Comparative Study of Raw and Dehydrated Boletus edulis Mushrooms by Hot Air and Centrifugal Vacuum Processes: Functional Properties and Fatty Acid and Aroma Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom: Samples and Preparation
2.2. Loose and Tapped Bulk Density, Hausner Ratio
2.3. Solubility, Water Solubility Index (WSI), and Rehydration Ratio
2.4. Determination of Emulsifying Properties
2.5. Determination of Fatty Acids by GC–MS Analysis
2.6. Extraction and Analysis of Volatile Compounds by Headspace In-Tube Extraction Coupled with Gas Chromatography–Mass Spectrometry (HS-ITEX/GC–MS)
2.7. Statistical Analysis
3. Results and Discussion
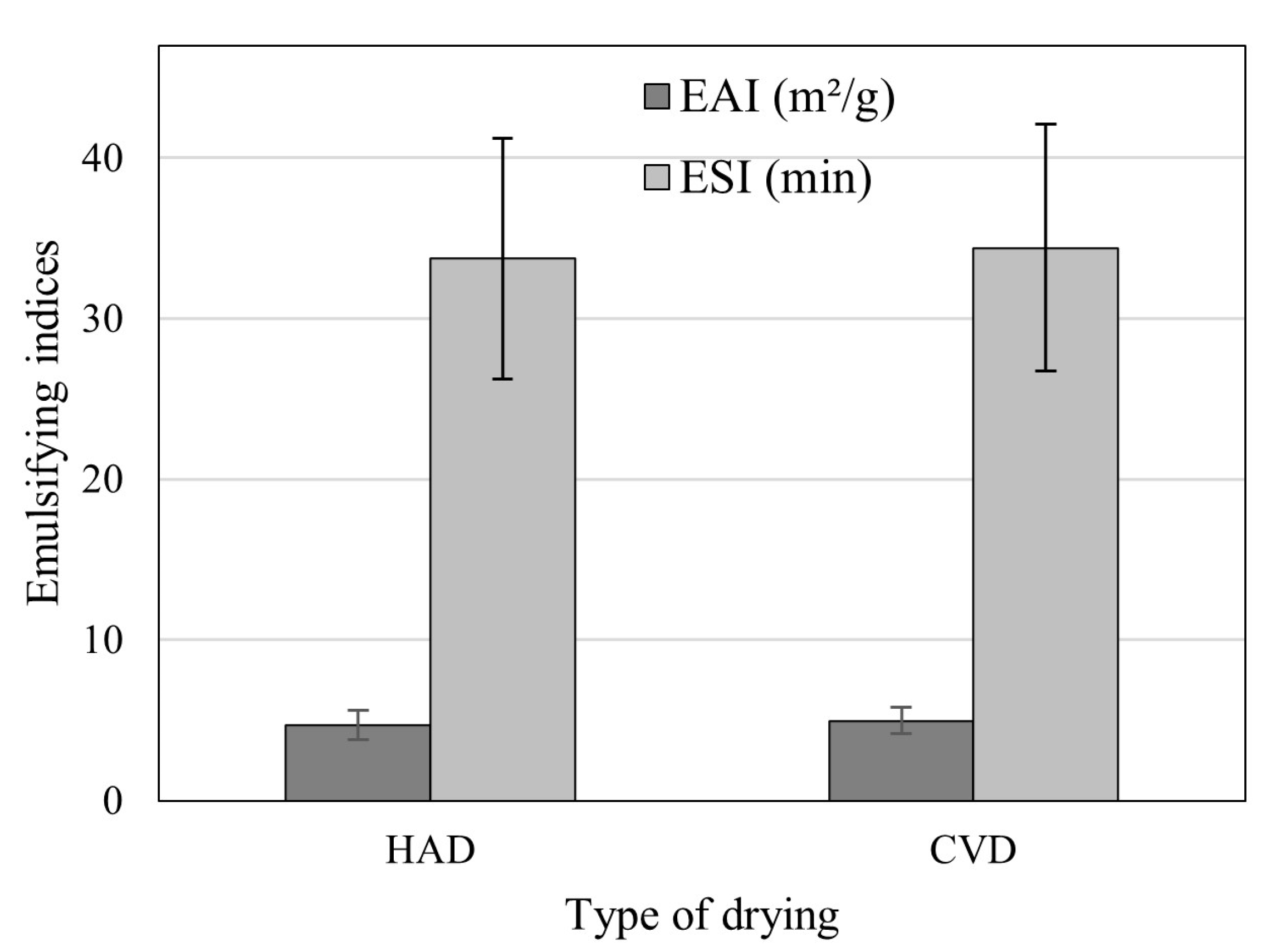
3.1. Physical and Emulsifying Properties of B. edulis POWDERS
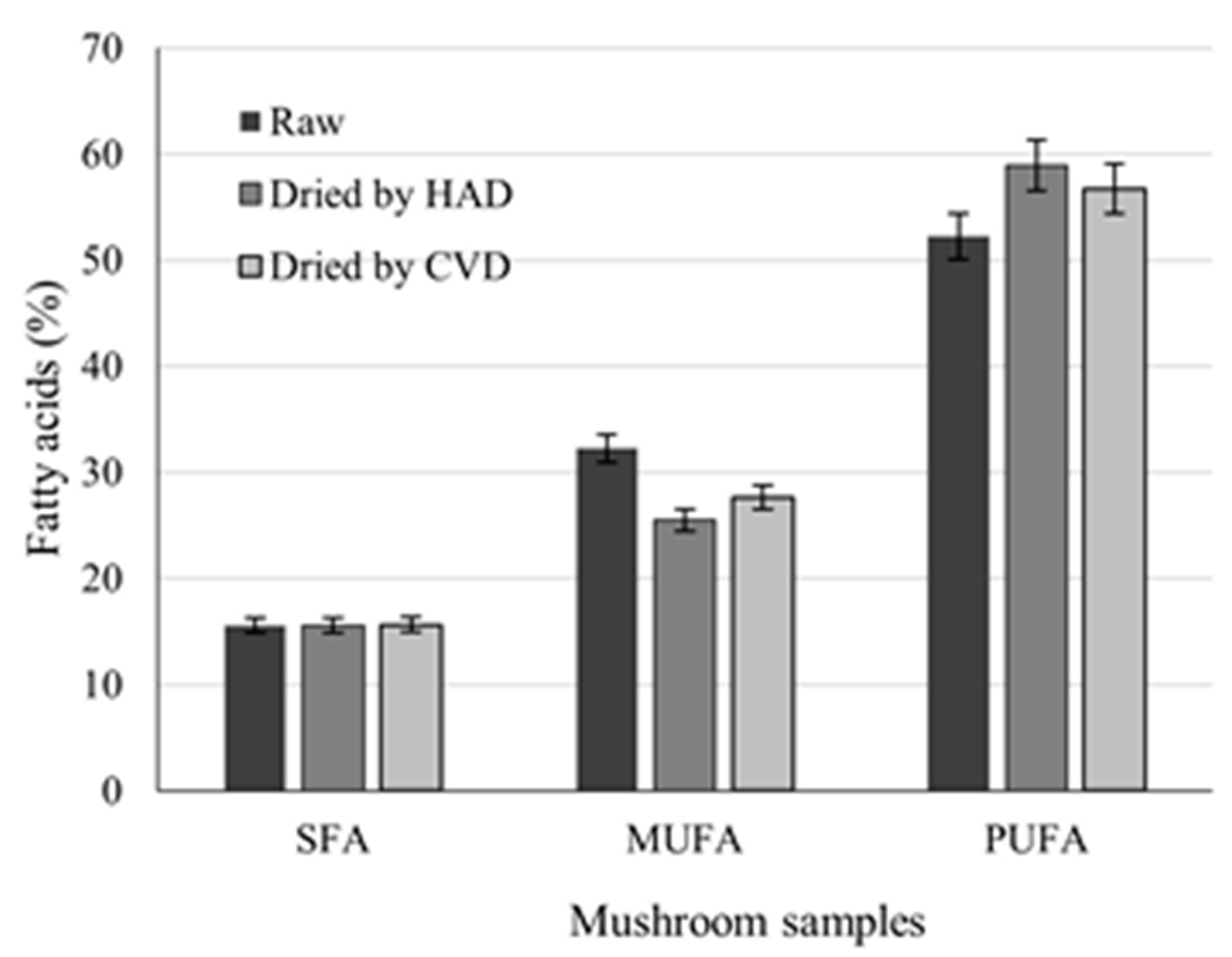
3.2. Fatty Acid Content of Raw and Dried B. edulis Mushrooms by GC–MS Analysis
3.3. Profile of Volatile Aroma Compounds in Raw and Dried B. edulis Mushrooms by HS-ITEX/GC–MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Walde, S.G.; Velu, V.; Jyothirmayi, T.; Math, R.G. Effects of pretreatments and drying methods on dehydration of mushroom. J. Food Eng. 2006, 74, 108–115. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.; Singh, G. Effect of different pretreatments on the quality of mushrooms during solar drying. J. Food Sci. Technol. 2013, 50, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zecchi, B.; Clavijo, L.; Martínez Garreiro, J.; Gerla, P. Modeling and minimizing process time of combined convective and vacuum drying of mushrooms and parsley. J. Food Eng. 2011, 104, 49–55. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Sande, D.; de Oliveira, G.P.; e Moura, M.A.F.; de Almeida Martins, B.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Vidyashri, S.; Laksminarayanan, A.; Rajeshkumar, S.; Lakshmi, T. Antioxidant and antiinflammatory activity of chitosan encapsulated omega 3-6-9. Plant. Cell. Biotechnol. Mol. Biol. 2020, 21, 69–74. [Google Scholar]
- Galán-Arriero, I.; Serrano-Muñoz, D.; Gómez-Soriano, J.; Goicoechea, C.; Taylor, J.; Velasco, A.; Ávila-Martín, G. The role of Omega-3 and Omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1629–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R. Optical purity of (R)-(-)-1-octen-3-ol in the aroma of various species of edible mushrooms. Food Chem. 2004, 86, 113–118. [Google Scholar] [CrossRef]
