomposition of methane over solution combustion synthesis derived catalyst: Synthesis of hydrogen and carbon nanofibers. Int. J. Energy Res. 2022, 46, 11957–11971. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; Kuvshinov, G.G. Nanofibrous carbon with herringbone structure as an effective catalyst of the H2S selective oxidation. Carbon 2010, 48, 2004–2012. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; Kuvshinov, G.G. New effective catalysts based on mesoporous nanofibrous carbon for selective oxidation of hydrogen sulfide. Appl. Catal. B Environ. 2009, 85, 180–191. [Google Scholar] [CrossRef]
- Su, D.S.; Centi, G. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173. [Google Scholar] [CrossRef]
- Brester, A.E.; Golovakhin, V.V.; Novgorodtseva, O.N.; Lapekin, N.I.; Shestakov, A.A.; Ukhina, A.V.; Prosanov, I.Y.; Maksimovskii, E.A.; Popov, M.V.; Bannov, A.G. Chemically Treated Carbon Nanofiber Materials for Supercapacitors. Dokl. Chem. 2021, 501, 264–269. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental remediation applications of carbon nanotubes and graphene oxide: Adsorption and catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, E.; Boczkowska, A.; Kurzydlowski, K.J.; Rosca, I.D.; Van Hoa, S. The effect of carbon nanotubes on epoxy matrix nanocomposites. J. Therm. Anal. Calorim. 2013, 111, 1019–1024. [Google Scholar] [CrossRef]
