Evaluation of the Effects of a 50 Hz Electric Field on Brain Tissue by Immunohistochemical Method, and on Blood Tissue by Biochemical, Physiological and Comet Method
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Groups
2.2. EF Creation
2.3. Histopathological Examinations
2.4. Immunohistochemical Studies
2.5. DNA Damage Determination with Comet Assay Method
3. Research Findings
3.1. Biochemical Findings
3.2. Whole Blood Findings
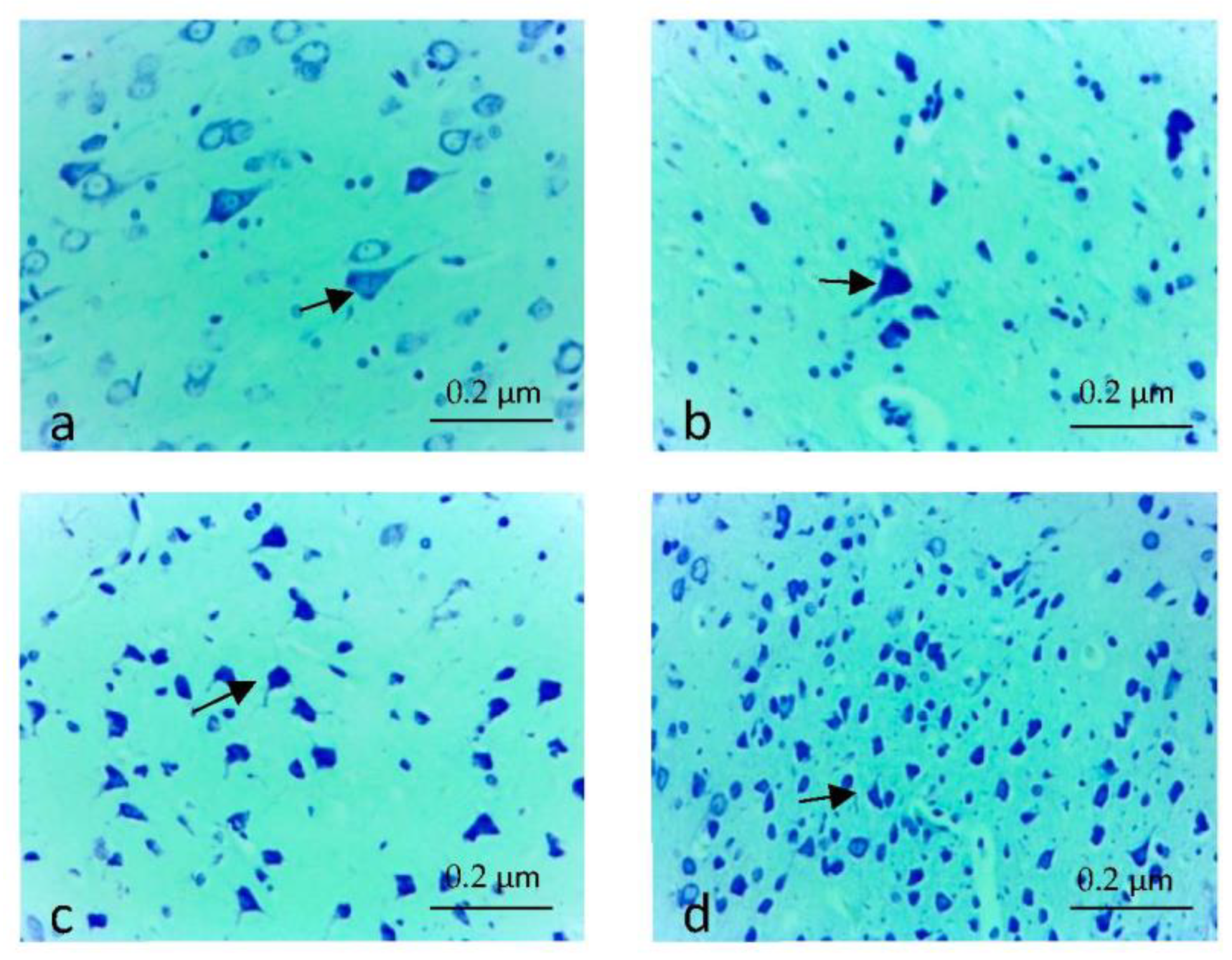
3.3. Histopathological Findings
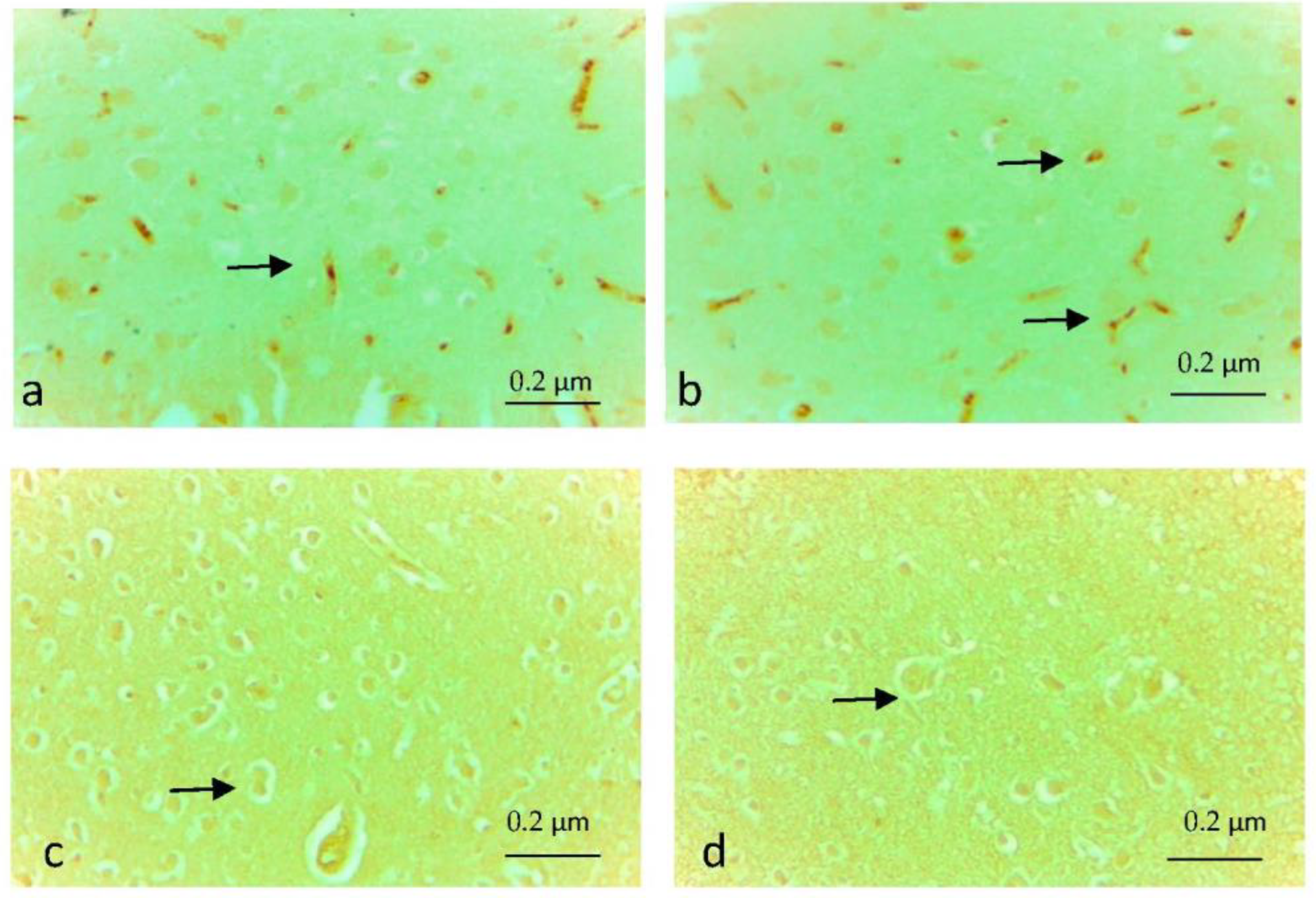
3.4. Immunohistochemical Findings
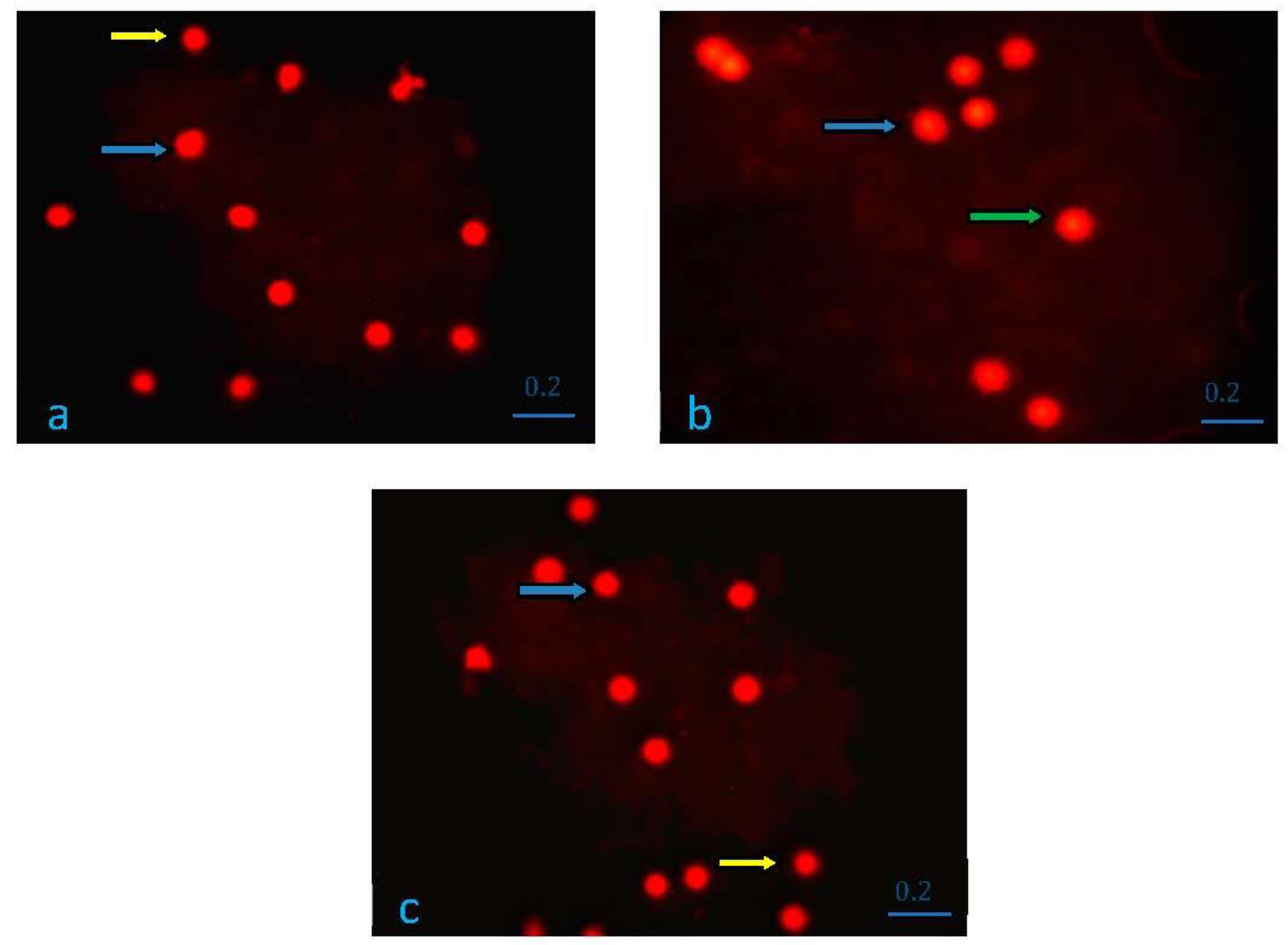
3.5. Findings of DNA Damage Determination by Comet Assay Method
3.6. Oxidant and Antioxidant Findings
4. Discussion and Conclusions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harakawa, S.; Nedachi, T.; Suzuki, H. Extremely low-frequency electric field suppresses not only induced stress response but also stress-related tissue damage in mice. Sci. Rep. 2020, 10, 20930. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Zhang, Y.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Study of electrical stimulation with different electric-field intensities in the regulation of the differentiation of PC12 cells. ACS Chem. Neurosci. 2018, 10, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Bahodirovich, N.B.; Karimovich, H.D. Assessment of behavior and biochemical parameters of blood in experimental animals under conditions of a technogenic rotating electric field. Bull. Sci. Educ. 2020, 23–2, 6–10. [Google Scholar]