- Dijkstra, F.Y.; Wikén, T.O. Studies on mushroom flavours. Z. Für Leobensmittel-Unters. Und-Forsch. 1976, 160, 255–2620. [Google Scholar] [CrossRef] [PubMed]
- Pennerman, K.K.; Yin, G.; Bennett, J.W. Health effects of small volatile compounds from East Asian medicinal mushrooms. Mycobiology 2015, 43, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.; Tăușan, I.; Drăghici, O.; Soare, A.; Oancea, S. Influence of Convective and Vacuum-Type Drying on Quality, Microstructural, Antioxidant and Thermal Properties of Pretreated Boletus edulis Mushrooms. Molecules 2022, 27, 4063. [Google Scholar] [CrossRef]
- Atalar, İ.; Kurt, A.; Saricaoğlu, F.T.; Gül, O.; Gençcelep, H. Agglomerated mushroom (Agaricus bisporus) powder: Optimization of top spray fluidized bed agglomeration conditions. J. Food Process. Eng. 2021, 44, e13687. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A. Gelatinization of Corn Grits by Roll Cooking, Extrusion Cooking and Steaming. Starch Stärke 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Kantrong, H.; Tansakul, A.; Mittal, G.S. Drying characteristics and quality of shiitake mushroom undergoing microwave-vacuum drying and microwave-vacuum combined with infrared drying. J. Food Sci. Technol. 2014, 51, 3594–3608. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Zhao, H.; Zhao, M.; Cui, C.; Liu, L. Physicochemical Properties of Soy Protein Isolates-Acacia Gum Conjugates. Czech J. Food Sci. 2011, 29, 129–136. [Google Scholar] [CrossRef]
- Christie, W.W. Gas chromatography and lipids. Phytochemistry 1989, 28, 3251–3252. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcas, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J. Powder properties in food production systems. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Eds.; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 285–308. [Google Scholar] [CrossRef]
- Mishra, M.; Kandasamy, P.; Shukla, R.N.; Kumar, A. Convective Hot-air Drying of Green Mango: Influence of Hot Water Blanching and Chemical Pretreatments on Drying Kinetics and Physicochemical Properties of Dried Product. Int. J. Fruit. Sci. 2021, 21, 732–757. [Google Scholar] [CrossRef]