- Zhao, C.; Ji, L.; Liu, H.; Hu, G.; Zhang, S.; Yang, M.; Yang, Z. Functionalized carbon nanotubes containing isocyanate groups. J. Solid State Chem. 2004, 177, 4394–4398. [Google Scholar] [CrossRef]
- Bannov, A.G.; Nazarenko, O.B.; Maksimovskii, E.A.; Popov, M.V.; Berdyugina, I.I.S. Thermal behavior and flammability of epoxy composites based on multi-walled carbon nanotubes and expanded graphite: A comparative study. Appl. Sci. 2020, 10, 6928. [Google Scholar] [CrossRef]
- Bannov, A.G.; Uvarov, N.F.; Shilovskaya, S.M.; Kuvshinov, G.G. Effect of the preparation methods on electrical properties of epoxy resin/carbon nanofiber composites. Nanotechnologies Russ. 2012, 7, 169–177. [Google Scholar] [CrossRef]
- Bannov, A.G.; Prášek, J.; Jašek, O.; Zajíčková, L. Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material. Sensors 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N. A review on chemiresistive gas sensors based on carbon nanotubes: Device and technology transformation. Sens. Actuators A Phys. 2018, 283, 174–186. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Sol–gel synthesis of titanium oxide supported nickel catalysts for hydrogen and carbon production by methane decomposition. J. Power Sources 2015, 280, 467–475. [Google Scholar] [CrossRef]