- Peña, A.F.; Devine, J.; Doronin, A.; Meglinski, I. Imaging of the interaction of low-frequency electric fields with biological tissues by optical coherence tomography. Opt. Lett. 2013, 38, 2629–2631. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyn, K.; Demidov, V.; Vuong, B.; Harduar, M.K.; Sun, C.; Yang VX, D.; Doganay, O.; Toronov, V.; Xu, Y. Imaging the electro-kinetic response of biological tissues with optical coherence tomography. Opt. Lett. 2013, 38, 2572–2574. [Google Scholar] [CrossRef]
- Peña, A.F.; Doronin, A.; Tuchin, V.V.; Meglinski, I. Monitoring of interaction of low-frequency electric field with biological tissues upon optical clearing with optical coherence tomography. J. Biomed. Opt. 2014, 19, 086002. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef]
- Peng, X.; Nie, Y.; Wu, J.; Huang, Q.; Cheng, Y. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4/NF-κB signaling pathway in high-fat diet rats. Biochem. Biophys. Res. Commun. 2015, 462, 245–250. [Google Scholar] [CrossRef]
- Anderson, L.E.; Morris, J.E. Large Granular lymphocytic leukemia in rats exposed to intermittent 60 Hz magnetic fields. Bioelectromagnetics 2001, 22, 185–193. [Google Scholar] [CrossRef]
- Miller, C.J. The measurements of electric fields in live line Working. IEEE Trans. Power Appar. Syst. 1967, PAS-86, 493–498. [Google Scholar] [CrossRef]
- Takuma, T.; Kawamoto, T.; Sunaga, Y. Analysis of calibration arrangements for AC field strength meters. IEEE Trans. Power Appar. Syst. 1985, PAS-104, 489–496. [Google Scholar] [CrossRef]
- Bancroft, J.D.İ.; Steven, A.; Turner, D.R. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA; Edinburg, UK, 1996; pp. 126–129. [Google Scholar]