- Shams, R.; Singh, J.; Dash, K.K.; Dar, A.H.; Nayik, G.A.; Ansari, M.J.; Hemeg, H.A.; Ahmed, A.E.M.; Shaikh, A.M.; Kovács, B. Effect of Maltodextrin and Soy Protein Isolate on the Physicochemical and Flow Properties of Button Mushroom Powder. Front. Nutr. 2022, 9, 908570. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan Koç, G. The effect of different drying techniques and microwave finish drying on the powder properties of the red pepper powder (Capsicum annuum L.). J. Food Sci. Technol. 2020, 57, 4576–4587. [Google Scholar] [CrossRef]
- Grabowski, J.A.; Truong, V.D.; Daubert, C.R. Spray-drying of amylase hydrolyzed sweetpotato puree and physicochemical properties of powder. J. Food Sci. 2006, 71, E209–E217. [Google Scholar] [CrossRef]
- Bhandari, B.; Bansal, N.; Zhang, M.; Schuck, P. Handbook of Food Powders Processes and Properties; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Jin, M.; Xie, Y.; Xie, P.; Zheng, Q.; Wei, T.; Guo, L.; Lin, J.; Ye, Z.; Zou, Y. Physicochemical and functional properties of Pleurotus geesteranus proteins. Food Res. Int. 2022, 162, 111978. [Google Scholar] [CrossRef]
- Ashraf Khan, A.; Gani, A.; Masoodi, F.A.; Mushtaq, U.; Silotry Naik, A. Structural, rheological, antioxidant, and functional properties of β–glucan extracted from edible mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus attrimentarius. Bioact. Carbohydr. Diet. Fibre 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Gałgowska, M.M.; Pietrzak-Fiećko, R.; Gałgowska, M.; Bakuła, S. Fatty acid composition in wild Boletus edulis from Poland. Ital. J. Food Sci. 2016, 28, 402–411. [Google Scholar] [CrossRef]
- Tenyang, N.; Ponka, R.; Tiencheu, B.; Djikeng, F.T.; Womeni, H.M. Effect of Traditional Drying Methods on Proximate Composition, Fatty Acid Profile, and Oil Oxidation of Fish Species Consumed in the Far-North of Cameroon. Glob. Chall. 2020, 4, 2000007. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M. Essential polyunsaturated fatty acids, inflammation, atherosclerosis and cardiovascular diseases. In Inflammation in the Pathogenesis of Chronic Diseases. Subcellular Biochemistry; Harris, R.E., Ed.; Springer: Dordrecht, The Natherland, 2007; pp. 283–297. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Marszałkiewicz, S.; Siger, A.; Gawrysiak-Witulska, M.; Kmiecik, D.; Rudzińska, M. The effect of drying temperature of milk thistle seeds on quality and bioactive compounds in the lipid fraction. J. Food Sci. Technol. 2020, 57, 4003–4013. [Google Scholar] [CrossRef]