- Maksimova, T.A.; Mishakov, I.V.; Bauman, Y.I.; Ayupov, A.B.; Mel’gunov, M.S.; Dmitrachkov, A.M.; Nartova, A.V.; Stoyanovskii, V.O.; Vedyagin, A.A. Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine. Materials 2022, 15, 8239. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Ding, J.-R.; Kim, K.-S.; Manukyan, K.V.; Mukasyan, A.S. Combustion synthesis of zero-, one-, two- and three-dimensional nanostructures: Current trends and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 79–118. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B Environ. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Pervikov, A.V.; Pustovalov, A.V.; Afonnikova, S.D.; Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A. Synthesis and structure of NiCu and NiAl electroexplosive nanoparticles for production of carbon nanofibers. Powder Technol. 2023, 415, 118164. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Khort, A.; Roslyakov, S.; Loginov, P. Solution combustion synthesis of single-phase bimetallic nanomaterials. Nano-Struct. Nano-Objects 2021, 26, 100727. [Google Scholar] [CrossRef]
- Khort, A.; Podbolotov, K.; Serrano-García, R.; Gun’ko, Y. One-Step Solution Combustion Synthesis of Cobalt Nanopowder in Air Atmosphere: The Fuel Effect. Inorg. Chem. 2018, 57, 1464–1473. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Meshkani, F.; Rezaei, M. Thermocatalytic decomposition of methane over mesoporous nanocrystalline promoted Ni/MgO·Al2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 16476–16488. [Google Scholar] [CrossRef]
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv. 2020, 10, 40882–40893. [Google Scholar] [CrossRef]
- Kuvshinov, D.G.; Kurmashov, P.B.; Bannov, A.G.; Popov, M.V.; Kuvshinov, G.G. Synthesis of Ni-based catalysts by hexamethylenetetramine-nitrates solution combustion method for co-production of hydrogen and nanofibrous carbon from methane. Int. J. Hydrogen Energy 2019, 44, 16271–16286. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane dissociation to COx-free hydrogen and carbon nanofiber over Ni-Cu/Al2O3 catalysts. Fuel 2017, 195, 88–96. [Google Scholar] [CrossRef]
- Yang, R.-X.; Chuang, K.-H.; Wey, M.-Y. Effects of Nickel Species on Ni/Al2O3 Catalysts in Carbon Nanotube and Hydrogen Production by Waste Plastic Gasification: Bench- and Pilot-Scale Tests. Energy Fuels 2015, 29, 8178–8187. [Google Scholar] [CrossRef]
- Garcia, A.B.; Cameán, I.; Suelves, I.; Pinilla, J.L.; Lázaro, M.J.; Palacios, J.M.; Moliner, R. The graphitization of carbon nanofibers produced by the catalytic decomposition of natural gas. Carbon 2009, 47, 2563–2570. [Google Scholar] [CrossRef]