- Sainouchi, M.; Tada, M.; Fitrah, Y.A.; Hara, N.; Tanaka, K.; Idezuka, J.; Aida, I.; Nakajima, T.; Miyashita, A.; Akazawa, K.; et al. Brain TDP-43 pathology in corticobasal degeneration: Topographical correlation with neuronal loss. Neuropathol. Appl. Neurobiol. 2022, 48, e12786. [Google Scholar] [CrossRef]
- Higami, Y.; Shimokawa, I.; Okimoto, T.; Ikeda, T. Vulnerability to Oxygen Radicals is More İmportant than İmpaired Repair in Hepatocytic Deoxyribonucleic Acid Damage in Aging. Lab. Investig. J. Tech. Methods Pathol. 1994, 71, 650–656. [Google Scholar]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Alkis, M.E.; Akdag, M.Z.; Dasdag, S. Effects of low-intensity microwave radiation on oxidant-antioxidant parameters and DNA damage in the liver of rats. Bioelectromagnetics 2021, 42, 76–85. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics 1997, 18, 156–165. [Google Scholar] [CrossRef]
- Güler, G.; Seyhan, N.; Arıcıoğlu, A. Effects of static and 50 Hz alternating electric fields on superoxide dismutase activity and TBARS levels in guinea pigs. Gen Physiol. Biophys. 2006, 25, 177–193. [Google Scholar]
- Rezaei-Tavirani, M.; Hasanzadeh, H.; Seyyedi, S.; Ghoujeghi, F.; Semnani, V.; Zali, H. Proteomic analysis of extremely low-frequency electromagnetic Field (ELF-EMF) with different intensities in rats hippocampus. Arch. Neurosci. 2018, 5, e62954. [Google Scholar] [CrossRef]
- Türközer, Z.; Güler, G.; Seyhan, N. Effects of expoure to 50 Hz electric field at different strengths on oxidative stress and antioxidant enzyme activities in the brain tissue of guinea pigs. Int. J. Radiat. Biol. 2008, 84, 581–590. [Google Scholar] [CrossRef]
- Salford, L.G.; Brun, A.E.; Elberhardt, J.L.; Malmgren, L.; Persson BR, R. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ. Health Perspect. 2003, 111, 881–883. [Google Scholar] [CrossRef]