- Yuan, C.; Bloch, K. Conversion of Oleic Acid to Linoleic Acid. J. Biol. Chem. 1961, 236, 1277–1279. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.D.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ghafoor, K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018, 55, 190–197. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, M.; Xiao, Z.; Chen, F.; Zhu, J.; Zhu, G. Effect of fatty acids profile with thermal oxidation of chicken fat on characteristic aroma of chicken flavors assessed by gas chromatography-mass spectrometry and descriptive sensory analysis. Food Sci. Technol. Res. 2016, 22, 245–254. [Google Scholar] [CrossRef]
- Mc Gorrin, R. Character impact compounds: Flavors and off-flavors in foods. In Flavor, Fragrance, and Odor Analysis; Marsili, R., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 207–262. [Google Scholar]
- Leffingwell, J.C.; Alford, E.D. Volatile Constituents of the Giant Puffball Mushroom (Calvatia gigantea). Leffingwell Rep. 2011, 4, 1–17. [Google Scholar]
- Lu, X.; Fan, C.; He, W.; Deng, J.; Yin, H. Sulfur-containing amino acid methionine as the precursor of volatile organic sulfur compounds in algea-induced black bloom. J. Environ. Sci. 2013, 25, 33–43. [Google Scholar] [CrossRef]
- Husson, F.; Krumov, K.N.; Cases, E.; Cayot, P.; Bisakowski, B.; Kermasha, S.; Belin, J.M. Influence of medium composition and structure on the biosynthesis of the natural flavour 1-octen-3-ol by Penicillium camemberti. Process. Biochem. 2005, 40, 1395–1400. [Google Scholar] [CrossRef]
| Sample | Moisture (%) | Loose Density (g/cm3) | Tapped Density (g/cm3) | HR | Solubility (g/100 g Water) | WSI (%) | RR (g/g DM) |
|---|---|---|---|---|---|---|---|
| Dried by HAD | 6.52 | 0.885 ± 0.007 | 1.050 ± 0.037 | 1.186 | 0.266 ± 0.025 | 24.688 ± 2.670 | 4.020 ± 0.216 |
| Dried by CVD | 5.89 | 0.765 ± 0.057 | 1.047 ± 0.074 | 1.368 | 0.316 ± 0.015 | 28.525 ± 1.475 | 3.012 ± 0.441 |
| No. | Common Name of Fatty Acids (Shorthand and Omega Type) | Content (%) | ||
|---|---|---|---|---|
| Raw Sample | Dried by HAD | Dried by CVD | ||