- Venugopal, A.; Naveen Kumar, S.; Ashok, J.; Hari Prasad, D.; Durga Kumari, V.; Prasad, K.B.S.; Subrahmanyam, M. Hydrogen production by catalytic decomposition of methane over Ni/SiO2. Int. J. Hydrogen Energy 2007, 32, 1782–1788. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. COx-free hydrogen and carbon nanofibers production by methane decomposition over nickel-alumina catalysts. Korean J. Chem. Eng. 2016, 33, 490–499. [Google Scholar] [CrossRef]
- Ahmed, W.; Noor El-Din, M.R.; Aboul-Enein, A.A.; Awadallah, A.E. Effect of textural properties of alumina support on the catalytic performance of Ni/Al2O3 catalysts for hydrogen production via methane decomposition. J. Nat. Gas Sci. Eng. 2015, 25, 359–366. [Google Scholar] [CrossRef]
- Al-Fatesh, A.-S.; Barama, S.; Ibrahim, A.-A.; Barama, A.; Khan, W.-U.; Fakeeha, A. Study of Methane Decomposition on Fe/MgO-Based Catalyst Modified by Ni, Co, and Mn Additives. Chem. Eng. Commun. 2017, 204, 739–749. [Google Scholar] [CrossRef]
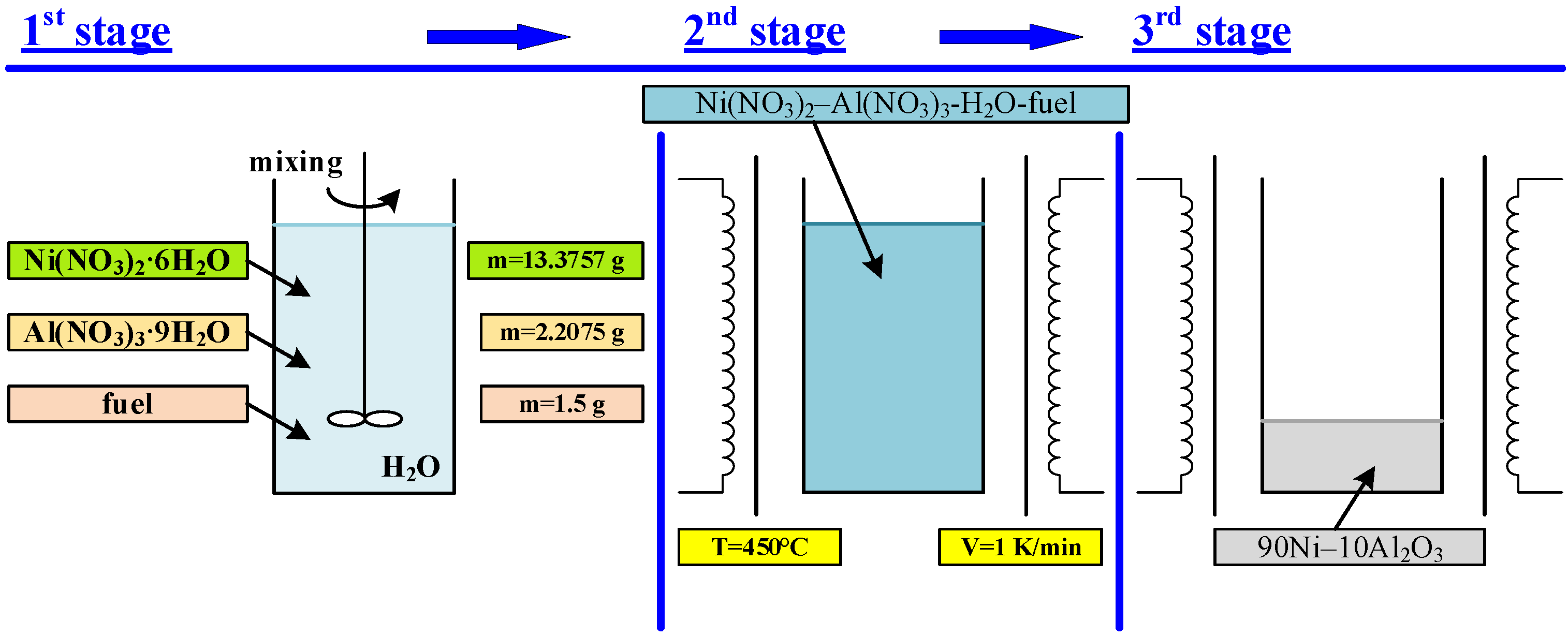
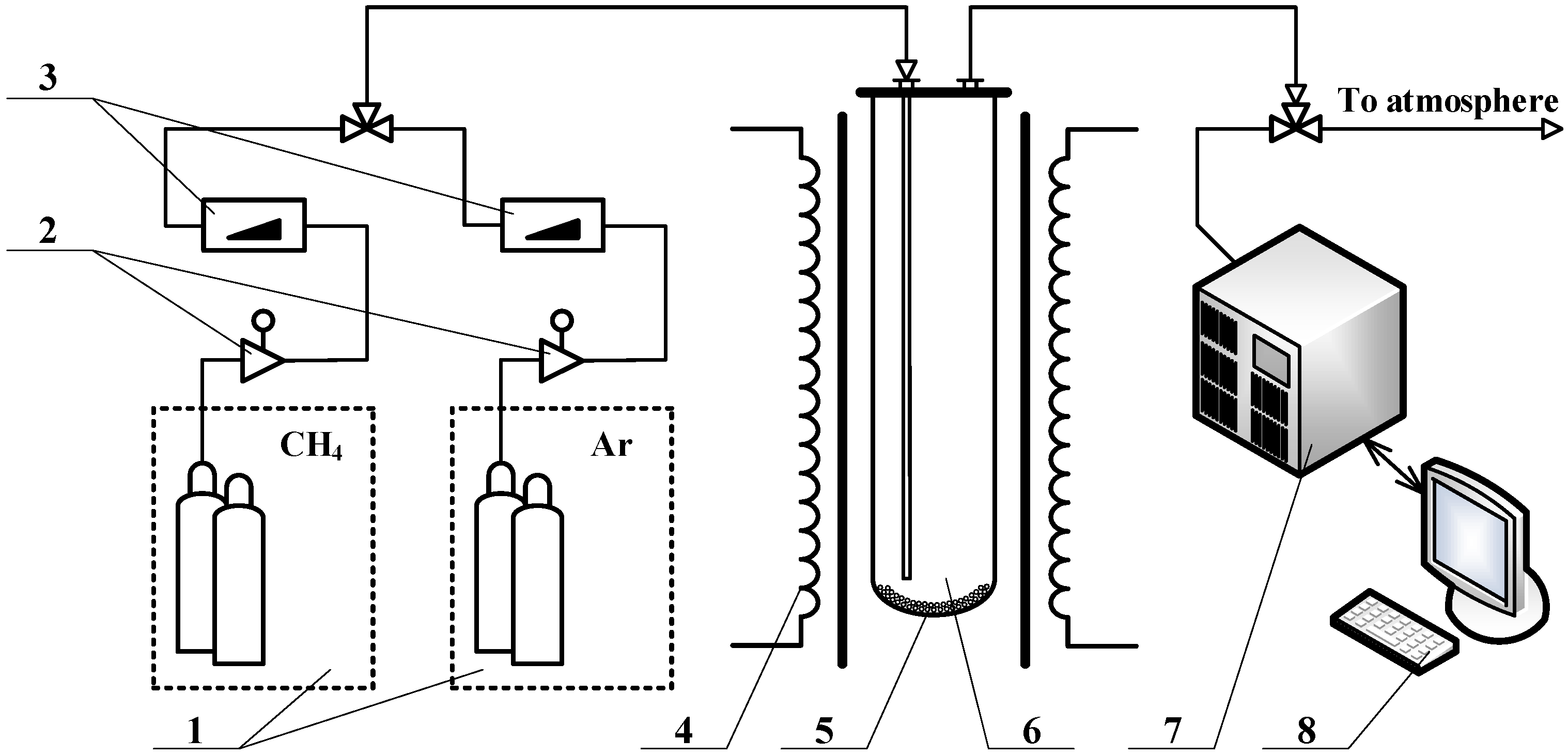
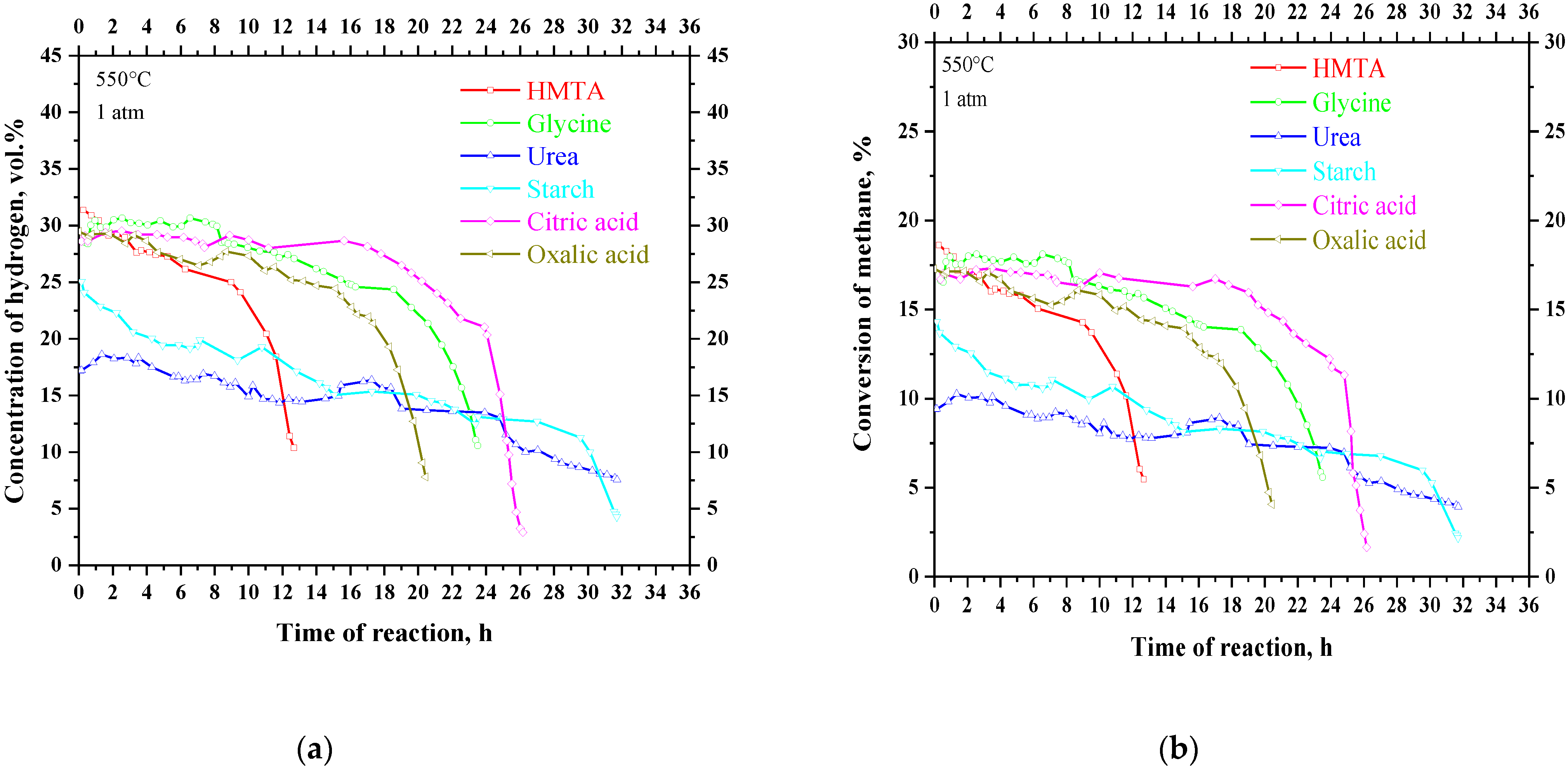
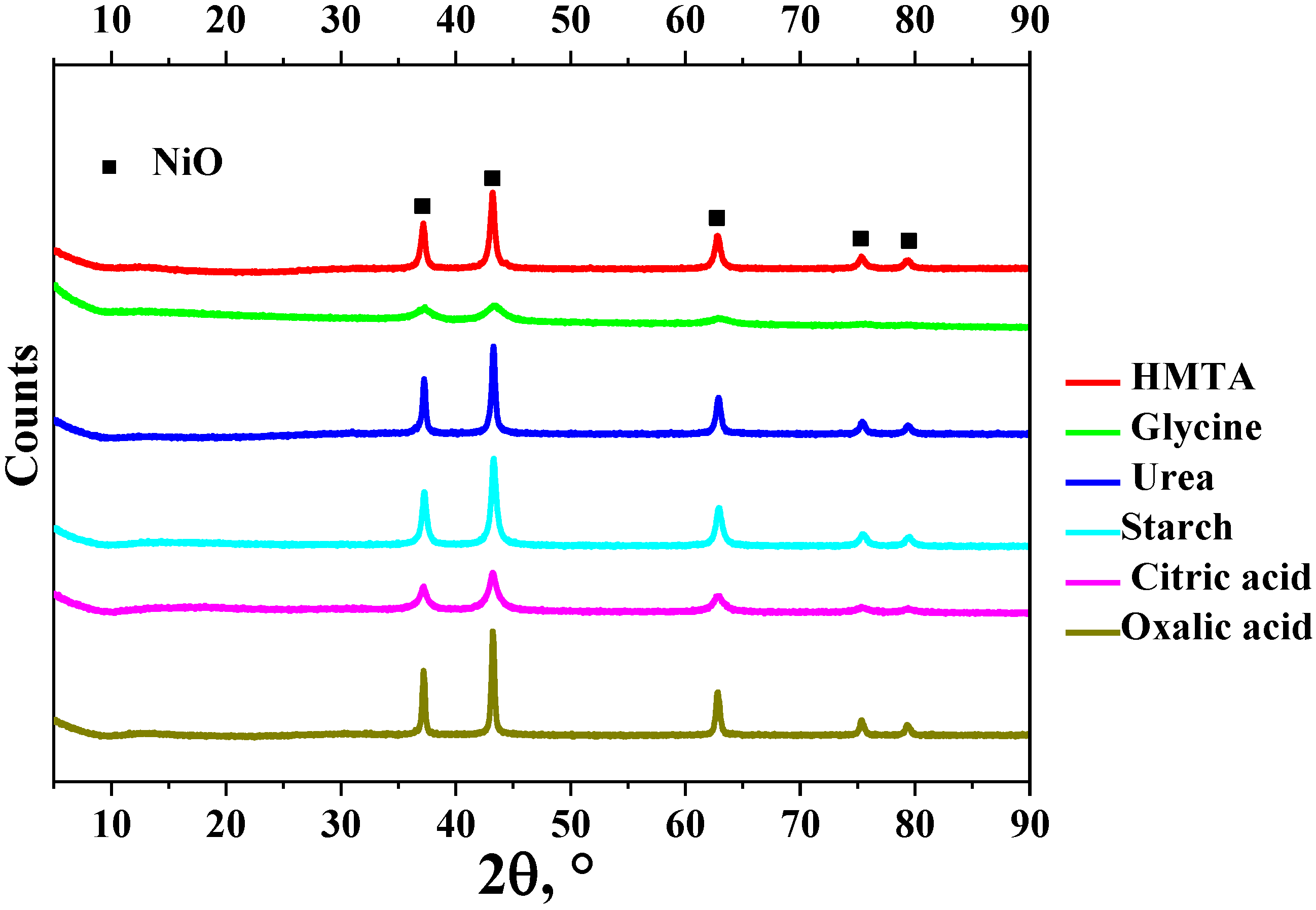
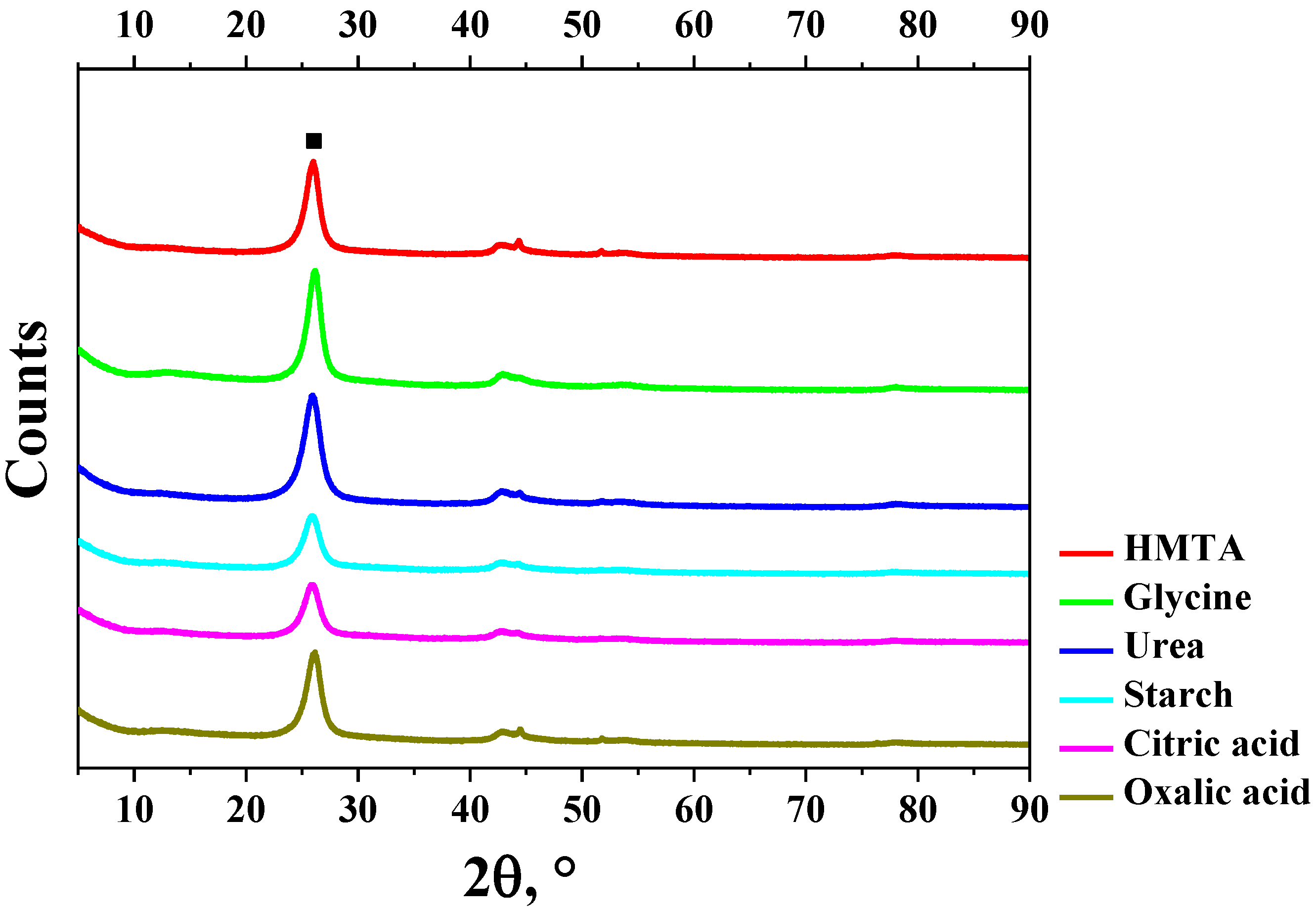