- Uzar, E.; Yılmaz, H.R.; Yılmaz, M.; Uz, E.; Yürekli, V.A.; Dündar, B.; Koyunuoğlu, H.R.; Çömlekçi, S. Effects of 50 Hz electric field on malondyaldehide and nitric oxide levels in spinal cord of rats at prenatal plus postnatal period. Turk. J. Med. Sci. 2011, 41, 65–72. [Google Scholar] [CrossRef]
- Falone, S.; Mirabilio, A.; Carbone, M.C.; Zimmitti, V.; Loreto, S.D.; Mariggio, M.A.; Mancinelli, R. Chronic exposure to 50 Hz magnetic fields causes a significant weakening of antioxidant defence systems in aged rat brain. Int. J. Biochem. Cell Biol. 2008, 40, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Samano, J.M.; Duran PV, T.; Oropeza MA, J.; Diaz, L.V. Effect of acute extreely low frequency electromagnetic field exposure on the antioxidant status and lipid levels in rat brain. Arch. Med. Res. 2012, 43, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Qin, Y.; Qi, L.; Fang, Q.; Zhao, L.; Chen, S.; Li, Q.; Zhang, D.; Wang, L. Juglone exerts antitumor effect in ovarian cancer cells. Iran. J. Basic Med. Sci. 2015, 18, 544–548. [Google Scholar]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Qu, X.R.; Jiang, F.Y.; Sui, D.Y. Juglone, from Juglans mandshruica maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 trought a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012, 50, 590–596. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Luo, J.; Zhao, X.; Xu, J.; Zhu, W.; Jiang, Y.; Fang, F. Proliferation inhibition and apoptosis induction of juglone on human cervical cancer caski cells. J. Hyg. Res. 2014, 43, 959–971. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]