| 1 | Caproic acid (C6:0) | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.02 ± 0.01 |
| 2 | Caprylic acid (C8:0) | 0.04 ± 0.01 | 0.14 ± 0.04 | 0.02 ± 0.01 |
| 3 | Capric acid (C10:0) | 0.04 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.01 |
| 4 | Myristic acid (C14:0) | 0.24 ± 0.02 | 0.39 ± 0.04 | 0.22 ± 0.02 |
| 5 | Pentadecanoic acid (C15:0) | 0.39 ± 0.04 | 0.39 ± 0.04 | 0.30 ± 0.03 |
| 6 | Palmitic acid (C16:0) | 11.31 ± 0.62 | 11.68 ± 0.64 | 12.49 ± 0.69 |
| 7 | Hypogeic acid (C16:1 n-9) | 0.21 ± 0.03 | 0.24 ± 0.04 | 0.22 ± 0.03 |
| 8 | Palmitoleic acid (C16:1 n-7) | 0.63 ± 0.03 | 0.69 ± 0.03 | 0.74 ± 0.03 |
| 9 | Margaric acid (C17:0) | 0.13 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.01 |
| 10 | Stearic acid (C18:0) | 2.39 ± 0.11 | 1.64 ± 0.07 | 1.75 ± 0.08 |
| 11 | Oleic acid (C18:1 n-9) | 28.75 ± 1.28 | 22.31 ± 0.99 | 23.94 ± 1.07 |
| 12 | Vaccenic acid (C18:1 n-7) | 1.95 ± 0.09 | 1.66 ± 0.07 | 2.07 ± 0.09 |
| 13 | Linoleic acid (C18:2 n-6) | 51.91 ± 2.08 | 58.52 ± 2.34 | 56.45 ± 2.26 |
| 14 | α-linolenic acid (C18:3 n-3) | 0.08 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| 15 | Arachidic acid (C20:0) | 0.34 ± 0.02 | 0.35 ± 0.04 | 0.19 ± 0.02 |
| 16 | Gondoic acid (C20:1 n-9) | 0.30 ± 0.05 | 0.00 ± 0.00 | 0.25 ± 0.04 |
| 17 | Eicosadienoic acid (C20:2 n-6) | 0.22 ± 0.02 | 0.38 ± 0.04 | 0.25 ± 0.03 |
| 18 | Behenic acid (C22:0) | 0.30 ± 0.06 | 0.42 ± 0.08 | 0.25 ± 0.05 |
| 19 | Erucic acid (C22:1 n-9) | 0.16 ± 0.03 | 0.22 ± 0.04 | 0.17 ± 0.03 |
| 20 | Lignoceric acid (C24:0) | 0.31 ± 0.03 | 0.34 ± 0.03 | 0.25 ± 0.03 |
| 21 | Nervonic acid (C24:1 n-9) | 0.23 ± 0.03 | 0.36 ± 0.05 | 0.24 ± 0.04 |
| No. | Compound | Content (%) | ||
|---|---|---|---|---|
| Raw Sample | Dried by HAD | Dried by CVD | ||
| Alcohols | ||||
| 1 | 3-Methyl-1-butanol | nd | 0.28 ± 0.04 | 0.37 ± 0.03 |
| 2 | 1,7-Octadien-3-ol | 0.03 ± 0.001 | nd | nd |
| 3 | 1-Octanol | nd | 0.16 ± 0.01 | nd |
| 4 | 1-Octen-3-ol | 70.5 ± 1.13 | 91.71 ± 1.32 | 91.25 ± 1.41 |
| 5 | €-2-Octen-1-ol | 0.04 ± 0.001 | 0.20 ± 0.01 | nd |
| 6 | (Z)-2-Octen-1-ol | 1.12 ± 0.03 | 2.48 ± 0.09 | 2.99 ± 0.03 |
| 7 | Octen-1-ol, acetate | nd | 0.22 ± 0.02 | nd |
| 8 | (Z)-3-Octen-1-ol, acetate | nd | 0.07 ± 0.001 | nd |
| Aldehydes | ||||