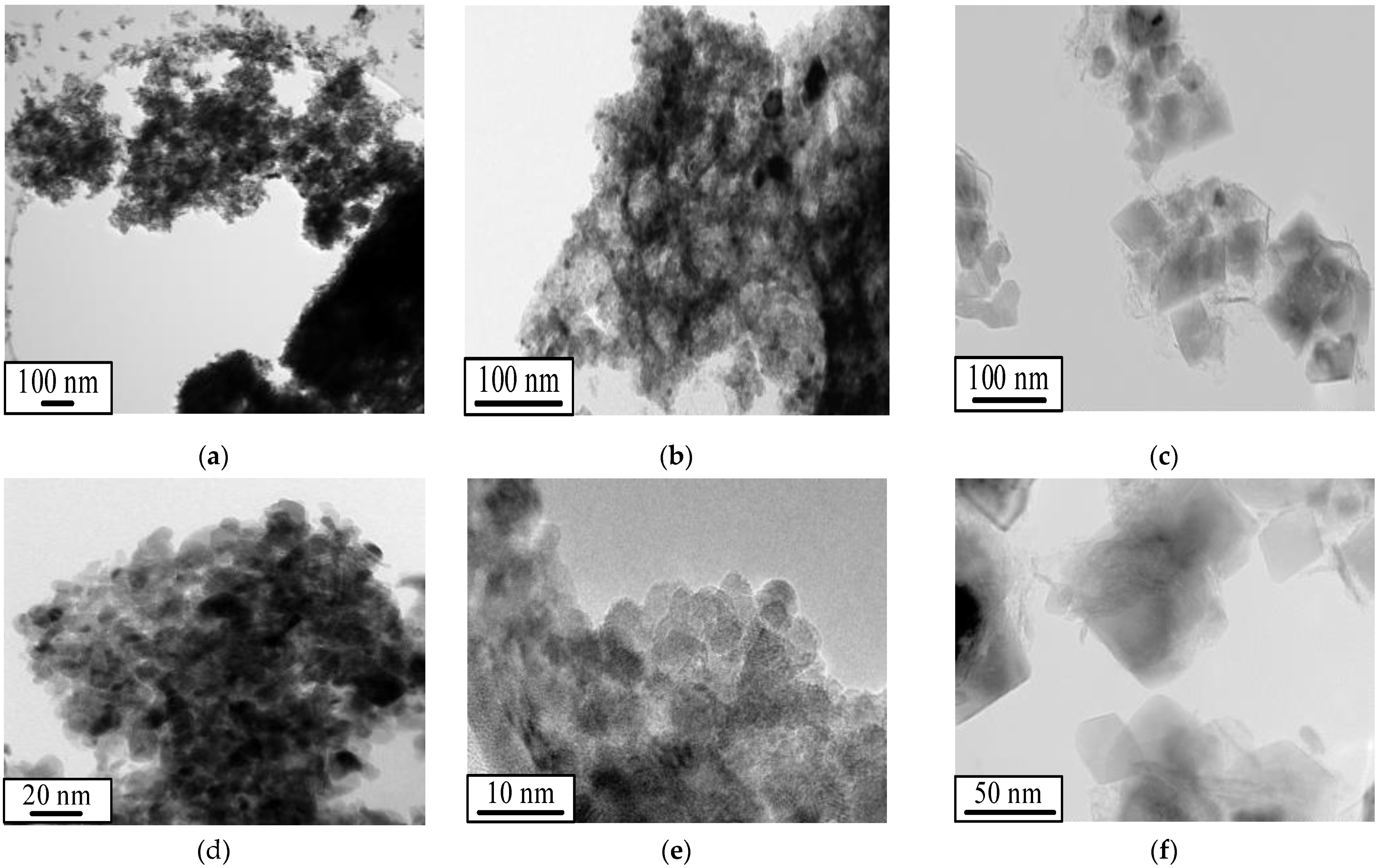
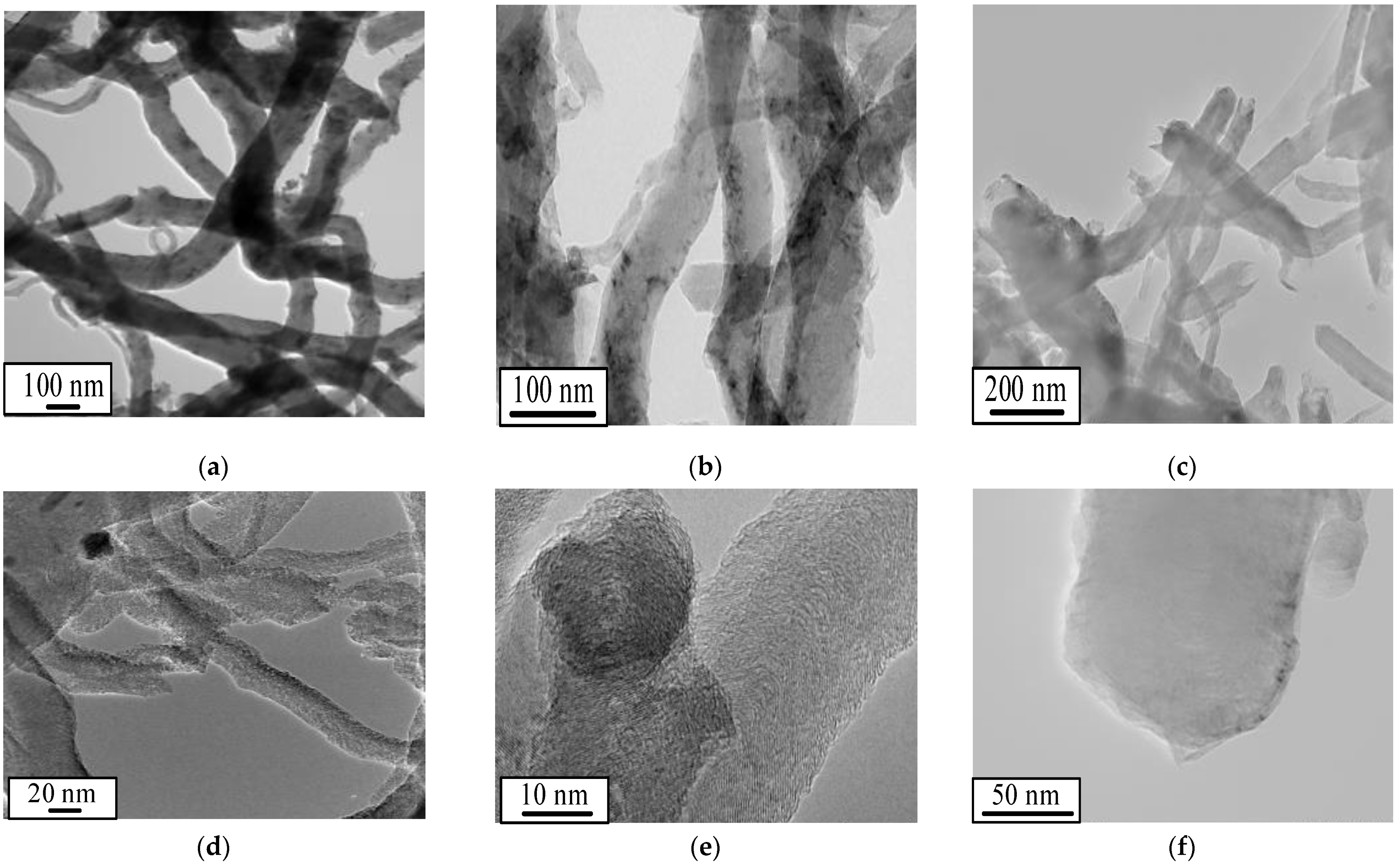
| Sample | Fuel Excess (φ) | Parameters of Catalyst Preparation in Furnace | Results of Synthesis (450 °C, 1 atm) | |||
|---|---|---|---|---|---|---|
| Temperature of Calcination, K | Heating Rate V, K/min | Total Time of Reaction t, h | Specific Yield of Hydrogen (YH2), mol/gcat | Specific Yield of CNFs (YC), g/gcat | ||
| HMTA | 0.70 | 723 | 1 | 12.7 | 9.5 | 69.1 |
| Glycine | 0.33 | 23.5 | 15.4 | 154.7 | ||
| Urea | 0.27 | 31.7 | 11.3 | 100.9 | ||
| Starch | 0.41 | 31.7 | 12.8 | 95.7 | ||
| Citric acid | 0.26 | 26.2 | 17.1 | 171.3 | ||
| Oxalic acid | 0.06 | 20.4 | 11.9 | 120.9 | ||
| Sample | Fuel Excess (φ) | Properties of Catalysts * | Properties of CNFs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| XRD | BET | XRD | BET | ||||||
| Lav (NiO), nm | Ssp, m2/g | Vtotal, cm3/g | Lpore, nm | Lav, nm | Ssp, m2/g | Vtotal, cm3/g | Lpore, nm | ||
| HMTA | 0.70 | 39.5 | 11 | 0.05 | 19.6 | 39.4 | 51 | 0.12 | 9.7 |
| Glycine | 0.33 | 16.6 | 153 | 0.50 | 13.1 | 21.4 | 61 | 0.24 | 15.5 |
| Urea | 0.27 | 37.2 | 51 | 0.21 | 16.7 | 22.9 | 86 | 0.16 | 7.8 |
| Starch | 0.41 | 35.8 | 81 | 0.27 | 13.5 | 17.7 | 86 | 0.17 | 8.0 |
| Citric acid | 0.26 | 20.9 | 94 | 0.23 | 9.9 | 21.9 | 80 | 0.16 | 7.9 |