| Biochemical | EF | EF + Juglone | Control | p Value |
|---|---|---|---|---|
| Glucose | 200.658 ± 23.155 | 241.761 ± 30.186 | 261.947 ± 11.757 | 0.164 |
| Urea | 0.108 ± 0.124 | 0.943 ± 0.012 | 0.133 ± 0.106 | 0.030 |
| AST | 167.738 ± 10.153 | 140.172 ± 7.178 | 102.586 ± 7.253 | 0.000 |
| ALT | 64.107 ± 3.810 | 61.114 ± 4.279 | 54.698 ± 5.812 | 0.155 |
| D. Bil. | 0.040 ± 0.005 | 0.0350 ± 0.003 | 0.036 ± 0.003 | 0.753 |
| İnd. Bil. | 0.068 ± 0.014 | 0.058 ± 0.008 | 0.975 ± 0.041 | 0.022 |
| BUN | 26.285 ± 1.062 | 24.714 ± 0.944 | 23.500 ± 0.779 | 0.126 |
| Whole Blood | EF | EF + Juglone | Control | p Value |
|---|---|---|---|---|
| WBC | 1.466 ± 0.817 | 1.675 ± 0.425 | 1.457 ± 0.419 | 0.956 |
| HGB | 14.666 ± 1.383 | 14.200 ± 0.192 | 13.885 ± 0.247 | 0.477 |
| HCT | 47.566 ± 5.261 | 43.550 ± 0.771 | 43.200 ± 0.922 | 0.223 |
| PLT | 430.333 ± 108.728 | 771.000 ± 45.910 | 759.000 ± 16.567 | 0.985 |
| RBC | 6.093 ± 1.766 | 8.003 ± 0.124 | 7.908 ± 0.161 | 0.053 |
| MCV | 56.700 ± 1.442 | 54.400 ± 0.380 | 54.628 ± 0.753 | 0.121 |
| MCH | 20.333 ± 2.018 | 17.737 ± 0187 | 17.585 ± 0.233 | 0.984 |
| Groups | EF | EF + Juglone | Control | p Value |
|---|---|---|---|---|
| Neuron Counts | 10.583 ± 0.633 | 17.500 ± 0.596 | 17.583 ± 0.652 | 0.000 |
| Groups | EF | EF + Juglone | Control | p Value |
|---|---|---|---|---|
| Caspase 3 immune positive cell density | 1.250 ± 0.163 | 0.125 ± 0.125 | 0.125 ± 0.125 | 0.000 |
| Tnf-α immune positive cell density | 1.250 ± 0.163 | 0.125 ± 0.125 | 0.125 ± 0.125 | 0.000 |
| Groups | Comet Score | ||
|---|---|---|---|
| Average ± SS | Ortanca (Min–Max) | p Value | |
| Group I (K) | 43.12 ± 11.60 | 43 (23–61) | 0.001 |