| 9 | Benzaldehyde | 0.20 ± 0.008 | 0.10 ± 0.001 | nd |
| 10 | Benzene acetaldehyde | nd | 0.30 ± 0.01 | 0.20 ± 0.02 |
| 11 | Dodecanal | 0.03 ± 0.001 | nd | nd |
| 12 | 2-Ethyl-2-hexenal | nd | 0.28 ± 0.03 | nd |
| 13 | 2-Ethyl-trans-2-butenal | 0.29 ± 0.01 | nd | nd |
| 14 | Heptanal | nd | 0.39 ± 0.39 | 0.41 ± 0.02 |
| 15 | Hexanal | 0.41 ± 0.01 | 0.98 ± 0.04 | 1.44 ± 0.02 |
| 16 | 2-Methyl-2-butenal | 11.88 ± 0.15 | nd | nd |
| 17 | 2-Methyl-2-hexenal | 0.12 ± 0.02 | nd | nd |
| 18 | Nonanal | 0.07 ± 0.001 | nd | nd |
| 19 | (E)-2-Octenal | 5.74 ± 0.06 | nd | nd |
| 20 | (E)-2-Pentenal | 0.16 ± 0.02 | 1.61 ± 0.02 | nd |
| Ketones | ||||
| 21 | 2-Heptanone | nd | 0.61 ± 0.02 | 0.59 ± 0.04 |
| 22 | 3-Octanone | 0.34 ± 0.05 | nd | nd |
| 23 | 1-Octen-3-one | 9.06 ± 0.11 | nd | nd |
| Others (hydrocarbons, sesquiterpenes, sulfur compounds, etc.) | ||||
| 24 | Caryophyllene | nd | 0.07 ± 0.001 | nd |
| 25 | Dimethyl disulfide | nd | nd | 1.59 ± 0.02 |
| 26 | D-Limonene | nd | 0.52 ± 0.04 | 0.35 ± 0.04 |
| 27 | 2-n-Pentyl-furan | nd | nd | 0.82 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, S.; Popa, M.; Socaci, S.A.; Dulf, F.V. Comparative Study of Raw and Dehydrated Boletus edulis Mushrooms by Hot Air and Centrifugal Vacuum Processes: Functional Properties and Fatty Acid and Aroma Profiles. Appl. Sci. 2023, 13, 3630. https://doi.org/10.3390/app13063630
Oancea S, Popa M, Socaci SA, Dulf FV. Comparative Study of Raw and Dehydrated Boletus edulis Mushrooms by Hot Air and Centrifugal Vacuum Processes: Functional Properties and Fatty Acid and Aroma Profiles. Applied Sciences. 2023; 13(6):3630. https://doi.org/10.3390/app13063630
Chicago/Turabian StyleOancea, Simona, Miruna Popa, Sonia Ancuța Socaci, and Francisc Vasile Dulf. 2023. "Comparative Study of Raw and Dehydrated Boletus edulis Mushrooms by Hot Air and Centrifugal Vacuum Processes: Functional Properties and Fatty Acid and Aroma Profiles" Applied Sciences 13, no. 6: 3630. https://doi.org/10.3390/app13063630
APA StyleOancea, S., Popa, M., Socaci, S. A., & Dulf, F. V. (2023). Comparative Study of Raw and Dehydrated Boletus edulis Mushrooms by Hot Air and Centrifugal Vacuum Processes: Functional Properties and Fatty Acid and Aroma Profiles. Applied Sciences, 13(6), 3630. https://doi.org/10.3390/app13063630