| Oxalic acid | 0.06 | 40.2 | 49 | 0.30 | 24.4 | 25.9 | 74 | 0.14 | 7.6 |
| Catalyst | Preparation Technique | Lav(NiO), nm | Ssp, m2/g | Vtotal, cm3/г | Lpore, nm | Ref. |
|---|---|---|---|---|---|---|
| 90Ni/10Al2O3 | solution combustion synthesis (citric acid fuel) | 20.9 | 94 | 0.23 | 9.97 | This work |
| 90Ni/10Al2O3 | solution combustion synthesis (glycine fuel) | 16.6 | 153 | 0.50 | 13.12 | |
| 90Ni/10Al2O3 | solution combustion synthesis (HMTA fuel) | 46.3 | 107 | 0.25 | 20.96 | [15] |
| 90Ni/SiO2 | impregnation | 40 | – | – | – | [41] |
| 60Ni-10Al2O3 | impregnation | 26.6 | 66.1 | 0.13 | 7.2 | [42] |
| 60Ni-10Al2O3 | impregnation | 36.9 | 58.7 | 0.67 | 3.92 | [43] |
| 50Ni/Al2O3 | impregnation | 24.5 | 89 | 0.2 | 7.93 | [38] |
| Ref. | Catalyst | Preparation Technique | Inlet Gas | Parameters of Process | Conversion of Hydrogen x, % Initial/Maximum | Yield of Carbon Yc, g/gcat |
|---|---|---|---|---|---|---|
| [6] | Ni/SiO2 | wet impregnation | (1:4) CH4/N2 | 550 °C | 19/28 | - |
| [38] | Ni-Cu/Al2O3 | wet impregnation | (3:7) CH4/N2 | 750 °C | 84/85 | - |
| [42] | 50% Ni/Al2O3 | wet impregnation | (3:7) CH4/N2 | 625 °C, 1 bar | n/a/54 | - |
| [15] | 90%Ni/Al2O3 | solution combustion synthesis | Pure CH4 | 535 °C, 1 bar | n/a | 268.3 |