| Group II (EF) | 72.25 ± 17.95 | 76 (35–91) | |
| Group III (Juglone) | 33.62 ± 8.61 | 31 (23–51) | |
| Group I (K) | Group II (Electric Field) | Group III (EF + Juglone) | p | |
|---|---|---|---|---|
| MDA | 1.59 ± 0.37 | 1.62 ± 0.58 | 1.60 ± 0.62 | AD |
| CAT | 0.36 ± 0.16 | 0.33 ± 0.23 | 0.34 ± 0.27 | AD |
| SOD | 0.90 ± 0.19 | 0.86 ± 0.03 | 0.82 ± 0.25 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şenol, N.; Kaya, E.; Coşkun, Ö.; Aslankoç, R.; Çömlekçi, S. Evaluation of the Effects of a 50 Hz Electric Field on Brain Tissue by Immunohistochemical Method, and on Blood Tissue by Biochemical, Physiological and Comet Method. Appl. Sci. 2023, 13, 3276. https://doi.org/10.3390/app13053276
Şenol N, Kaya E, Coşkun Ö, Aslankoç R, Çömlekçi S. Evaluation of the Effects of a 50 Hz Electric Field on Brain Tissue by Immunohistochemical Method, and on Blood Tissue by Biochemical, Physiological and Comet Method. Applied Sciences. 2023; 13(5):3276. https://doi.org/10.3390/app13053276
Chicago/Turabian StyleŞenol, Nurgül, Erşan Kaya, Özlem Coşkun, Rahime Aslankoç, and Selçuk Çömlekçi. 2023. "Evaluation of the Effects of a 50 Hz Electric Field on Brain Tissue by Immunohistochemical Method, and on Blood Tissue by Biochemical, Physiological and Comet Method" Applied Sciences 13, no. 5: 3276. https://doi.org/10.3390/app13053276
APA StyleŞenol, N., Kaya, E., Coşkun, Ö., Aslankoç, R., & Çömlekçi, S. (2023). Evaluation of the Effects of a 50 Hz Electric Field on Brain Tissue by Immunohistochemical Method, and on Blood Tissue by Biochemical, Physiological and Comet Method. Applied Sciences, 13(5), 3276. https://doi.org/10.3390/app13053276