| [44] | 3%Ni-15%Fe/MgO | co-precipitation | (1.5:1) CH4/N2 | 700 °C, | 64/73 | - |
| This work | 90%Ni/Al2O3 | solution combustion synthesis | Pure CH4 | 550 °C, 1 bar | 16/18 | 171.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmashov, P.B.; Ukhina, A.V.; Manakhov, A.; Ishchenko, A.V.; Maksimovskii, E.A.; Bannov, A.G. Solution Combustion Synthesis of Ni/Al2O3 Catalyst for Methane Decomposition: Effect of Fuel. Appl. Sci. 2023, 13, 3962. https://doi.org/10.3390/app13063962
Kurmashov PB, Ukhina AV, Manakhov A, Ishchenko AV, Maksimovskii EA, Bannov AG. Solution Combustion Synthesis of Ni/Al2O3 Catalyst for Methane Decomposition: Effect of Fuel. Applied Sciences. 2023; 13(6):3962. https://doi.org/10.3390/app13063962
Chicago/Turabian StyleKurmashov, Pavel B., Arina V. Ukhina, Anton Manakhov, Arkady V. Ishchenko, Evgenii A. Maksimovskii, and Alexander G. Bannov. 2023. "Solution Combustion Synthesis of Ni/Al2O3 Catalyst for Methane Decomposition: Effect of Fuel" Applied Sciences 13, no. 6: 3962. https://doi.org/10.3390/app13063962
APA StyleKurmashov, P. B., Ukhina, A. V., Manakhov, A., Ishchenko, A. V., Maksimovskii, E. A., & Bannov, A. G. (2023). Solution Combustion Synthesis of Ni/Al2O3 Catalyst for Methane Decomposition: Effect of Fuel. Applied Sciences, 13(6), 3962. https://doi.org/10.3390/app13063962








